Expression Analysis of a Novel Oxidoreductase Glutaredoxin 2 in Black Tiger Shrimp, Penaeus monodon
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Collection
2.3. Ammonia-N Stress
2.4. In Vivo Experiment
2.5. Extraction of RNA and Synthesis of cDNA
2.6. Cloning the Full-Length cDNA of PmGrx2
2.7. mRNA Expression by Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. Ammonia-N Stress on PmGrx2-Interfered Shrimps
2.9. Correlation Analysis between the SNPs of PmGrx2 and Ammonia-N-Stress-Tolerance Trait
3. Results
3.1. Identification and Characterization of the PmGrx2 Nucleotide Sequence
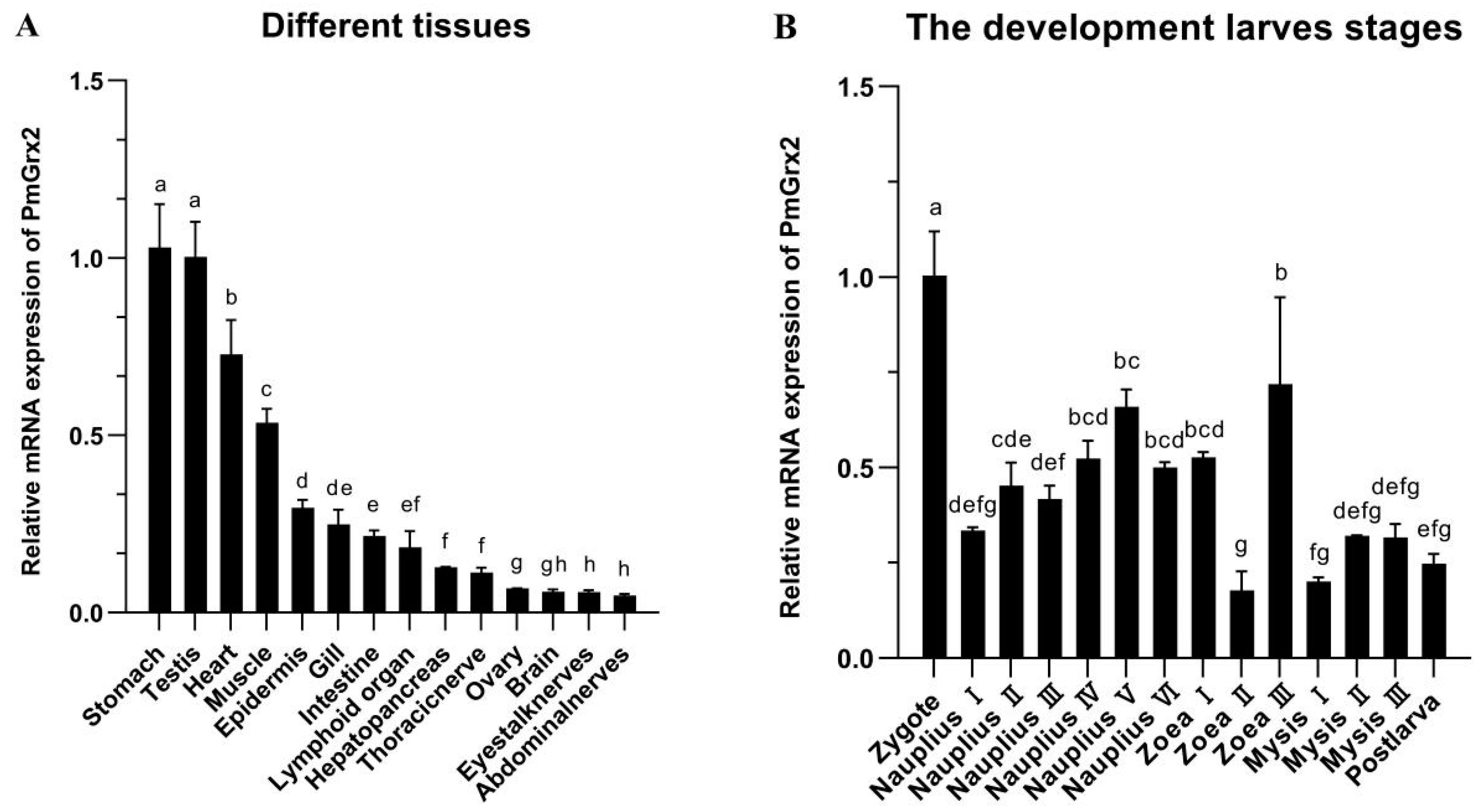
3.2. mRNA Expression of PmGrx2 in Different Tissues and Developmental Stage
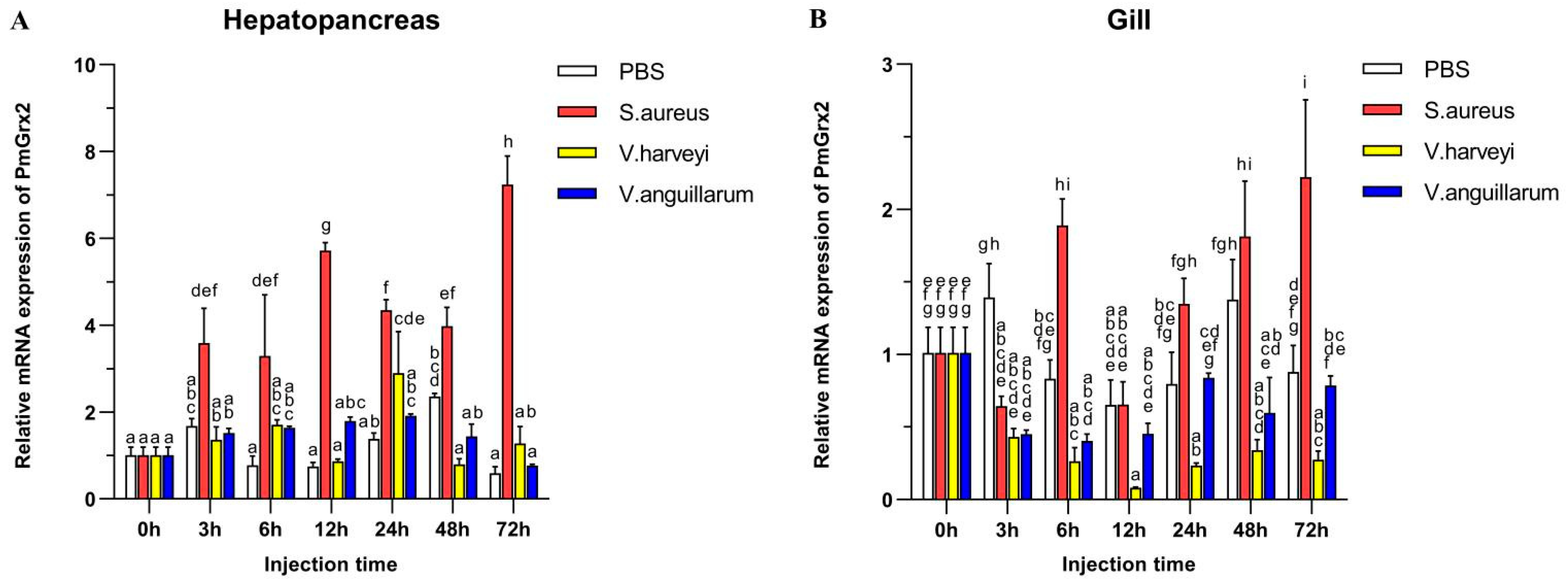
3.3. Exploration of PmGrx2 Transcription of Hepatopancreas and Gills on the Heels of Bacterial Challenge
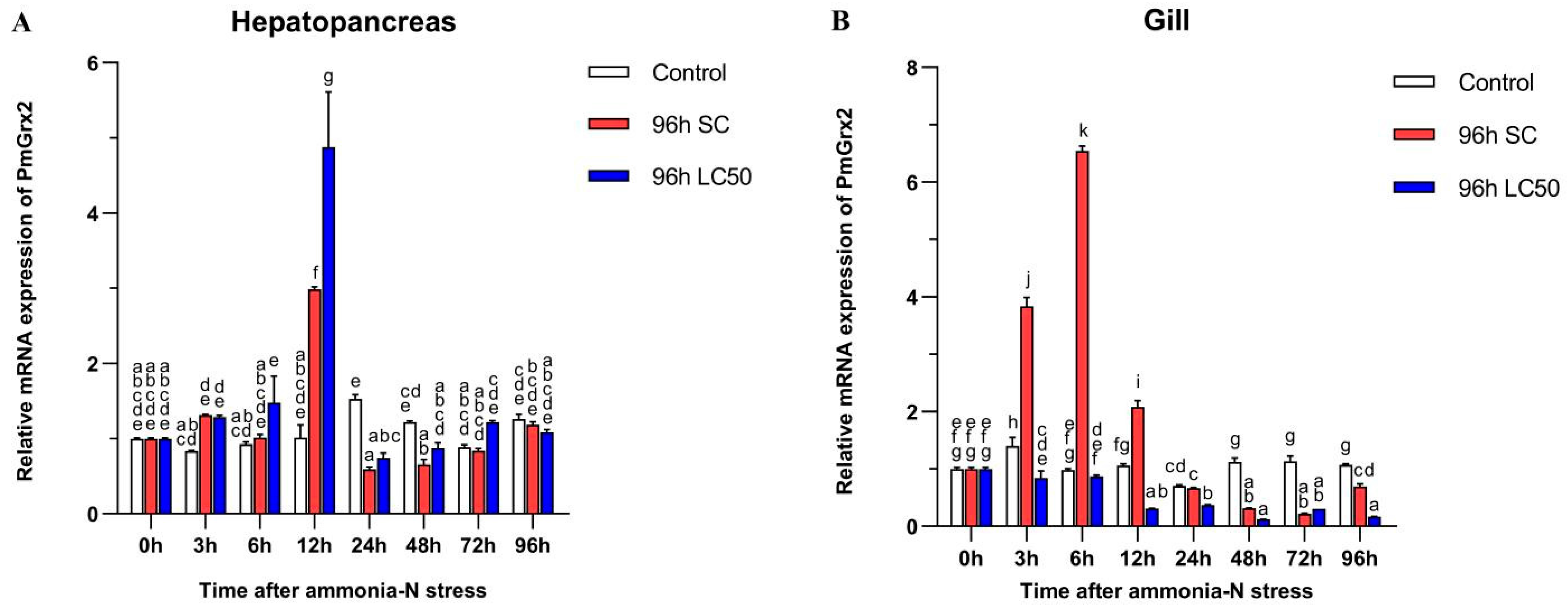
3.4. Exploration of PmGrx2 Transcription of Hepatopancreas and Gill under Ammonia-N Stress
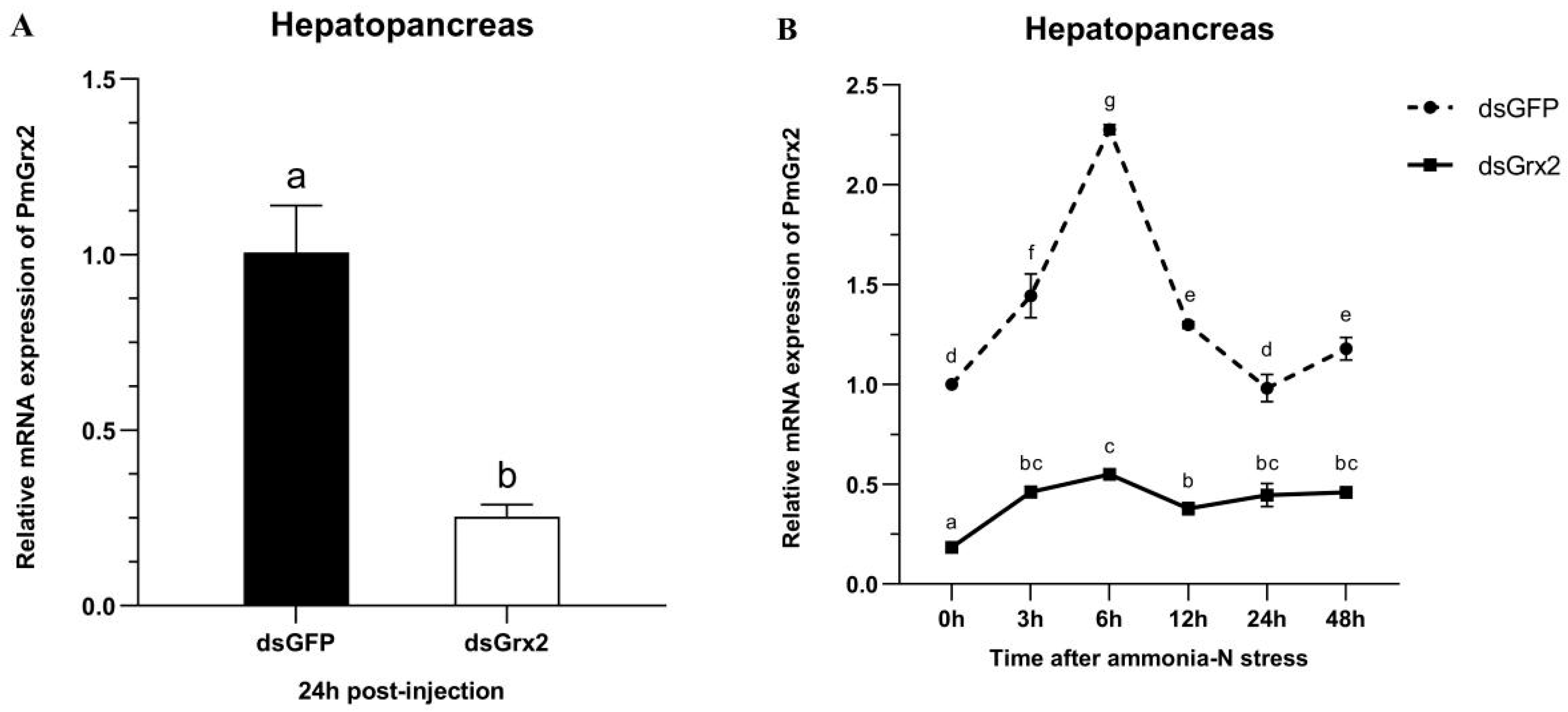
3.5. The Interference Efficiency of dsGrx2 in the Hepatopancreas
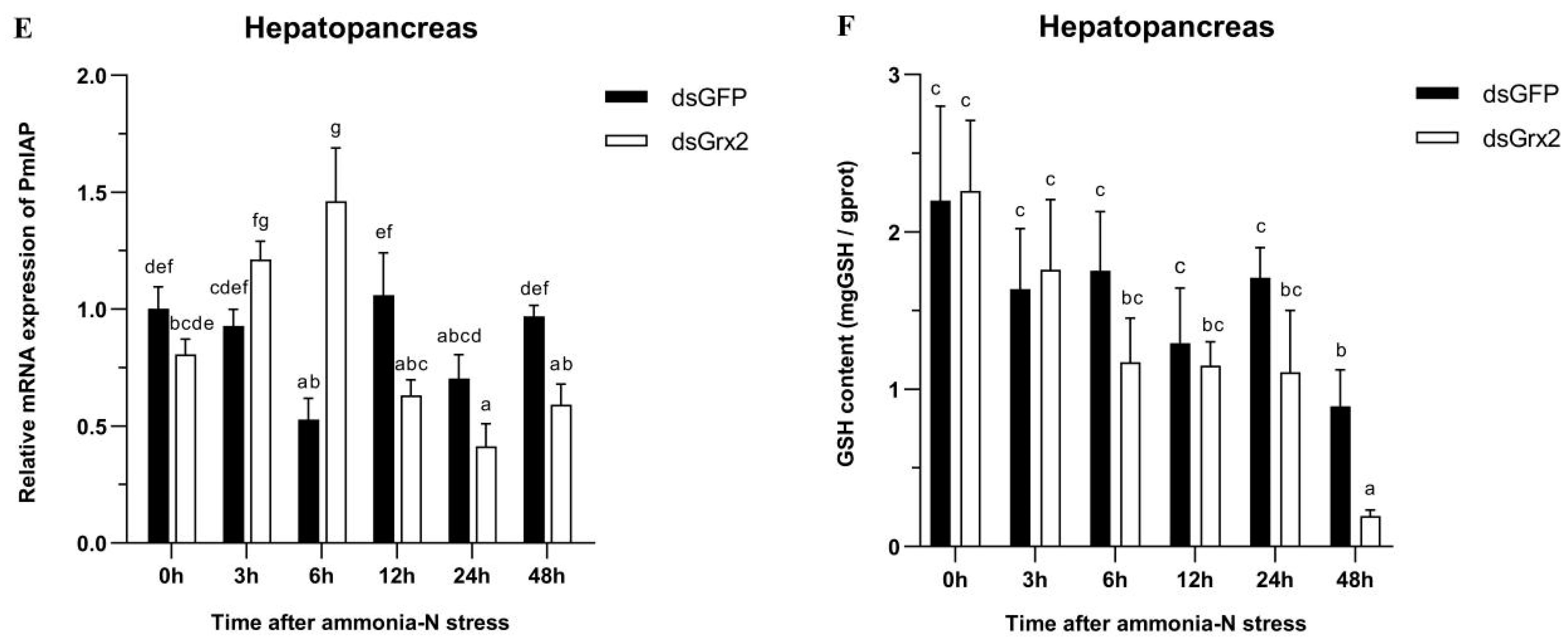
3.6. GSH Content and mRNA Expression of Related Genes in the Hepatopancreas of dsRNA-Injected Shrimps under Ammonia-N Stress
3.7. Correlation Analysis between the SNPs of PmGrx2 and Ammonia-N-Stress-Tolerance Trait
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmgren, A.; Johansson, C.; Berndt, C.; Lönn, M.E.; Hudemann, C.; Lillig, C.H. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem. Soc. Trans. 2005, 33, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.P.; Fernandes, P.A.; Ramos, M.J. Similarities and differences in the thioredoxin superfamily. Prog. Biophys. Mol. Biol. 2006, 91, 229–248. [Google Scholar] [CrossRef]
