Determination of Maximum Oil Yield, Quality Indicators and Absorbance Spectra of Hulled Sunflower Seeds Oil Extraction under Axial Loading
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Determination of Moisture Content
2.2. Determination of Sample Oil Content
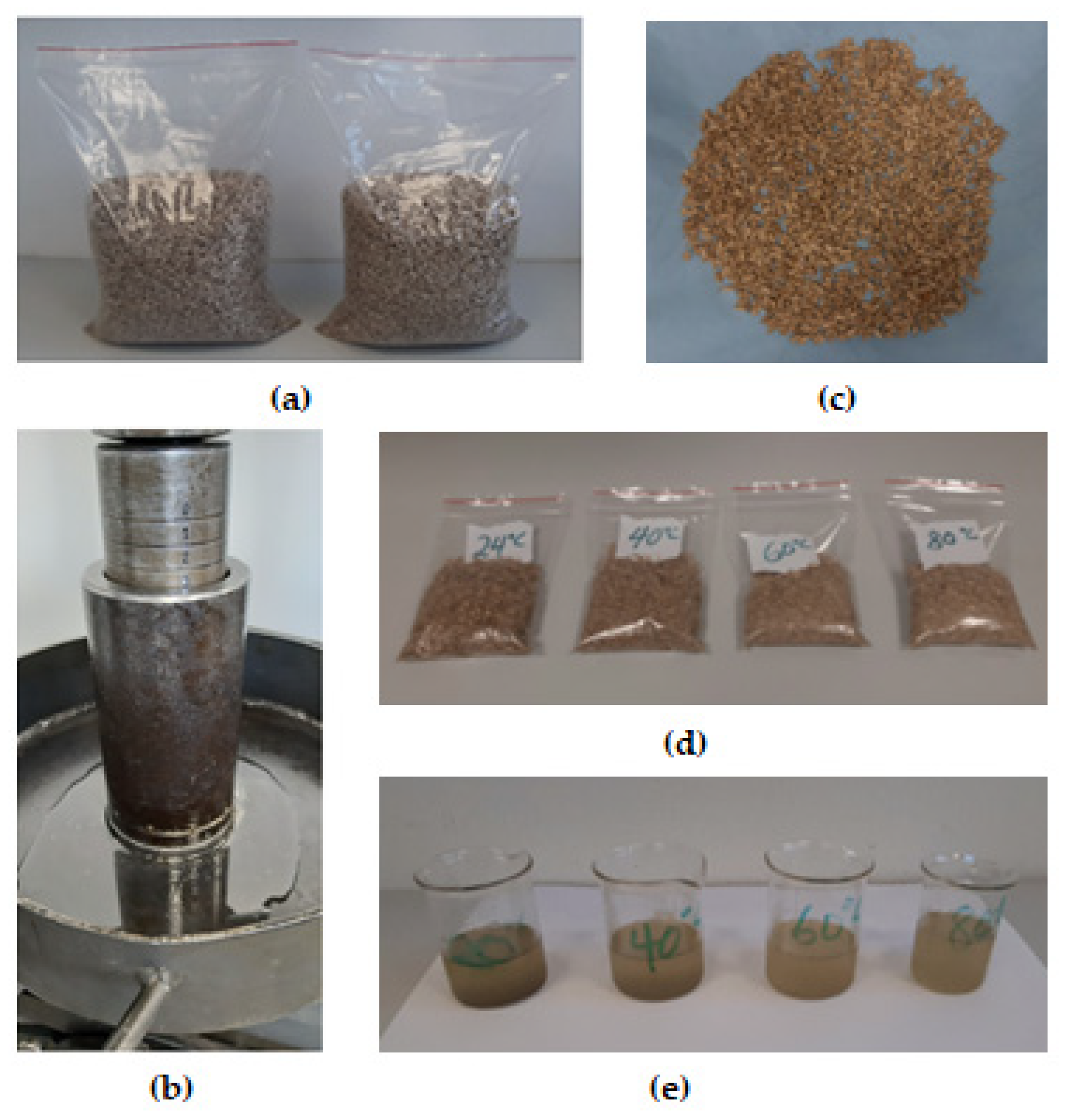
2.3. Samples Pretreatment
2.4. Oil Extraction under Uniaxial Process
2.5. Determination of Oils Quality Parameters
2.6. Measurement of UV Spectral Parameters
2.7. Statistical Analyses
3. Results
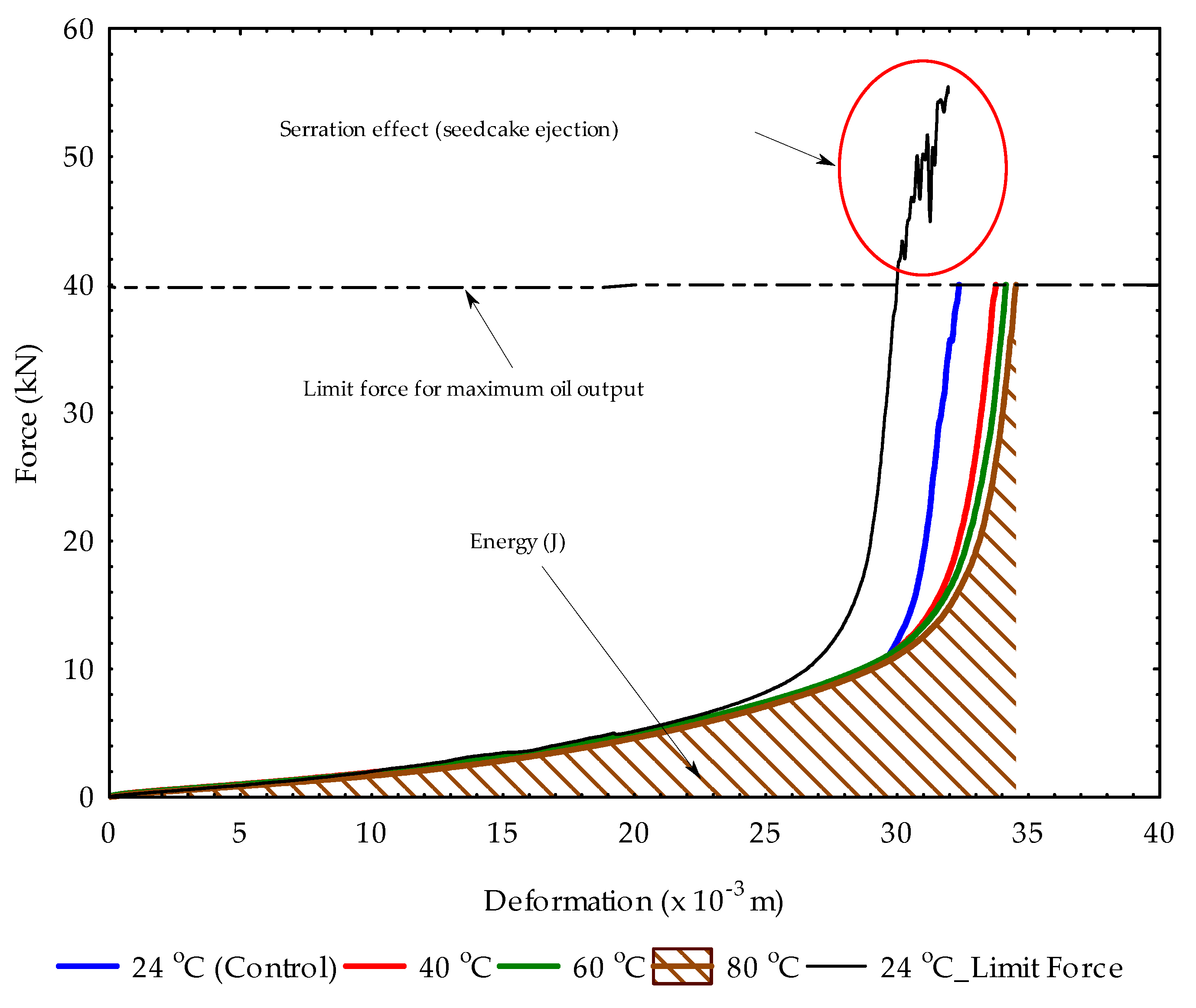
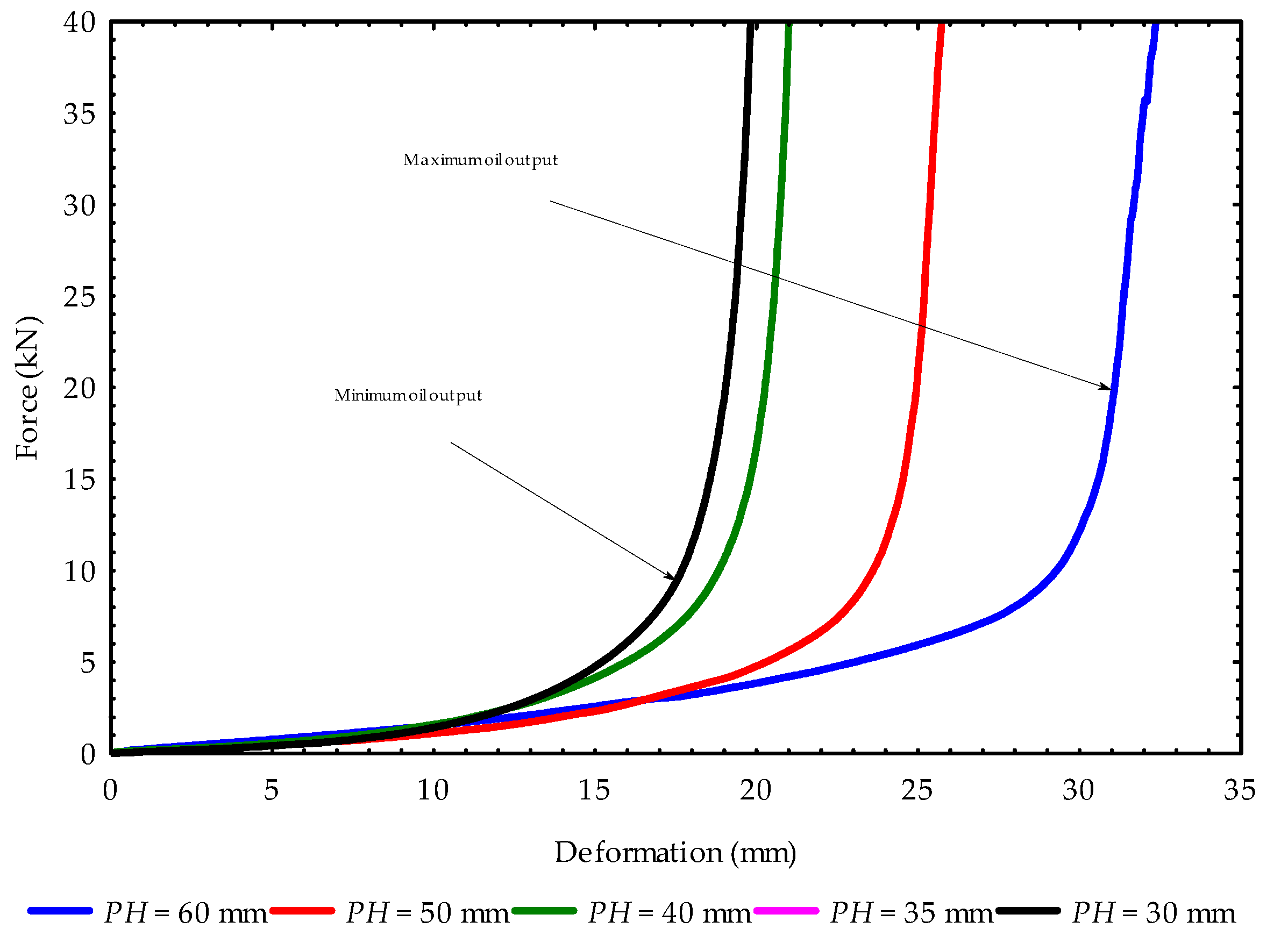
3.1. Compression Curves at Initial and Repeated Pressings
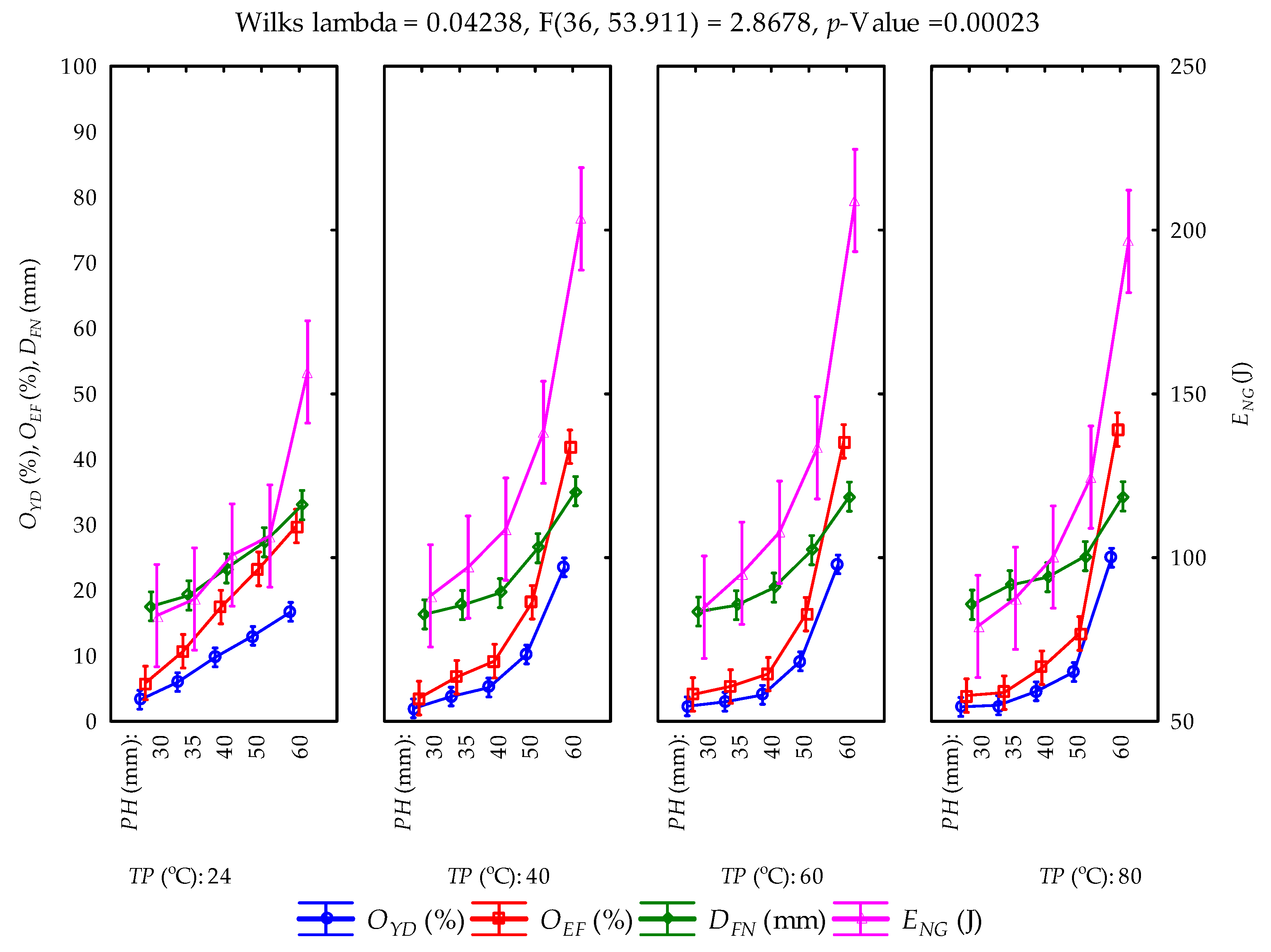
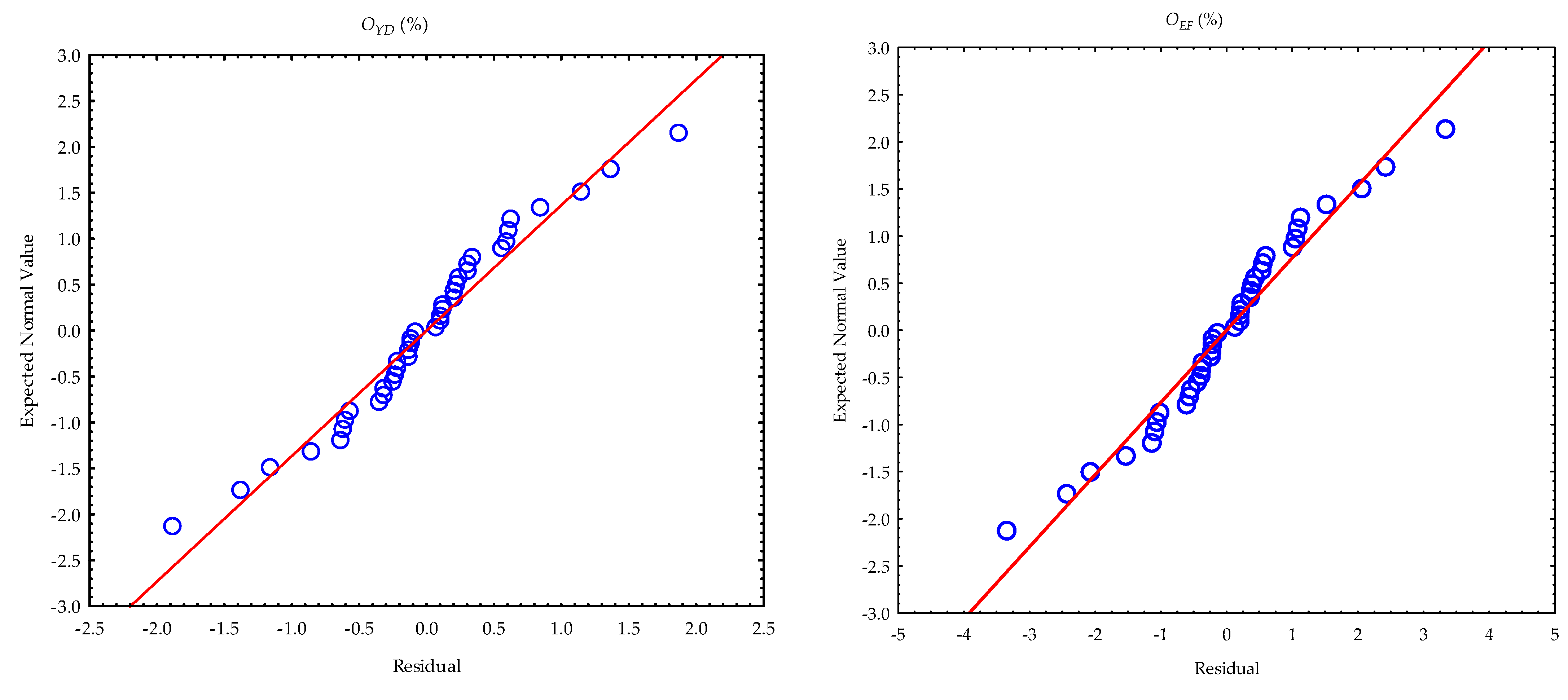
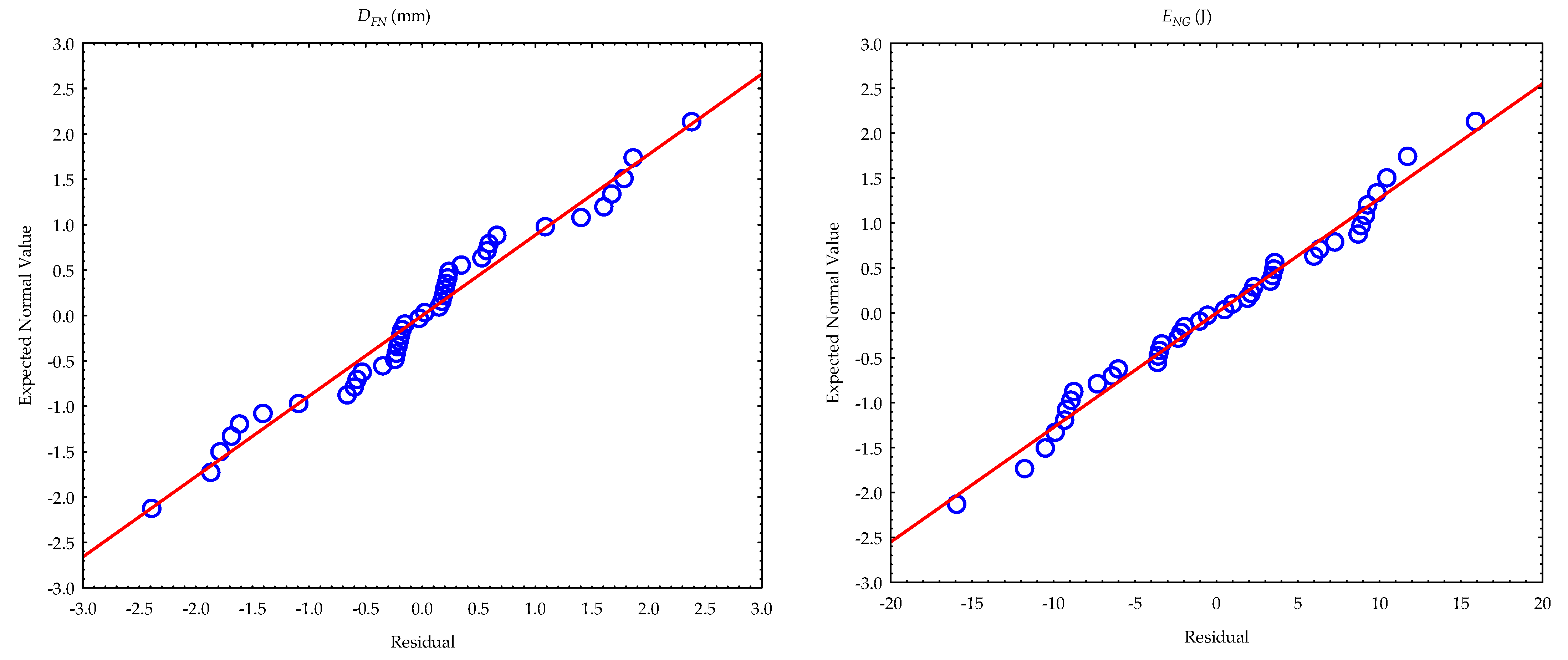
3.2. Calculated Parameters and Statistical Evaluation
3.3. Quality Indicators of Oils and Statistical Evaluation
3.4. UV Spectral Properties of Oils
3.5. Supplementary Materials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.L.; Inglett, G.E. Oil and Tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Chatepa, L.E.C.; Uluko, H.; Masamba, K. Comparison of oil quality extracted from selected conventional and non conventional sources of vegetable oil from Malawi. Afr. J. Biotechnol. 2019, 18, 171–180. [Google Scholar]
- Gotor, A.A.; Rhazi, L. Effects of refining process on sunflower oil minor components: A review. OCL 2016, 23, D207. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Nogueira, R.; Nunes, L.M. Quantitative assessment of the valorization of used cooking oils in 23 countries. Waste Manag. 2018, 78, 611–620. [Google Scholar] [CrossRef]
- Mitrea, L.; Teleky, B.-E.; Leopold, L.-F.; Nemes, S.-A.; Plamada, D.; Dulf, F.V.; Pop, I.-D.; Vodnar, D.C. The physicochemical properties of five vegetable oils exposed at high temperature for a short-time-interval. J. Food Compost. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- Ng, T.-T.; So, P.K.; Zheng, B.O.; Yao, Z.-P. Rapid screening of mixed edible oils and gutter oils by matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chim. Acta 2015, 884, 70–76. [Google Scholar] [CrossRef]
- Rifna, E.J.; Pandiselvam, R.; Kothakota, A.; Rao, K.V.S.; Dwivedi, M.; Kumar, M.; Thirumdas, R.; Ramesh, S.V. Advanced process analytical tools for identification of adulterants in edible oils—A review. Food Chem. 2022, 369, 130898. [Google Scholar] [CrossRef]
- Dimitrijevic, A.; Horn, R. Sunflower hybrid breeding: From markers to genomic selection. Front. Plant Sci. 2018, 8, 2238. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.L.; Quiroz, F.J.; Carrera, A.D. Detection of Plasmopara halstedii in sunflower seeds: A case study using molecular testing. J. Saudi Soc. Agric. Sci. 2021, 20, 395–400. [Google Scholar]
