Influence of Moisture in Quartzite on the Lining Properties and Efficiency of Industrial-Frequency Induction Crucible Furnaces
Abstract
:1. Introduction
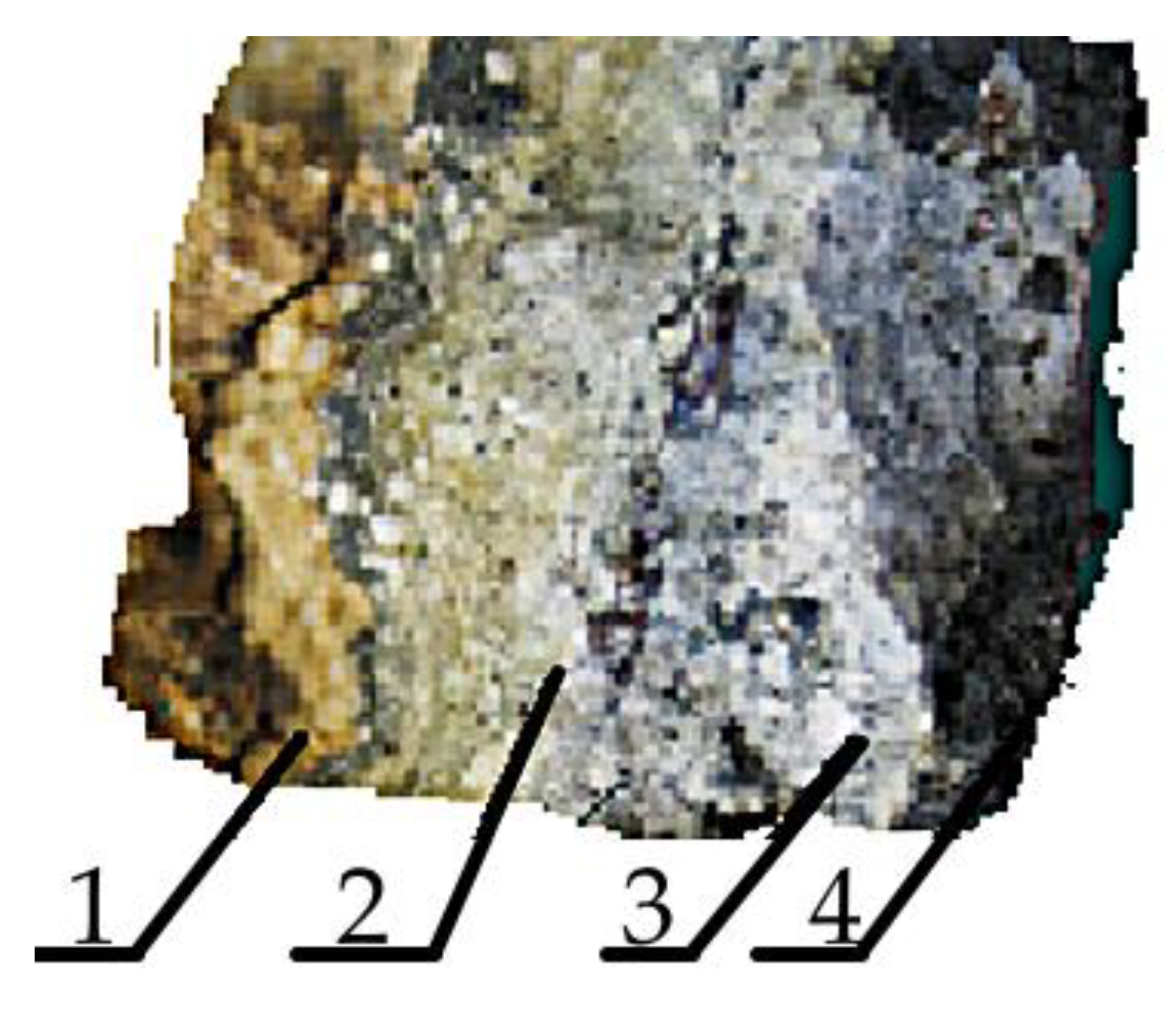
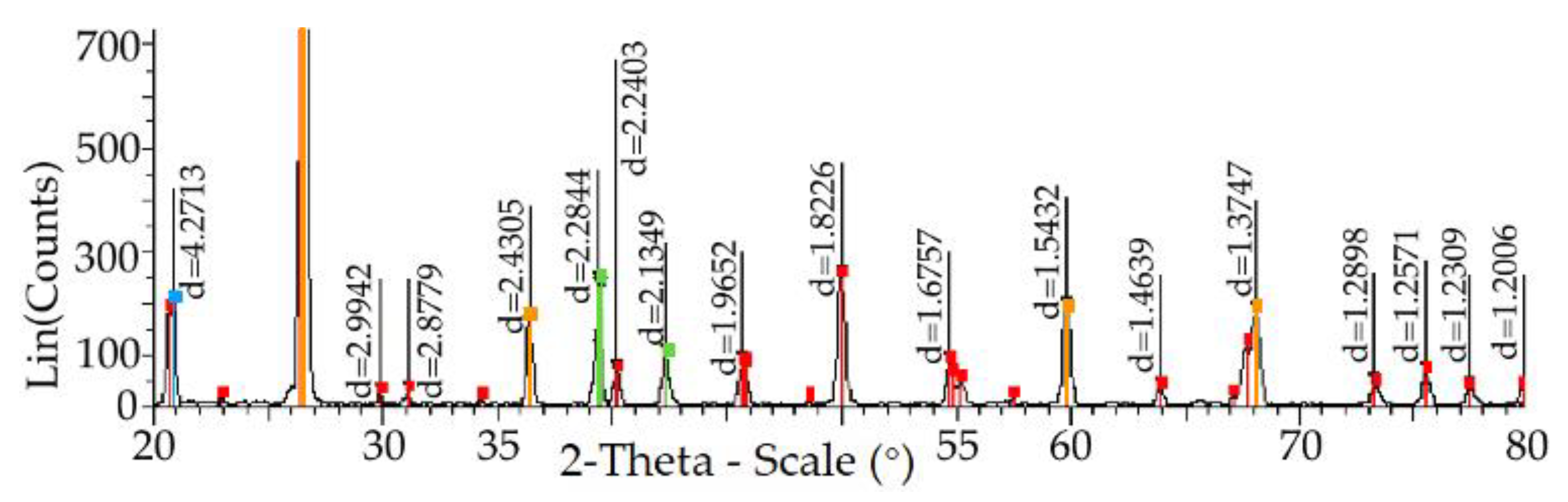
- Studies have found that the minimum amount of GLI leads to trisimite, and the maximum is cristobalite, which is detected after its temperature treatment during drying and the further exposure to temperature melting conditions;
- Impurities detected by the X-ray fluorescent wavodispersion spectrometer Shimadzu XRF-1800 in the amount of 1.149% are found not to be mineralizers, but despite this, when using a traditional proclamator at 800 °C, the phase transformation