Insight into the Mechanism of Salt-Induced Oxidative Stress Tolerance in Soybean by the Application of Bacillus subtilis: Coordinated Actions of Osmoregulation, Ion Homeostasis, Antioxidant Defense, and Methylglyoxal Detoxification
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Stress Treatments
2.2. Measurement of Root Length, Shoot Length, and Stem Diameter
2.3. Estimation of Fresh Weight and Dry Weight
2.4. Quantification of Photosynthetic Pigments
2.5. Estimation of Leaf Relative Water Content
2.6. Determination of Proline Content
2.7. Estimation of Electrolyte Leakage
2.8. Assessment of Hydrogen Peroxide Content
2.9. Quantification of Malondialdehyde Content
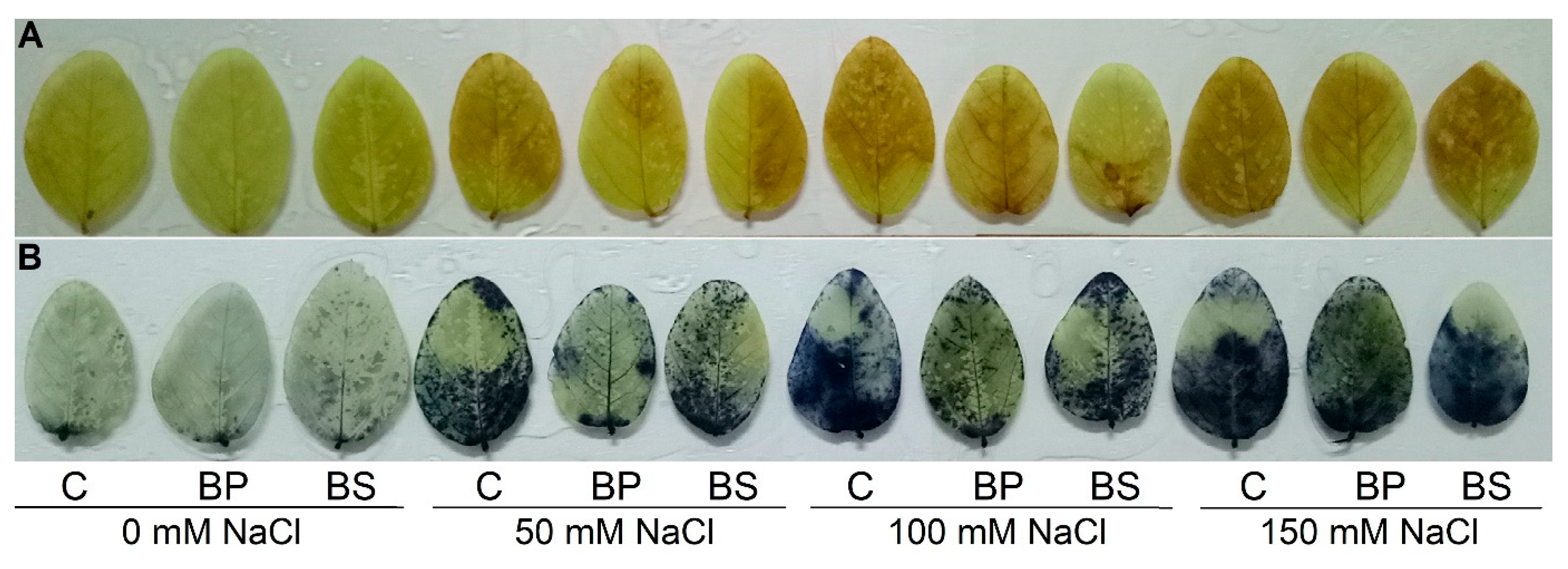
2.10. Histochemical Detection of Hydrogen Peroxide and Superoxide Anion
2.11. Estimation of Methylglyoxal Content
2.12. Quantification of Na+, K+, and Ca2+ Content
2.13. Determination of Ascorbate and Glutathione Content
