Drug Molecular Immobilization and Photofunctionalization of Calcium Phosphates for Exploring Theranostic Functions
Abstract
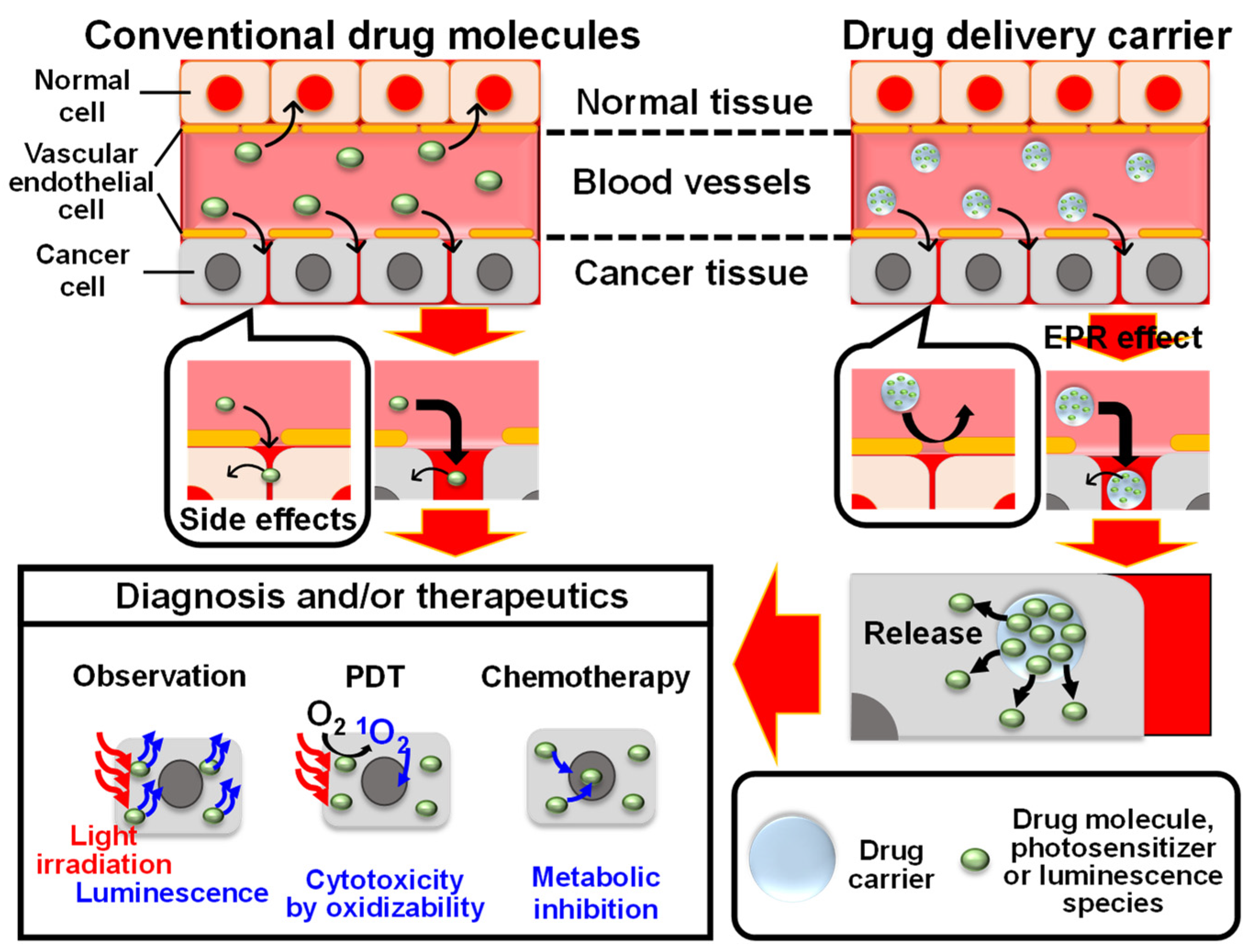
1. Introduction
2. Photofunctionalization of Calcium Phosphates Based on Biomimetic Surface Engineering
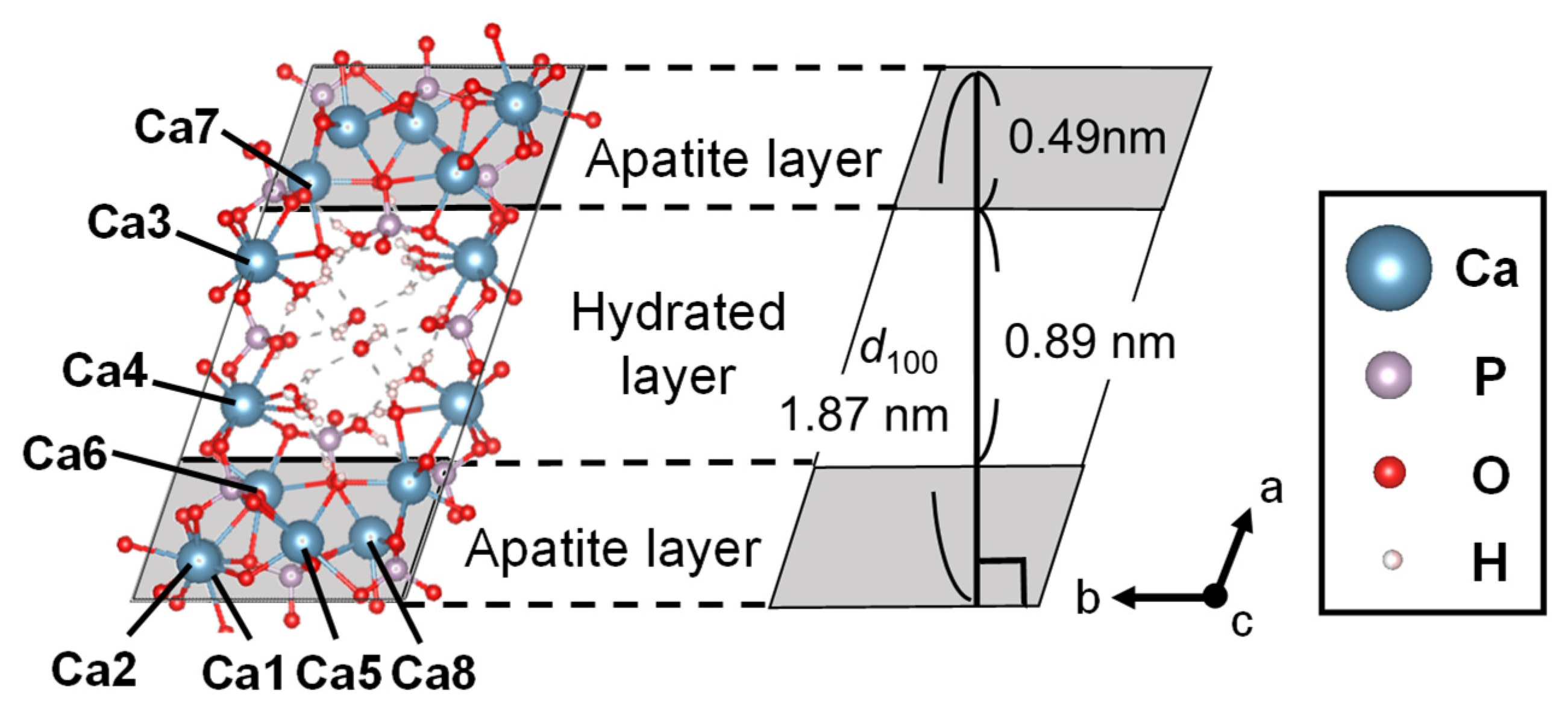
2.1. Octacalcium Phosphate (OCP)
2.1.1. Features
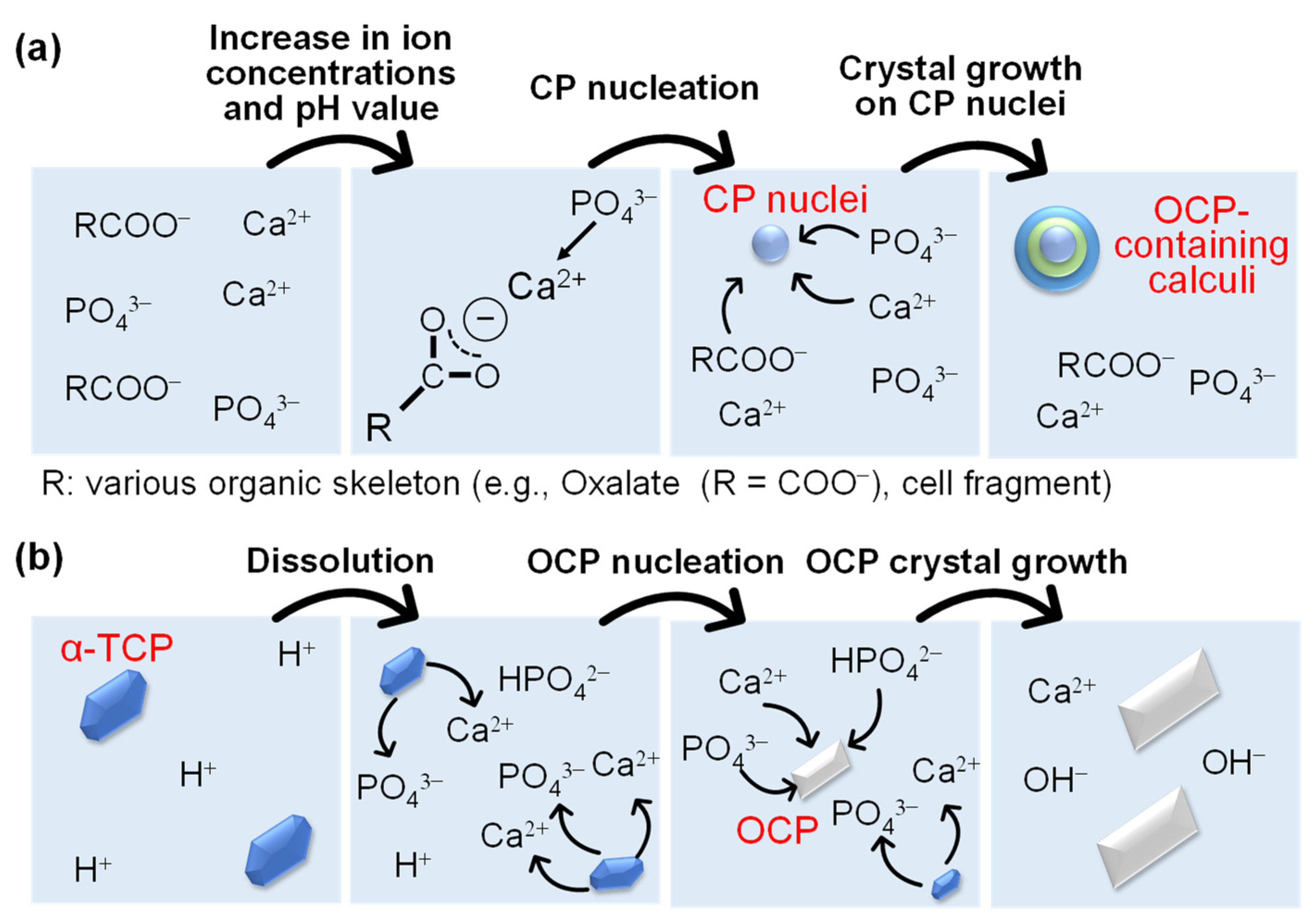
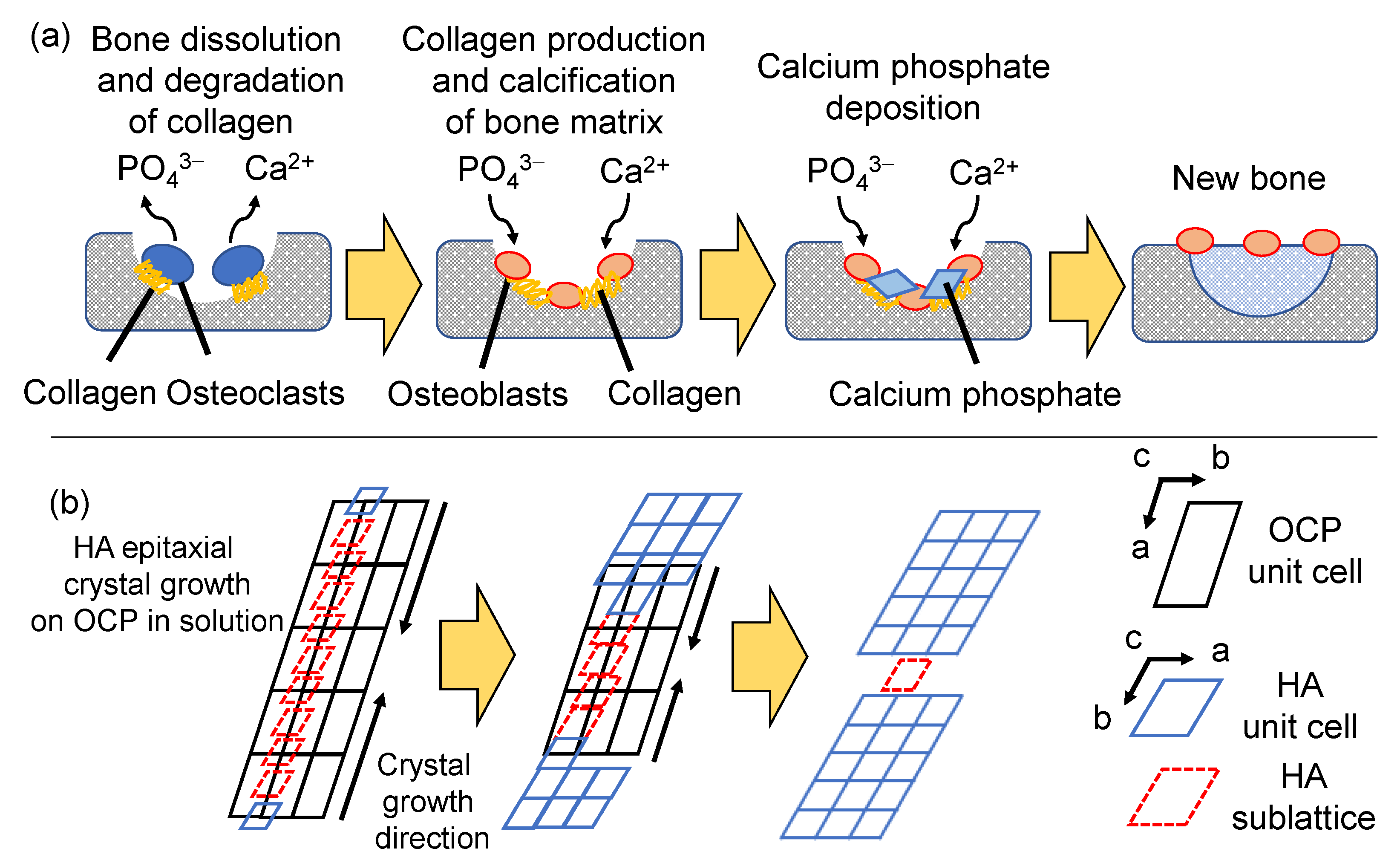
2.1.2. In Vivo and In Vitro Formation Processes
2.1.3. Hybridization with Organic Molecules in the Formation Processes
2.1.4. Photofunctionalization Based on the Biomimetic Hybridization
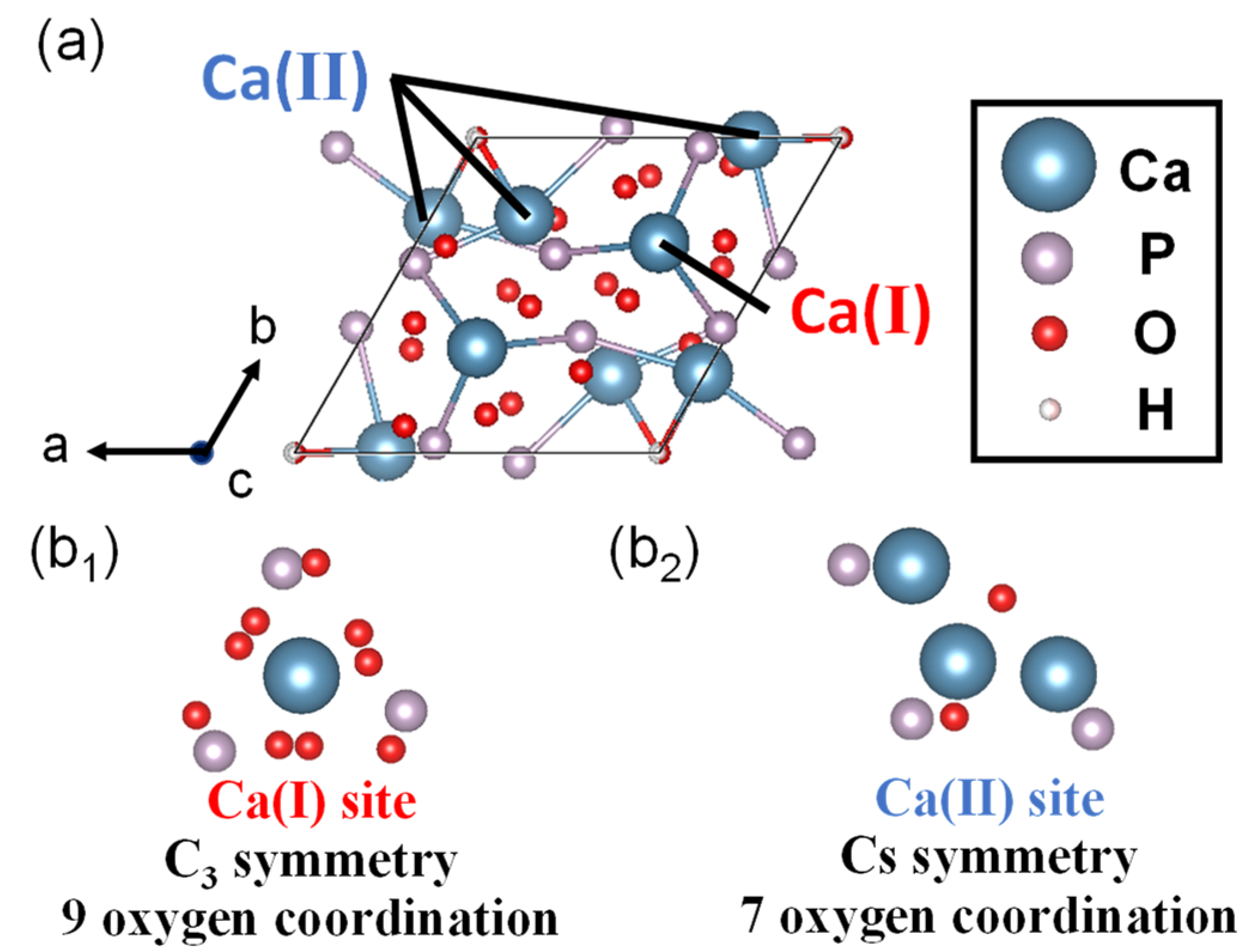
2.2. Hydroxyapatite
2.2.1. Features
2.2.2. In Vivo and In Vitro Formation Processes
2.2.3. Hybridization with Organic Molecules in the Formation Process
2.2.4. Photofunctionalization Based on the Biomimetic Hybridization
3. Utilization of Europium(III) (Eu3+) Ion Doped in CPs
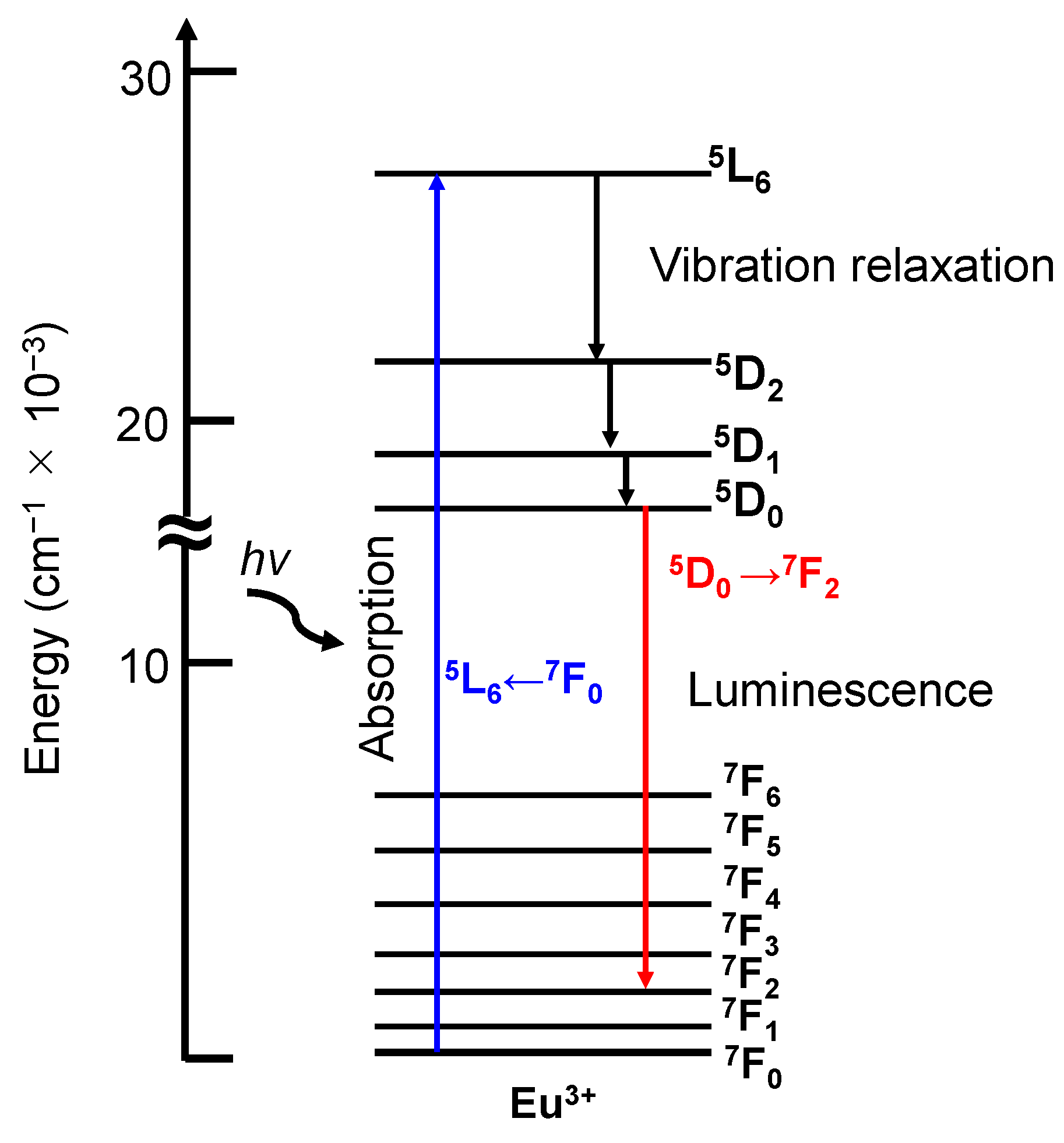
3.1. Photophysical Properties of Eu3+ Ion
3.2. Doping System in CPs
3.3. Bioimaging Application with OCP
4. Utilization of Methylene Blue Adsorbed on HA
4.1. Photophysical and Photochemical Properties of Methylene Blue
4.2. Immobilization on HA
4.3. Therapeutic Application
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Funkhouser, J. Reinventing Pharma: The Theranostic Revolution. Curr. Drug Discov. 2002, 2, 17–19. [Google Scholar]
- Bryson, J.M.; Fichter, K.M.; Chu, W.J.; Lee, J.H.; Li, J.; Madsen, L.A.; McLendon, P.M.; Reineke, T.M. Polymer Beacons for Luminescence and Magnetic Resonance Imaging of DNA Delivery. Proc. Natl. Acad. Sci. USA 2009, 106, 16913–16918. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Son, S.; An, J.; Kim, I.; Choi, M.; Kong, N.; Tao, W.; Kim, J.S. Nanoscale Porous Organic Polymers for Drug Delivery and Advanced Cancer Theranostics. Chem. Soc. Rev. 2021, 50, 12883–12896. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.S.; Patel, G.; Kushwah, V.; Jain, S.; Banerjee, U.C. Facile Development of Biodegradable Polymer-Based Nanotheranostics: Hydrophobic Photosensitizers Delivery, Fluorescence Imaging and Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2019, 193, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.D.; Kamaly, N.; Kalber, T.L.; Brody, L.P.; Sahuri, M.; Shamsaei, E.; Miller, A.D.; Bell, J.D. Novel Multifunctional Nanoparticle Mediates SiRNA Tumour Delivery, Visualisation and Therapeutic Tumour Reduction in vivo. J. Control. Release 2011, 149, 111–116. [Google Scholar] [CrossRef]
- Kono, K.; Nakashima, S.; Kokuryo, D.; Aoki, I.; Shimomoto, H.; Aoshima, S.; Maruyama, K.; Yuba, E.; Kojima, C.; Harada, A.; et al. Multi-Functional Liposomes Having Temperature-Triggered Release and Magnetic Resonance Imaging for Tumor-Specific Chemotherapy. Biomaterials 2011, 32, 1387–1395. [Google Scholar] [CrossRef]
- Kaida, S.; Cabral, H.; Kumagai, M.; Kishimura, A.; Terada, Y.; Sekino, M.; Aoki, I.; Nishiyama, N.; Tani, T.; Kataoka, K. Visible Drug Delivery by Supramolecular Nanocarriers Directing to Single-Platformed Diagnosis and Therapy of Pancreatic Tumor Model. Cancer Res. 2010, 70, 7031–7041. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum Dots versus Organic Dyes as Fluorescent Labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Tan, W.B.; Jiang, S.; Zhang, Y. Quantum-Dot Based Nanoparticles for Targeted Silencing of HER2/Neu Gene via RNA Interference. Biomaterials 2007, 28, 1565–1571. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Lin, J.; Fu, L.H.; Huang, P. Calcium-Based Biomaterials for Diagnosis, Treatment, and Theranostics. Chem. Soc. Rev. 2018, 47, 357–403. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Fang, R.H.; Zhang, L. Lipid- and Polymer-Based Nanostructures for Cancer Theranostics. Theranostics 2012, 2, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.S.; Mandal, N.; Pharm, M.T.; Patel, G.; Kirar, S.; Reddy, Y.N.; Pharm, M.S.; Kushwah, V.; Jain, S.; Kalia, Y.N.; et al. Co-Administration of Zinc Phthalocyanine and Quercetin via Hybrid Nanoparticles for Augmented Photodynamic Therapy. Nanomed. Nanotechnol. Biol. Med. 2021, 33, 102368. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zeng, Y.; Chen, N.; Zhang, M.; Wang, Y.; Pan, Z. A Facile and Universal Method for Preparing Polyethylene Glycol-Metal Hybrid Nanoparticles and Their Application in Tumor Theranostics. Adv. Healthc. Mater. 2022, 11, 2200044. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Chandra, S. Inorganic Hybrid Nanoparticles in Cancer Theranostics: Understanding Their Combinations for Better Clinical Translation. Mater. Today Chem. 2020, 18, 100381. [Google Scholar] [CrossRef]
- Gonçalves, M.S.T. Fluorescent Labeling of Biomolecules with Organic Probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. Biomaterials A Review of NIR Dyes in Cancer Targeting and Imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K.; et al. Uniform Mesoporous Dye-Doped Silica Nanoparticles Decorated with Multiple Magnetite Nanocrystals for Simultaneous Enhanced Magnetic Resonance Imaging, Fluorescence Imaging, and Drug Delivery. J. Am. Chem. Soc. 2010, 132, 552–557. [Google Scholar] [CrossRef]
- Lash, T.D.; Hayes, M.J.; Spence, J.D.; Muckey, M.A.; Ferrence, G.M.; Szczepura, L.F. Conjugated Macrocycles Related to the Porphyrins. 21. Synthesis, Spectroscopy, Electrochemistry, and Structural Characterization of Carbaporphyrins. J. Org. Chem. 2002, 67, 4860–4874. [Google Scholar] [CrossRef] [PubMed]