of the quartzite remains unchanged. At 600 °C, a polymorphic transformation occurs, which results in the emergence of a tridimite elementary cell, and cristobalite cells appear only at 1550 °C;
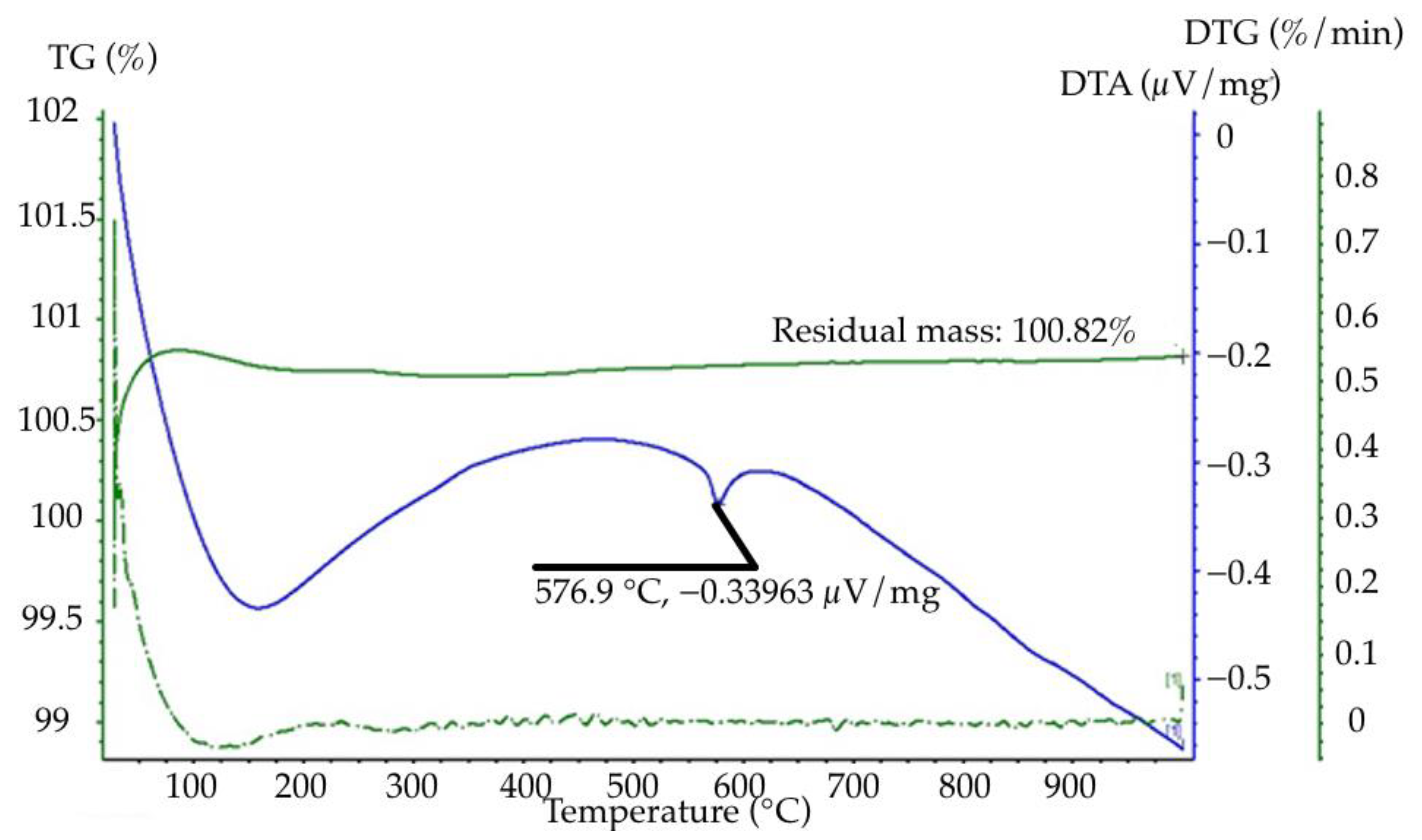
- Studies using the derivative STA 449 F1 Jupiter showed that free water is removed from the quartzite at temperatures of 170–200 °C. This is characterized by the first endoeffect derivative and refers to the loss of adsorbed (physically bound) water. The second endoeffect is observed at a temperature of 570 °C and relates to polymorphic transformation leading to alpha-quartz phase formation in beta-quartz. It is accompanied by the beginning of the process of removing chemically bound water and is not accompanied by a change in mass;