2.14. Extraction of Enzyme and Estimation of Free Protein
2.15. Determination of Enzymatic Activities
2.16. Statistical Analysis
3. Results
3.1. Plant Growth and Biomass
3.2. Photosynthetic Pigments
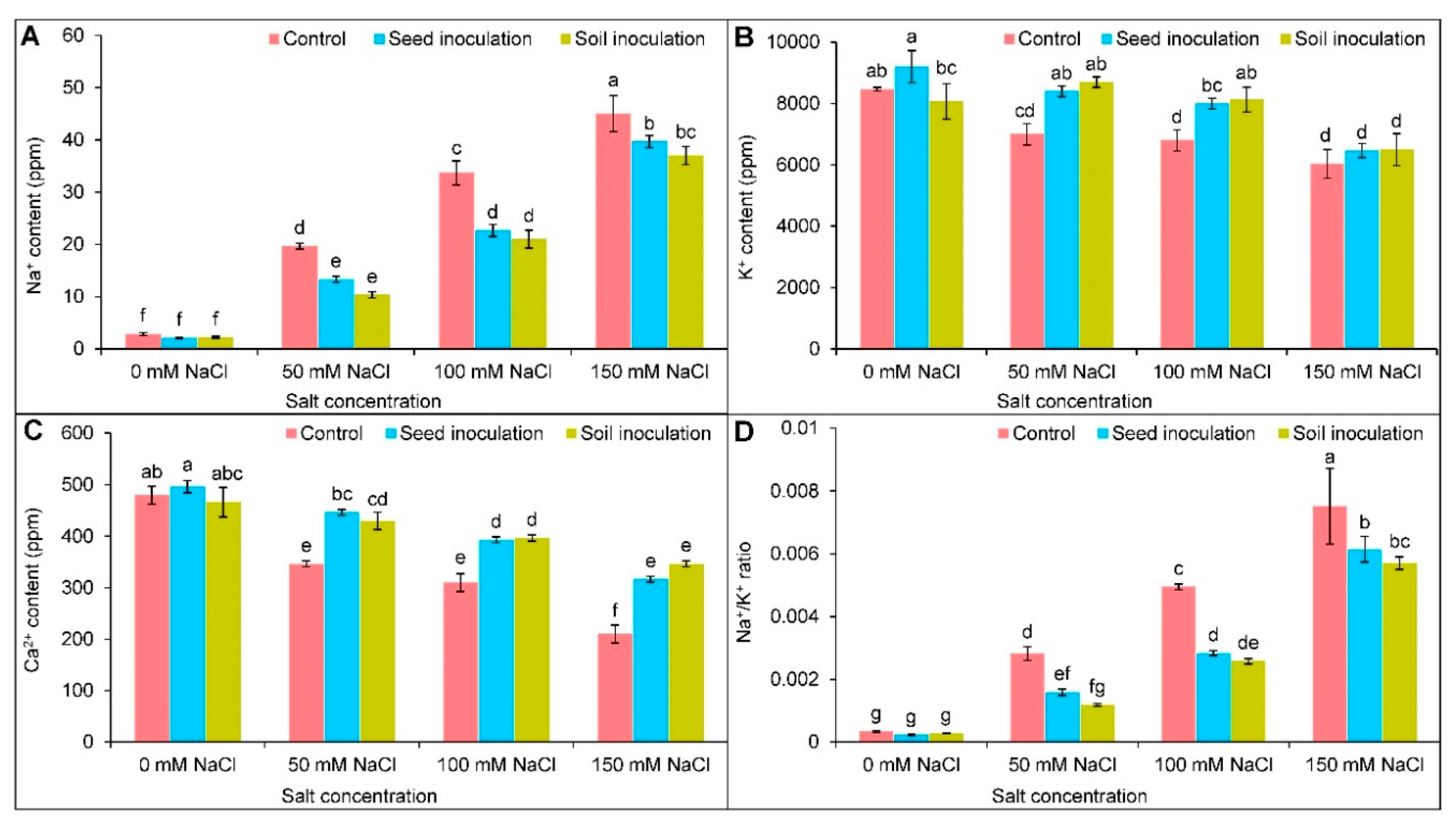
3.3. Ion Contents
3.4. Physiological Attributes
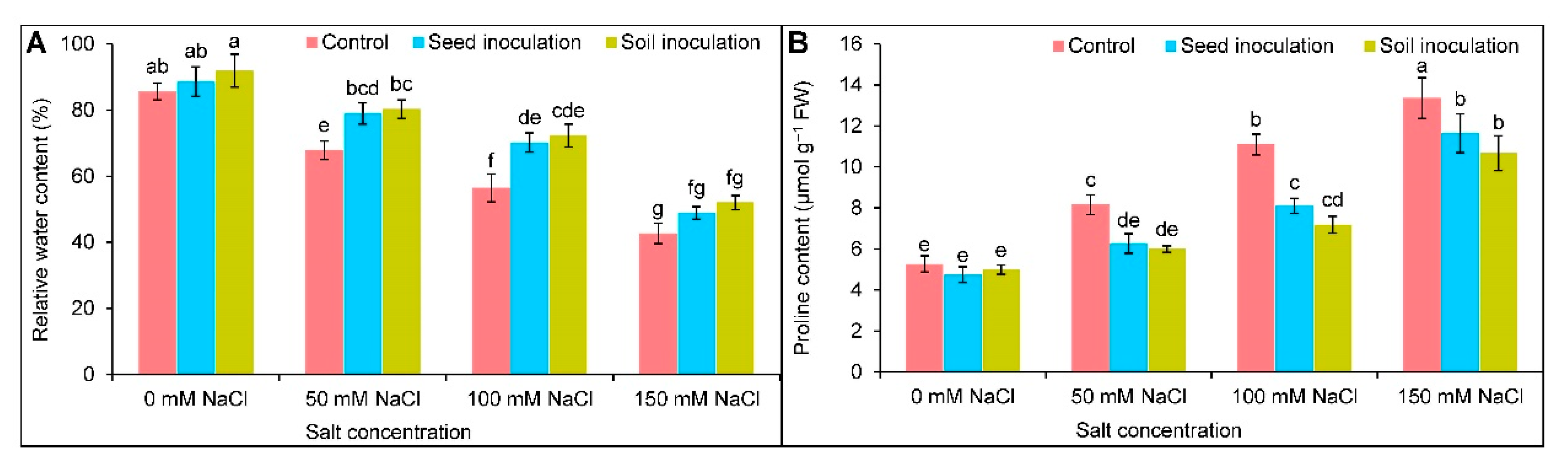
3.4.1. Relative Water Content
3.4.2. Proline Content
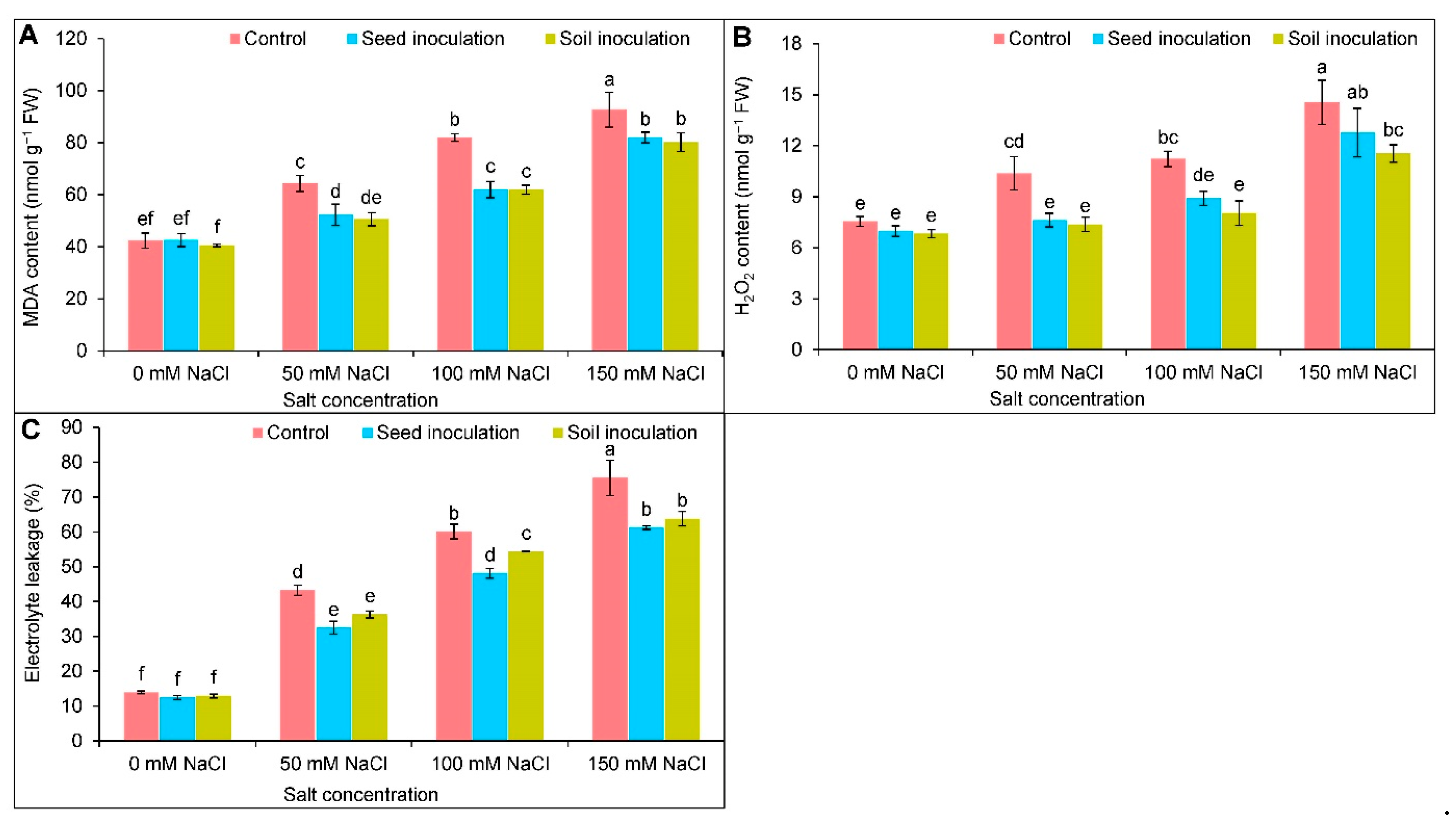
3.5. Oxidative Stress Indicators
3.6. Ascorbate-Glutathione Pool
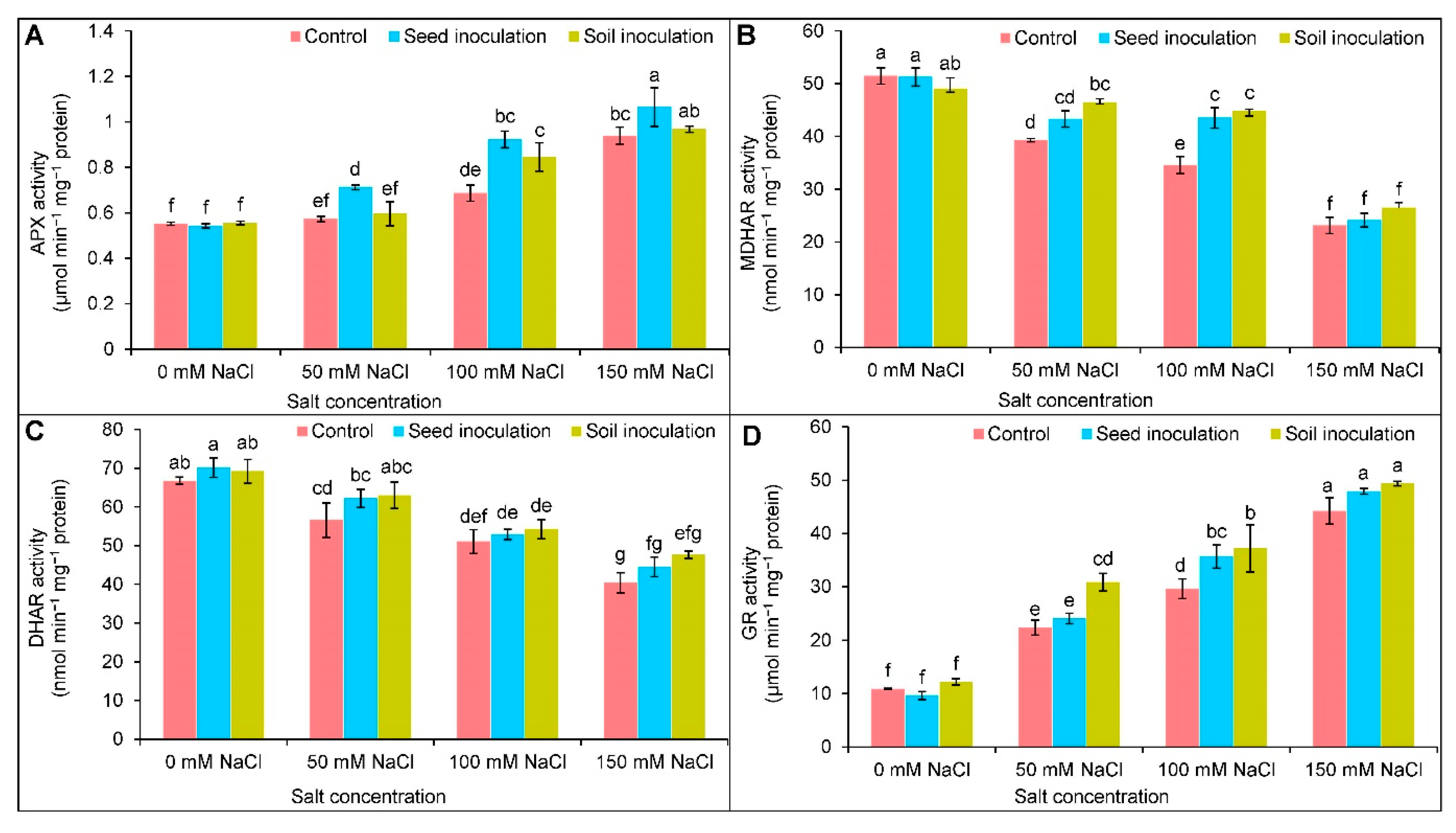
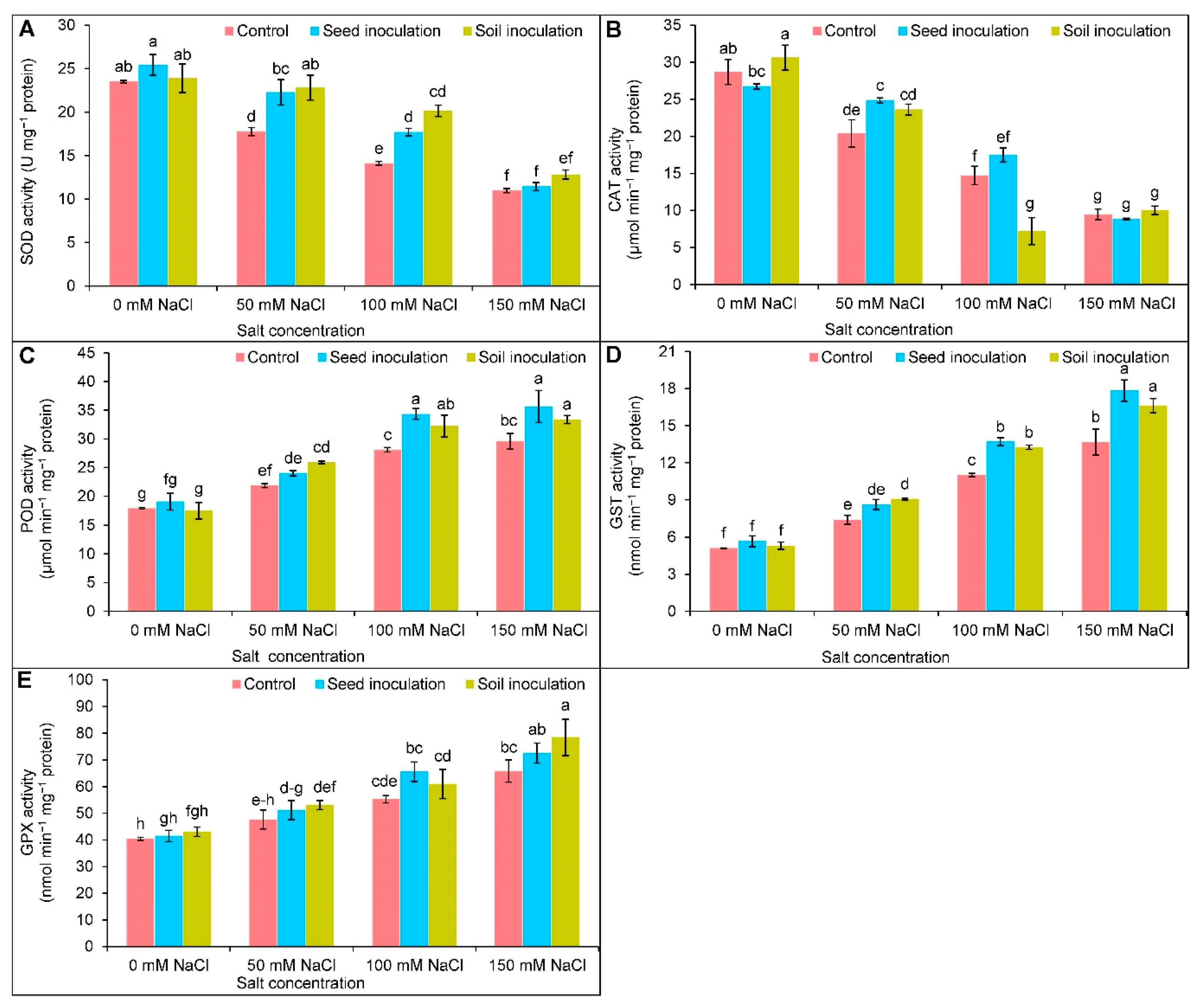
3.7. Antioxidant Enzymes Activities
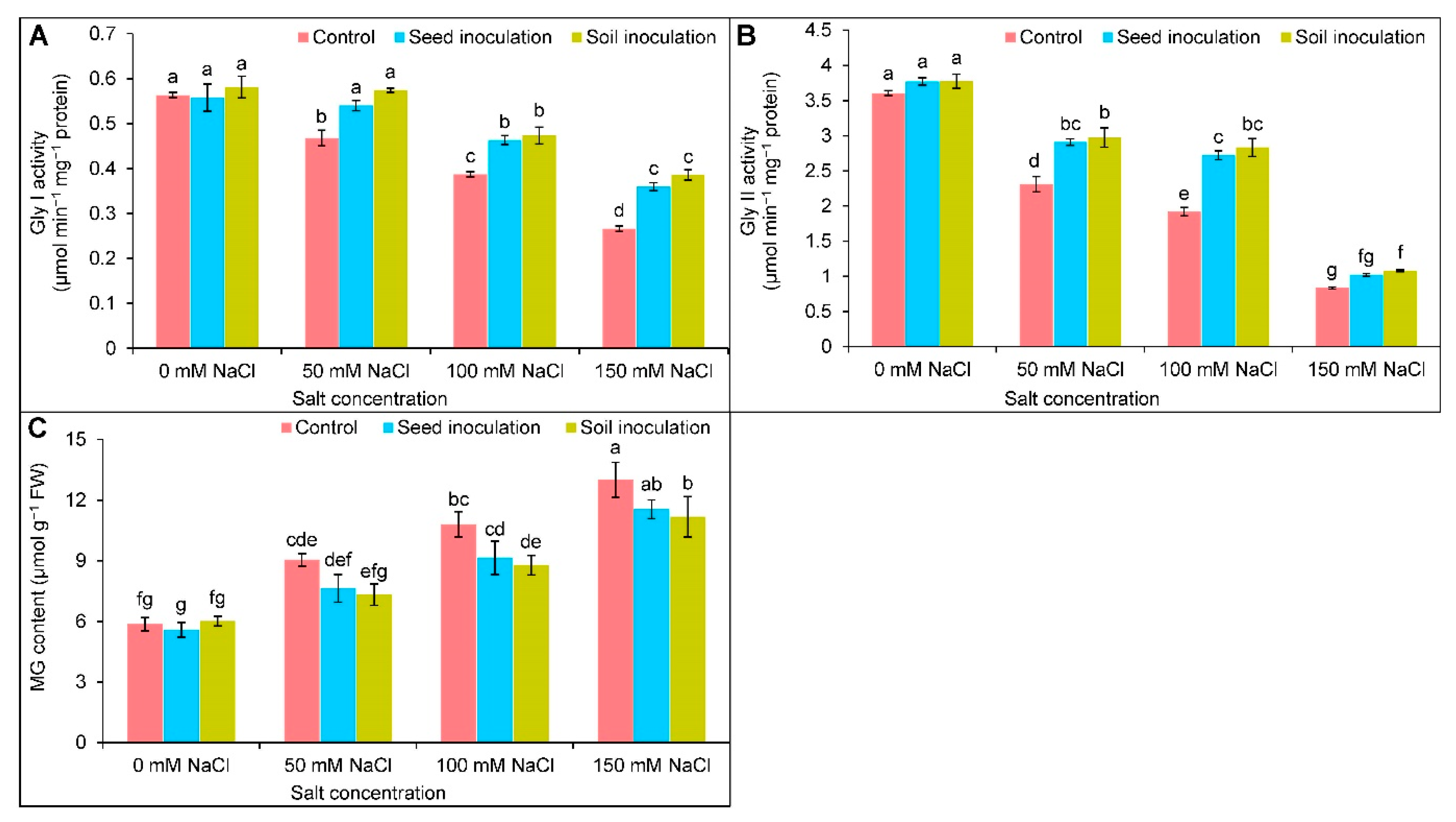
3.8. Glyoxalase System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity inhibits rice seed germination by reducing α-amylase activity via decreased bioactive gibberellin content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Roman, V.J.; den Toom, L.A.; Gamiz, C.C.; van der