- Safonova, E.A.; Martynov, A.G.; Nefedov, S.E.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. A Molecular Chameleon: Reversible pH- and Cation-Induced Control of the Optical Properties of Phthalocyanine-Based Complexes in the Visible and Near-Infrared Spectral Ranges. Inorg. Chem. 2016, 55, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Si, W.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Organic Dye Based Nanoparticles for Cancer Phototheranostics. Small 2018, 14, 1704247. [Google Scholar] [CrossRef] [PubMed]
- Górecki, T.; Patonay, G.; Strekowski, L.; Chin, R.; Salazar, N. Synthesis of Novel Near-infrared Cyanine Dyes for Metal Ion Determination. J. Heterocycl. Chem. 1996, 33, 1871–1876. [Google Scholar] [CrossRef]
- Lu, C.; Das, S.; Magut, P.K.S.; Li, M.; El-Zahab, B.; Warner, I.M. Irradiation Induced Fluorescence Enhancement in PEGylated Cyanine-Based NIR Nano- and Mesoscale GUMBOS. Langmuir 2012, 28, 14415–14423. [Google Scholar] [CrossRef]
- He, X.; Gao, J.; Gambhir, S.S.; Cheng, Z. Near-Infrared Fluorescent Nanoprobes for Cancer Molecular Imaging: Status and Challenges. Trends Mol. Med. 2010, 16, 574–583. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent Quantum Dots for Multiplexed Biological Detection and Imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Lim, E.K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The Role of Apoptosis in Response to Photodynamic Therapy: What, Where, Why, and How. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [PubMed]
- Ang, J.M.; Riaz, I.B.; Kamal, M.U.; Paragh, G.; Zeitouni, N.C. Photodynamic Therapy and Pain: A Systematic Review. Photodiagnosis Photodyn. Ther. 2017, 19, 308–344. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Del Giglio, A.; De Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene Blue in Photodynamic Therapy: From Basic Mechanisms to Clinical Applications. Photodiagnosis Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Hirose, M.; Yoshida, Y.; Horii, K.; Hasegawa, Y.; Shibuya, Y. Efficacy of Antimicrobial Photodynamic Therapy with Rose Bengal and Blue Light against Cariogenic Bacteria. Arch. Oral Biol. 2021, 122, 105024. [Google Scholar] [CrossRef]
- Thakur, N.S.; Bhaumik, J.; Kirar, S.; Banerjee, U.C. Development of Gold-Based Phototheranostic Nanoagents through a Bioinspired Route and Their Applications in Photodynamic Therapy. Sustain. Chem. Eng. 2017, 5, 7950–7960. [Google Scholar] [CrossRef]
- Junqueira, H.C.; Severino, D.; Dias, L.G.; Gugliotti, M.S.; Baptista, M.S. Modulation of Methylene Blue Photochemical Properties Based on Adsorption at Aqueous Micelle Interfaces. Phys. Chem. Chem. Phys. 2002, 4, 2320–2328. [Google Scholar] [CrossRef]
- Timko, B.P.; Whitehead, K.; Gao, W.W.; Kohane, D.S.; Farokhzad, O.; Anderson, D.; Langer, D. Advances in Drug Delivery. Annu. Rev. Mater. Res. 2011, 41, 1–20. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Hao, X.; Hu, X.; Zhang, C.; Chen, S.; Li, Z.; Yang, X.; Liu, H. Hybrid Mesoporous Silica-Based Drug Carrier Nanostructures with Improved Degradability by Hydroxyapatite. ACS Nano 2015, 9, 9614–9625. [Google Scholar] [CrossRef]
- Kester, M.; Heakal, Y.; Fox, T.; Sharma, A.; Robertson, G.P.; Morgan, T.T.; Altinoǧlu, E.I.; Tabaković, A.; Parette, M.R.; Rouse, S.M.; et al. Calcium Phosphate Nanocomposite Particles for in vitro Imaging and Encapsulated Chemotherapeutic Drug Delivery to Cancer Cells. Nano Lett. 2008, 8, 4116–4121. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Popa, A.; Zhou, S.; Zhu, J.; Samia, A.C.S. Iron Oxide-Loaded Hollow Mesoporous Silica Nanocapsules for Controlled Drug Release and Hyperthermia. Chem. Commun. 2013, 49, 11436–11438. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, C.; Gao, M.; Hu, A.; Liu, Z. Remotely Controlled Red Blood Cell Carriers for Cancer Targeting and Near-Infrared Light-Triggered Drug Release in Combined Photothermal—Chemotherapy. Adv. Funtional Mater. 2015, 25, 2386–2394. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, I.; Delgado-Lopez, J.M.; Duran-Olivencia, M.A.; Iafisco, M.; Tampieri, A.; Colangelo, D.; Prat, M.; Gomez-Morales, J. pH-Responsive Delivery of Doxorubicin from Citrate—Apatite Nanocrystals with Tailored Carbonate Content. Langmuir 2013, 29, 8213–8221. [Google Scholar] [CrossRef]
- Lelli, M.; Roveri, N.; Marzano, C.; Hoeschele, J.D.; Curci, A.; Margiotta, N.; Gandin, V.; Natile, G. Hydroxyapatite Nanocrystals as a Smart, pH Sensitive, Delivery System for Kiteplatin. Dalt. Trans. 2016, 45, 13187–13195. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, C.; Liao, S.; Lin, Y.; Tang, H.; Liu, S.; Lai, I.; Wu, K.C. Cosynthesis of Cargo-Loaded Hydroxyapatite/Alginate Core—Shell Nanoparticles (HAP@Alg) as pH-Responsive Nanovehicles by a Pre- Gel Method. ACS Appl. Mater. Interfaces 2012, 4, 6720–6727. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Mathew, M.; Brown, W.E.; Schroeder, L.W.; Dickens, B. Crystal Structure of Octacalcium Bis(Hydrogenphosphate) Tetrakis(Phosphate)Pentahydrate, Ca8(HPO4)2(PO4)4·5H2O. J. Crystallogr. Spectrosc. Res. 1988, 18, 235–250. [Google Scholar] [CrossRef]
- Monma, H.; Nishimura, Y.; Okura, T. Characterization of Layer-Structured Octacalcium Phosphate/Dicarboxylate Composite. Phosphorus Res. Bull. 2005, 18, 127–134. [Google Scholar] [CrossRef]
- Fernandez, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Calcium Phosphate Bone Cements for Clinical Applications-Part I: Solution Chemistry. J. Mater. Sci. Mater. Med. 1999, 10, 169–176. [Google Scholar] [CrossRef]
