Biological Evaluation of 3-Azaspiro[Bicyclo[3.1.0]Hexane-2,5′-Pyrimidines] as Potential Antitumor Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Antiproliferative Effect of Synthesized Compounds against Cancer Cell Lines
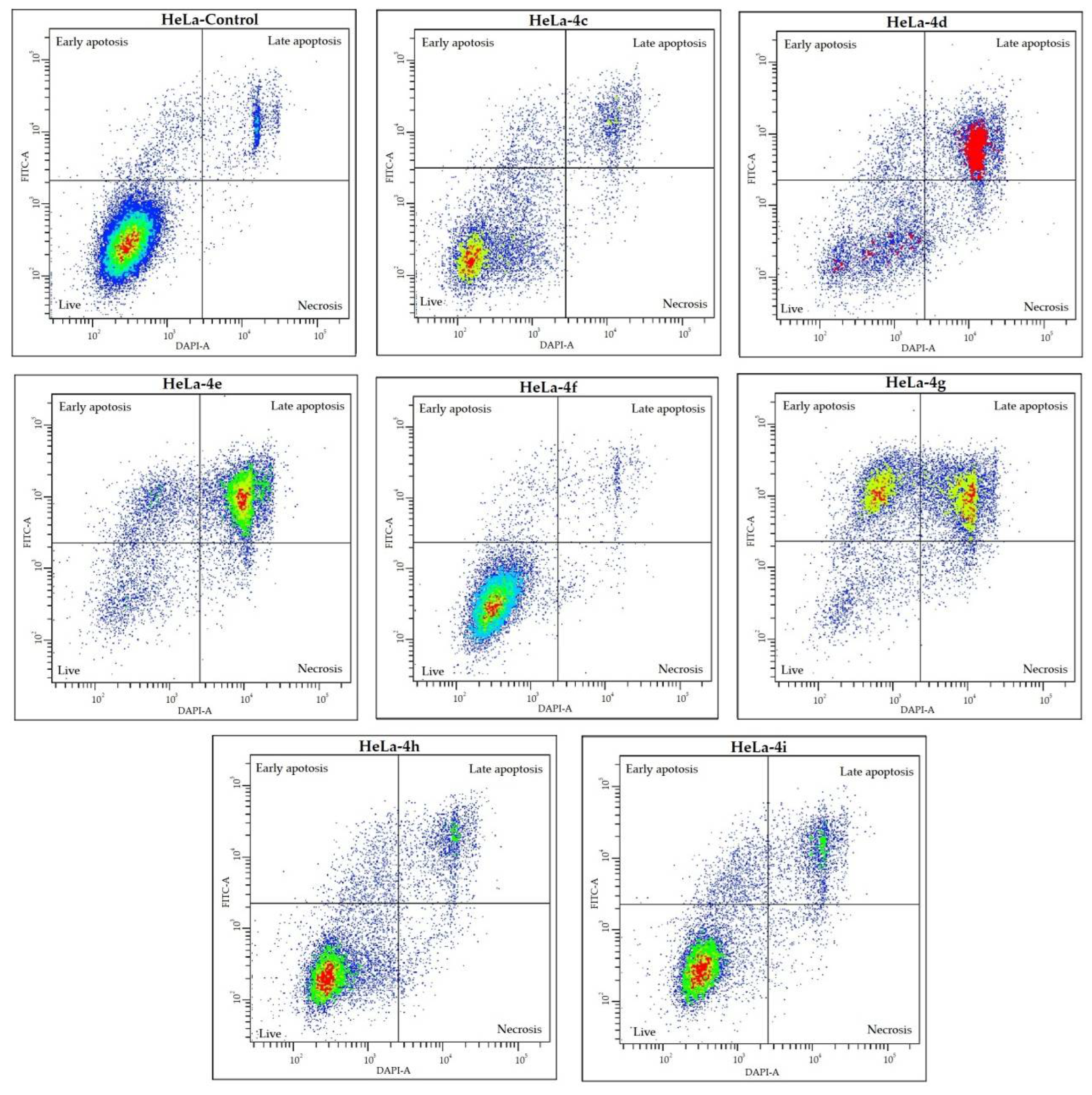
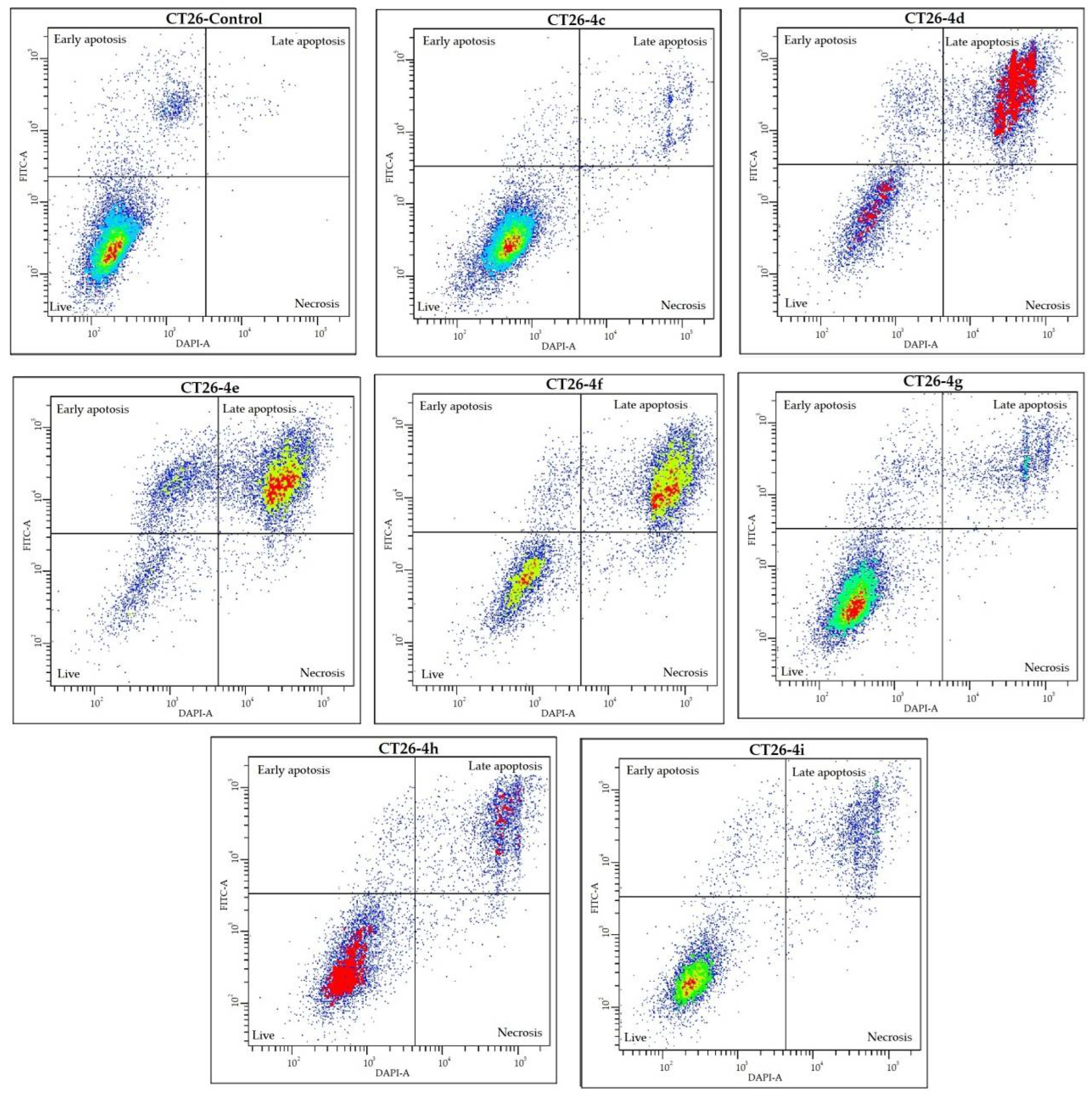
2.3. Cell Death Analysis
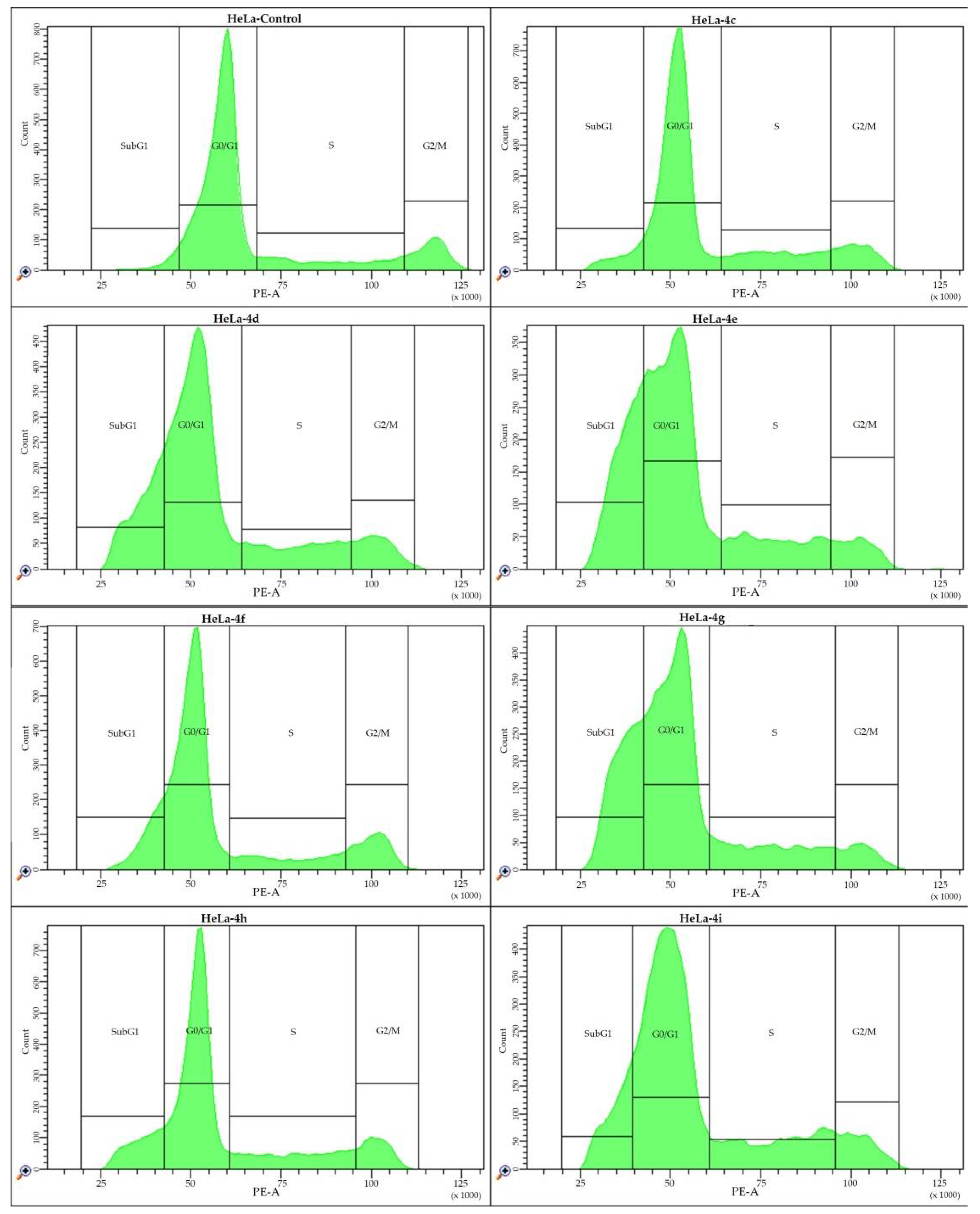
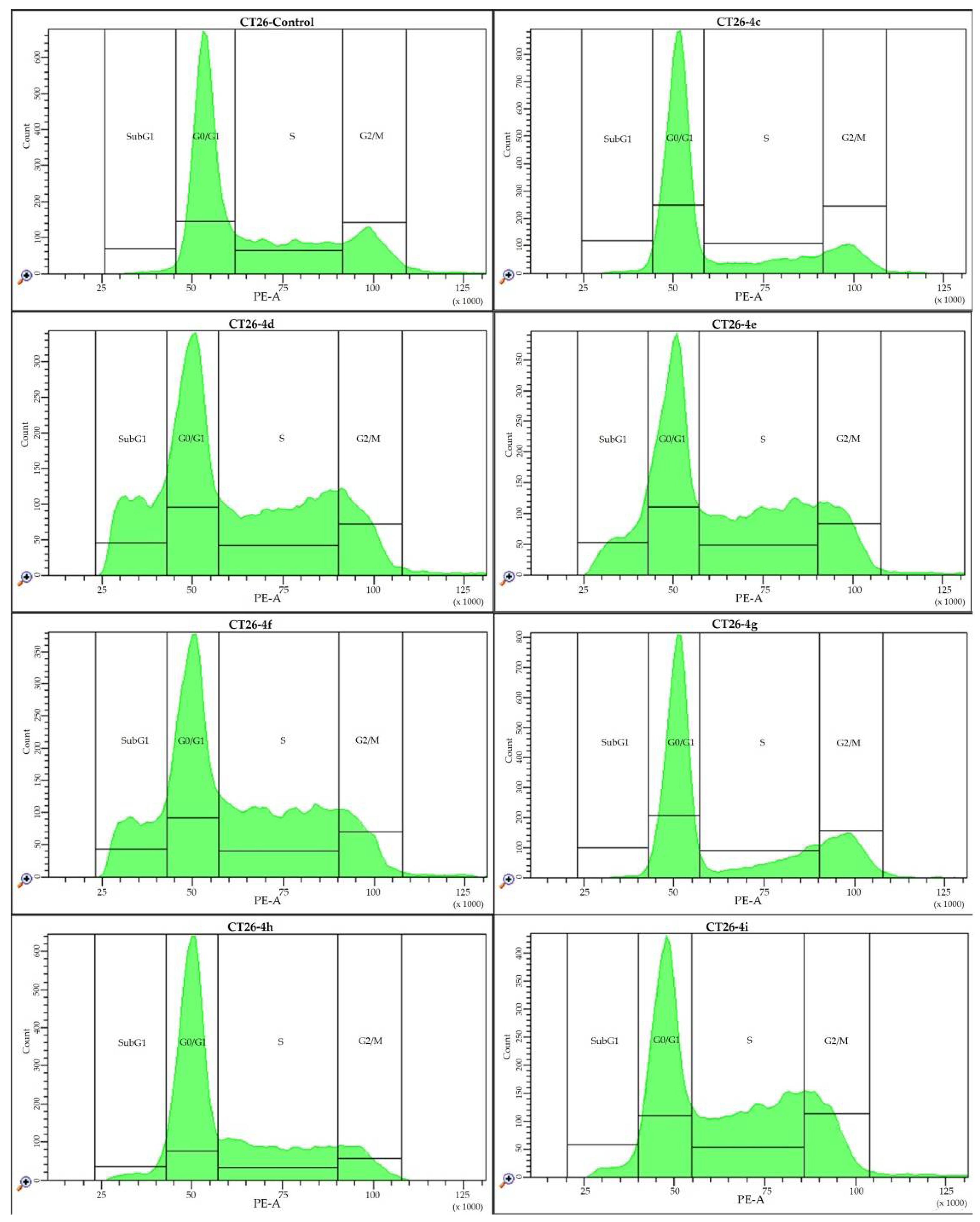
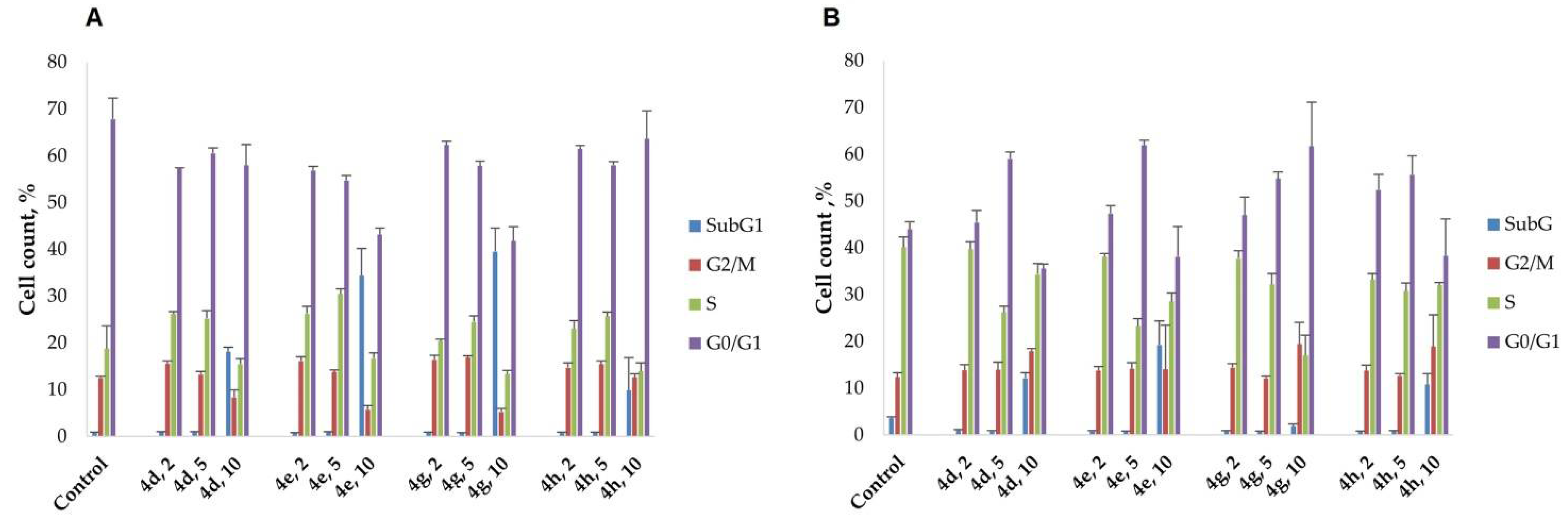
2.4. Cell Cycle Analysis
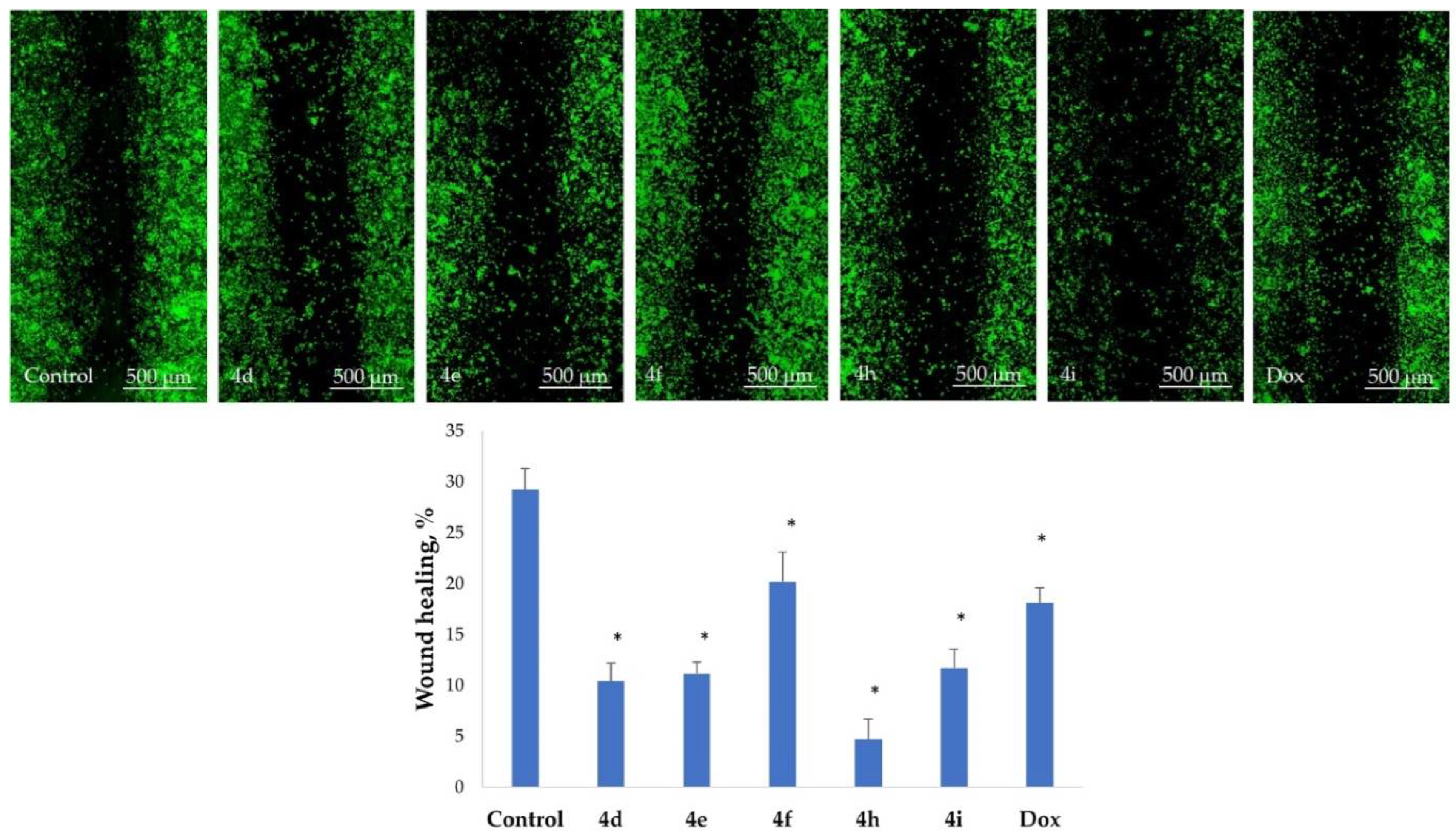
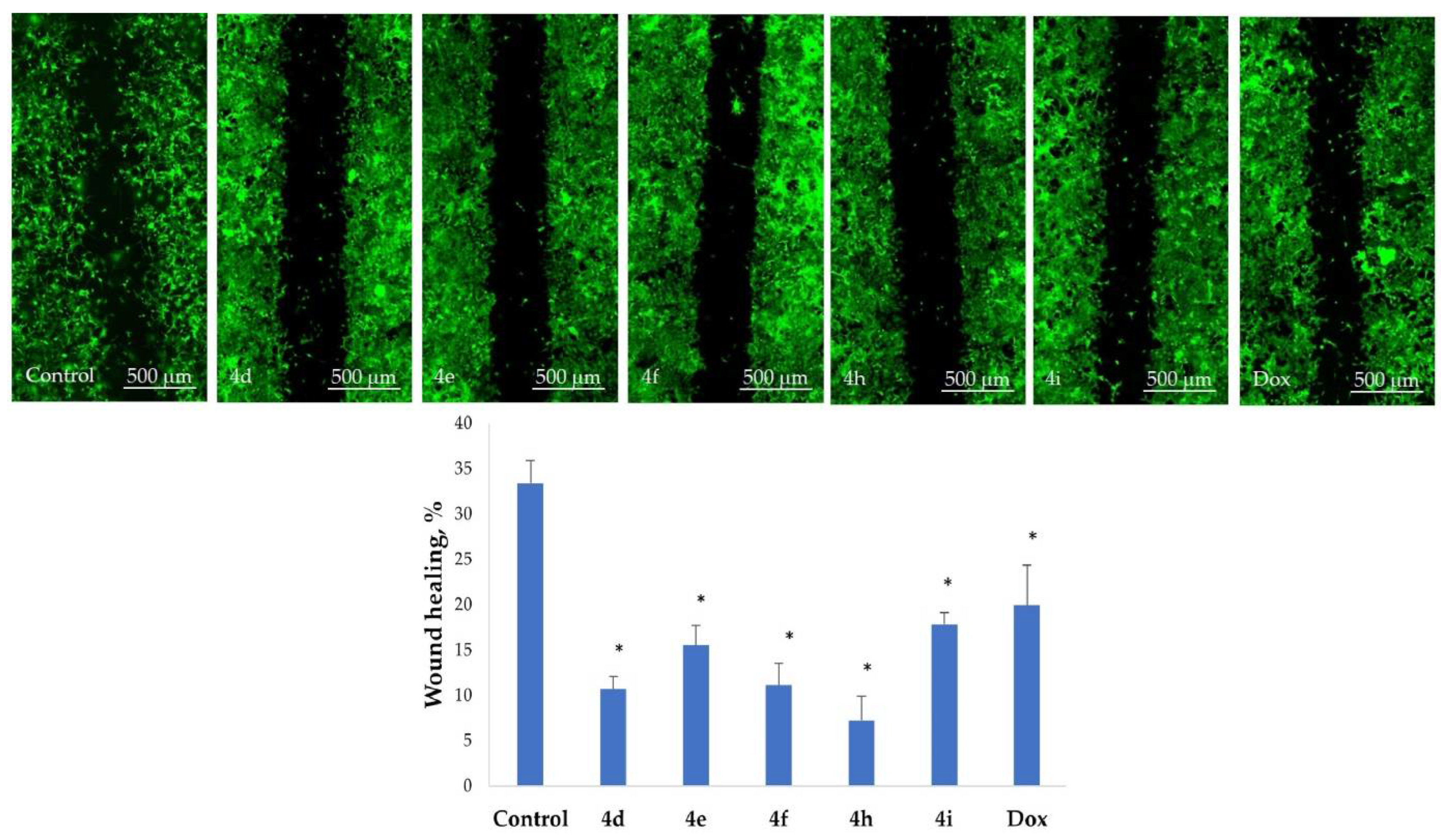
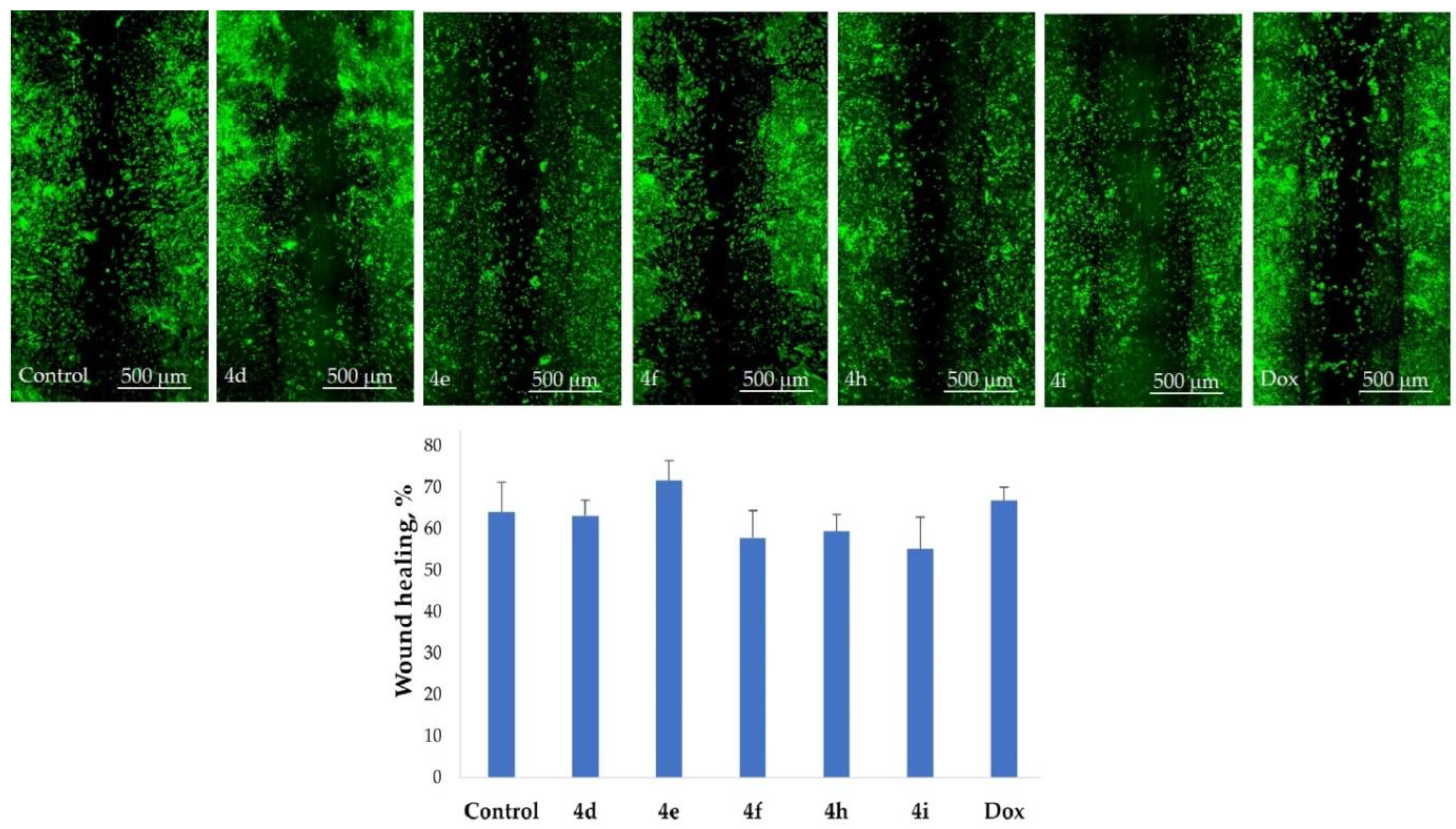
2.5. Inhibition of Cell Motility Evaluated by Scratch Test
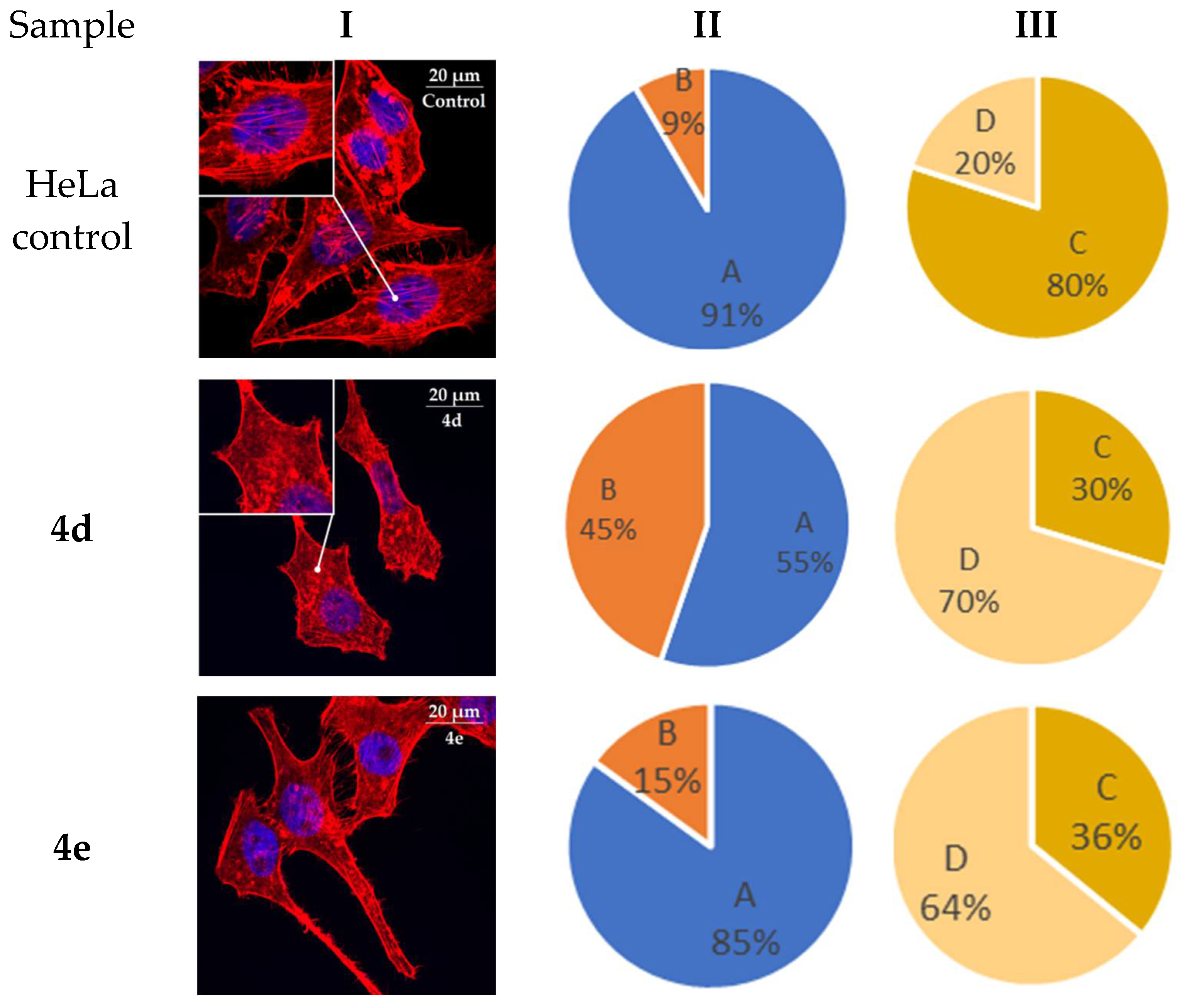
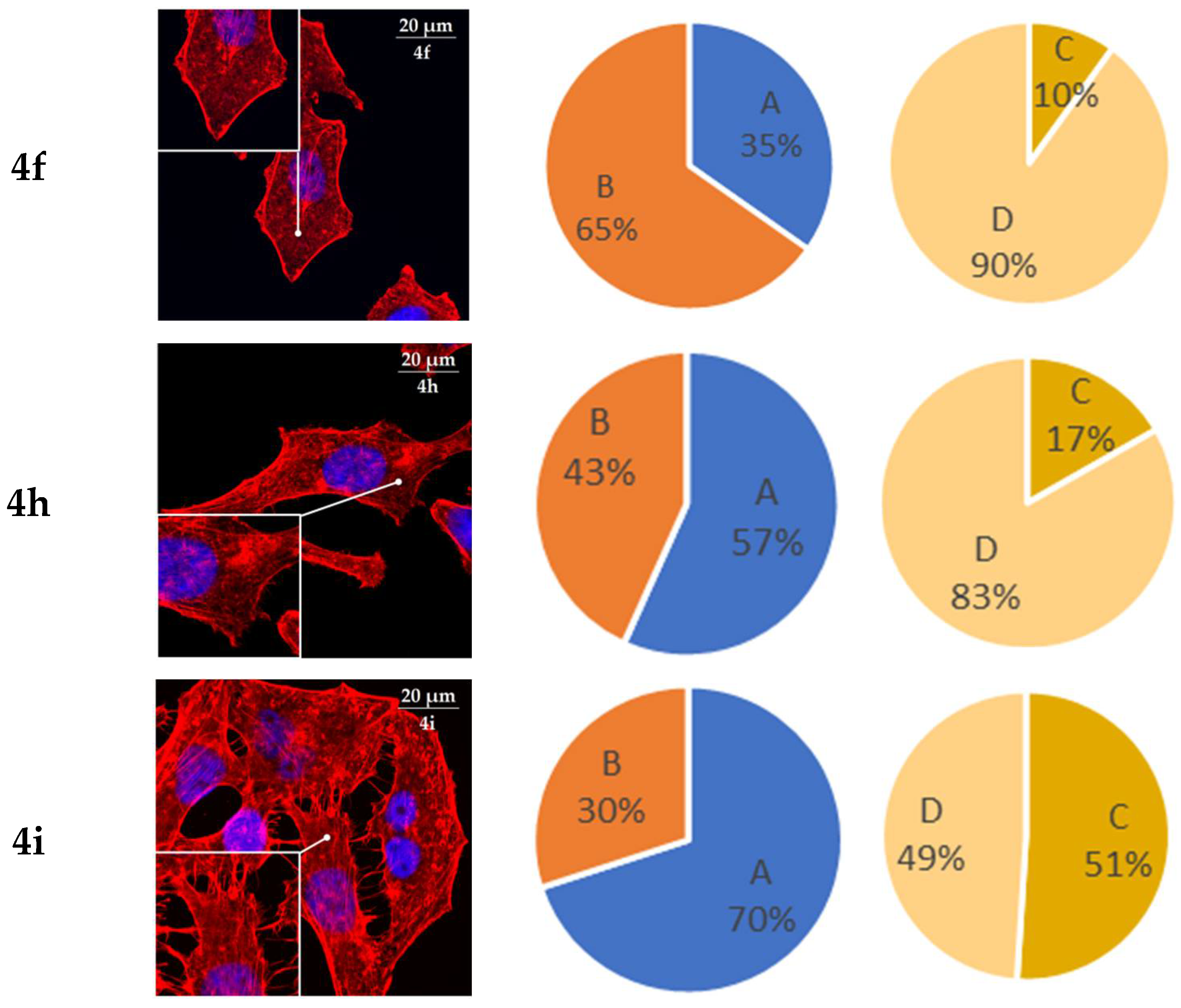
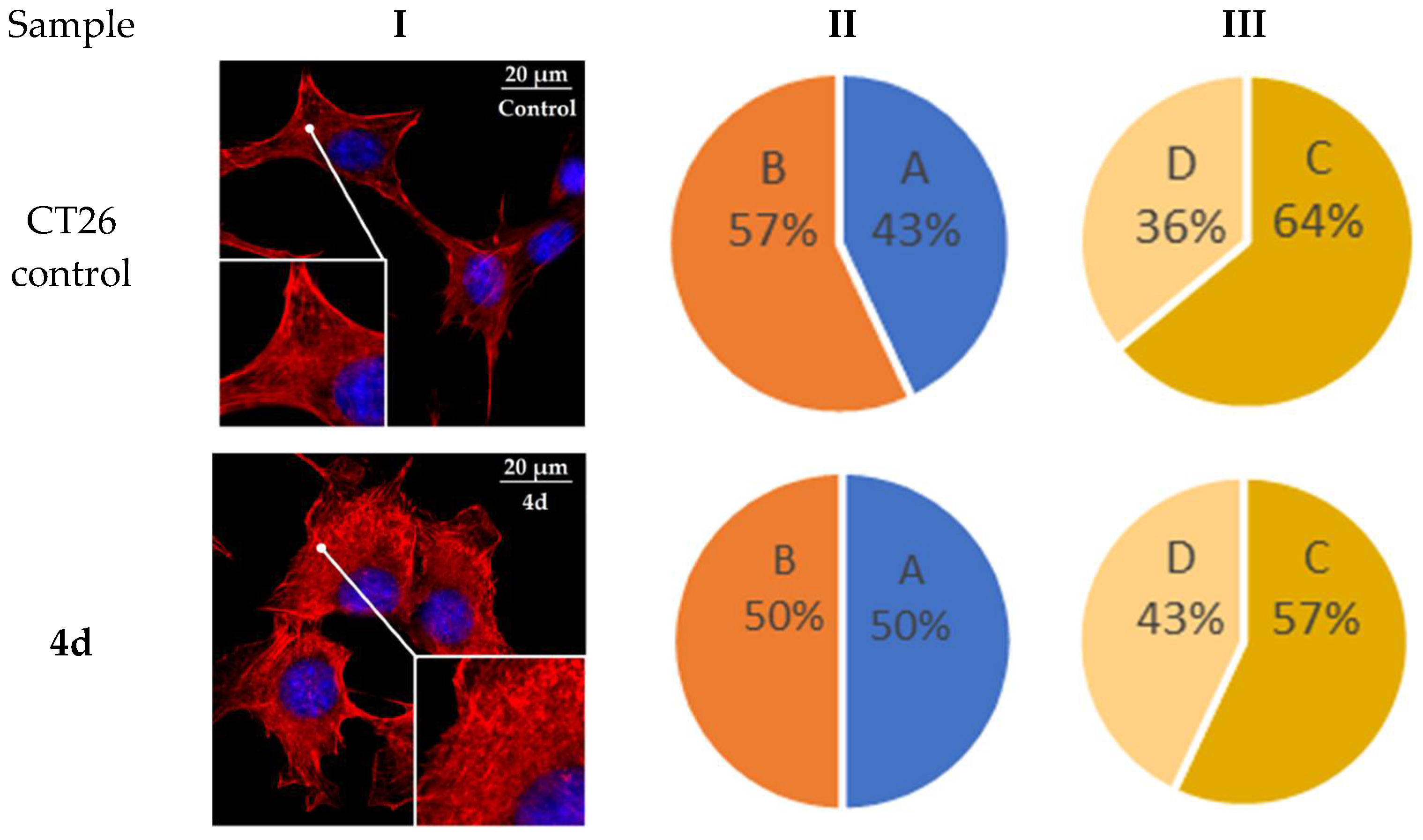
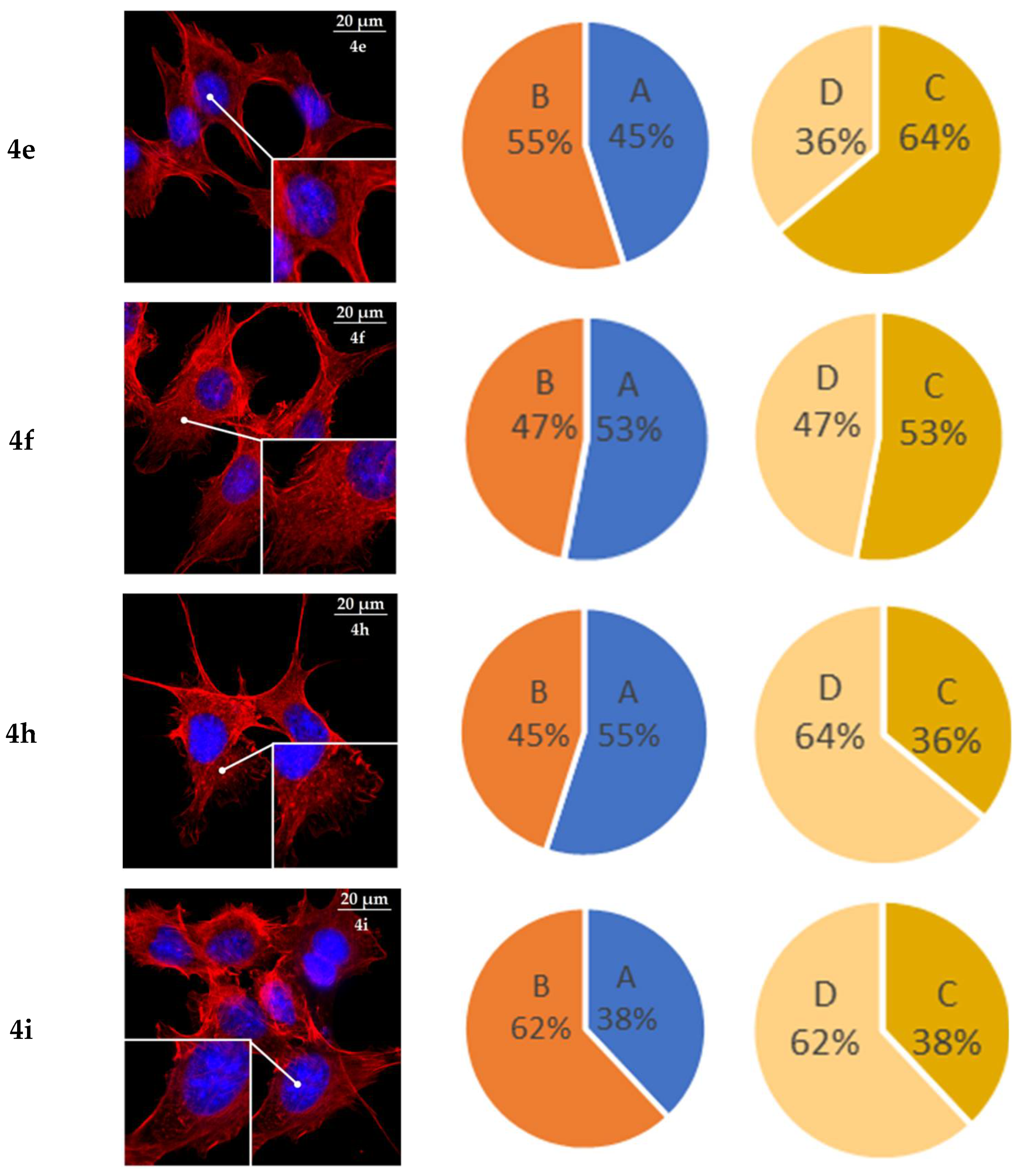
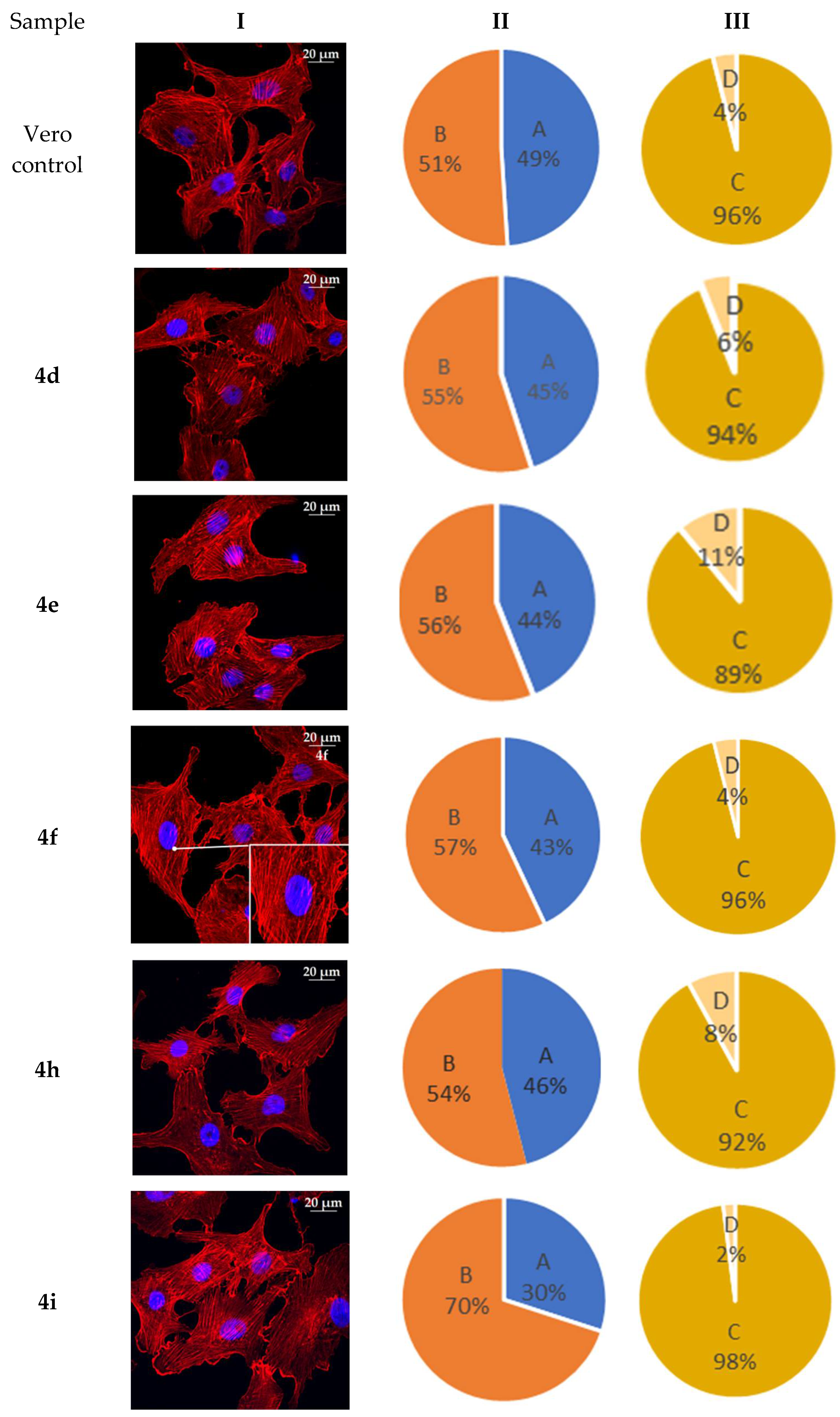
2.6. Actine Cytoskeleton Changes
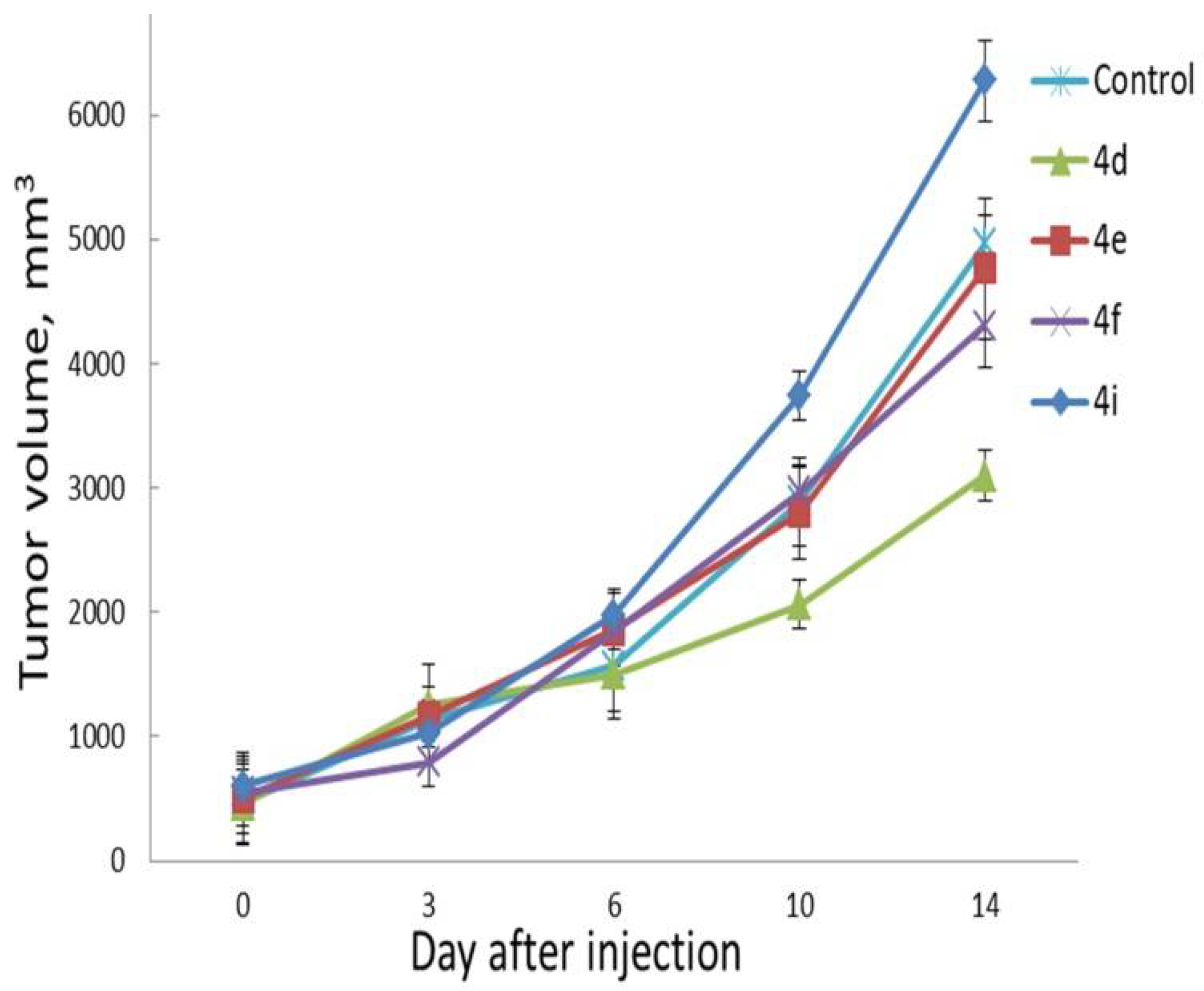
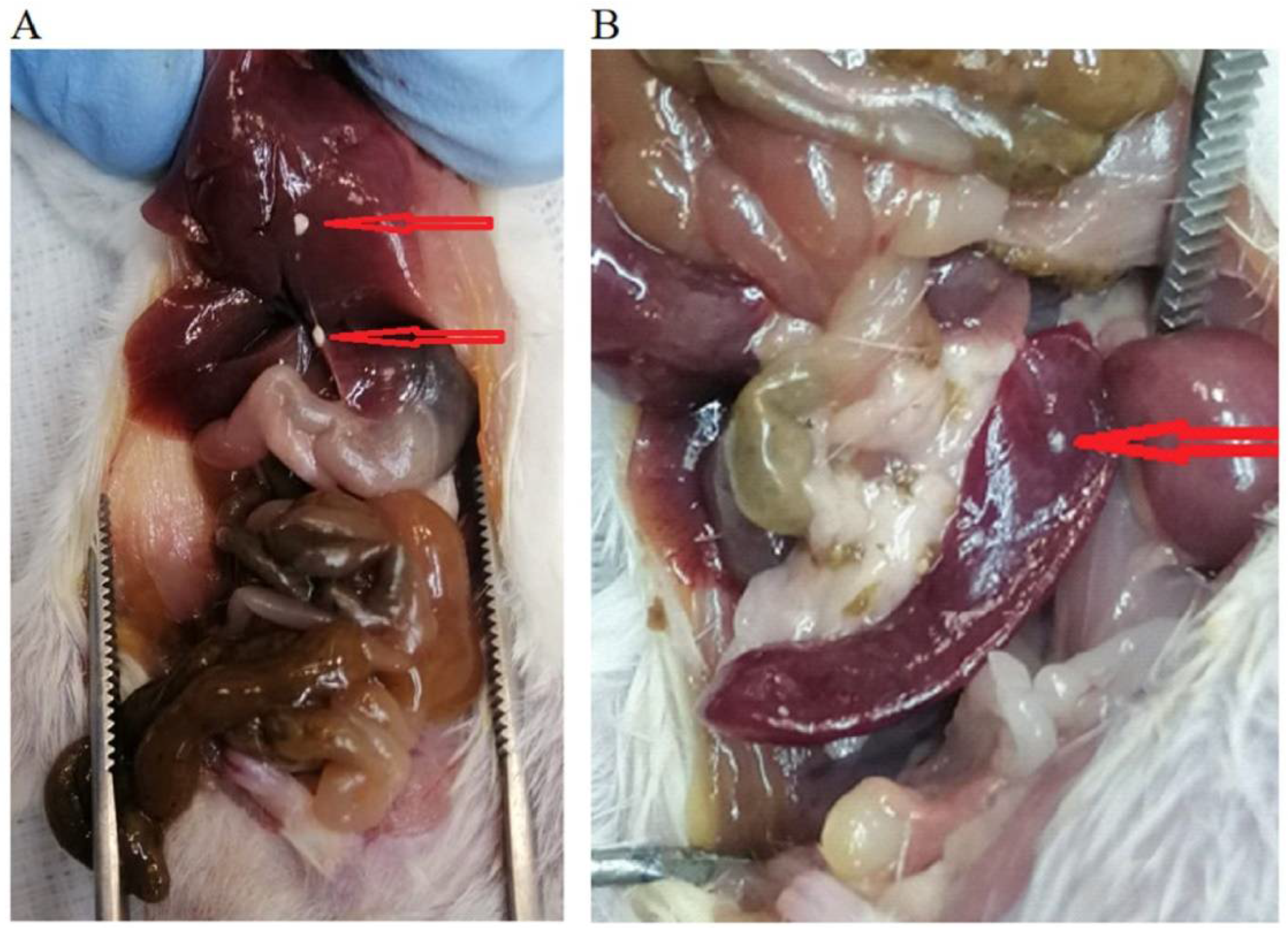
2.7. In Vivo Evaluation
3. Materials and Methods
3.1. Cell Culture and Culturing Conditions
3.2. Cell Proliferation Assay
3.3. Cell Distribution over the Different Phases of the Cell Cycle
3.4. Annexin V-FITC/DAPI Staining Assay
3.5. Actin Cytoskeleton Staining
3.6. Evaluation of Cell Motility by Scratch Test
3.7. Laboratory Animals and Ethics Statement
3.8. Assessment of Anti-Tumor Effect In Vivo
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Srinivasulu, V.; Schilf, P.; Ibrahim, S.; Khanfar, M.A.; Sieburth, S.M.; Omar, H.; Sebastian, A.; AlQawasmeh, R.A.; O’Connor, M.J.; Al-Tel, T.H. Multidirectional desymmetrization of pluripotent building block en route to diastereoselective synthesis of complex nature-inspired scaffolds. Nat. Commun. 2018, 9, 4989. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Manjal, S.K.; Rawal, R.K.; Kumar, K. Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg. Med. Chem. 2017, 25, 4533–4552. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Honmura, Y.; Uesugi, S.; Fukushi, E.; Tanaka, K.; Maeda, H.; Kimura, K.; Nehira, T.; Hashimoto, M. Cyclohelminthol X, a Hexa-Substituted Spirocyclopropane from Helminthosporium velutinum yone96: Structural Elucidation, Electronic Circular Dichroism Analysis, and Biological Properties. J. Org. Chem. 2017, 82, 5574–5582. [Google Scholar] [CrossRef]
- Netz, N.; Opatz, T. A Modular Formal Total Synthesis of (±)-Cycloclavine. J. Org. Chem. 2016, 81, 1723–1730. [Google Scholar] [CrossRef]