- Dean, C. Coconut oil for Alzheimer’s. J. Am. Acad. Physician Assist. 2012, 25, 1–5. [Google Scholar]
- Sandupama, P.; Munasinghe, D.; Jayasinghe, M. Coconut oil as a therapeutic treatment for alzheimer’s disease: A review. J. Future Foods. 2022, 2, 41–52. [Google Scholar] [CrossRef]
- Kumar, K.A.; Viswanathan, K. Study of UV transmission through a few edible oils and chicken oil. J. Spectrosc. 2013, 2013, 540417. [Google Scholar]
- Liu, R.; Lu, M.; Zhang, Z.; Chang, M.; Wang, X. Evaluation of the antioxidant properties of micronutrients in different vegetable oils. Eur. J. Lipid Sci. Technol. 2020, 122, 1900079. [Google Scholar] [CrossRef]
- Cai, Z.; Li, K.; Lee, W.J.; Reaney, M.T.J.; Zhang, N.; Wang, Y. Recent progress in the thermal treatment of oilseeds and oil oxidative stability: A review. Fundam. Res. 2021, 6, 767–784. [Google Scholar] [CrossRef]
- Savoire, R. Screw pressing applications to oilseeds. Ref. Modul. Food Sci. 2017. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoiselle, J.L.; Vorobiev, E. Mechanical continuous oil expression from oilseeds. A review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Singh, K.K.; Wiesenborn, D.; Kangas, N.; Tostenson, K. Screw pressing characteristics of dehulled crambe seed. Trans. ASABE 2004, 47, 199–204. [Google Scholar] [CrossRef]
- Bogaert, L.; Mathieu, H.; Mhemdi, H.; Vorobiev, E. Characterization of oilseeds mechanical expression in an instrumented pilot screw press. Ind. Crops. Prod. 2018, 121, 106–113. [Google Scholar] [CrossRef]
- Mpagalile, J.J.; Clarke, B. Effect of processing parameters on coconut oil expression efficiencies. Int. J. Food Sci. Nutr. 2005, 56, 125–132. [Google Scholar] [CrossRef]
- Karaj, S.; Muller, J. Optimizing mechanical oil extraction of Jatropha curcas L. seeds with respect to press capacity, oil recovery and energy efficiency. Ind. Crops. Prod. 2011, 34, 1010–1016. [Google Scholar] [CrossRef]
- Romuli, S.; Karaj, S.; Latif, S.; Muller, J. Performance of mechanical co-extraction of Jatropha curcas L. kernels with rapeseed, maize or soybean with regard to oil recovery, press capacity and product quality. Ind. Crops. Prod. 2017, 104, 81–90. [Google Scholar] [CrossRef]
- Divisova, M.; Herak, D.; Kabutey, A.; Sigalingging, R.; Svatonova, T. Deformation curve characteristics of rapeseeds and sunflower seeds under compression loading. Sci. Agric. Bohem. 2014, 45, 180–186. [Google Scholar]
- Munson-Mcgee, S.H. D-optimal experimental designs for uniaxial expression. J. Food Process Eng. 2014, 37, 248–256. [Google Scholar] [CrossRef]
- Demirel, C.; Kabutey, A.; Herák, D.; Sedlaček, A.; Mizera, Č.; Dajbych, O. Using Box–Behnken Design Coupled with Response Surface Methodology for Optimizing Rapeseed Oil Expression Parameters under Heating and Freezing Conditions. Processes 2022, 10, 490. [Google Scholar] [CrossRef]