- Kapoor, D.; Sharma, R.; Handa, N.; Kaur, H.; Rattan, A.; Yadav, P.; Gautam, V.; Kaur, R.; Bhardwaj, R. Redox homeostasis in plants under abiotic stress: Role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front. Environ. Sci. 2015, 3, 13. [Google Scholar] [CrossRef]
- Holmgreen, A. Glutathione dependent synthesis of deoxyribonucleotides. J. Biol. Chem. 1979, 254, 3622–3638. [Google Scholar] [CrossRef]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 2008, 1780, 1304–1317. [Google Scholar] [CrossRef]
- Mondal, S.; Kumar, V.; Singh, S.P. Phylogenetic distribution and structural analyses of cyanobacterial glutaredoxins (Grxs). Comput. Biol. Chem. 2020, 84, 107141. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, W.; Chen, P.; Yuan, X.; Li, X.; Zhang, T.; Chen, S. Identification and expression analyzes of CC-type glutaredoxin in cucumber (Cucumis sativus L.) under abiotic stress. Sci. Hortic. 2021, 289, 110417. [Google Scholar] [CrossRef]
- Bräutigam, L.; Johansson, C.; Kubsch, B.; McDonough, M.A.; Bill, E.; Holmgren, A.; Berndt, C. An unusual mode of iron-sulfur-cluster coordination in a teleost glutaredoxin. Biochem. Biophys. Res. Commun. 2013, 436, 491–496. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Holmgren, A. Glutaredoxins: Glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid. Redox Signal. 2004, 6, 63–74. [Google Scholar] [CrossRef]