- Moisture has been found to act as a mineralizer, and its condition influences the formation of specific phases. This assumption was made in the work [39]. Temperature treatment at 800 °C results in the complete removal of free water and a significant amount of chemically bonded water, including GLI, which reduces the efficiency of the structural conversion of quartzite to cristobalite and promotes the formation of tridymite;
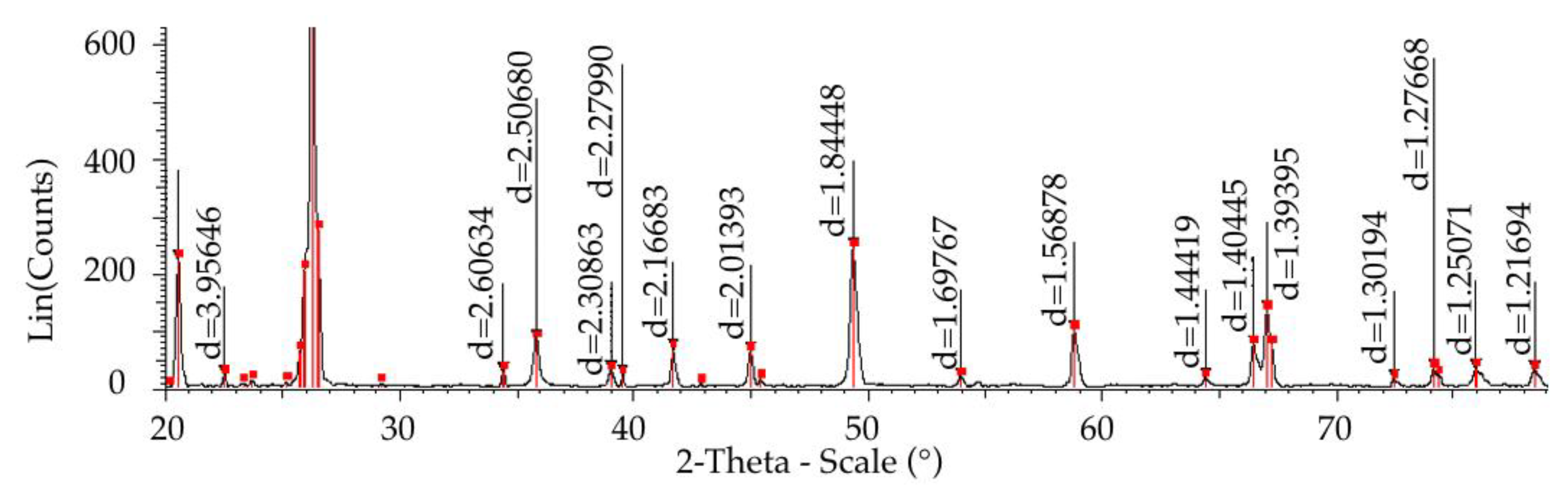
- Drying at 200 °C results only in the removal of free water and contributes to the process of crystallization. Cristobalite begins to appear at 870 °C; the tridimite phase is not detected even at 1550 °C;