- Gupta, R.K.; Das, S.K. Fracture resistance of sunflower seed and kernel to compressive loading. J. Food Eng. 2000, 46, 1–8. [Google Scholar] [CrossRef]
- Kabutey, A.; Mizera, Č.; Dajbych, O.; Hrabě, P.; Herák, D.; Demirel, C. Modelling and Optimization of Processing Factors of Pumpkin Seeds Oil Extraction under Uniaxial Loading. Processes 2021, 9, 540. [Google Scholar] [CrossRef]
- Kabutey, A.; Herak, D.; Mizera, C.; Hrabe, P. Compressive loading experiment of non-roasted bulk oil palm kernels at varying pressing factors. Int. Agrophysics 2018, 32, 357–363. [Google Scholar] [CrossRef]
- Erum, Z.; Rehana, S.; Mehwish, A.H.; Anjum, Y. Study of Physicochemical Properties of Edible Oil and Evaluation of Frying Oil Quality by Fourier Transform-Infrared (FT-IR) Spectroscopy. Arab. J. Chem. 2014, 10, 3870–3876. [Google Scholar]
- Herchi, W.; Ammar, K.B.; Bouali, I.; Abdallah, I.B.; Guetet, A.; Boukhchina, S. Heating effects on physicochemical characteristics and antioxidant activity of flaxseed hull oil (Linum usitatissimum L.). Food Sci. Technol. 2016, 36, 97–102. [Google Scholar] [CrossRef]
- Tang, T.S. 8 Analysis of oleochemicals. Oleochem. Manu. Appl. 2001, 4, 227. [Google Scholar]
- de Souza, T.R.P.; Olenka, L.; Peternella, W.S. A study of degradation in vegetable oils by exposure to sunlight using Fourier Transform Infrared Spectroscopy. Mater. Sci. Appl. 2020, 11, 678–691. [Google Scholar] [CrossRef]
- Amadi, B.A.; Lele, K.C.; Duru, M.K.C. Extraction and characterization of vegetable oils from legume and palmae; using African Oil Bean (Pentaclethra macrophylla) and ‘Akwu Ojukwu’ (Elais guineensis) respectively. Am. J. Biol. Life Sci. 2013, 1, 7–10. [Google Scholar]
- Katkade, M.B.; Syed, H.M.; Andhale, R.R.; Farooqui, A.S. Comparative study of physicochemical properties of different edible vegetable oils. J. Sci. Agric. Eng. 2018, 8, 235–238. [Google Scholar]
- Adeyanju, J.A.; Ogunlakin, G.O.; Adekunle, A.A.; Alawode, G.E.; Majekolagbe, O.S. Optimization of oil extraction from coconut using response surface methodology. J. Chem. Pharm. Res. 2016, 8, 374–381. [Google Scholar]
- Ifa, L.; Syarif, T.; Sartia, S.; Juliani, J.; Nurdjannah, N.; Kusuma, H.S. Techno-economics of coconut coir bioadsorbent utilization on free fatty acid level reduction in crude palm oil. Heliyon 2022, 8, e09146. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR determination of free fatty acids in vegetable oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef]
- Cammerer, B.; Kroh, L.W. Shelf life of linseeds and peanuts in relation to roasting. Food Sci. Technol. 2009, 42, 545–549. [Google Scholar] [CrossRef]