- Liu, H.-W.; Walsh, C.T. The Chemistry of the Cyclopropyl Group; Rappoport, Z., Ed.; Wiley-VCH: New York, NY, USA, 1987; p. 959. [Google Scholar]
- Chen, P.; Zhu, C.; Zhu, R.; Lin, Z.; Wu, W.; Jiang, H. Synthesis of 3-azabicyclo[3.1.0]hexane derivatives via palladium-catalyzed cyclopropanation of maleimides with N-tosylhydrazones: Practical and facile access to CP-866,087. Org. Biomol. Chem. 2017, 15, 1228–1235. [Google Scholar] [CrossRef]
- Orri, M.; Abraham, L.; Giraldi, A. A Phase 2a Multicenter, Double-Blind, Placebo-Controlled, Crossover Trial to Investigate the Efficacy, Safety, and Toleration of CP-866,087 (a High-Affinity Mu-Opioid Receptor Antagonist) in Premenopausal Women Diagnosed with Female Sexual Arousal Disorder (FSAD). J. Sex. Med. 2013, 10, 2484–2496. [Google Scholar] [CrossRef]
- Lunn, G.; Banks, B.J.; Crook, R.; Feeder, N.; Pettman, A.; Sabnis, Y. Discovery and synthesis of a new class of opioid ligand having a 3-azabicyclo[3.1.0]hexane core. An example of a ‘magic methyl’ giving a 35-fold improvement in binding. Bioorganic Med. Chem. Lett. 2011, 21, 4608–4611. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, Z.; Ye, F.; Ma, J.; Xu, Z.; Bai, X.; Li, L.; Xu, L. Highly efficient desymmetrization of cyclopropenes to azabicyclo[3.1.0]hexanes with five continuous stereogenic centers by copper-catalyzed [3 + 2] cycloadditions. Org. Chem. Front. 2018, 5, 2759–2764. [Google Scholar] [CrossRef]
- Runyon, S.P.; Kormos, C.M.; Gichinga, M.G.; Mascarella, S.W.; Navarro, H.A.; Deschamps, J.R.; Imler, G.H.; Carroll, F.I. Design, synthesis, and biological evaluation of structurally rigid analogues of 4-(3-hydroxyphenyl)piperidine opioid receptor antagonists. J. Org. Chem. 2016, 81, 10383–10391. [Google Scholar] [CrossRef] [PubMed]
- Topczewski, J.J.; Cabrera, P.J.; Saper, N.I.; Sanford, M.S. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 2016, 531, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Moffat, D.; Patel, S.; Day, F.; Belfield, A.; Donald, A.; Rowlands, M.; Wibawa, J.; Brotherton, D.; Stimson, L.; Clark, V.; et al. Discovery of 2-(6-{[(6-Fluoroquinolin-2-yl)methyl]amino}bicyclo[3.1.0]hex-3-yl)-N-hydroxypyrimidine-5-carboxamide (CHR-3996), a Class I Selective Orally Active Histone Deacetylase Inhibitor. J. Med. Chem. 2010, 53, 8663–8678. [Google Scholar] [CrossRef]