- Fernandez, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Calcium Phosphate Bone Cements for Clinical Applications-Part II: Precipitate Formation during Setting Reactions. J. Mater. Sci. Mater. Med. 1999, 10, 177–183. [Google Scholar] [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T.; Nakamura, M.; Zhao, B.; Honda, Y.; Kamijo, R. Bone Formation Enhanced by Implanted Octacalcium Phosphate Involving Conversion into Ca-Deficient Hydroxyapatite. Biomaterials 2006, 27, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Anada, T.; Masuda, T.; Honda, Y.; Sakai, Y.; Kato, Y.; Kamakura, S.; Echigo, S.; Suzuki, O. The Effect of Synthetic Octacalcium Phosphate in a Collagen Scaffold on the Osteogenicity of Mesenchymal Stem Cells. Eur. Cells Mater. 2011, 22, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.; Smith, J.P.; Lehr, J.R.; Frazier, A.W. Crystallographic and Chemical Relations between Octacalciumphosphate and Hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Crane, N.J.; Popescu, V.; Morris, M.D.; Steenhuis, P.; Ignelzi, M.A. Raman Spectroscopic Evidence for Octacalcium Phosphate and Other Transient Mineral Species Deposited during Intramembranous Mineralization. Bone 2006, 39, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.G.A.; Barry, J.C.; Shields, C.P.; Glena, R.; Featherstone, J.D.B. Crystal Morphology, Composition, and Dissolution Behavior of Carbonated Apatites Prepared at Controlled pH and Temperature. J. Colloid Interface Sci. 1989, 130, 467–479. [Google Scholar] [CrossRef]
- Miake, Y.; Shimoda, S.; Fukae, M.; Aoba, T. Epitaxial Overgrowth of Apatite Crystals on the Thin-Ribbon Precursor at Early Stages of Porcine Enamel Mineralization. Calcif. Tissue Int. 1993, 53, 249–256. [Google Scholar] [CrossRef]
- Iijima, M.; Moriwaki, Y.; Takagi, T.; Moradian-Oldak, J. Effects of Bovine Amelogenins on the Crystal Morphology of Octacalcium Phosphate in a Model System of Tooth Enamel Formation. J. Cryst. Growth 2001, 222, 615–626. [Google Scholar] [CrossRef]
- Iijima, M.; Fan, D.; Bromley, K.M.; Sun, Z.; Moradian-Oldak, J. Tooth Enamel Proteins Enamelin and Amelogenin Cooperate to Regulate the Growth Morphology of Octacalcium Phosphate Crystals. Cryst. Growth Des. 2010, 10, 4815–4822. [Google Scholar] [CrossRef]
- Cheng, P.T. Octacalcium Phosphate Formation in vitro: Implications for Bone Formation. Calcif. Tissue Int. 1985, 37, 91–94. [Google Scholar] [CrossRef]
- Iijima, M.; Tohda, H.; Suzuki, H.; Yanagisawa, T.; Moriwaki, Y. Effects of F- on Apatite-Octacalcium Phosphate Intergrowth and Crystal Morphology in a Model System of Tooth Enamel Formation. Calcif. Tissue Int. 1992, 50, 357–361. [Google Scholar] [CrossRef]
- Graham, S.; Brown, P.W. Reactions of Octacalcium Phosphate to Form Hydroxyapatite. J. Cryst. Growth 1996, 165, 106–115. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauzá, A.; García-Ferragut, L. Biopathological Crystallization: A General View about the Mechanisms of Renal Stone Formation. Adv. Colloid Interface Sci. 1998, 74, 169–194. [Google Scholar] [CrossRef]
- Sheng, X.; Ward, M.D.; Wesson, J.A. Adhesion between Molecules and Calcium Oxalate Crystals: Critical Interactions in Kidney Stone Formation. J. Am. Chem. Soc. 2003, 125, 2854–2855. [Google Scholar] [CrossRef]
- Coe, F.L.; Evan, A.P.; Worcester, E.M.; Lingeman, J.E. Three Pathways for Human Kidney Stone Formation. Urol. Res. 2010, 38, 147–160. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Rapid Communication Preparation of Octacalcium Phosphate (OCP): A Direct Fast Method. Calcif. Tissue Int. 1985, 37, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Nakamura, M.; Miyasaka, Y.; Kagayama, M.; Sakurai, M. Bone Formation on Synthetic Precursors of Hydroxyapatite. Tohoku J. Exp. Med. 1991, 164, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, X.; Gan, Y.; Gao, C.; Zhang, X.; Ting, K.; Wu, B.M.; Gou, Z. Facile Synthesis of Octacalcium Phosphate Nanobelts: Growth Mechanism and Surface Adsorption Properties. J. Phys. Chem. C 2010, 114, 6265–6271. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Rubini, K.; Bigi, A. Collapsed Octacalcium Phosphate Stabilized by Ionic Substitutions. Cryst. Growth Des. 2010, 10, 3612–3617. [Google Scholar] [CrossRef]
- Arellano-Jiménez, M.J.; García-García, R.; Reyes-Gasga, J. Synthesis and Hydrolysis of Octacalcium Phosphate and Its Characterization by Electron Microscopy and X-Ray Diffraction. J. Phys. Chem. Solids 2009, 70, 390–395. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Mou, C.Y.; Chan, J.C.C. Solid-State NMR Study of the Transformation of Octacalcium Phosphate to Hydroxyapatite: A Mechanistic Model for Central Dark Line Formation. J. Am. Chem. Soc. 2006, 128, 6909–6918. [Google Scholar] [CrossRef]
- Monma, H. Preparation of Octacalcium Phosphate by the Hydrolysis of α-Tricalcium Phosphate. J. Mater. Sci. 1980, 15, 2428–2434. [Google Scholar] [CrossRef]
- Yokoi, T.; Goto, T.; Kitaoka, S. Transformation of Dicalcium Phosphate Dihydrate into Octacalcium Phosphate with Incorporated Dicarboxylate Ions. J. Ceram. Soc. Jpn. 2018, 126, 462–468. [Google Scholar] [CrossRef]
- Sugiura, Y.; Saito, Y.; Endo, T.; Makita, Y. Effect of the Ionic Radius of Alkali Metal Ions on Octacalcium Phosphate Formation via Different Substitution Modes. Cryst. Growth Des. 2019, 19, 4162–4171. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Ohtsuki, C.; Takahashi, A.; Tanihara, M. Effect of Silane-Coupling Treatment on Thermal Decomposition of Octacalcium Phosphate. J. Soc. Mater. Sci. Jpn. 2006, 55, 881–884. [Google Scholar] [CrossRef][Green Version]
- Kamitakahara, M.; Ito, N.; Murakami, S.; Watanabe, N.; Ioku, K. Hydrothermal Synthesis of Hydroxyapatite from Octacalcium Phosphate: Effect of Hydrothermal Temperature. J. Ceram. Soc. Jpn. 2009, 117, 385–387. [Google Scholar] [CrossRef]
- Suzuki, K.; Honda, Y.; Anada, T.; Handa, T.; Miyatake, N.; Takahashi, A.; Hosaka, M.; Imaizumi, H.; Itoi, E.; Suzuki, O. Stimulatory Capacity of an Octacalcium Phosphate/Gelatin Composite on Bone Regeneration in a Rabbit Tibia Defect Model. Phosphorus Res. Bull. 2012, 26, 53–58. [Google Scholar] [CrossRef]