Pijl, N.; Visser, R.G.F.; van Loo, E.N.; Gerard van der Linden, C. Differential responses to salt stress in ion dynamics, growth and seed yield of European quinoa varieties. Environ. Exp. Bot. 2020, 177, 104146. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Saad, A.M.; El-Saadony, M.T.; Merwad, A.R.M.; Rady, M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020, 30, 101878. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Khojah, E.; Samra, B.N.; Fujita, M.; Nahar, K. Biochar and chitosan regulate antioxidant defense and methylglyoxal detoxification systems and enhance salt tolerance in jute (Corchorus olitorius L.). Antioxidants 2021, 10, 2017. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuaĉ, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 9, 384. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Perreault, R.; Laforest-Lapointe, I. Plant-microbe interactions in the phyllosphere: Facing challenges of the anthropocene. ISME J. 2022, 16, 339–345. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Khan, M.A.; Hamayun, M.; Asaf, S.; Khan, M.; Yun, B.-W.; Kang, S.-M.; Lee, I.-J. Rhizospheric Bacillus spp. rescues plant growth under salinity stress via regulating gene expression, endogenous hormones, and antioxidant system of Oryza sativa L. Front. Plant Sci. 2021, 12, 665590. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, F.; Mickan, B.; Wang, D.; Wang, W. Physiological, proteomic, and metabolomic analysis provide insights into Bacillus sp.-mediated salt tolerance in wheat. Plant Cell Rep. 2022, 41, 95–118. [Google Scholar] [CrossRef]
- Baek, D.; Rokibuzzaman, M.; Khan, A.; Kim, M.C.; Park, H.J.; Yun, D.-J.; Chung, Y.R. Plant-growth promoting Bacillus oryzicola YC7007 modulates stress-response gene expression and provides protection from salt stress. Front. Plant Sci. 2020, 10, 1646. [Google Scholar] [CrossRef]