- Murata, H.; Ihara, Y.; Nakamura, H.; Yodoi, J.; Sumikawa, K.; Kondo, T. Glutaredoxin Exerts an Antiapoptotic Effect by Regulating the Redox State of Akt. J. Biol. Chem. 2003, 278, 50226–50233. [Google Scholar] [CrossRef]
- Fan, R.; Jiang, S.; Li, Y.; Yang, Q.; Jiang, S.; Huang, J.; Yang, L.; Chen, X.; Zhou, F. Molecular Characterization and Expression Analysis of Glutaredoxin 5 in Black Tiger Shrimp (Penaeus monodon) and Correlation Analysis Between the SNPs of PmGrx5 and Ammonia-N Stress Tolerance Trait. Front. Mar. Sci. 2022, 9, 909827. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Huang, J.; Yang, L.; Jiang, S.; Yang, Q.; He, J.; Jiang, S. Transcriptome reveals involvement of immune defense, oxidative imbalance, and apoptosis in ammonia-stress response of the black tiger shrimp (Penaeus monodon). Fish Shellfish. Immunol. 2018, 83, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Gürel, T. The Larval Development of Penaeus semisulcatus (de Hann, 1850) (Decapoda: Penaeidae). J. Fish. Aquat. Sci. 2005, 22, 195–199. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Su, T.; Zhou, F.; Yang, L.; Huang, J. The toxicity of ammonia-N on Penaeus monodon and immune parameters. J. Shanghai Ocean. Univ. 2012, 21, 358–362. [Google Scholar]
- Zhou, K.; Zhou, F.; Huang, J.; Yang, Q.; Jiang, S.; Qiu, L.; Yang, L.; Zhu, C.; Jiang, S. Characterization and Expression Analysis of a Chitinase Gene (PmChi-4) From Black Tiger Shrimp (Penaeus monodon) Under Pathogen Infection and Ambient Ammonia Nitrogen Stress. Fish Shellfish. Immunol. 2017, 62, 31–40. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, S.; Huang, J.; Zhou, F.; Yang, Q.; Jiang, S.; Yang, L. C-type lectin response to bacterial infection and ammonia nitrogen stress in tiger shrimp (Penaeus monodon). Fish Shellfish. Immunol. 2019, 90, 188–198. [Google Scholar] [CrossRef]