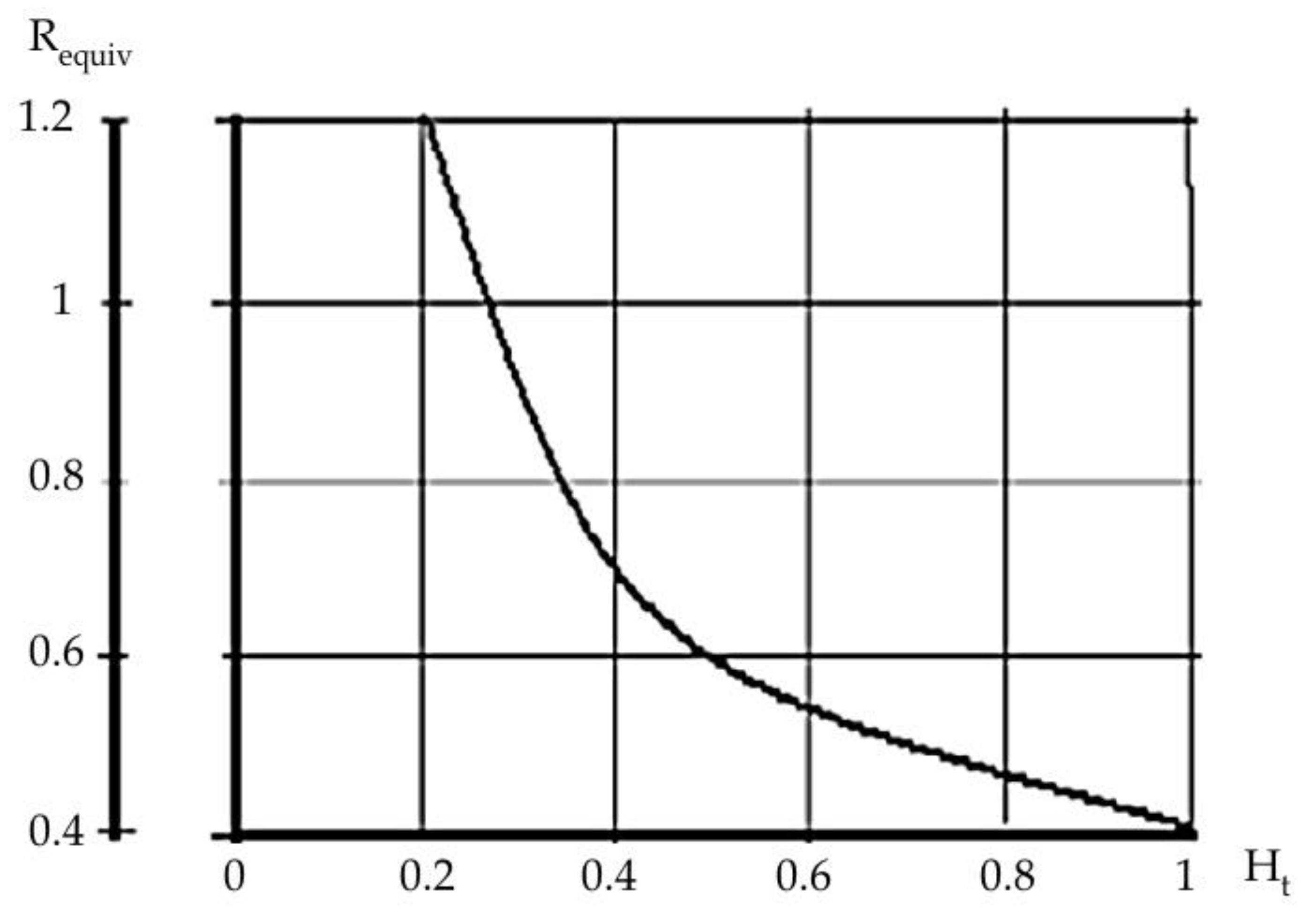
- It has been determined that tridymit, within the temperature range of 1000–1550 °C (these are the working temperatures at the smelting of synthetic cast iron from metal filling consisting of up to 90% steel scrap), allows for volume changes in the limit of 9% and cristobalite in the limit of 15%.
2. Materials and Methods
2.1. Formulation of the Problem
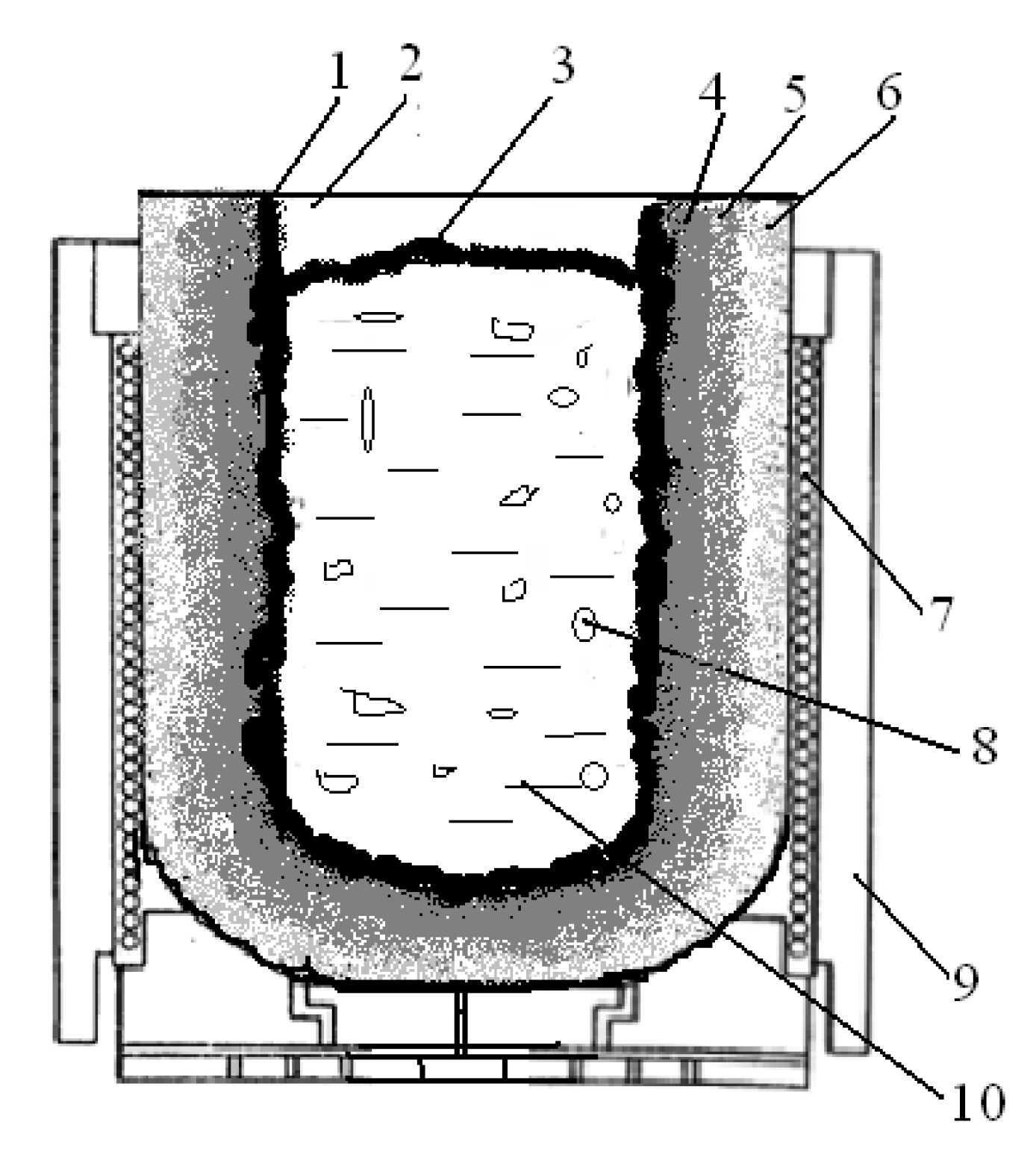
2.2. Description of the Field Experiment
- The remainder is not grid 2, including 6–15;
- The balance on the grid is 3.2, not more than 5;
- Pass through the grid 05, including 50–59;
- Pass through grid 01-31-41.