- Henry, S.; Anand, J.P.; Twarozynski, J.J.; Brinkel, A.C.; Pogozheva, I.D.; Sears, B.F.; Jutkiewicz, E.M.; Traynor, J.R.; Mosberg, H.I. Aromatic–Amine Pendants Produce Highly Potent and Efficacious Mixed Efficacy μ-Opioid Receptor (MOR)/δ-Opioid Receptor (DOR) Peptidomimetics with Enhanced Metabolic Stability. J. Med. Chem. 2020, 63, 1671–1683. [Google Scholar] [CrossRef]
- Lunn, G.; Roberts, L.R.; Content, S.; Critcher, D.J.; Douglas, S.; Fenwick, A.E.; Gethin, D.M.; Goodwin, G.; Greenway, D.; Greenwood, S.; et al. SAR and biological evaluation of 3-azabicyclo[3.1.0]hexane derivatives as μ opioid ligands. Bioorganic Med. Chem. Lett. 2012, 22, 2200–2203. [Google Scholar] [CrossRef] [PubMed]
- Appel, N.M.; Li, S.H.; Holmes, T.H.; Acri, J.B. Dopamine D3 receptor antagonist (GSK598809) potentiates the hypertensive effects of cocaine in conscious, freely-moving dogs. J. Pharmacol. Exp. Ther. 2015, 354, 484–492. [Google Scholar] [CrossRef]
- Skepper, C.K.; Armstrong, D.; Balibar, C.J.; Bauer, D.; Bellamacina, C.; Benton, B.M.; Bussiere, D.; De Pascale, G.; De Vicente, J.; Dean, C.R.; et al. Topoisomerase Inhibitors Addressing Fluoroquinolone Resistance in Gram-Negative Bacteria. J. Med. Chem. 2020, 63, 7773–7816. [Google Scholar] [CrossRef]
- Komine, T.; Kojima, A.; Asahina, Y.; Saito, T.; Takano, H.; Shibue, T.; Fukuda, Y. Synthesis and Structure−Activity Relationship Studies of Highly Potent Novel Oxazolidinone Antibacterials. J. Med. Chem. 2008, 51, 6558–6562. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Ucha-Udabe, R.; Alamo, C. The history of barbiturates a century after their clinical introduction. Neuropsychiatr. Dis. Treat. 2005, 1, 329–343. [Google Scholar]
- Oliva, A.; De Cillis, G.; Grams, F.; Livi, V.; Zimmermann, G.; Menta, E.; Krell, H.-W. Barbituric Acid Derivatives with Antimetastatic and Antitumor Activity. U.S. Patent 6335332 B1, 1 January 2002. [Google Scholar]
- Bhaskarachar, R.K.; Revanasiddappa, V.G.; Hegde, S.; Balakrishna, J.P.; Reddy, S.Y. Design, synthesis and anticancer activity of functionalized spiro-quinolines with barbituric and thiobarbituric acids. Med. Chem. Res. 2014, 24, 3516–3528. [Google Scholar] [CrossRef]
- King, S.B.; Stratford, E.; Craig, C.; Fifer, E.K. Synthesis and pharmacological evaluation of spiro-analogues of 5-benzyl-5-ethyl barbituric acid. Pharm. Res. 1995, 12, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Monforte, M.T.; Miceli, N.; Raneri, E. Anticonvulsant and sedative effects of some 5-substituted bromopyrazolinic spirobarbiturates. Farmaco 2001, 56, 459–461. [Google Scholar] [CrossRef]