- Dunnen, J.; Dalgleish, R.; Maglott, D.; Hart, R.; Greenblatt, M.; Jordan, J.; Roux, A.; Smith, T.; Antonarakis, S.; Taschner, P.E.M. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef]
- Malik, W.A.; Wang, X.; Wang, X.; Shu, N.; Cui, R.; Chen, X.; Wang, D.; Lu, X.; Yin, Z.; Wang, J.; et al. Genome-wide expression analysis suggests glutaredoxin genes response to various stresses in cotton. Int. J. Biol. Macromol. 2020, 153, 470–491. [Google Scholar] [CrossRef]
- Lisy, S.; Rothamel, K.; Ascano, M. RNA Binding Proteins as Pioneer Determinants of Infection: Protective, Proviral, or Both? Viruses 2021, 13, 2172. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Shi, X.; Wang, C.; Wang, F.; Fan, J.; Shen, F.; Xu, J.; Bao, W.; Liu, M.; et al. Critical role of bacterial isochorismatase in the autophagic process induced by Acinetobacter baumannii in mammalian cells. FASEB J. 2016, 30, 3563–3577. [Google Scholar] [CrossRef]
- Maghames, C.M.; Lobato-Gil, S.; Perrin, A.; Trauchessec, H.; Rodriguez, M.S.; Urbach, S.; Marin, P.; Xirodimas, D.P. NEDDylation promotes nuclear protein aggregation and protects the Ubiquitin Proteasome System upon proteotoxic stress. Nat. Commun. 2018, 9, 4376. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Knaus, U.G. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 2018, 11, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Goroshinskaya, I.; Surikova, E.; Frantsiyants, E.; Neskubina, I.; Pogorelova, Y.; Medvedeva, D.; Maslov, A.; Kit, O. Redox forms of glutathione mark the aggressiveness of stomach cancer. Ann. Oncol. 2018, 29, v4. [Google Scholar] [CrossRef]