2.3. Experimental Research Methodology
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Legemza, J.; Findorák, R.; Bul’ko, B.; Briančin, J. New Approach in Research of Quartzes and Quartzites for Ferroalloys and Silicon Production. Metal 2021, 11, 670. [Google Scholar] [CrossRef]
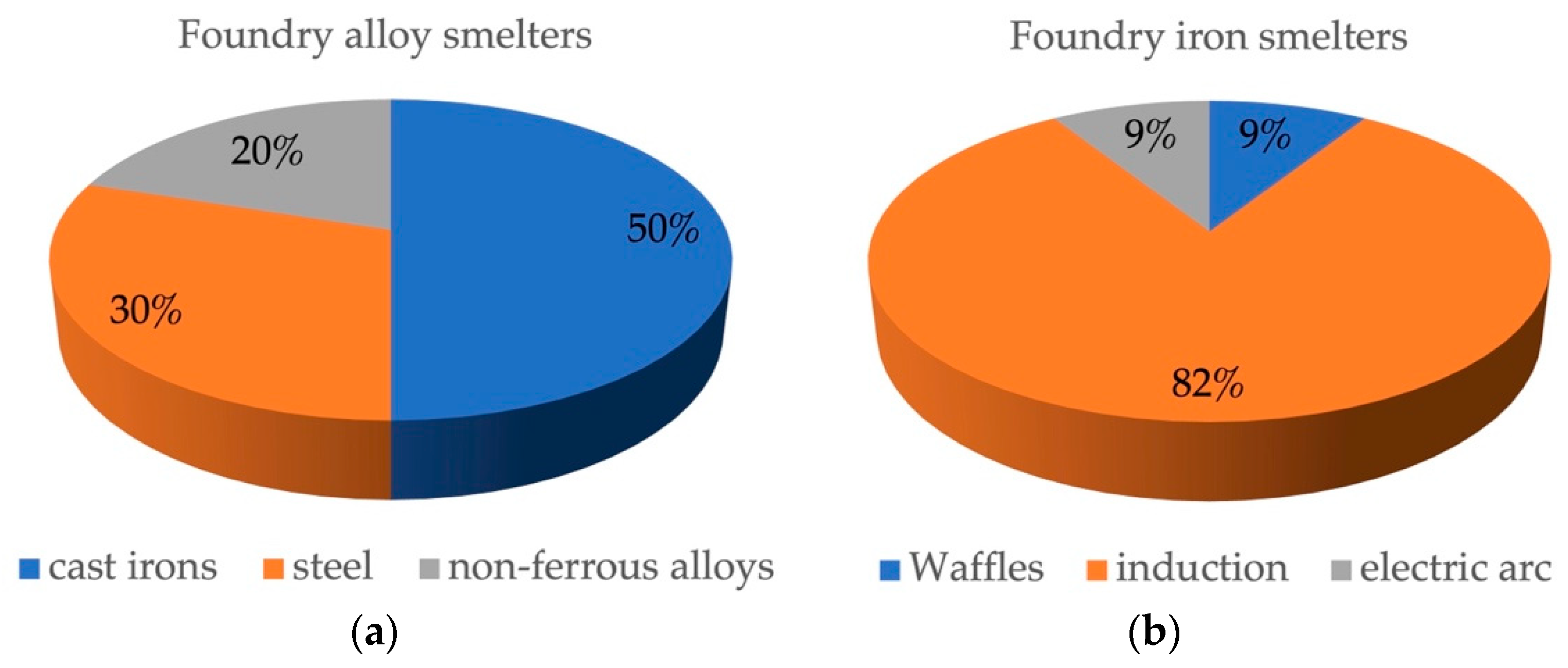
- Mirzaev, B.; Bozarova, M. Status and Prospects of Foundry Production in Russia. In Proceedings of the Innovative Approaches in Modern Science, Materials of the CVIII International Scientific and Practical Conference, Moscow, Russia, 21 December 2021; pp. 92–95. [Google Scholar]
- Adetunji, O.; Ojo, S.S.; Oyetunji, A.; Itua, N.; Adetunji, O.; Ojo, S.S.; Oyetunji, A.; Itua, N. Melting Time Prediction Model for Induction Furnace Melting Using Specific Thermal Consumption from Material Charge Approach. J. Miner. Mater. Charact. Eng. 2020, 9, 61–74. [Google Scholar] [CrossRef]
- Sassa, V.S. Lining of Induction Melting Furnaces and Mixers; Energoatomizdat: Moscow, Russia, 1983. [Google Scholar]
- Kostyukova, A.P. Institutional Method as the Basis for Monitoring the Induction Melting Furnace. Adv. Mod. Sci. 2017, 4, 19–23. [Google Scholar]
- Druzhevsky, M.A.; Subdue, B. Lining of Induction Melting Furnaces with Materials Based on Quartzite. Liteynoye Proizv. 2010, 4, 33–38. [Google Scholar]
- Futas, P.; Pribulova, A.; Petrik, J.; Pokusova, M.; Junakova, A. The Study of Synthetic Cast Iron Quality Made from Steel Scrap. In Proceedings of the 18th International Multidisciplinary Scientific GeoConference SGEM 2018, Vienna, Austria, 3–7 December 2018; pp. 321–329. [Google Scholar]
- Nelub, V.; Gantimurov, A.; Borodulin, A. Economic Analysis of Data Protection in Systems with Complex Architecture Using Neural Network Methods. Econ. Ann. 2020, 185, 178–188. [Google Scholar] [CrossRef]
- Edalati, K.; Akhlaghi, F.; Nili-Ahmadabadi, M. Influence of SiC and FeSi Addition on the Characteristics of Gray Cast Iron Melts Poured at Different Temperatures. J. Mater. Process. Technol. 2005, 160, 183–187. [Google Scholar] [CrossRef]
- Zavertkin, A.S. Development of the Composition of the Lining of Induction Furnaces from Pervouralsk Quartzite and the Practice of Its Application. Proc. Kola Sci. Cent. Russ. Acad. Sci. 2015, 44, 532–534. [Google Scholar]
- Nelyub, V.A. The Effect of Copper and Zinc Coatings on the Properties of Carbon Fibers and Composites Based on Them. Polym. Sci. Ser. D 2021, 14, 260–264. [Google Scholar] [CrossRef]
- Fireproof Mass for Printed Lining. Patent 2011647 from 30.04.1994. Available online: https://i.moscow/patents/RU2011647C1_19940430 (accessed on 4 September 2022).
- Silica Refractory Mass. Patent 2127234 from 25.04.1979. Available online: https://patentdb.ru/patent/2127234 (accessed on 4 September 2022).
- Volodichev, O.I. Mineralogy, Petrology and Minerageny of Precambrian Complexes in Karelia, 1st ed.; Karelian Research Centre Russian Academy of Sciences Institute of Geology: Petrozavodsk, Russia, 2007; pp. 1–124. [Google Scholar]
- Kukartsev, V.A. Application of Pervouralsky Quartzite in Acid Lining for Induction Iron-Smelting Furnaces. Liteyshchik Russ. 2012, 2, 35–37. [Google Scholar]