- Monma, H. The Incorporation of Dicarboxylates into Octacalcium Bis(Hydrogenphosphate) Tetrakis(Phosphate) Pentahydrate. Bull. Chem. Soc. Jpn. 1984, 57, 599–600. [Google Scholar] [CrossRef]
- Monma, H.; Goto, M. Complexes of Apatitic Layered Compound Ca8(HPO4)2(PO4)4·5H2O with Dicarboxylates. J. Incl. Phenom. 1984, 2, 127–134. [Google Scholar] [CrossRef]
- Marković, M.; Fowler, B.O.; Brown, W.E. Octacalcium Phosphate Carboxylates. 1. Preparation and Identification. Chem. Mater. 1993, 5, 1401–1405. [Google Scholar] [CrossRef]
- Fowler, B.O.; Marković, M.; Brown, W.E. Octacalcium Phosphate. 3. Infrared and Raman Vibrational Spectra. Chem. Mater. 1993, 5, 1417–1423. [Google Scholar] [CrossRef]
- Li, Y.; Reid, D.G.; Duer, M.J.; Chan, J.C.C. Solid State NMR—An Indispensable Tool in Organic-Inorganic Biocomposite Characterization; Refining the Structure of Octacalcium Phosphate Composites with the Linear Metabolic Di-Acids Succinate and Adipate. Solid State Nucl. Magn. Reson. 2018, 95, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.; Duer, M.J.; Ashbrook, S.E.; Griffin, J.M. Applications of NMR Crystallography to Problems in Biomineralization: Refinement of the Crystal Structure and 31P Solid-State NMR Spectral Assignment of Octacalcium Phosphate. J. Am. Chem. Soc. 2012, 134, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- Monma, H. Thermal Properties of Layer-Structured Calcium Phosphate Intercalated with Succinate and Methylsuccinate Lons. Gypsum Lime 1990, 229, 12–17. [Google Scholar]
- Aoki, S.; Sakamoto, K.; Yamaguchi, S.; Nakahira, A. Syntheses of Octacalcium Phosphate Containing Dicarboxylic Acids and Effects of the Side Groups on the Crystal Growth of Octacalcium Phosphate. J. Ceram. Soc. Jpn. 2000, 108, 909–914. [Google Scholar] [CrossRef]
- Yokoi, T.; Kamitakahara, M.; Ohtsuki, C. Continuous Expansion of the Interplanar Spacing of Octacalcium Phosphate by Incorporation of Dicarboxylate Ions with a Side Chain. Dalt. Trans. 2015, 44, 7943. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Kamitakahara, M.; Kawashita, M.; Ohtsuki, C. Formation of Organically Modified Octacalcium Phosphate in Solutions Containing Various Amounts of Benzenedicarboxylic Acids. J. Ceram. Soc. Japan 2013, 121, 219–225. [Google Scholar] [CrossRef]
- Yamada, I.; Tagaya, M. Immobilization of 2,2′-Bipyridine-5,5′-Dicarboxylic Acid in Layered Octacalcium Phosphate. Colloids Interface Sci. Commun. 2019, 30, 100182. [Google Scholar] [CrossRef]
- Yokoi, T.; Machida, S.; Sugahara, Y.; Hashimoto, M.; Kitaoka, S. Enantioselective Incorporation of Dicarboxylate Guests by Octacalcium Phosphate †. Chem. Commun. 2017, 53, 6524–6527. [Google Scholar] [CrossRef]
- Yokoi, T.; Goto, T.; Hara, M.; Sekino, T.; Seki, T.; Kamitakahara, M.; Ohtsuki, C.; Kitaoka, S.; Takahashi, S.; Kawashita, M. Incorporation of Tetracarboxylate Ions into Octacalcium Phosphate for the Development of Next-Generation Biofriendly Materials. Commun. Chem. 2021, 4, 2–10. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal Structure of Hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale Hydroxyapatite Particles for Bone Tissue Engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite–Polymer Biocomposites for Bone Regeneration: A Review of Current Trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- Lowe, B.; Hardy, J.G.; Walsh, L.J. Optimizing Nanohydroxyapatite Nanocomposites for Bone Tissue Engineering. ACS Omega 2020, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, C.; Johansson, K.; Philipson, L. Hydroxyapatite Chromatography and Formamide Denaturation of Adenovirus DNA. J. Virol. 1973, 12, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Makitsuru, K.; Nakazawa, H.; Hara, S.; Suehiro, T.; Yamamoto, A.; Hiraide, T.; Ogawa, T. Separation of Double-Strand DNA Fragments by High-Performance Liquid Chromatography Using a Ceramic Hydroxyapatite Column. Anal. Chim. Acta 1999, 386, 69–75. [Google Scholar] [CrossRef]
- Kawasaki, T.; Takahashi, S.; Ideda, K. Hydroxyapatite High-performance Liquid Chromatography: Column Performance for Proteins. Eur. J. Biochem. 1985, 152, 361–371. [Google Scholar] [CrossRef]
- Cummings, L.J.; Snyder, M.A.; Brisack, K. Chapter 24 Protein Chromatography on Hydroxyapatite Columns. Methods Enzymol. 2009, 463, 387–404. [Google Scholar]
- Ibrahim, M.; Labaki, M.; Giraudon, J.M.; Lamonier, J.F. Hydroxyapatite, a Multifunctional Material for Air, Water and Soil Pollution Control: A Review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Pai, S.; Kini, M.S.; Selvaraj, R. A Review on Adsorptive Removal of Dyes from Wastewater by Hydroxyapatite Nanocomposites. Environ. Sci. Pollut. Res. 2021, 28, 11835–11849. [Google Scholar] [CrossRef]
- Ishikawa, K. Carbonate Apatite Bone Replacement: Learn from the Bone. J. Ceram. Soc. Jpn. 2019, 127, 595–601. [Google Scholar] [CrossRef]
- Liu, D.M.; Troczynski, T.; Tseng, W.J. Water-Based Sol-Gel Synthesis of Hydroxyapatite: Process Development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef]
- Feng, W.; Mu-Sen, L.; Yu-Peng, L.; Yong-Xin, Q. A Simple Sol-Gel Technique for Preparing Hydroxyapatite Nanopowders. Mater. Lett. 2005, 59, 916–919. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcilum Phosphates. Angew. Chemie Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Posner, A.S.; Betts, F. Synthetic Amorphous Calcium Phosphate and Its Relation to Bone Mineral Structure. Acc. Chem. Res. 1975, 8, 273–281. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.K. Recent Advances in the Synthesis, Functionalization and Biomedical Applications of Hydroxyapatite: A Review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef]