- Fraser, W.; Suckling, C.J.; Wood, H.C.S. Latent inhibitors. Part 7. Inhibition of dihydro-orotate dehydrogenase by spirocyclopropanobarbiturates. J. Chem. Soc. Perkin Trans. 1 1990, 11, 3137–3144. [Google Scholar] [CrossRef]
- Kim, S.-H.; Pudzianowski, A.T.; Leavitt, K.J.; Barbosa, J.; McDonnell, P.A.; Metzler, W.J.; Rankin, B.M.; Liu, R.; Vaccaro, W.; Pitts, W. Structure-based design of potent and selective inhibitors of collagenase-3 (MMP-13). Bioorganic Med. Chem. Lett. 2005, 15, 1101–1106. [Google Scholar] [CrossRef]
- Taylor, S.N.; Marrazzo, J.; Batteiger, B.E.; Hook, E.W., III; Seña, A.C.; Long, J.; Wierzbicki, M.R.; Kwak, H.; Johnson, S.M.; Lawrence, K.; et al. Single-dose zoliflodacin (ETX0914) for treatment of urogenital gonorrhea. N. Engl. J. Med. 2018, 379, 1835–1845. [Google Scholar] [CrossRef]
- Damião Gouveia, A.C.; Unemo, M.; Jensen, J.S. In vitro activity of zoliflodacin (ETX0914) against macrolide-resistant, fluoroquinolone-resistant and antimicrobial-susceptible Mycoplasma genitalium strains. J. Antimicrob. Chemother. 2018, 73, 1291–1294. [Google Scholar] [CrossRef]
- Wang, S.; Filatov, A.S.; Lozovskiy, S.V.; Shmakov, S.V.; Khoroshilova, O.V.; Larina, A.G.; Selivanov, S.I.; Boitsov, V.M.; Stepakov, A.V. Construction of Spiro[3-azabicyclo[3.1.0]hexanes] via 1,3-Dipolar Cycloaddition of 1,2-Diphenylcyclopropenes to Ninhydrin-Derived Azomethine Ylides. Synthesis 2021, 53, 2114–2132. [Google Scholar] [CrossRef]
- Filatov, A.S.; Knyazev, N.A.; Molchanov, A.P.; Panikorovsky, T.L.; Kostikov, R.R.; Larina, A.G.; Boitsov, V.M.; Stepakov, A.V. Synthesis of Functionalized 3-Spiro[cyclopropa[a]pyrrolizine]-and 3-Spiro[3-azabicyclo[3.1.0]hexane]oxindoles from Cyclopropenes and Azomethine Ylides via [3 + 2]-Cycloaddition. J. Org. Chem. 2017, 82, 959–975. [Google Scholar] [CrossRef]
- Filatov, A.S.; Knyazev, N.A.; Shmakov, S.V.; Bogdanov, A.A.; Ryazantsev, M.N.; Shtyrov, A.A.; Starova, G.L.; Molchanov, A.P.; Larina, A.G.; Boitsov, V.M.; et al. Concise Synthesis of Tryptanthrin Spiro Analogues with In Vitro Antitumor Activity Based on One-Pot, Three-Component 1,3-Dipolar Cycloaddition of Azomethine Ylides to Cyclopropenes. Synthesis 2019, 51, 713–729. [Google Scholar] [CrossRef]
- Filatov, A.S.; Knyazev, N.A.; Ryazantsev, M.N.; Suslonov, V.V.; Larina, A.G.; Molchanov, A.P.; Kostikov, R.R.; Boitsov, V.M.; Stepakov, A.V. A highly diastereoselective one-pot three-component 1,3-dipolar cycloaddition of cyclopropenes with azomethine ylides generated from 11H-indeno[1,2- b]-quinoxalin-11-ones. Org. Chem. Front. 2018, 5, 595–605. [Google Scholar] [CrossRef]