- Adejumo, B.A.; Inaede, S.G.; Adamu, T.S. Effect of moisture content on the yield and characteristics of oil from valiculatum and V. calycinium. Pac. Sci. 2013, 48, 458–463. [Google Scholar]
- Okene, E.O.; Evbuomwan, B.O. Solvent extraction and characteristics of oil from coconut seeds using alternative solvents. Int. J. Eng. Technol. Res. 2014, 2, 135–138. [Google Scholar]
- Atinafu, D.G.; Bedemo, B. Estimation of total free fatty acid and cholesterol content in some commercial edible oils in Ethiopia, Bahir DAR. J. Cereals Oilseeds 2011, 2, 71–76. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physicochemical properties of cold pressed sunflower, peanut, rapeseed, mustard and olive oils grown in the Eastern Mediterranean region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Fats, Oils and Related Products, 2nd ed.; Food and Agriculture Organizaation of the United Nations: Rome, Italy, 2001; Volume 8. [Google Scholar]
- ISI. Indian Standard Methods for Analysis of Oilseeds. In Indian Standard Institute; IS: 3579; ISI: New Delhi, India, 1966. [Google Scholar]
- Blahovec, J. Agromatereials Study Guide; Czech University of Life Sciences Prague: Prague, Czech Republic, 2008. [Google Scholar]
- Keneni, Y.G.; Hvoslef-Eide, A.K.T.; Marchetti, J.M. Mathematical modelling of the drying kinetics of Jatropha curcas L. seed. Ind. Crops. Prod. 2019, 132, 12–20. [Google Scholar] [CrossRef]
- Niu, L.; Li, J.; Chen, M.S.; Xu, Z.F. Determination of oil contents in Sacha inchi (Plukenetia volubilis) seeds at different developmental stages by two methods: Soxhlet extraction and time-domain nuclear magnetic resonance. Ind. Crops. Prod. 2014, 56, 187–190. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crops. Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Gürdil, A.K.G.; Kabutey, A.; Selvi, K.C.; Hrabe, P.; Herak, D.; Frankova, A. Investigation of heating and freezing pretreatments of mechanical, chemical and spectral properties of bulk sunflower seeds and oil. Processes 2020, 8, 411. [Google Scholar] [CrossRef]
- Deli, S.; Farah Masturah, M.; Tajul Aris, Y.; Wan Nadiah, W.A. The effects of physical parameters of the screw press oil expeller on oil yield from Nigella sativa L. seeds. Int. Food Res. J. 2011, 18, 1367–1373. [Google Scholar]
- Chanioti, S.; Constantina, T. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Lysiak, G. Fracture toughness of pea: Weibull analysis. J. Food Eng. 2007, 83, 436–443. [Google Scholar] [CrossRef]
- Demirel, C.; Kabutey, A.; Herák, D.; Hrabě, P.; Mizera, Č.; Dajbych, O. Optimizing Uniaxial Oil Extraction of Bulk Rapeseeds: Spectrophotometric and Chemical Analyses of the Extracted Oil under Pretreatment Temperatures and Heating Intervals. Processes 2021, 9, 1755. [Google Scholar] [CrossRef]