- Sans, J.; Sanz, V.; Puiggalí, J.; Turon, P.; Alemán, C. Controlled Anisotropic Growth of Hydroxyapatite by Additive-Free Hydrothermal Synthesis. Cryst. Growth Des. 2021, 21, 748–756. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Y.J. Large-Scale Automated Production of Highly Ordered Ultralong Hydroxyapatite Nanowires and Construction of Various Fire-Resistant Flexible Ordered Architectures. ACS Nano 2016, 10, 11483–11495. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis Method of Hydroxyapatite: A Review. Mater. Today Proc. 2019, 29, 233–239. [Google Scholar] [CrossRef]
- Kumar, R.; Prakash, K.H.; Cheang, P.; Khor, K.A. Temperature Driven Morphological Changes of Chemically Precipitated Hydroxyapatite Nanoparticles. Langmuir 2004, 20, 5196–5200. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Heshajin, M.S.; Kazemzadeh, A.; Zakeri, M. Synthesis of Nanocrystalline Hydroxyapatite by Using Precipitation Method. J. Alloys Compd. 2007, 430, 330–333. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for Bone Tissue Regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K. Self-Organization Mechanism in a Bone-like Hydroxyapatite/Collagen Nanocomposite Synthesized in vitro and Its Biological Reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef]
- Wahl, D.A.; Czernuszka, J.T. Collagen-Hydroxyapatite Composites for Hard Tissue Repair. Eur. Cells Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and Bone Regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef]
- Chang, M.C.; Ko, C.C.; Douglas, W.H. Preparation of Hydroxyapatite-Gelatin Nanocomposite. Biomaterials 2003, 24, 2853–2862. [Google Scholar] [CrossRef]
- Li, D.; Sun, H.; Jiang, L.; Zhang, K.; Liu, W.; Zhu, Y.; Fangteng, J.; Shi, C.; Zhao, L.; Sun, H.; et al. Enhanced Biocompatibility of PLGA Nanofibers with Gelatin/Nano-Hydroxyapatite Bone Biomimetics Incorporation. ACS Appl. Mater. Interfaces 2014, 6, 9402–9410. [Google Scholar] [CrossRef]
- Madhumathi, K.; Binulal, N.S.; Nagahama, H.; Tamura, H.; Shalumon, K.T.; Selvamurugan, N.; Nair, S.V.; Jayakumar, R. Preparation and Characterization of Novel β-Chitin-Hydroxyapatite Composite Membranes for Tissue Engineering Applications. Int. J. Biol. Macromol. 2009, 44, 1–5. [Google Scholar] [CrossRef]
- Chang, C.; Peng, N.; He, M.; Teramoto, Y.; Nishio, Y.; Zhang, L. Fabrication and Properties of Chitin/Hydroxyapatite Hybrid Hydrogels as Scaffold Nano-Materials. Carbohydr. Polym. 2013, 91, 7–13. [Google Scholar] [CrossRef]
- Pighinelli, L.; Kucharska, M. Chitosan-Hydroxyapatite Composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef]
- Zaharia, A.; Muşat, V.; Anghel, E.M.; Atkinson, I.; Mocioiu, O.C.; Buşilă, M.; Pleşcan, V.G. Biomimetic Chitosan-Hydroxyapatite Hybrid Biocoatings for Enamel Remineralization. Ceram. Int. 2017, 43, 11390–11402. [Google Scholar] [CrossRef]
- Sui, G.; Yang, X.; Mei, F.; Hu, X.; Chen, G.; Deng, X.; Ryu, S. Poly-L-Lactic Acid/Hydroxyapatite Hybrid Membrane for Bone Tissue Regeneration. J. Biomed. Mater. Res. Part A 2007, 82A, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Muddana, H.S.; Morgan, T.T.; Adair, J.H.; Butler, P.J. Photophysics of Cy3-Encapsulated Calcium Phosphate Nanoparticles. Nano Lett. 2009, 9, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Oltolina, F.; Gregoletto, L.; Colangelo, D.; Gómez-Morales, J.; Delgado-López, J.M.; Prat, M. Monoclonal Antibody-Targeted Fluorescein-5-Isothiocyanate-Labeled Biomimetic Nanoapatites: A Promising Fluorescent Probe for Imaging Applications. Langmuir 2015, 31, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, H.; Younssi, S.A.; Ouammou, M.; Gurlo, A.; Bekheet, M.F. Azo Dye Adsorption on an Industrial Waste-Transformed Hydroxyapatite Adsorbent: Kinetics, Isotherms, Mechanism and Regeneration Studies. J. Environ. Chem. Eng. 2020, 8, 103807. [Google Scholar] [CrossRef]
- Paziresh, F.; Salem, A.; Salem, S. Super Effective Recovery of Industrial Wastewater Contaminated by Multi-Disperse Dyes through Hydroxyapatite Produced from Eggshell. Sustain. Chem. Pharm. 2021, 23, 100501. [Google Scholar] [CrossRef]
- Ignjatović, N.L.; Mančić, L.; Vuković, M.; Stojanović, Z.; Nikoli, M.G.; Škapin, S.; Jovanov, S.; Veselinović, L.; Vuk, U.; Lazić, S.; et al. Rare-Earth (Gd3+, Yb3+/Tm3+, Eu3+) Co-Doped Hydroxyapatite as Magnetic, up-Conversion and down-Conversion Materials for Multimodal Imaging. Sci. Rep. 2019, 9, 16305. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Stoica, A.E.; Vasile, B.S. Luminescent Hydroxyapatite Doped with Rare Earth Elements for Biomedical Applications. Nanomaterials 2019, 9, 239. [Google Scholar] [CrossRef]
- Gu, M.; Li, W.; Jiang, L.; Li, X. Recent Progress of Rare Earth Doped Hydroxyapatite Nanoparticles: Luminescence Properties, Synthesis and Biomedical Applications. Acta Biomater. 2022, 148, 22–43. [Google Scholar] [CrossRef]
- Long, M.; Hong, F.; Li, W.; Li, F.; Zhao, H.; Lv, Y.; Li, H. Size-Dependent Microstructure and Europium Site Preference Influence Fluorescent Properties of Eu3+-Doped Ca10(PO4)6(OH)2 Nanocrystal. J. Lumin. 2008, 128, 428–436. [Google Scholar] [CrossRef]
- Diniz, J.R.; Correa, J.R.; Moreira, D.D.A.; Fontenele, R.S.; De Oliveira, A.L.; Abdelnur, P.V.; Dutra, J.D.L.; Freire, R.O.; Rodrigues, M.O.; Neto, B.A.D. Water-Soluble Tb3+ and Eu3+ Complexes with Ionophilic (Ionically Tagged) Ligands as Fluorescence Imaging Probes. Inorg. Chem. 2013, 52, 10199–10205. [Google Scholar] [CrossRef]