- Stoyanova, M.V.; Novikov, A.D.; Borodulin, A.S. Evaluation Construction Made of Polymer Composite Materials by Molding Using Reusable Flexible Punches Production Profitability. IOP Conf. Ser. Mater. Sci. Eng. 2020, 934, 012068. [Google Scholar] [CrossRef]
- Ananiev, Y.S.; Ananyeva, L.G.; Dolgov, I.V.; Korobeinikov, A.F.; Korovkin, M.V. Search, Evaluation and Enrichment of Quartz Raw Materials for High Technologies. Bull. Tomsk Polytech. Univ. Geores. Eng. 2001, 304, 123–130. [Google Scholar]
- Rajan, H.; Uchida, H.; Bryan, D.L.; Swaminathan, R.; Downs, R.T.; Hall-Wallace, M. Building the American Mineralogist Crystal Structure Database: A Recipe for Construction of a Small Internet Database. Geoinform. Data Knowl. Geol. Soc. Am. Spec. Pap. 2006, 397, 73–80. [Google Scholar] [CrossRef]
- Kosenko, N.F.; Smirnova, M.A. Mechanostimulated Polymorphic Transitions of Quartz. Refract. Tech. Ceram. 2012, 7, 7–13. [Google Scholar]
- Riposan, I.; Chisamera, M.; Stan, S. Enhanced Quality in Electric Melt Grey Cast Irons. ISIJ Int. 2013, 53, 1683–1695. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Abkaryan, A.K.; Temnykh, V.I.; Kukartsev, V.V.; Tynchenko, V.S.; Kukartsev, A.V. The Changes of Pervouralsky Quartzite Crystal Lattice under Heating from up 25 to 600 °C. Novyye Ogneup. 2021, 1, 34–39. [Google Scholar] [CrossRef]
- Hanagiri, S.; Matsui, T.; Shimpo, A.; Aso, S.; Inuzuka, T.; Matsuda, T.; Sakaki, S.; Nakagawa, H. Recent Improvement of Recycling Technology for Refractories. Shinnittetsu Giho 2008, 388, 93–98. [Google Scholar]
- Kukartsev, V.A.; Kukartsev, V.V.; Kukartsev, A.V. Effect of the Temperature Treatment of Quartzite on the Lining Resistance of Commercial-Frequency Induction Crucible Furnaces. Refract. Ind. Ceram. 2018, 59, 252–256. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist Crystal Structure Database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Ringdalen, E. Changes in Quartz During Heating and the Possible Effects on Si Production. JOM 2015, 67, 484–492. [Google Scholar] [CrossRef]
- Pryanishnikov, V.P. Silica System; Stroyizdat: Leningrad, Russia, 1971; Volume 238. [Google Scholar]
- Novakovic, R.; Radic, S.M.; Ristic, M.M. Kinetics and Mechanism of Quartz-Tridymite Transformation. Nterceram Interceram 1986, 35, 29–30. [Google Scholar]
- Breneman, R.C.; Halloran, J.W. Kinetics of Cristobalite Formation in Sintered Silica. J. Am. Ceram. Soc. 2014, 97, 2272–2278. [Google Scholar] [CrossRef]
- Shchiptsov, V.V.; Perepelitsyn, V.A.; Grishenkov, E.E.; Enenko, V.P.; Zavertkin, A.S. Pervoural’skii and Karel’skii Quartzites for the Lining of Crucible-Type Induction Furnaces. Refract. Ind. Ceram. 2003, 44, 67–74. [Google Scholar] [CrossRef]
- Nikiforova, E.M.; Eromasov, R.G.; Simonova, N.S.; Vasilyeva, M.N.; Taskin, V.Y. Phase Transformations in the Siliceous Rocks-Mineralizer System. Mod. Probl. Sci. Educ. 2012, 1, 218–219. [Google Scholar]
- Li, W.; Xu, C.; Xie, A.; Chen, K.; Yang, Y.; Liu, L.; Zhu, S. Microstructure Study of Phase Transformation of Quartz in Potassium Silicate Glass at 900 °C and 1000 °C. Crystals 2021, 11, 1481. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Kukartsev, V.V.; Tynchenko, V.S.; Bukhtoyarov, V.V.; Tynchenko, V.V.; Sergienko, R.B.; Bashmur, K.A.; Lysyannikov, A.V. The Technology of Using Liquid Glass Mixture Waste for Reducing the Harmful Environmental Impact. Materials 2022, 15, 1220. [Google Scholar] [CrossRef] [PubMed]