- Filatov, A.S.; Wang, S.; Khoroshilova, O.V.; Lozovskiy, S.V.; Larina, A.G.; Boitsov, V.M.; Stepakov, A.V. Stereo- and Regioselective 1,3-Dipolar Cycloaddition of the Stable Ninhydrin-Derived Azomethine Ylide to Cyclopropenes: Trapping of Unstable Cyclopropene Dipolarophiles. J. Org. Chem. 2019, 84, 7017–7036. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, N.A.; Shmakov, S.V.; Pechkovskaya, S.A.; Filatov, A.S.; Stepakov, A.V.; Boitsov, V.M.; Filatova, N.A. Identification of Spiro-Fused [3-azabicyclo[3.1.0]hexane]oxindoles as Potential Antitumor Agents: Initial In Vitro Evaluation of Anti-Proliferative Effect and Actin Cytoskeleton Transformation in 3T3 and 3T3-SV40 Fibroblast. Int. J. Mol. Sci. 2021, 22, 8264. [Google Scholar] [CrossRef] [PubMed]
- Latypova, D.K.; Shmakov, S.V.; Pechkovskaya, S.A.; Filatov, A.S.; Stepakov, A.V.; Knyazev, N.A.; Boitsov, V.M. Identification of Spiro-Fused Pyrrolo[3,4-a]pyrrolizines and Tryptanthrines as Potential Antitumor Agents: Synthesis and In Vitro Evaluation. Int. J. Mol. Sci. 2021, 22, 11997. [Google Scholar] [CrossRef] [PubMed]
- Surget, S.; Khoury, M.P.; Bourdon, J.C. Uncovering the role of p53 splice variants in human malignancy: A clinical perspective. OncoTargets Ther. 2014, 7, 57–68. [Google Scholar] [CrossRef]
- Taylor, W.R.; DePrimo, S.E.; Agarwal, A.; Agarwal, M.L.; Schonthal, A.H.; Katula, K.S.; Stark, G.R. Mechanisms of G2 arrest in response to overexpression of p53. Mol. Biol. Cell 1999, 10, 3607–3622. [Google Scholar] [CrossRef]
- Filatov, A.S.; Selivanov, S.I.; Shmakov, S.V.; Larina, A.G.; Boitsov, V.M.; Stepakov, A.V. An Experimental and Theoretical Study of the 1,3-Dipolar Cycloaddition of Alloxan-Derived Azomethine Ylides to Cyclopropenes. Synthesis 2022, 54, 1803–1816. [Google Scholar] [CrossRef]
- Stuelten, C.H.; Parent, C.A.; Montell, D.J. Cell motility in cancer invasion and metastasis: Insights from simple model organisms. Nat. Rev. Cancer 2018, 18, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers—Assembly, dynamics and biological roles. J. Cell. Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef]
- Sahai, E.; Marshall, C.J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell. Biol. 2003, 5, 711–719. [Google Scholar] [CrossRef]
- Spano, A.; Monaco, G.; Barni, S.; Sciola, L. Cisplatin treatment of NIH/3T3 cultures induces a form of autophagic death in polyploid cells. Histol. Histopathol. 2008, 23, 717–730. [Google Scholar] [CrossRef]