- Shao, G.; Han, R.; Ma, Y.; Tang, M.; Xue, F.; Sha, Y.; Wang, Y. Bionanoprobes with Excellent Two-Photon-Sensitized Eu3+ Luminescence Properties for Live Cell Imaging. Chem. A Eur. J. 2010, 16, 8647–8651. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L. One-Pot Syntheses and Cell Imaging Applications of Poly(Amino Acid) Coated LaVO4:Eu3+ Luminescent Nanocrystals. Inorg. Chem. 2013, 52, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yao, T.; Zheng, X.; Hui, J.; Fan, D. Eu3+/Tb3+-Doped Whitlockite Nanocrystals: Controllable Synthesis, Cell Imaging, and the Degradation Process in the Bone Reconstruction. Nano Res. 2022, 15, 1303–1309. [Google Scholar] [CrossRef]
- Liu, J.; Tian, X.; Luo, N.; Yang, C.; Xiao, J.; Shao, Y.; Chen, X.; Yang, G.; Chen, D.; Li, L. Sub-10 Nm Monoclinic Gd2O3:Eu3+ Nanoparticles as Dual-Modal Nanoprobes for Magnetic Resonance and Fluorescence Imaging. Langmuir 2014, 30, 13005–13013. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Z.; Li, L.; Zhang, L.Y.; Wang, T.T.; Wang, C.G.; Su, Z.M. Facile Fabrication of Hollow Mesoporous Eu3+-Doped Gd2O3 Nanoparticles for Dual-Modal Imaging and Drug Delivery. Dye. Pigment. 2015, 123, 8–15. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, W.; Zhang, J.; Li, Y.; Zhou, G.; Jia, G. Uniform Mesoporous CaSiO3:Eu3+ Nanospheres: Template-Directed Synthesis, Luminescence and Sustained Drug Release Properties. Dye. Pigment. 2017, 136, 427–433. [Google Scholar] [CrossRef]
- Wang, Y.B.; He, L.; Zhou, B.C.; Tang, F.; Fan, J.; Wang, D.Q.; Lu, A.H.; Li, W.C. Hydroxyapatite Nanorods Rich in [Ca-O-P] Sites Stabilized Ni Species for Methane Dry Reforming. Ind. Eng. Chem. Res. 2021, 60, 15064–15073. [Google Scholar] [CrossRef]
- Wang, Q.N.; Weng, X.F.; Zhou, B.C.; Lv, S.P.; Miao, S.; Zhang, D.; Han, Y.; Scott, S.L.; Schüth, F.; Lu, A.H. Direct, Selective Production of Aromatic Alcohols from Ethanol Using a Tailored Bifunctional Cobalt-Hydroxyapatite Catalyst. ACS Catal. 2019, 9, 7204–7216. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.B.; Kumar, G.S.; Kolesnikov, E.; Janarthanan, G.; Pillai, M.M.; Rajendran, S.; Boobalan, S.; Gorshenkov, M.V.; Kuznetsov, D. Ascorbic Acid-Assisted Microwave Synthesis of Mesoporous Ag-Doped Hydroxyapatite Nanorods from Biowaste Seashells for Implant Applications. ACS Appl. Bio Mater. 2019, 2, 2280–2293. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and Characterization of Zn-Doped Hydroxyapatite: Scaffold Application, Antibacterial and Bioactivity Studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef]
- Jenifer, A.; Senthilarasan, K.; Arumugam, S.; Sivaprakash, P.; Sagadevan, S.; Sakthivel, P. Investigation on Antibacterial and Hemolytic Properties of Magnesium-Doped Hydroxyapatite Nanocomposite. Chem. Phys. Lett. 2021, 771, 138539. [Google Scholar] [CrossRef]
- Kaygili, O.; Dorozhkin, S.V.; Ates, T.; Al-Ghamdi, A.A.; Yakuphanoglu, F. Dielectric Properties of Fe Doped Hydroxyapatite Prepared by Sol-Gel Method. Ceram. Int. 2014, 40, 9395–9402. [Google Scholar] [CrossRef]
- Dasgupta, S.; Banerjee, S.S.; Bandyopadhyay, A.; Bose, S. Zn- and Mg-Doped Hydroxyapatite Nanoparticles for Controlled Release of Protein. Langmuir 2010, 26, 4958–4964. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, K.; Park, S.P.; Lee, J.H.; Jung, H.S. Aspartic Acid-Assisted Synthesis of Multifunctional Strontium-Substituted Hydroxyapatite Microspheres. Cryst. Growth Des. 2016, 16, 4318–4326. [Google Scholar] [CrossRef]
- Escudero, A.; Calvo, M.E.; Rivera-ferna, S. Microwave-Assisted Synthesis of Biocompatible Europium-Doped Calcium Hydroxyapatite and Fluoroapatite Luminescent Nanospindles Functionalized with Poly(Acrylic Acid). Langmuir 2013, 29, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Doat, A.; Fanjul, M.; Pellé, F.; Hollande, E.; Lebugle, A. Europium-Doped Bioapatite: A New Photostable Biological Probe, Internalizable by Human Cells. Biomaterials 2003, 24, 3365–3371. [Google Scholar] [CrossRef]
- Yang, C.; Yang, P.; Wang, W.; Wang, J.; Zhang, M.; Lin, J. Solvothermal Synthesis and Characterization of Ln (Eu3+, Tb3+) Doped Hydroxyapatite. J. Colloid Interface Sci. 2008, 328, 203–210. [Google Scholar] [CrossRef]
- Jiménez-Flores, Y.; Suárez-Quezada, M.; Rojas-Trigos, J.B.; Lartundo-Rojas, L.; Suárez, V.; Mantilla, A. Characterization of Tb-Doped Hydroxyapatite for Biomedical Applications: Optical Properties and Energy Band Gap Determination. J. Mater. Sci. 2017, 52, 9990–10000. [Google Scholar] [CrossRef]
- Terraschke, H.; Rothe, M.; Tsirigoni, A.; Lindenberg, P.; Arana, L.R. In Situ Luminescence Analysis: A New Light on Monitoring Calcium Phosphate Phase Transitions. Inorg. Chem. Front. 2017, 4, 1157–1165. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Dai, H.; Li, S. Synthesis and Luminescence of Eu3+ Doped Hydroxyapatite Nanocrystallines: Effects of Calcinations and Eu3+ Content. J. Lumin. 2013, 135, 281–287. [Google Scholar] [CrossRef]
- Kumar, G.S.; Thamizhavel, A.; Yokogawa, Y.; Kalkura, S.N.; Girija, E.K. Synthesis, Characterization and in vitro Studies of Zinc and Carbonate Co-Substituted Nano-Hydroxyapatite for Biomedical Applications. Mater. Chem. Phys. 2012, 134, 1127–1135. [Google Scholar] [CrossRef]
- Matsunaga, K. First-Principles Study of Substitutional Magnesium and Zinc in Hydroxyapatite and Octacalcium Phosphate. J. Chem. Phys. 2008, 128, 245101. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Murata, H. Strontium Substitution in Bioactive Calcium Phosphates: A First-Principles Study. J. Phys. Chem. B 2009, 113, 3584–3589. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; He, F.; Ye, J. Synthesis and Structure of Iron- and Strontium-Substituted Octacalcium Phosphate: Effects of Ionic Charge and Radius. J. Mater. Chem. B 2016, 4, 1712–1719. [Google Scholar] [CrossRef]