- Shultana, R.; Zuan, A.T.K.; Yusop, M.R.; Saud, H.M.; El-Shehawi, A.M. Bacillus tequilensis strain ‘UPMRB9’ improves biochemical attributes and nutrient accumulation in different rice varieties under salinity stress. PLoS ONE 2021, 16, e0260869. [Google Scholar] [CrossRef]
- Maslennikova, D.; Lastochkina, O. Contribution of ascorbate and glutathione in endobacteria Bacillus subtilis-mediated drought tolerance in two Triticum aestivum L. genotypes contrasting in drought sensitivity. Plants 2021, 10, 2557. [Google Scholar] [CrossRef]
- Misra, S.; Chauhan, P.S. ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar]
- Ji, C.; Tian, H.; Wang, X.; Song, X.; Ju, R.; Li, H.; Gao, Q.; Li, C.; Zhang, P.; Li, J.; et al. Bacillus subtilis HG-15, a halotolerant rhizoplane bacterium, promotes growth and salinity tolerance in wheat (Triticum aestivum). BioMed Res. Int. 2022, 2022, 9506227. [Google Scholar] [CrossRef]
- BARI (Bangladesh Agricultural Research Institute). Krishi Projukti Hatboi; Bangladesh Agricultural Research Institute: Gazipur, Bangladesh, 2021. (In Bengali) [Google Scholar]
- Arnon, D.T. Copper enzymes in isolated chloroplasts. Polyphenoloxidase. Beta Vulgaris. J. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teari, D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Hemeda, H.M.; Klein, B.P. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Elia, A.C.; Galarini, R.; Taticchi, M.I.; Dorr, A.J.M.; Mantilacci, L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol. Environ. Saf. 2003, 55, 162–167. [Google Scholar] [CrossRef]
- Principato, G.B.; Rosi, G.; Talesa, V.; Govannini, E.; Uolila, L. Purification and characterization of two forms of glyoxalase II from rat liver and brain of Wistar rats. Biochim. Biophys. Acta 1987, 911, 349–355. [Google Scholar] [CrossRef]
- CoStat. CoStat-Statistics Software, Version 6.400; CoHort Software: Monterey, CA, USA, 2008.