- Bonello, T.T.; Stehn, J.R.; Gunning, P.W. New approaches to targeting the actin cytoskeleton for chemotherapy. Future Med. Chem. 2009, 1, 1311–1331. [Google Scholar] [CrossRef] [PubMed]
- Northcott, J.M.; Dean, I.S.; Mouw, J.K.; Weaver, V.M. Feeling Stress: The Mechanics of Cancer Progression and Aggression. Front. Cell. Dev. Biol. 2018, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Mouw, J.K.; Weaver, V.M. Forcing form and function: Biomechanical regulation of tumor evolution. Trends Cell. Biol. 2011, 21, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Brayford, S.; Schevzov, G.; Vos, J.; Gunning, P. The Role of the Actin Cytoskeleton in Cancer and Its Potential Use as a Therapeutic Target. In The Cytoskeleton in Health and Disease; Schatten, H., Ed.; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Babushkina, A.A.; Dogadina, A.V.; Egorov, D.M.; Piterskaia, J.L.; Shtro, A.A.; Nikolaeva, Y.V.; Galochkina, A.V.; Kornev, A.A.; Boitsov, V.M. Efficient synthesis and evaluation of antiviral and antitumor activity of novel 3-phosphonylated thiazolo[3,2-a]oxopyrimidines. Med. Chem. Res. 2021, 30, 2203–2215. [Google Scholar] [CrossRef]
- Aseervatham, J. Cytoskeletal Remodeling in Cancer. Biology 2020, 9, 385. [Google Scholar] [CrossRef]
- Reddy, V.G.; Reddy, T.S.; Nayak, V.L.; Prasad, B.; Reddy, A.P.; Ravikumar, A.; Taj, S.; Kamal, A. Design, synthesis and biological evaluation of N-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-1,3-diphenyl-1Hpyrazole-4-carboxamides as CDK1/Cdc2 inhibitors. Eur. J. Med. Chem. 2016, 122, 164–177. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. 1972, 3, 59–61. [Google Scholar]

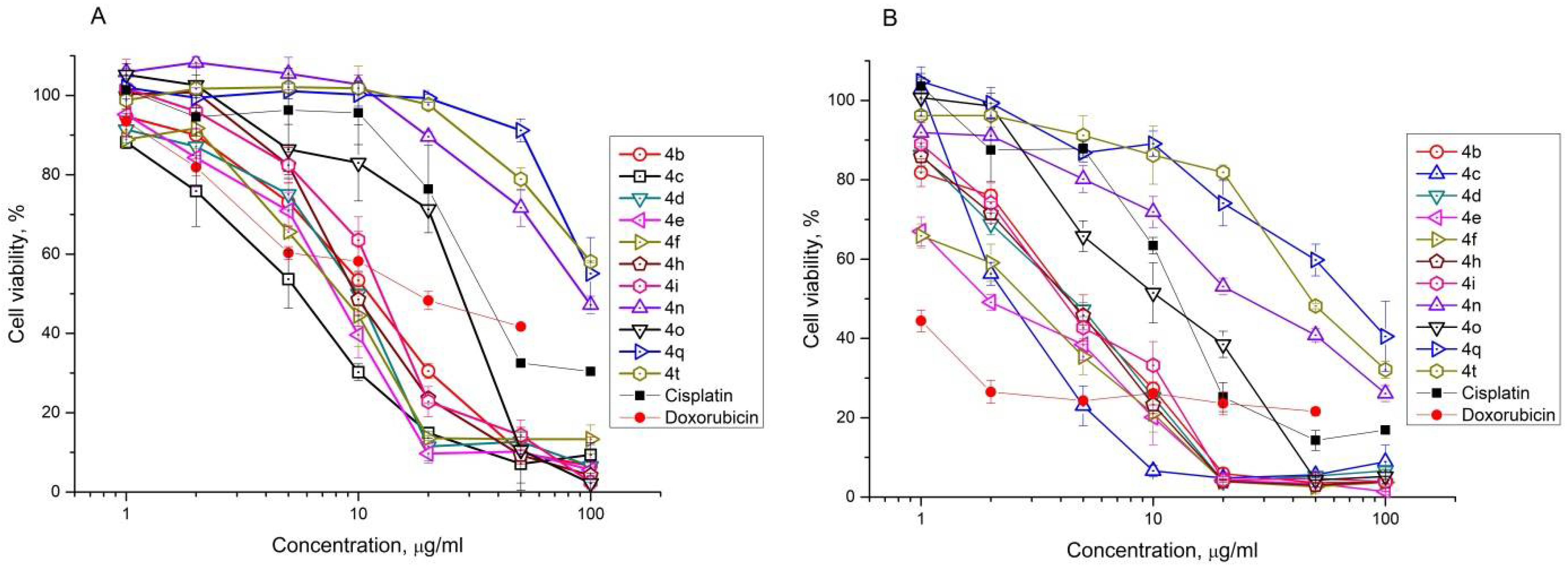
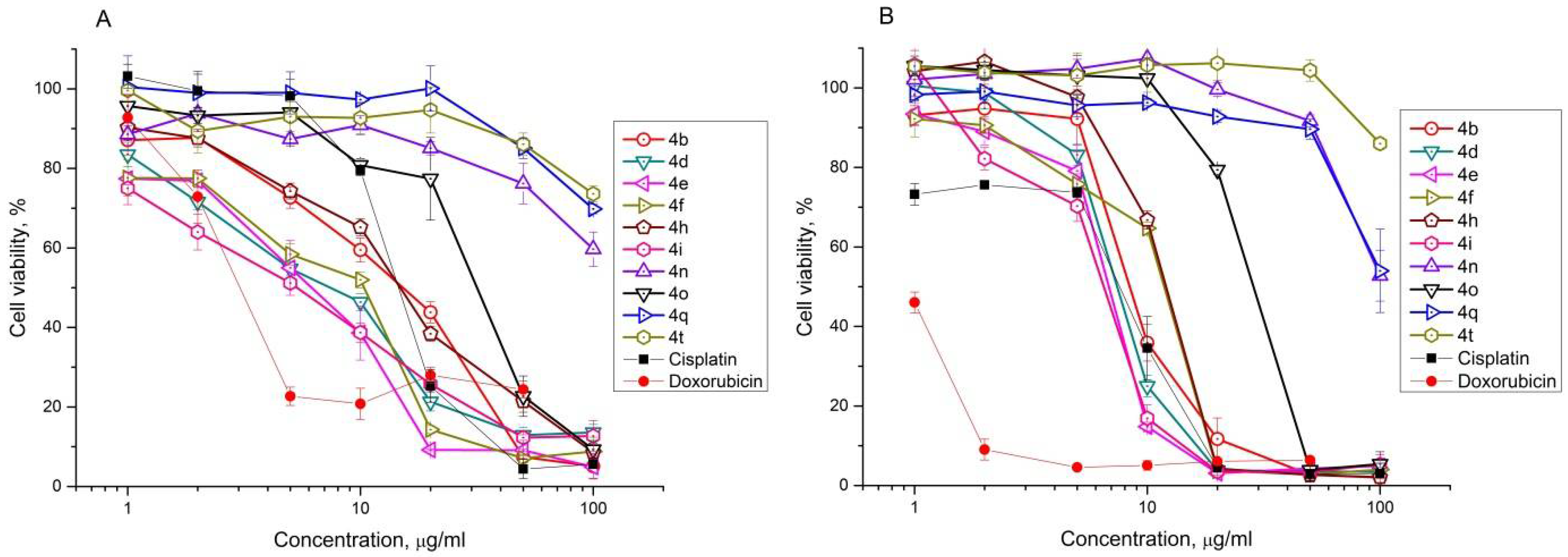
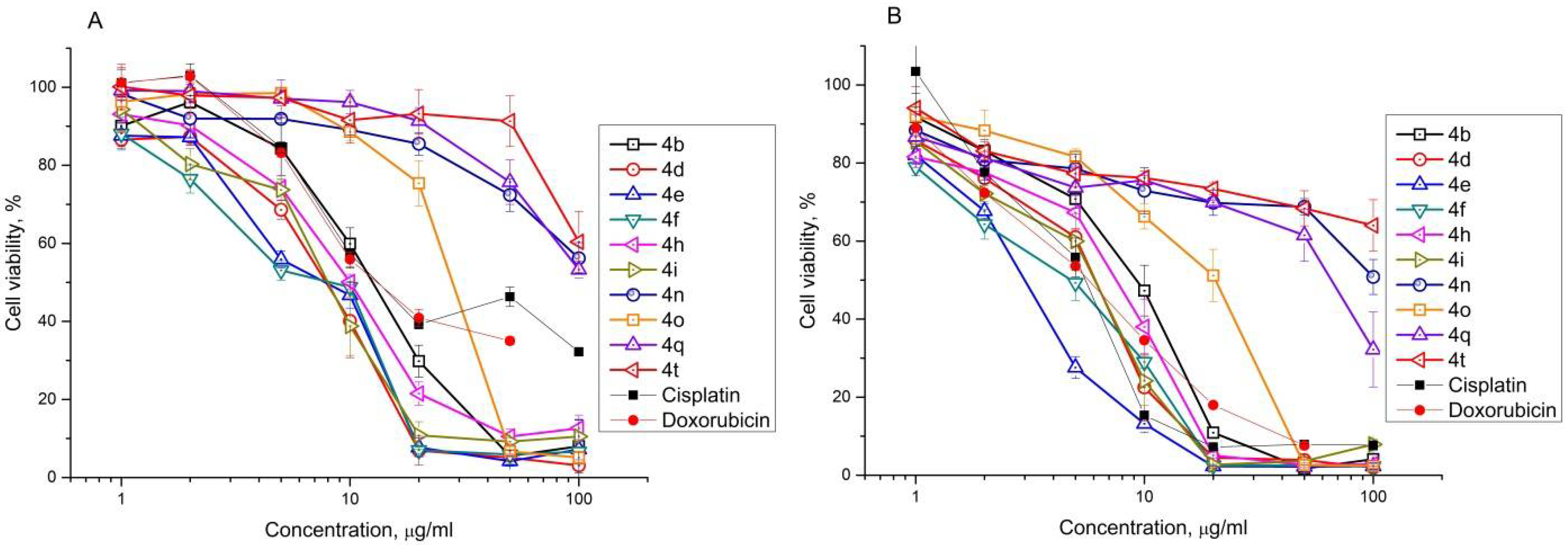
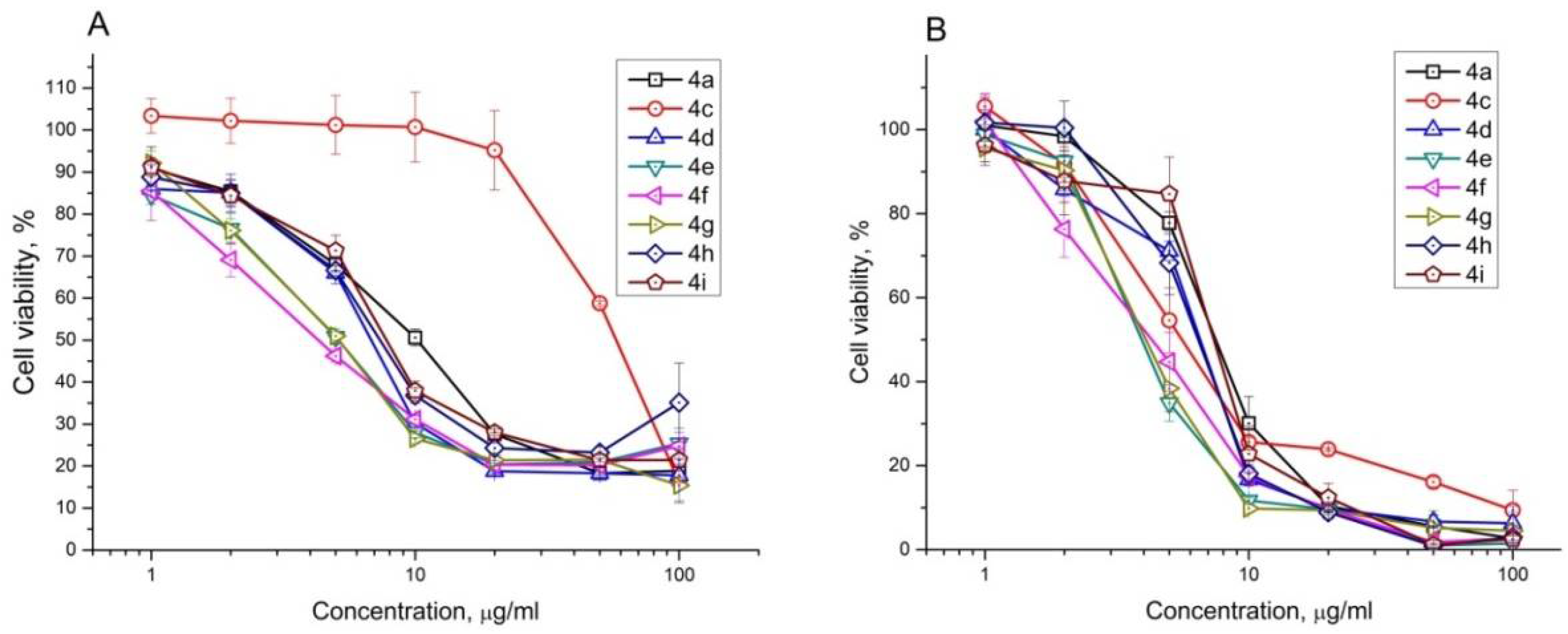

| Compound | IC50, μM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K562 | HeLa | CT26 | Jurkat | Vero | ||||||||||
| 24 h | 72 h | SI | 24 h | 72 h | SI | 24 h | 72 h | SI | 24 h | 72 h | SI | 24 h | 72 h | |
| 4a | 47 ± 8 | 14 ± 1 | 1.0 | 125 ± 18 | 70 ± 4 | 0.2 | 64 ± 7 | 50 ± 7 | 0.3 | 24 ± 2 | 19 ± 5 | 0.8 | 50 ± 14 | 14 ± 2 |
| 4b | 25 ± 5 | 9 ± 2 | 2.4 | 34 ± 2 | 18 ± 2 | 1.2 | 30 ± 2 | 21 ± 2 | 1.0 | - | - | - | 55 ± 7 | 21 ± 2 |
| 4c | 12 ± 2 | 5 ± 2 | 3.2 | 21 ± 2 | 13 ± 2 | 1.3 | 27 ± 2 | 20 ± 2 | 0.8 | 149 ± 33 | 15 ± 2 | 1.1 | 47 ± 11 | 16 ± 2 |