- Orozco, F.D.A.; Sousa, A.C.; Araujo, M.C.U.; Domini, C.E. A new flow UV-Vis kinetics spectrophotometric method based on photodegradative reaction for determining the oxidative stability of biodiesel. Fuel 2020, 26, 116–197. [Google Scholar]
- Statsoft Inc. STATISTICA for Windows; Statsoft Inc.: Tulsa, OK, USA, 2013. [Google Scholar]
- Khan, L.M.; Hanna, M.A. Expression of oil from oilseeds—A Review. J. Agric. Eng. Res. 1983, 28, 495–503. [Google Scholar] [CrossRef]
- Li, W.G.; Sun, X.L.; Zu, Y.G.; Zhao, X.H. Optimization peony seed oil extraction process at suitable temperature and physicochemical property analysis. Bull. Botan. Res. 2020, 40, 73–78. [Google Scholar]
- Deng, R.; Gao, J.; Yi, J.; Liu, P. Could peony seeds oil become a high-quality edible vegetable oils? The nutritional and phytochemistry profiles, extraction, health benefits, safety and value-added-products. Food Res. Int. 2022, 156, 111200. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, D.; Adjei, F.; Tulashie, S.K.; Adukpoh, K.E.; Agbolegbe, R.E.; Gawou, G.; Manu, G.P. Quality evaluation of different repeatedly heated vegetable oils for deep-frying of yam fries. Meas. Food. 2022, 7, 100035. [Google Scholar] [CrossRef]
- Hoffmann, G. The Chemistry and Technology of Edible Oils and Fats and Their High-Fat Products; Academic Press: New York, NY, USA, 1989; pp. 63–68. [Google Scholar]
- Reuber, M. New Technologies for processing Crambe abyssinica. M.S. Thesis, Iowa State University, Ames, IA, USA, 1992. [Google Scholar]
- Singh, K.K.; Wiesenborn, D.P.; Tostenson, K.; Kangas, N. Influence of moisture content and cooking on screw pressing of crambe seed. JAOCS 2002, 79, 165–170. [Google Scholar] [CrossRef]
- Choo, W.S.; Birch, E.J.; Dufour, J.P. Physicochemical and stability characteristics of flaxseed oils during pan-heating. JAOCS 2007, 84, 735–740. [Google Scholar] [CrossRef]
- Ajai, A.I.; Adedokun, T.A.; Inobeme, A.; Jacob, J.O. Determination of physicochemical properties of selected vegetable oils in Minna. FUW Trends Sci. Technol. J. 2016, 1, 475–478. [Google Scholar]
- Vicentini-Polette, C.M.; Ramos, P.R.; Goncalves, C.B.; De Oliveira, A.L. Determination of free fatty acids in crude vegetable oil samples obtained by high-pressure processes. Food Chem. X 2021, 12, 100166. [Google Scholar] [CrossRef]
- Eke-Ejiofor, J.; Beleya, E.A.; Allen, J.E. Effect of variety on the quality parameters of crude soybean oil. Am. J. Food Sci. Technol. 2021, 9, 69–75. [Google Scholar]
- Nduka, J.K.C.; Omozuwa, P.O.; Imanah, O.E. Effect of heating on the physiological properties of selected vegetable oils. Arab. J. Chem. 2021, 14, 103063. [Google Scholar] [CrossRef]