- Nelyub, V.A.; Fedorov, S.Y.; Malysheva, G.V.; Berlin, A.A. Properties of Carbon Fibers after Applying Metal Coatings on Them by Magnetron Sputtering Technology. Fibre Chem. 2021, 53, 252–257. [Google Scholar] [CrossRef]
- Wahl, F.M.; Grim, R.E.; Graf, R.B. Phase Transformations in Silica as Examined by Continuous X-ray Diffraction|American Mineralogist|GeoScienceWorld. Am. Mineral. J. Earth Planet. Mater. 1961, 46, 196–208. [Google Scholar]
- Benarieb, I.; Duyunova, V.A.; Oglodkov, M.S.; Puchkov, Y.A.; Pakhomkin, S.I. Quench Factor Analysis for Predicting Precipitation Hardening of Sheets from Aluminum Alloy V-1341 of the Al–Mg–Si System. Met. Sci. Heat Treat. 2022, 63, 583–589. [Google Scholar] [CrossRef]
- Voronin, A.S.; Fadeev, Y.V.; Makeev, M.O.; Mikhalev, P.A.; Osipkov, A.S.; Provatorov, A.S.; Ryzhenko, D.S.; Yurkov, G.Y.; Simunin, M.M.; Karpova, D.V.; et al. Low Cost Embedded Copper Mesh Based on Cracked Template for Highly Durability Transparent EMI Shielding Films. Materials 2022, 15, 1449. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Cherepanov, A.I.; Kukartsev, V.V.; Tynchenko, V.S.; Bukhtoyarov, V.V.; Popov, A.M.; Sergienko, R.B.; Tynchenko, S.V. X-ray Diffraction Phase Analysis of Changes in the Lattice of Pervouralsk Quartzite upon Heating. Minerals 2022, 12, 233. [Google Scholar] [CrossRef]
- Platonov, B.P.; Akimenko, A.D.; Bagutskaya, S.M. Induction Furnaces for Cast Iron Melting; Mashinostroyeniye: Moscow, Russia, 1976. [Google Scholar]
- Satr, P. New Refractory Technology for Melting Cast Iron Alloys and Alloy Steel in Induction Furnaces. Liteyshchik Russ. 2019, 1, 11–15. [Google Scholar]
- Kukartsev, V. Increase in the Life of the Industrial Induction Crucible Furnace at Temperatures above 1550 C. Technol. Mech. Eng. 2014, 1, 5–6. [Google Scholar]
- Zavertkin, A.S. Effect of Quartzite Heat Treatment on Induction Furnace Lining Failure Mechanism. Refract. Ind. Ceram. 2019, 60, 67–70. [Google Scholar] [CrossRef]
- Kukartsev, V.A.; Trunova, A.I.; Kukartsev, A.V. Thermal Analysis of Quartzite Used to Line a Crucible-Equipped Industrial-Frequency Induction Furnace. Refract. Ind. Ceram. 2014, 55, 220–222. [Google Scholar] [CrossRef]
- He, J.; Jusnes, K.F.; Tangstad, M. Phase Transformation in Quartz at Elevated Temperatures. Asp. Min. Miner. Sci. 2021, 6, 691–699. [Google Scholar] [CrossRef]
- Shakirov, K.M.; Sokolov, G.S.; Nelyub, V.A. Research of Transversal Properties of Winding Basalt Plastics Based on Basalt Fiber with Experimental Lubricants. J. Phys. Conf. Ser. 2021, 1990, 012044. [Google Scholar] [CrossRef]
- Orlov, M.A.; Nelyub, V.A.; Kalinnikov, A.N.; Borodulin, A.S. Modeling Basalt Fibers Wetting Processes Used in the Basalt Rebar Production. J. Phys. Conf. Ser. 2021, 1990, 012042. [Google Scholar] [CrossRef]
- Quartzite Ground Pervoural Dynasty Plant for Induction Furnace Crucifixes. TC 14-8-542-87 from 01.01.1988. Available online: https://www.standards.ru/document/3230296.aspx (accessed on 11 September 2022).