| 4d | 21 ± 4 | 9 ± 2 | 1.8 | 17 ± 2 | 15 ± 2 | 1.1 | 17 ± 2 | 13 ± 2 | 1.3 | 15 ± 2 | 13 ± 2 | 1.3 | 48 ± 4 | 16 ± 2 |
| 4e | 17 ± 2 | 4 ± 2 | 2.5 | 13 ± 2 | 13 ± 4 | 0.8 | 10 ± 2 | 6 ± 2 | 1.7 | 12 ± 2 | 9 ± 2 | 1.2 | 23 ± 2 | 10 ± 2 |
| 4f | 17 ± 4 | 6 ± 2 | 1.7 | 19 ± 2 | 23 ± 6 | 0.5 | 17 ± 2 | 10 ± 2 | 1.0 | 11 ± 2 | 9 ± 2 | 1.2 | 48 ± 6 | 10 ± 2 |
| 4g | 19 ± 2 | 10 ± 2 | 1.4 | 15 ± 2 | 13 ± 2 | 1.1 | 22 ± 2 | 12 ± 2 | 1.2 | 11 ± 2 | 8 ± 2 | 1.8 | 33 ± 6 | 14 ± 2 |
| 4h | 20 ± 4 | 10 ± 2 | 2.2 | 28 ± 2 | 24 ± 6 | 1.0 | 22 ± 2 | 16 ± 2 | 1.4 | 18 ± 4 | 13 ± 4 | 1.7 | 31 ± 4 | 22 ± 4 |
| 4i | 26 ± 4 | 8 ± 4 | 2.9 | 10 ± 2 | 12 ± 6 | 2.0 | 16 ± 2 | 12 ± 2 | 2.0 | 19 ± 2 | 14 ± 4 | 1.7 | 97 ± 12 | 23 ± 4 |
| Cisplatin | 46 ± 8 | 14 ± 2 | 0.3 | 16 ± 4 | 6 ± 1 | 0.7 | 24 ± 4 | 5 ± 1 | 0.8 | - | - | - | 14 ± 1 | 4 ± 1 |
| Doxorubicin | 16 ± 3 | 1.2 ± 0.3 | 2.0 | 4 ± 1 | 0.5 ± 0.1 | 4.8 | 19 ± 3 | 5.5 ± 0.3 | 0.5 | - | - | - | 13 ± 2 | 2.4 ± 0.4 |
| A | HeLa | Live Cells, % | Early Apoptotic Cells, % | Late Apoptotic Cells, % | Necrotic Cells, % |
| Control | 89.0 ± 0.7 | 4.5 ± 0.7 | 5.9 ± 0.4 | 0.5 ± 0.3 | |
| 4c | 81.0 ± 4.4 * | 5.6 ± 1.3 | 1.9 ± 2.8 * | 2.2 ± 0.5 * | |
| 4d | 46.8 ± 18.4 * | 11.6 ± 0.2 * | 38.1 ± 16.8 * | 3.6 ± 1.8 * | |
| 4e | 15.0 ± 2.7 * | 18.1 ± 3.1 * | 61.3 ± 3.8 * | 5.6 ± 2.6 * | |
| 4f | 90.9 ± 1.6 | 2.9 ± 0.1 * | 4.1 ± 0.5 * | 2.1 ± 1.3 * | |
| 4g | 17.1 ± 3.5 * | 38.5 ± 1.9 * | 38.0 ± 6.9 * | 6.3 ± 2.8 * | |
| 4h | 72.2 ± 0.4 * | 9.8 ± 1.0 * | 15.3 ± 0.5 * | 1.9 ± 0.2 * | |
| 4i | 67.9 ± 2.4 * | 12.6 ± 0.8 * | 17.3 ± 1.4 * | 2.1 ± 0.9 * | |
| B | CT26 | Live Cells, % | Early Apoptotic Cells, % | Late Apoptotic Cells, % | Necrotic Cells, % |
| Control | 88.5 ± 1.1 | 10.5 ± 0.7 | 0.9 ± 0.4 | 0.0 ± 0.0 | |
| 4c | 89.8 ± 0.5 | 1.9 ± 0.3 * | 6.4 ± 1.0 * | 1.8 ± 0.8 * | |
| 4d | 32.8 ± 3.0 * | 9.6 ± 0.5 | 56.4 ± 2.4 * | 1.1 ± 0.3 * | |
| 4e | 13.5 ± 0.4 * | 23.4 ± 0.8 * | 61.6 ± 0.5 * | 1.4 ± 0.1 * | |
| 4f | 34.1 ± 3.8 * | 6.2 ± 1.2 * | 57.8 ± 3.1 * | 1.9 ± 0.5 * | |
| 4g | 84.2 ± 0.3 * | 5.5 ± 0.1 * | 10.0 ± 0.3 * | 0.3 ± 0.1 * | |
| 4h | 70.3 ± 0.6 * | 4.8 ± 0.5 * | 24.0 ± 0.7 * | 0.9 ± 0.3 * | |
| 4i | 76.2 ± 0.3 * | 4.0 ± 0.1 * | 19.5 ± 0.5 * | 0.4 ± 0.1 * |
| A | HeLa | Control | 4c | 4d | 4e | 4f | 4g | 4h | 4i |
| G0/G1 (%) | 73.1 ± 2.6 | 63.3 ± 2.2 * | 58.0 ± 4.4 * | 43.1 ± 1.4 * | 61.5 ± 3.3 * | 41.8 ± 3.0 * | 63.6 ± 6.8 * | 51.8 ± 4.0 * | |