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Munns, R. Plant adaptations to salt and water stress: Differences and commonalities. Adv. Bot. Res. 2011, 57, 1–32. [Google Scholar]
- Bhise, K.K.; Bhagwat, P.K.; Dandge, P.B. Plant growth-promoting characteristics of salt tolerant Enterobacter cloacae strain KBPD and its efficacy in amelioration of salt stress in Vigna radiata L. J. Plant Growth Regul. 2017, 36, 215–226. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef]
- Kubi, H.A.A.; Khan, M.A.; Adhikari, A.; Imran, M.; Kang, S.-M.; Hamayun, M.; Lee, I.-J. Silicon and plant growth-promoting rhizobacteria Pseudomonas psychrotolerans CS51 mitigates salt stress in Zea mays L. Agriculture 2021, 11, 272. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, C.; Feng, Q.; Liou, R.-M.; Lin, Y.-F.; Qiao, J.; Lu, Y.; Chang, Y. The mechanisms of sodium chloride stress mitigation by salt-tolerant plant growth promoting rhizobacteria in wheat. Agronomy 2022, 12, 543. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Adhikari, A.; Al-Sadi, A.M.; Kang, S.-M.; Kim, L.-R.; Lee, I.-J. Rhizospheric Bacillus amyloliquefaciens protects Capsicum annuum cv. Geumsugangsan from multiple abiotic stresses via multifarious plant growth-promoting attributes. Front. Plant Sci. 2021, 12, 669693. [Google Scholar] [CrossRef]
- Kao, W.Y.; Tsai, T.T.; Shih, C.N. Photosynthetic gas exchange and chlorophyll a fluorescence of three wild soybean species in response to NaCl treatments. Photosynthetica 2003, 41, 415–419. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef]
- Ansari, M.; Shekari, F.; Mohammadi, M.H.; Juhos, K.; Végvári, G.; Biró, B. Salt-tolerant plant growth-promoting bacteria enhanced salinity tolerance of salt-tolerant alfalfa (Medicago sativa L.) cultivars at high salinity. Acta Physiol. Plant. 2019, 41, 195. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: A review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef]
- Yasmin, H.; Naeem, S.; Bakhtawar, M.; Jabeen, Z.; Nosheen, A.; Naz, R.; Keyani, R.; Mumtaz, S.; Hassan, M.N. Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L.) against salinity stress. PLoS ONE 2020, 15, e0231348. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Shultana, R.; Zuan, A.T.K.; Yusop, M.R.; Saud, H.M.; Ayanda, A.F. Effect of salt-tolerant bacterial inoculations on rice seedlings differing in salt-tolerance under saline soil conditions. Agronomy 2020, 10, 1030. [Google Scholar] [CrossRef]
- Barragán, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; De Luca, A.; Fernández, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef]
- Ghosh, D.; Gupta, A.; Mohapatra, S.A. Comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World J. Microbiol. Bioethanol. 2019, 35, 90. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. A halotolerant bacterium Bacillus licheniformis HSW-16 augments induced systemic tolerance to salt stress in wheat plant (Triticum aestivum). Front Plant Sci. 2016, 7, 1890. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Yang, Y. Germination, growth, osmotic adjustment and ionic balance of wheat in response to saline and alkaline stresses. Soil Sci. Plant Nutr. 2009, 55, 667–679. [Google Scholar] [CrossRef]
- Sahile, A.A.; Khan, M.A.; Hamayun, M.; Imran, M.; Kang, S.-M.; Lee, I.-J. Novel Bacillus cereus strain, ALT1, enhance growth and strengthens the antioxidant system of soybean under cadmium stress. Agronomy 2021, 11, 404. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Hasanuzzaman, M.; Khan, M.I.R.; Nahar, K.; Fujita, M. β-Aminobutyric acid pretreatment confers salt stress tolerance in Brassica napus L. by modulating reactive oxygen species metabolism and methylglyoxal detoxification. Plants 2020, 9, 241. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Puthiyottil, P.; Akkara, Y. Pre treatment with Bacillus subtilis mitigates drought induced photo-oxidative damages in okra by modulating antioxidant system and photochemical activity. Physiol. Mol. Biol. Plants 2021, 27, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S.H. An introduction to antioxidants and their roles in plant stress tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 1–23. [Google Scholar]
- Rafiq, K.; Akram, M.S.; Shahid, M.; Qaisar, U.; Rashid, N. Enhancement of salt tolerance in maize (Zea mays L.) using locally isolated Bacillus sp. SR-2-1/1. Biologia 2020, 75, 1425–1436. [Google Scholar] [CrossRef]