- Lönn, M.E.; Hudemann, C.; Berndt, C.; Cherkasov, V.; Capani, F.; Holmgren, A.; Lillig, C.H. Expression pattern of human glutaredoxin 2 isoforms: Identification and characterization of two testis/cancer cell-specific isoforms. Antioxid. Redox Signal. 2008, 10, 547–557. [Google Scholar] [CrossRef]
- Fujii, J.; Iuchi, Y.; Matsuki, S.; Ishii, T. Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J. Androl. 2003, 3, 231–242. [Google Scholar]
- Beinert, H. Iron-sulfur proteins: Ancient structures, still full of surprises. J. Biol. Inorg. Chem. 2000, 5, 2–15. [Google Scholar] [CrossRef]
- Bräutigam, L.; Jensen, L.D.E.; Poschmann, G.; Nyström, S.; Bannenberg, S.; Dreij, K.; Lepka, K.; Prozorovski, T.; Montano, S.J.; Aktas, O.; et al. Glutaredoxin regulates vascular development by reversible glutathionylation of sirtuin 1. Proc. Natl. Acad. Sci. USA 2013, 110, 20057–20062. [Google Scholar] [CrossRef]
- Berndt, C.; Poschmann, G.; Stühler, K.; Holmgren, A.; Bräutigam, L. Zebrafish heart development is regulated via glutaredoxin 2 dependent migration and survival of neural crest cells. Redox Biol. 2014, 2, 673–678. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Xuan, J.Y.; McBride, S.; Maharsy, W.; Thorn, S.; Holterman, C.E.; Kennedy, C.R.J.; Rippstein, P.; deKemp, R.; da Silva, J.; et al. Glutaredoxin-2 is required to control oxidative phosphorylation in cardiac muscle by mediating deglutathionylation reactions. J. Biol. Chem. 2014, 289, 14812–14828. [Google Scholar] [CrossRef]