- Tiffour, I.; Dehbi, A.; Mourad, A.-H.I.; Belfedal, A. Synthesis and characterization of a new organic semiconductor material. Mater. Chem. Phys. 2016, 178, 49–56. [Google Scholar] [CrossRef]
- Gonzalez, M.F.; Barbero, G.F.; Alvarez, J.A.; Ruiz, A.; Palma, M.; Ayuso, J. Authentication of virgin olive oil by a novel curve resolution approach combined with visible spectroscopy. Food Chem. 2007, 220, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xin, Y.; Wan, X. Spectral detection technology of vegetable oil: Spectral analysis of porphyrins and terpenoids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 119965. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Standard deviations and standard errors. BJM 2005, 331, 903. [Google Scholar] [CrossRef]


| (°C) |
Oil Yield, (%) |
Oil Expression Efficiency, (%) |
Deformation, (mm) |
Energy, (J) | |
|---|---|---|---|---|---|
| 60 a | 24 * | 16.725 ± 0.349 | 29.815 ± 0.622 | 33.020 ± 0.933 | 156.698 ± 5.048 |
| 50 b | 13.060 ± 1.929 | 23.281 ± 3.439 | 27.350 ± 2.277 | 106.598 ± 14.802 | |
| 40 b | 9.791 ± 0.801 | 17.454 ± 1.427 | 23.355 ± 3.373 | 100.788 ± 22.546 | |
| 35 b | 6.003 ± 2.652 | 10.701 ± 4.727 | 19.225 ± 0.813 | 87.367 ± 12.951 | |
| 30 b | 3.291 ± 0.292 | 5.866 ± 0.520 | 17.560 ± 0.283 | 82.260 ± 10.296 | |
| Sum ± SD | 48.869 ± 6.023 | 87.117 ± 10.736 | 120.510 ± 7.679 | 533.709 ± 65.644 | |
| 60 a | 40 | 23.521 ± 0.897 | 41.930 ± 1.599 | 35.155 ± 1.987 | 203.426 ± 16.646 |
| 50 b | 10.206 ± 1.635 | 18.194 ± 2.914 | 26.445 ± 0.318 | 138.270 ± 13.150 | |
| 40 b | 5.169 ± 0.442 | 9.214 ± 0.788 | 19.600 ± 0.212 | 108.731 ± 9.006 | |
| 35 b | 3.793 ± 0.295 | 6.761 ± 0.525 | 17.765 ± 2.638 | 97.129 ± 4.719 | |
| 30 b | 2.000 ± 0.179 | 3.565 ± 0.319 | 16.355 ± 2.524 | 88.329 ± 2.730 | |
| Sum ± SD | 44.689 ± 3.448 | 79.665 ± 6.146 | 115.320 ± 7.769 | 635.883 ± 46.252 | |
| 60 a | 60 | 23.986 ± 0.171 | 42.759 ± 0.305 | 34.305 ± 0.304 | 209.032 ± 13.975 |
| 50 b | 9.183 ± 0.105 | 16.370 ± 0.188 | 26.150 ± 0.269 | 133.532 ± 12.344 | |
| 40 b | 4.053 ± 0.432 | 7.225 ± 0.770 | 20.425 ± 0.488 | 107.773 ± 12.566 | |
| 35 b | 2.990 ± 0.310 | 5.330 ± 0.552 | 17.740 ± 1.541 | 95.251 ± 3.264 | |
| 30 b | 2.312 ± 0.166 | 4.122 ± 0.296 | 16.775 ± 0.841 | 84.848 ± 8.473 | |
| Sum ± SD | 42.523 ± 1.184 | 75.805 ± 2.111 | 115.395 ± 3.444 | 630.435 ± 50.621 | |
| 60 a | 80 | 24.988 ± 0.835 | 44.545 ± 1.489 | 34.355 ± 0.247 | 196.548 ± 1.426 |
| 50 b | 7.530 ± 1.211 | 13.424 ± 2.159 | 25.230 ± 2.376 | 124.590 ± 0.744 | |
| 40 b | 4.599 ± 0.866 | 8.198 ± 1.543 | 22.025 ± 0.035 | 100.181 ± 3.053 | |
| 35 b | 2.470 ± 0.166 | 4.402 ± 0.296 | 20.790 ± 0.339 | 87.584 ± 4.897 | |
| 30 b | 2.212 ± 0.479 | 3.943 ± 0.853 | 17.810 ± 0.750 | 79.030 ± 5.062 | |