- Skamnitskaya, L.S.; Svetova, E.N.; Shanina, S.N. The Effect of Fluid Inclusions on the Vein Quartz Grade. Obogashchenie Rud 2019, 2019, 20–26. [Google Scholar] [CrossRef]
- Zavertkin, A.S. Development of a Lining Made from Karelian Quartzites for Crucible Induction Furnaces. Refract. Ind. Ceram. 2010, 50, 400–405. [Google Scholar] [CrossRef]

| Name | Amount (%) | |
|---|---|---|
| Business Averages | Achieved in Some Industries | |
| Shelf life | 64.6 | 82.2 |
| Rejects | 2.3 | 2.9 |
| Ungar | 2 | 2 |
| Sprues and arrived | 29.5 | 8.9 |
| Irretrievable losses | 1.6 | 2 |
| Plums and scrap | - | 2 |
| Result | 100 | 100 |
| Metal Content | Content of Constituents (%) | |
|---|---|---|
| Traditional Composition | Efficiency-Enhancing | |
| Zumpf (liquid residue) | 30–33 | 10–20 |
| Production return | 15–32 | 0 |
| Cast iron removable, and cast iron scrap | 10–15 | 0 |
| Steel scrap | 25–30 | 78–88 |
| Ferromanganese/ferrosilicon | 0.5–1 | 1–1.5 |
| Carburizer | 0.5–0.6 | 1–2 |
| Indicative cost (thousands of rubles) | 39.1 | 22.9 |
| Chemical Composition of PKMVI-3B Quartzite | Content (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | TiO2 | Fe2O3 | P2O5 | MnO | CaO | Na2O | K2O | |
| On Technical Condition | 97.5 | 1.1 | - | - | - | 0.6 | - | - | - | - | - |
| Spectrometer of dried sample at 200 °C | 96.84 | 0.882 | 0.099 | 0.029 | 0.265 | 1.03 | 0.017 | 0.041 | 0.099 | 0.125 | 0.474 |
| Lattice Parameter | Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 Crude | 200/800 | 25/30 Dry | 200 | 600 | 870 | 1000 | 1470 | 1550 | |
| davg, (Å) | 2.676 | 2.908/ 3.012 | 2.814/ 2.7574 | 2.8340/ 2.9012 | 2.7913/ 3.0066 | 2.9277/ 3.0545 | 2.9796/ 3.1048 | 3.0384/ 3.156 | 3.2619/ 3.2156 |
| Vavg, (Å3) | 115.4 | 116.35/ 129.85 | 119.1/ 114.83 | 116.55/ 115.41 | 117.47/ 647.47 | 125.86/ 1653.02 | 124.06/ 1722.83 | 124.04/ 1742.69 | 143.65/ 1606.96 |
| Davg, (g/cm3) | 2.590 | 2.552/ 2.249 | 2.5971/ 2.601 | 2.552/ 2.592 | 2.333/ 2.502 | 2.292/ 2.2685 | 2.291/ 2.266 | 2.229/ 2.265 | 2.227/ 2.258 |
| Mavg, (g/mol) | 60.08 | 60.08/ 51.01 | 60.08/ 60.08 | 60.08/ 60.08 | 55.16/ 60.08 | 53.91/ 58.38 | 53.66/ 58.63 | 53.66/ 58.79 | 54.41/ 58.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukartsev, V.A.; Cherepanov, A.I.; Kukartsev, V.V.; Tynchenko, V.S.; Kurashkin, S.O.; Tynchenko, V.V.; Sergienko, R.B.; Bashmur, K.A.; Boyko, A.A.; Bukhtoyarov, V.V. Influence of Moisture in Quartzite on the Lining Properties and Efficiency of Industrial-Frequency Induction Crucible Furnaces. Metals 2022, 12, 1515. https://doi.org/10.3390/met12091515
Kukartsev VA, Cherepanov AI, Kukartsev VV, Tynchenko VS, Kurashkin SO, Tynchenko VV, Sergienko RB, Bashmur KA, Boyko AA, Bukhtoyarov VV. Influence of Moisture in Quartzite on the Lining Properties and Efficiency of Industrial-Frequency Induction Crucible Furnaces. Metals. 2022; 12(9):1515. https://doi.org/10.3390/met12091515
Chicago/Turabian StyleKukartsev, Viktor Alekseevich, Aleksandr Ivanovich Cherepanov, Vladislav Viktorovich Kukartsev, Vadim Sergeevich Tynchenko, Sergei Olegovich Kurashkin, Valeriya Valerievna Tynchenko, Roman Borisovich Sergienko, Kirill Aleksandrovich Bashmur, Andrei Anatolevich Boyko, and Vladimir Viktorovich Bukhtoyarov. 2022. "Influence of Moisture in Quartzite on the Lining Properties and Efficiency of Industrial-Frequency Induction Crucible Furnaces" Metals 12, no. 9: 1515. https://doi.org/10.3390/met12091515
APA StyleKukartsev, V. A., Cherepanov, A. I., Kukartsev, V. V., Tynchenko, V. S., Kurashkin, S. O., Tynchenko, V. V., Sergienko, R. B., Bashmur, K. A., Boyko, A. A., & Bukhtoyarov, V. V. (2022). Influence of Moisture in Quartzite on the Lining Properties and Efficiency of Industrial-Frequency Induction Crucible Furnaces. Metals, 12(9), 1515. https://doi.org/10.3390/met12091515