- Soliman, M.H.; Abdulmajeed, A.M.; Alhaithloul, H.; Alharbi, B.M.; El-Esawi, M.A.; Hasanuzzaman, M.; Elkelish, A. Saponin biopriming positively stimulates antioxidants defense, osmolytes metabolism and ionic status to confer salt stress tolerance in soybean. Acta Physiol. Plant. 2020, 42, 114. [Google Scholar] [CrossRef]
- Khan, M.L.; Bilal, S.; Halo, B.A.; Al-Harrasi, A.; Khan, A.R.; Waqas, M.; Al-Thani, G.S.; Al-Amri, I.; Al-Rawahi, A.; Lee, I.-J. Bacillus amyloliquefaciens BSL16 improves phytoremediation potential of Solanum lycopersicum during copper stress. J. Plant Interact. 2017, 12, 550–559. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Thangappan, S.; Uthandi, S. Plant growth-promoting Bacillus sp. cahoots moisture stress alleviation in rice genotypes by triggering antioxidant defense system. Microbiol. Res. 2020, 239, 126518. [Google Scholar] [CrossRef] [PubMed]


| Salt Concentration (NaCl) | Bacterial Inoculation | Shoot Length (cm) | Root Length (cm) | Stem Diameter (mm) | Root FW (g Plant−1) | Shoot FW (g Plant−1) | Root DW (mg Plant−1) | Shoot DW (mg Plant−1) |
|---|---|---|---|---|---|---|---|---|
| 0 mM | Control | 36.95 ± 0.50 ab | 9.36 ± 0.26 ab | 1.87 ± 0.05 ab | 0.29 ± 0.01 a | 3.79 ± 0.11 ab | 55.4 ± 1.61 a | 735.8 ± 13.42 a |
| Seed inoculation | 39.52 ± 1.6 a | 9.64 ± 0.76 ab | 1.95 ± 0.14 a | 0.31 ± 0.02 a | 4.10 ± 0.18 a | 56.4 ± 2.78 a | 770.4 ± 39.14 a | |
| Soil inoculation | 39.80 ± 1.53 a | 10.43 ± 0.82 a | 1.95 ± 0.06 a | 0.31 ± 0.01 a | 3.99 ± 0.07 ab | 53.2 ± 1.97 a | 770.0 ± 25.43 a | |
| 50 mM | Control | 31.94 ± 2.94 bcd | 7.9 ± 0.22 cde | 1.71 ± 0.10 abc | 0.17 ± 0.01 de | 2.76 ± 0.17 e | 30.7 ± 0.38 cd | 379.2 ± 17.48 def |
| Seed inoculation | 35.35 ± 1.55 abc | 8.67 ± 0.50 bcd | 1.99 ± 0.13 a | 0.20 ± 0.012 bc | 3.19 ± 0.21 d | 35.0 ± 2.14 c | 478.3 ± 24.80 bc | |
| Soil inoculation | 36.25 ± 2.75 ab | 9.76 ± 0.86 ab | 1.83 ± 0.03 abc | 0.21 ± 0.01 b | 3.62 ± 0.08 bc | 41.0 ± 0.90 b | 542.2 ± 48.00 b | |
| 100 mM | Control | 28.23 ± 2.01 de | 7.22 ± 0.45 de | 1.56 ±0.01 c | 0.14 ± 0.01 ef | 2.06 ± 0.14 f | 23.0 ± 1.77 ef | 335.0 ± 13.26 efg |
| Seed inoculation | 34.98 ± 1.10 abc | 8.31 ± 0.60 bcd | 1.81 ± 0.03 abc | 0.19 ± 0.01 bcd | 2.90 ± 0.15 de | 32.4 ± 0.56 cd | 385.6 ± 20.15 de | |
| Soil inoculation | 34.46 ± 2.33 abc | 9.14 ± 0.77 abc | 1.82 ± 0.03 abc | 0.21 ± 0.01 bc | 3.26 ± 0.22 cd | 34.8 ± 3.02 c | 446.3 ± 23.11 cd | |
| 150 mM | Control | 25.75 ± 1.23 e | 6.63 ± 0.43 e | 1.23 ± 0.24 d | 0.12 ± 0.01 f | 1.01 ± 0.05 g | 22.1 ± 0.45 f | 196.6 ± 8.83 h |
| Seed inoculation | 30.47 ± 1.55 cde | 7.75 ± 0.27 cde | 1.60 ± 0.06 bc | 0.18 ± 0.01 cd | 1.74 ± 0.08 f | 27.8 ± 0.58 de | 294.5 ± 17.15 g | |
| Soil inoculation | 30.56 ± 1.52 cde | 8.6 ± 0.20 bcd | 1.54 ± 0.07 c | 0.16 ± 0.01 de | 1.69 ± 0.04 f | 31.0 ± 2.05 cd | 307.8 ± 27.30 fg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanuzzaman, M.; Raihan, M.R.H.; Nowroz, F.; Fujita, M. Insight into the Mechanism of Salt-Induced Oxidative Stress Tolerance in Soybean by the Application of Bacillus subtilis: Coordinated Actions of Osmoregulation, Ion Homeostasis, Antioxidant Defense, and Methylglyoxal Detoxification. Antioxidants 2022, 11, 1856. https://doi.org/10.3390/antiox11101856
Hasanuzzaman M, Raihan MRH, Nowroz F, Fujita M. Insight into the Mechanism of Salt-Induced Oxidative Stress Tolerance in Soybean by the Application of Bacillus subtilis: Coordinated Actions of Osmoregulation, Ion Homeostasis, Antioxidant Defense, and Methylglyoxal Detoxification. Antioxidants. 2022; 11(10):1856. https://doi.org/10.3390/antiox11101856
Chicago/Turabian StyleHasanuzzaman, Mirza, Md. Rakib Hossain Raihan, Farzana Nowroz, and Masayuki Fujita. 2022. "Insight into the Mechanism of Salt-Induced Oxidative Stress Tolerance in Soybean by the Application of Bacillus subtilis: Coordinated Actions of Osmoregulation, Ion Homeostasis, Antioxidant Defense, and Methylglyoxal Detoxification" Antioxidants 11, no. 10: 1856. https://doi.org/10.3390/antiox11101856
APA StyleHasanuzzaman, M., Raihan, M. R. H., Nowroz, F., & Fujita, M. (2022). Insight into the Mechanism of Salt-Induced Oxidative Stress Tolerance in Soybean by the Application of Bacillus subtilis: Coordinated Actions of Osmoregulation, Ion Homeostasis, Antioxidant Defense, and Methylglyoxal Detoxification. Antioxidants, 11(10), 1856. https://doi.org/10.3390/antiox11101856