| Sum ± SD | 41.798 ± 3.557 | 74.512 ± 6.341 | 120.210 ± 3.748 | 587.932 ± 15.183 | |
| Effect | Test | Value | F-Value | Effect df | Error df | p-Value |
|---|---|---|---|---|---|---|
| Intercept | Wilks lambda | 0.001 | 4413.75 | 3 | 18 | <0.05 |
| Pressing height, PH (mm) | Wilks lambda | 0.003 | 30.97 | 12 | 47.915 | <0.05 |
| Temperature, TP (°C) | Wilks lambda | 0.229 | 4.064 | 9 | 43.958 | <0.05 |
| PH × TP | Wilks lambda | 0.042 | 2.868 | 36 | 53.911 | <0.05 |
| Calculated Parameters | Multiple R | Multiple R2 | Adjusted R2 | F-Value | p-Value |
|---|---|---|---|---|---|
| (%) | 0.996 | 0.992 | 0.984 | 124.332 | <0.05 |
| (%) | 0.996 | 0.992 | 0.984 | 124.332 | <0.05 |
| (mm) | 0.986 | 0.972 | 0.945 | 35.974 | <0.05 |
| (J) | 0.983 | 0.967 | 0.935 | 30.524 | <0.05 |
| Cell No. | PH (mm) | TP (°C) | (%) * Means | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 30 | 40 | 2.000 | **** | |||||||||
| 4 | 30 | 80 | 2.212 | **** | |||||||||
| 3 | 30 | 60 | 2.312 | **** | **** | ||||||||
| 8 | 35 | 80 | 2.470 | **** | **** | ||||||||
| 7 | 35 | 60 | 2.990 | **** | **** | **** | |||||||
| 1 | 30 | 24 | 3.291 | **** | **** | **** | |||||||
| 6 | 35 | 40 | 3.793 | **** | **** | **** | **** | ||||||
| 11 | 40 | 60 | 4.053 | **** | **** | **** | **** | ||||||
| 12 | 40 | 80 | 4.599 | **** | **** | **** | |||||||
| 10 | 40 | 40 | 5.169 | **** | **** | ||||||||
| 5 | 35 | 24 | 6.003 | **** | **** | ||||||||
| 16 | 50 | 80 | 7.530 | **** | **** | ||||||||
| 15 | 50 | 60 | 9.183 | **** | **** | ||||||||
| 9 | 40 | 24 | 9.791 | **** | |||||||||
| 14 | 50 | 40 | 10.206 | **** | |||||||||
| 13 | 50 | 24 | 13.060 | **** | |||||||||
| 17 | 60 | 24 | 16.725 | **** | |||||||||
| 18 | 60 | 40 | 23.521 | **** | |||||||||
| 19 | 60 | 60 | 23.986 | **** | |||||||||
| 20 | 60 | 80 | 24.988 | **** |
| Cell No. | PH (mm) | TP (°C) | (J) * Means | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 30 | 80 | 79.030 | **** | ||||||
| 1 | 30 | 24 | 82.260 | **** | **** | |||||
| 3 | 30 | 60 | 84.848 | **** | **** | **** | ||||
| 5 | 35 | 24 | 87.367 | **** | **** | **** | ||||
| 8 | 35 | 80 | 87.584 | **** | **** | **** | ||||
| 2 | 30 | 40 | 88.329 | **** | **** | **** | ||||
| 7 | 35 | 60 | 95.251 | **** | **** | **** | ||||
| 6 | 35 | 40 | 97.129 | **** | **** | **** | ||||
| 12 | 40 | 80 | 100.181 | **** | **** | **** | **** | |||
| 9 | 40 | 24 | 100.788 | **** | **** | **** | **** | |||
| 13 | 50 | 24 | 106.598 | **** | **** | **** | ||||
| 11 | 40 | 60 | 107.773 | **** | **** | **** | ||||
| 10 | 40 | 40 | 108.731 | **** | **** | |||||
| 16 | 50 | 80 | 124.590 | **** | **** | |||||
| 15 | 50 | 60 | 133.532 | **** | ||||||
| 14 | 50 | 40 | 138.270 | **** | **** | |||||