- Bräutigam, L.; Schütte, L.D.; Godoy, J.R.; Prozorovski, T.; Gellert, M.; Hauptmann, G.; Holmgren, A.; Lillig, C.H.; Berndt, C. Vertebrate-specific glutaredoxin is essential for brain development. Proc. Natl. Acad. Sci. USA 2011, 108, 20532–20537. [Google Scholar] [CrossRef]
- Rőszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.J.; Tsui, T.K.N. Ammonia toxicity in fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, F.; Ling, R.; Liao, S.; Miao, Y.; Ye, C.; Wang, A. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, H.; Liu, B.; Zhu, K.; Guo, L.; Liu, B.; Zhang, N.; Yang, J.; Jiang, S.; Zhang, D. Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κb signaling pathway in golden pompano (Trachinotus ovatus). Ecotoxicol. Environ. Saf. 2021, 222, 112504. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Jiang, S.; Li, Y.; Yang, Q.; Jiang, S.; Yang, L.; Huang, J.; Zhou, F. Comprehensive expression analysis of the beta integrin from Penaeus monodon indicating its participation in innate immunity and ammonia nitrogen stress response. Fish Shellfish. Immunol. 2019, 98, 887–898. [Google Scholar] [CrossRef]
- Zheng, P.; Wang, L.; Wang, A.; Zhang, X.; Ye, J.; Wang, D.; Sun, J.; Li, J.; Lu, Y.; Xian, J. cDNA cloning and expression analysis of glutaredoxin (Grx) 2 in the Pacific white shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2018, 86, 662–671. [Google Scholar] [CrossRef]
- Trenzado, C.; Hidalgo, M.C.; García-Gallego, M.; Morales, A.E.; Furné, M.; Domezain, A.; Domezain, J.; Sanz, A. Antioxidant enzymes and lipid peroxidation in sturgeon Acipenser naccarii and trout Oncorhynchus mykiss. A comparative study. Aquaculture 2005, 254, 758–767. [Google Scholar] [CrossRef]