- Honda, Y.; Anada, T.; Morimoto, S.; Shiwaku, Y.; Suzuki, O. Effect of Zn2+ on the Physicochemical Characteristics of Octacalcium Phosphate and Its Hydrolysis into Apatitic Phases. Cryst. Growth Des. 2011, 11, 1462–1468. [Google Scholar] [CrossRef]
- Sugiura, Y.; Obika, H.; Horie, M.; Niitsu, K.; Makita, Y. Aesthetic Silver-Doped Octacalcium Phosphate Powders Exhibiting Both Contact Antibacterial Ability and Low Cytotoxicity. ACS Omega 2020, 5, 24434–24444. [Google Scholar] [CrossRef]
- Sugiura, Y.; Ono, F.; Nohara, M.; Horino, R.; Kutara, K.; Kanda, T.; Oowada, K.; Horie, M.; Makita, Y. Ag-Substituted Octacalcium Phosphate Blocks That Exhibit High Osteoconductivity and High Antibacterial Activity toward Various Pathogens. Mater. Today Commun. 2022, 30, 103130. [Google Scholar] [CrossRef]
- Mayer, I.; Jacobsohn, O.; Niazov, T.; Werckmann, J.; Iliescu, M.; Richard-plouet, M.; Burghaus, O.; Reinen, D. Manganese in Precipitated Hydroxyapatites. Eur. J. Inorg. Chem. 2003, 2003, 1445–1451. [Google Scholar] [CrossRef]
- Kishi, S.; Segawa, Y.; Yamaguchi, M. Histomorphological Confirmation of the Preventive Effect of β-Alanyl-L-Histidinato Zinc on Bone Loss in Ovariectomized Rats. Biol. Pharm. Bull. 1994, 17, 862–865. [Google Scholar] [CrossRef][Green Version]
- Hall, S.L.; Dimai, H.P.; Farley, J.R. Effects of Zinc on Human Skeletal Alkaline Phosphatase Activity in vitro. Calcif. Tissue Int. 1999, 64, 163–172. [Google Scholar] [CrossRef]
- Guggenbuhl, P.; Filmon, R.; Mabilleau, G.; Baslé, M.F.; Chappard, D. Iron Inhibits Hydroxyapatite Crystal Growth in vitro. Metabolism 2008, 57, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, W.; Li, F.; Perera, T.S.H.; Gan, L.; Han, Y.; Wang, X.; Li, S.; Dai, H. Luminescence Enhanced Eu3+/Gd3+ Co-Doped Hydroxyapatite Nanocrystals as Imaging Agents in vitro and in vivo. ACS Appl. Mater. Interfaces 2016, 8, 10212–10219. [Google Scholar] [CrossRef]
- Targonska, S.; Sikora, M.; Marycz, K.; Smieszek, A.; Wiglusz, R.J. Theranostic Applications of Nanostructured Silicate-Substituted Hydroxyapatite Codoped with Eu3+ and Bi3+ Ions-A Novel Strategy for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 6148–6160. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, P.; Zhu, Y.; Wu, J.; Cui, D. Biomaterials Multifunctional Eu3+/Gd3+ Dual-Doped Calcium Phosphate Vesicle-like Nanospheres for Sustained Drug Release and Imaging. Biomaterials 2012, 33, 6447–6455. [Google Scholar] [CrossRef] [PubMed]
- Heger, D.; Jirkovský, J.; Klán, P. Aggregation of Methylene Blue in Frozen Aqueous Solutions Studied by Absorption Spectroscopy. J. Phys. Chem. A 2005, 109, 6702–6709. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Yasukawa, A.; Kandori, K.; Ishikawa, T. FTIR Study on Incorporation of CO2 into Calcium Hydroxyapatite. J. Chem. Soc. Faraday Trans. 1998, 94, 1501–1505. [Google Scholar] [CrossRef]
- Selvam, S.; Sarkar, I. Bile Salt Induced Solubilization of Methylene Blue: Study on Methylene Blue Fluorescence Properties and Molecular Mechanics Calculation. J. Pharm. Anal. 2017, 7, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.J.G.; Verbeek, F.P.R.; Schaafsma, B.E.; Boonstra, M.C.; Van Der Vorst, J.R.; Liefers, G.J.; Van De Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L. Real-Time Intraoperative Detection of Breast Cancer Using near-Infrared Fluorescence Imaging and Methylene Blue. Eur. J. Surg. Oncol. 2014, 40, 850–858. [Google Scholar] [CrossRef]
- Spiller, W.; Kliesch, H.; Wöhrle, D.; Hackbarth, S.; Röder, B. Singlet Oxygen Quantum Yields of Different Photosensitizers in Polar Solvents and Micellar Solutions. J. Porphyr. Phthalocyanines 1998, 2, 145–158. [Google Scholar] [CrossRef]
- Fan, Z.; Dai, X.; Lu, Y.; Yu, E.; Brahmbatt, N.; Carter, N.; Tchouwou, C.; Singh, A.K.; Jones, Y.; Yu, H.; et al. Enhancing Targeted Tumor Treatment by near IR Light-Activatable Photodynamic-Photothermal Synergistic Therapy. Mol. Pharm. 2014, 11, 1109–1116. [Google Scholar] [CrossRef]
- Simon, T.; Potara, M.; Gabudean, A.M.; Licarete, E.; Banciu, M.; Astilean, S. Designing Theranostic Agents Based on Pluronic Stabilized Gold Nanoaggregates Loaded with Methylene Blue for Multimodal Cell Imaging and Enhanced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2015, 7, 16191–16201. [Google Scholar] [CrossRef] [PubMed]
- Isikawa, M.; Guidelli, E. Microfluidic Synthesis of Theranostic Nanoparticles with Near-Infrared Scintillation: Toward Next-Generation Dosimetry in X-Ray-Induced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Ramírez-García, G.; Banu, N.; Vallejo-Cardona, A.A.; Lugo-Fabres, P.; Camacho-Villegas, T.A.; Salas, P.; De la Rosa, E. Ligand-Targeted Theranostic Liposomes Combining Methylene Blue Attached Upconversion Nanoparticles for NIR Activated Bioimaging and Photodynamic Therapy against HER-2 Positive Breast Cancer. J. Lumin. 2021, 237, 118143. [Google Scholar] [CrossRef]
- Pentrák, M.; Czímerová, A.; Madejová, J.; Komadel, P. Changes in Layer Charge of Clay Minerals upon Acid Treatment as Obtained from Their Interactions with Methylene Blue. Appl. Clay Sci. 2012, 55, 100–107. [Google Scholar] [CrossRef]
- Czímerová, A.; Jankovič, L.; Bujdák, J. Effect of the Exchangeable Cations on the Spectral Properties of Methylene Blue in Clay Dispersions. J. Colloid Interface Sci. 2004, 274, 126–132. [Google Scholar] [CrossRef]
- Czímerová, A.; Bujdák, J.; Gáplovský, A. The Aggregation of Thionine and Methylene Blue Dye in Smectite Dispersion. Colloids Surf. A Physicochem. Eng. Asp. 2004, 243, 89–96. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Heughebaert, L. Clay Adsorbed Dyes: Methylene Blue on Laponite. Clay Miner. 1992, 27, 91–100. [Google Scholar] [CrossRef]