| S (%) | 12.5 ± 1.7 | 17.6 ± 1.2 * | 15.5 ± 1.1 * | 16.7 ± 1.2 * | 15.2 ± 1.2 * | 13.3 ± 0.8 | 13.9 ± 1.8 | 18.1 ± 2.4 * | |
| G2/M (%) | 12.2 ± 1.3 | 11.0 ± 1.5 | 8.4 ± 1.5 * | 5.7 ± 0.9 * | 12.8 ± 1.3 | 5.3 ± 0.7 * | 12.6 ± 0.8 | 9.9 ± 0.8 * | |
| SubG1 (%) | 2.3 ± 0.4 | 8.1 ± 1.9 * | 18.1 ± 1.0 * | 34.4 ± 5.8 * | 10.5 ± 2.0 * | 39.5 ± 5.0 * | 9.9 ± 7.8 * | 20.1 ± 1.3 * | |
| B | CT26 | Control | 4c | 4d | 4e | 4f | 4g | 4h | 4i |
| G0/G1 (%) | 44.0 ± 1.7 | 68.5 ± 3.0 * | 35.6 ± 1.0 * | 38.1 ± 6.4 * | 29.7 ± 1.4 * | 61.7 ± 9.4 * | 38.3 ± 7.9 * | 33.2 ± 0.6 * | |
| S (%) | 40.1 ± 1.5 | 16.3 ± 1.7 * | 34.4 ± 2.3 * | 28.5 ± 1.8 * | 31.9 ± 2.6 * | 17.0 ± 4.3 * | 32.0 ± 0.6 * | 37.2 ± 0.2 * | |
| G2/M (%) | 12.3 ± 0.9 | 13.2 ± 2.9 * | 17.9 ± 0.6 * | 14.1 ± 9.4 | 13.5 ± 0.9 * | 19.4 ± 4.7 * | 18.9 ± 6.8 * | 22.4 ± 1.7 * | |
| SubG1 (%) | 3.6 ± 0.6 | 2.0 ± 0.4 * | 12.2 ± 1.1 * | 19.3 ± 5.1 * | 24.9 ± 5.2 * | 1.9 ± 0.5 * | 10.8 ± 2.3 * | 7.2 ± 2.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmakov, S.V.; Latypova, D.K.; Shmakova, T.V.; Rubinshtein, A.A.; Chukin, M.V.; Zhuravskii, S.G.; Knyazev, N.A.; Stepakov, A.V.; Galagudza, M.M.; Boitsov, V.M. Biological Evaluation of 3-Azaspiro[Bicyclo[3.1.0]Hexane-2,5′-Pyrimidines] as Potential Antitumor Agents. Int. J. Mol. Sci. 2022, 23, 10759. https://doi.org/10.3390/ijms231810759
Shmakov SV, Latypova DK, Shmakova TV, Rubinshtein AA, Chukin MV, Zhuravskii SG, Knyazev NA, Stepakov AV, Galagudza MM, Boitsov VM. Biological Evaluation of 3-Azaspiro[Bicyclo[3.1.0]Hexane-2,5′-Pyrimidines] as Potential Antitumor Agents. International Journal of Molecular Sciences. 2022; 23(18):10759. https://doi.org/10.3390/ijms231810759
Chicago/Turabian StyleShmakov, Stanislav V., Diana K. Latypova, Tatiana V. Shmakova, Artem A. Rubinshtein, Mark V. Chukin, Sergei G. Zhuravskii, Nickolay A. Knyazev, Alexander V. Stepakov, Michael M. Galagudza, and Vitali M. Boitsov. 2022. "Biological Evaluation of 3-Azaspiro[Bicyclo[3.1.0]Hexane-2,5′-Pyrimidines] as Potential Antitumor Agents" International Journal of Molecular Sciences 23, no. 18: 10759. https://doi.org/10.3390/ijms231810759
APA StyleShmakov, S. V., Latypova, D. K., Shmakova, T. V., Rubinshtein, A. A., Chukin, M. V., Zhuravskii, S. G., Knyazev, N. A., Stepakov, A. V., Galagudza, M. M., & Boitsov, V. M. (2022). Biological Evaluation of 3-Azaspiro[Bicyclo[3.1.0]Hexane-2,5′-Pyrimidines] as Potential Antitumor Agents. International Journal of Molecular Sciences, 23(18), 10759. https://doi.org/10.3390/ijms231810759