- Hu, W.; Yao, C. Molecular and immune response characterizations of a novel AIF and cytochrome c in Litopenaeus vannamei defending against WSSV infection. Fish Shellfish. Immunol. 2016, 56, 84–95. [Google Scholar] [CrossRef]
- Zhu, J.; Du, R.; Liu, Q.; Luo, L.; Lin, S.; Zhang, H.; Chen, Y. Transcriptome analysis provides insights into the molecular mechanism of hepatocyte apoptosis in response to feeding restriction in juvenile largemouth bass Micropterus salmoides. Aquaculture 2022, 548, 737500. [Google Scholar] [CrossRef]
- Chaney, J.L.; Clark, P.L. Roles for Synonymous Codon Usage in Protein Biogenesis. Annu. Rev. Biophys. 2015, 44, 143–166. [Google Scholar] [CrossRef]
- Chen, K.; Guo, R.; Wei, C.C. Synonymous mutation rs2515641 affects CYP2E1 mRNA and protein expression and susceptibility to drug-induced liver injury. Pharmacogenomics 2020, 21, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Cheng, Y.Y.; Liu, S.C.; Xu, Y.X.; Gao, Y.; Wang, C.L.; Wang, Z.G.; Feng, T.Q.; Lu, G.H.; Song, J.; et al. A synonymous mutation in IGF-1 impacts the transcription and translation process of gene expression. Mol. Ther.-Nucleic Acids 2021, 26, 1446–1465. [Google Scholar] [CrossRef] [PubMed]

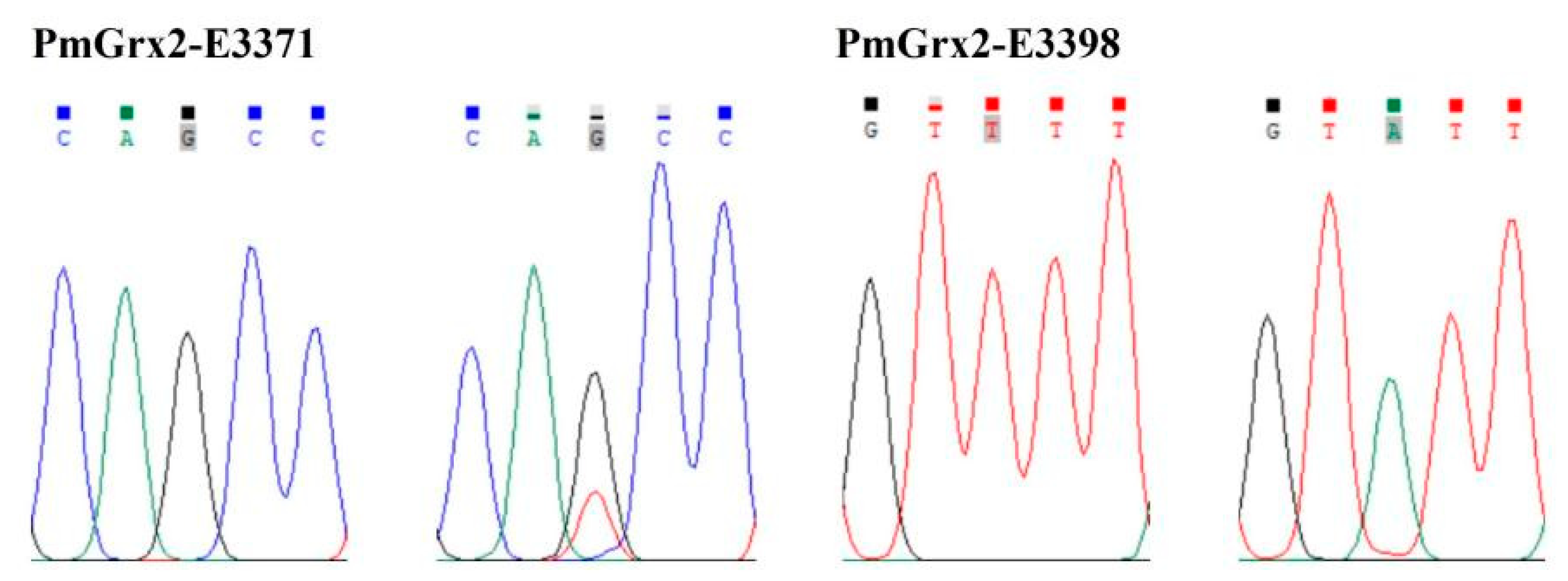
| SNPs | Position | Type of Base Mutation | Type of Protein Mutation |
|---|---|---|---|
| PmGrx2-E3371 | Exon 3 (371 bp) | c.192G > T | Mm p.Gln64His |
| PmGrx2-E3398 | Exon 3 (398 bp) | c.219T > A | Sm p.Val73= |
| SNPs | Ho | He | Ne | MAF | PIC | HWE |