| 17 | 60 | 24 | 156.698 | **** | ||||||
| 20 | 60 | 80 | 196.548 | **** | ||||||
| 18 | 60 | 40 | 203.426 | **** | ||||||
| 19 | 60 | 60 | 209.032 | **** |
| Temperature (°C) | Peroxide Value, PV (meq O2/kg Oil) | Acid Value, AV (mg KOH/g Oil) | Free Fatty Acid, FFA (mg KOH/g Oil) |
|---|---|---|---|
| 24 * | 6.898 ± 0.144 | 1.567 ± 0.350 | 0.783 ± 0.175 |
| 40 | 7.068 ± 0.403 | 1.998 ± 0.276 | 0.999 ± 0.138 |
| 60 | 7.290 ± 0.507 | 1.043 ± 0.166 | 0.521 ± 0.083 |
| 80 | 6.957 ± 0.347 | 1.698 ± 0.257 | 0.849 ± 0.128 |
| Chemical Properties | R2 | F-Value | p-Value |
|---|---|---|---|
| Peroxide value, PV (meq O2/kg) | 0.242 | 0.426 | >0.05 |
| Acid value, AV (mg KOH/g oil) | 0.765 | 4.351 | >0.05 |
| Free fatty acid, FFA (mg KOH/g oil) | 0.766 | 4.358 | >0.05 |
| Factors Effect | Parameter, A (-) a | Sum of Squares | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Intercept | 1.26584 | 27.176 | 27.176 | 801.688 | <0.05 |
| WL | −0.00156 | 10.890 | 10.890 | 321.260 | <0.05 |
| TP | 0.00214 | 1.369 | 1.369 | 40.379 | <0.05 |
| Factors Effect | Parameter, T (%) a | Sum of Squares | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Intercept | 1.34048 | 30.48 | 30.48 | 0.3858 | >0.05 |
| WL | 0.06468 | 18,362.42 | 18,362.42 | 232.4536 | <0.05 |
| TP | −0.11678 | 4059.91 | 4059.91 | 51.3952 | <0.05 |
| Factor Effects | Test | Value | F-Value | Effect df | Error df | p-Value |
|---|---|---|---|---|---|---|
| Intercept | Wilks lambda | 0.000124 | 1799571 | 2 | 447 | <0.05 |
| WL | Wilks lambda | 0.001361 | 212 | 110 | 894 | <0.05 |
| TP | Wilks lambda | 0.040675 | 590 | 6 | 894 | <0.05 |
| WP × TP | Wilks lambda | 0.056873 | 9 | 330 | 894 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabutey, A.; Herák, D.; Mizera, Č. Determination of Maximum Oil Yield, Quality Indicators and Absorbance Spectra of Hulled Sunflower Seeds Oil Extraction under Axial Loading. Foods 2022, 11, 2866. https://doi.org/10.3390/foods11182866
Kabutey A, Herák D, Mizera Č. Determination of Maximum Oil Yield, Quality Indicators and Absorbance Spectra of Hulled Sunflower Seeds Oil Extraction under Axial Loading. Foods. 2022; 11(18):2866. https://doi.org/10.3390/foods11182866
Chicago/Turabian StyleKabutey, Abraham, David Herák, and Čestmír Mizera. 2022. "Determination of Maximum Oil Yield, Quality Indicators and Absorbance Spectra of Hulled Sunflower Seeds Oil Extraction under Axial Loading" Foods 11, no. 18: 2866. https://doi.org/10.3390/foods11182866
APA StyleKabutey, A., Herák, D., & Mizera, Č. (2022). Determination of Maximum Oil Yield, Quality Indicators and Absorbance Spectra of Hulled Sunflower Seeds Oil Extraction under Axial Loading. Foods, 11(18), 2866. https://doi.org/10.3390/foods11182866