|---|---|---|---|---|---|---|
| PmGrx2-E3371 | 0.0125 | 0.0124 | 1.0126 | 0.0063 | 0.0123 | 0.9984 |
| PmGrx2-E3398 | 0.0000 | 0.0722 | 1.0778 | 0.0375 | 0.0696 | 0.0000 |
| SNPs | Genotype | Genotype Frequencies | χ2 Value | p-Value | |
|---|---|---|---|---|---|
| Sensitivity | Resistance | ||||
| PmGrx2-E3371 | GG | 1.0000 | 0.9750 | 2.0006 | 0.1572 |
| TG | 0.0000 | 0.0250 | |||
| PmGrx2-E3398 | AA | 0.0750 | 0.0000 | 6.0163 | 0.0142 * |
| TT | 0.9250 | 1.0000 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Li, Y.; Yang, Q.; Jiang, S.; Huang, J.; Yang, L.; Chen, X.; Zhou, F.; Jiang, S. Expression Analysis of a Novel Oxidoreductase Glutaredoxin 2 in Black Tiger Shrimp, Penaeus monodon. Antioxidants 2022, 11, 1857. https://doi.org/10.3390/antiox11101857
Fan R, Li Y, Yang Q, Jiang S, Huang J, Yang L, Chen X, Zhou F, Jiang S. Expression Analysis of a Novel Oxidoreductase Glutaredoxin 2 in Black Tiger Shrimp, Penaeus monodon. Antioxidants. 2022; 11(10):1857. https://doi.org/10.3390/antiox11101857
Chicago/Turabian StyleFan, Rui, Yundong Li, Qibin Yang, Song Jiang, Jianhua Huang, Lishi Yang, Xu Chen, Falin Zhou, and Shigui Jiang. 2022. "Expression Analysis of a Novel Oxidoreductase Glutaredoxin 2 in Black Tiger Shrimp, Penaeus monodon" Antioxidants 11, no. 10: 1857. https://doi.org/10.3390/antiox11101857
APA StyleFan, R., Li, Y., Yang, Q., Jiang, S., Huang, J., Yang, L., Chen, X., Zhou, F., & Jiang, S. (2022). Expression Analysis of a Novel Oxidoreductase Glutaredoxin 2 in Black Tiger Shrimp, Penaeus monodon. Antioxidants, 11(10), 1857. https://doi.org/10.3390/antiox11101857