- Chen, G.; Pan, J.; Han, B.; Yan, H. Adsorption of Methylene Blue on Montmorillonite. J. Dispers. Sci. Technol. 1999, 20, 1179–1187. [Google Scholar] [CrossRef]
- Cenens, J.; Schoonheydt, R.A. Visible Spectroscopy of Methylene Blue on Hectorite, Laponite B, and Barasym in Aqueous Suspension. Clays Clay Miner. 1988, 36, 214–224. [Google Scholar] [CrossRef]
- Wei, W.; Yang, L.; Zhong, W.H.; Li, S.Y.; Cui, J.; Wei, Z.G.; Control, P.; Normal, N. Fast Removal of Methylene Blue from Aqueous Solution by Adsorption onto Poorly Crystalline Hydroxyapatite. Dig. J. Nanomater. Biostructures 2015, 10, 1343–1363. [Google Scholar]
- Allam, K.; El Bouari, A.; Belhorma, B.; Bih, L. Removal of Methylene Blue from Water Using Hydroxyapatite Submitted to Microwave Irradiation. J. Water Resour. Prot. 2016, 08, 358–371. [Google Scholar] [CrossRef]
- Guesmi, Y.; Agougui, H.; Lafi, R.; Jabli, M.; Hafiane, A. Synthesis of Hydroxyapatite-Sodium Alginate via a Co-Precipitation Technique for Efficient Adsorption of Methylene Blue Dye. J. Mol. Liq. 2018, 249, 912–920. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Zeng, X.; Kang, Y.; Wang, J.; Xie, H.; Liu, Y.; Zhang, Y. Efficient Adsorption of Methylene Blue by Xanthan Gum Derivative Modified Hydroxyapatite. Int. J. Biol. Macromol. 2020, 151, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Zhang, Y.; Wang, G.; Li, S.; Han, R.; Wei, W. Reed Biochar Supported Hydroxyapatite Nanocomposite: Characterization and Reactivity for Methylene Blue Removal from Aqueous Media. J. Mol. Liq. 2018, 263, 53–63. [Google Scholar] [CrossRef]
- Schwiertz, J.; Wiehe, A.; Gräfe, S.; Gitter, B.; Epple, M. Calcium Phosphate Nanoparticles as Efficient Carriers for Photodynamic Therapy against Cells and Bacteria. Biomaterials 2009, 30, 3324–3331. [Google Scholar] [CrossRef]
- Nakayama, M.; Lim, W.Q.; Kajiyama, S.; Kumamoto, A.; Ikuhara, Y.; Kato, T.; Zhao, Y. Liquid-Crystalline Hydroxyapatite/Polymer Nanorod Hybrids: Potential Bioplatform for Photodynamic Therapy and Cellular Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 17759–17765. [Google Scholar] [CrossRef]
- Tada, D.B.; Rossi, L.M.; Leite, C.A.P.; Itri, R.; Baptista, M.S. Nanoparticle Platform to Modulate Reaction Mechanism of Phenothiazine Photosensitizers. J. Nanosci. Nanotechnol. 2010, 10, 3100–3108. [Google Scholar] [CrossRef]
- Valizadeh, S.; Rasoulifard, M.H.; Saeed, M.; Dorraji, S. Adsorption and Photocatalytic Degradation of Organic Dyes onto Crystalline and Amorphous Hydroxyapatite: Optimization, Kinetic and Isotherm Studies. Korean J. Chem. Eng. 2016, 33, 481–489. [Google Scholar] [CrossRef]
- El Boujaady, H.; El Rhilassi, A.; Bennani-ziatni, M.; El Hamri, R.; Taitai, A.; Lacout, J.L. Removal of a Textile Dye by Adsorption on Synthetic Calcium Phosphates. Desalination 2011, 275, 10–16. [Google Scholar] [CrossRef]
- Russin, T.J.; Kaiser, J.M.; Barth, B.M.; Eklund, P.C.; Kester, M.; Adair, J.H. Near-Infrared Emitting Fluorophore- for In vivo Imaging of Human Breast. ACS Nano 2008, 2, 2075–2084. [Google Scholar]
- Haedicke, K.; Kozlova, D.; Gräfe, S.; Teichgräber, U.; Epple, M.; Hilger, I. Multifunctional Calcium Phosphate Nanoparticles for Combining Near-Infrared Fluorescence Imaging and Photodynamic Therapy. Acta Biomater. 2015, 14, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lopera, A.A.; Montoya, A.; Vélez, I.D.; Robledo, S.M.; Garcia, C.P. Synthesis of Calcium Phosphate Nanostructures by Combustion in Solution as a Potential Encapsulant System of Drugs with Photodynamic Properties for the Treatment of Cutaneous Leishmaniasis. Photodiagnosis Photodyn. Ther. 2018, 21, 138–146. [Google Scholar] [CrossRef] [PubMed]


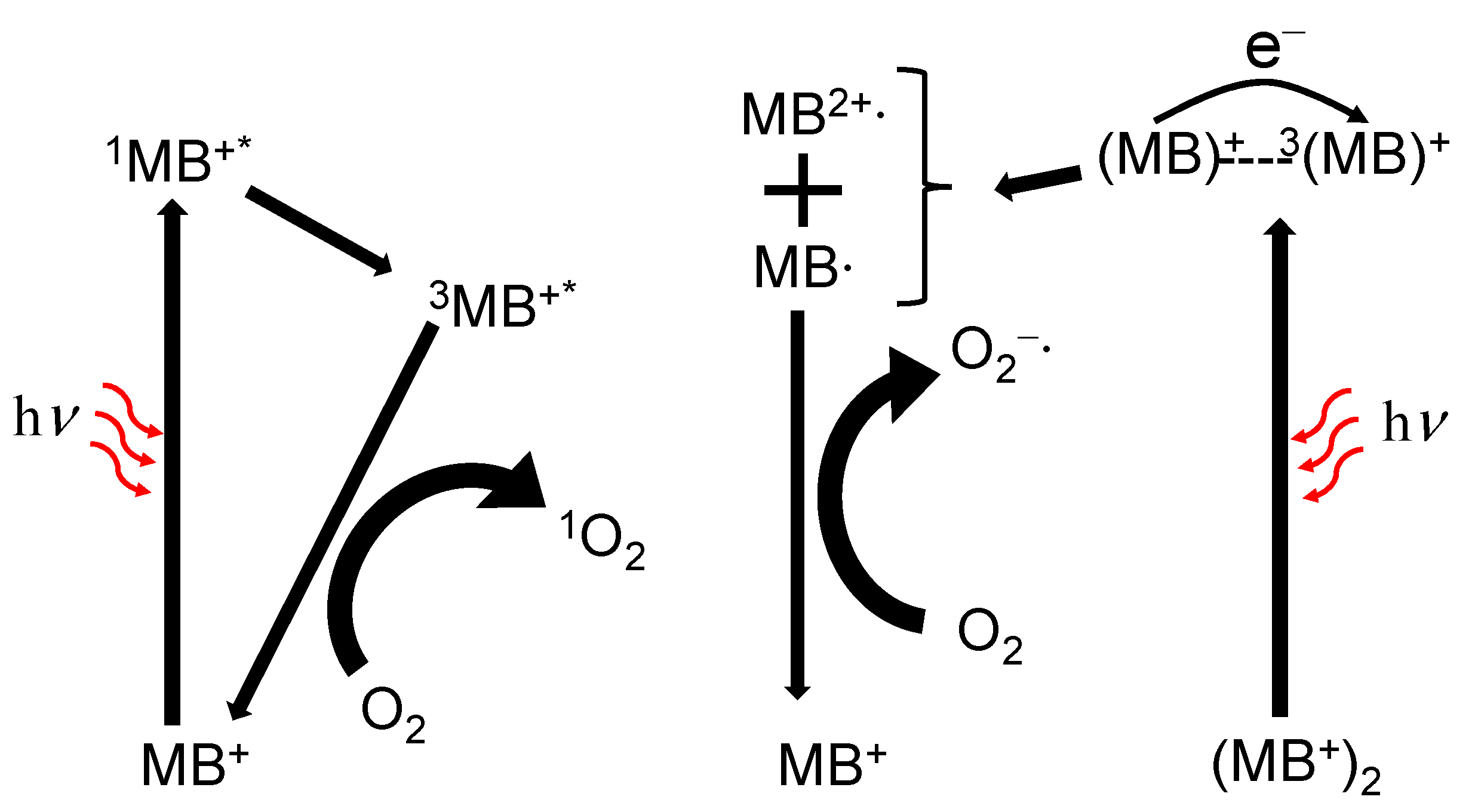
| Dicarboxylate Ions | Interlayer Distance (d100) | Reference |
|---|---|---|
| Malonate | 2.01 nm | [80] |
| Succinate | 2.14 nm | [49,80,81,82,83] |
| Adipate | 2.37 nm | [49,80] |
| 2-Methylsuccinate | 2.04 nm | [84,85] |
| 2-Mercaptosuccinate | 2.12 nm | [86] |
| Fumarate | 2.15 nm | [80] |
| 1,3-Benzenedicarboxylate | 2.30 nm | [87] |
| 2,2′-Bipyridine-5,5′-dicarboxylate | 2.49 nm | [88] |
| Substituted Ions | Ion Radius (Å) (8-Coordination) | Effect on OCP Property | In Vivo Role | Reference |
|---|---|---|---|---|
| Mg2+ | 0.89 | Inhibition of crystallization Decrease in thermal stability Decrease in crystal size | Influence on osteoblasts and osteoclasts activity | [153] |
| Sr2+ | 1.26 | Inhibition of crystallization Decrease in thermal stability Increase in crystal size | Presence in the bone, especially at the regions of high metabolic turnover | [154,155] |
| Zn2+ | 0.90 | Defect generation Decrease in hydrolysis rate Increase in amorphous phase generation | Stimulation of osteoblast activity (promote bone formation) | [153,156] |
| Fe3+ FeOH2+ Fe(OH)2+ | 0.78 - - | Lattice expansion | Essential elements for the cell | [155] |
| Ag+ | 1.28 | Facilitation of OCP generation | Antibacterial activity | [157,158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, I.; Shiba, K.; Galindo, T.G.P.; Tagaya, M. Drug Molecular Immobilization and Photofunctionalization of Calcium Phosphates for Exploring Theranostic Functions. Molecules 2022, 27, 5916. https://doi.org/10.3390/molecules27185916
Yamada I, Shiba K, Galindo TGP, Tagaya M. Drug Molecular Immobilization and Photofunctionalization of Calcium Phosphates for Exploring Theranostic Functions. Molecules. 2022; 27(18):5916. https://doi.org/10.3390/molecules27185916
Chicago/Turabian StyleYamada, Iori, Kota Shiba, Tania Guadalupe Peñaflor Galindo, and Motohiro Tagaya. 2022. "Drug Molecular Immobilization and Photofunctionalization of Calcium Phosphates for Exploring Theranostic Functions" Molecules 27, no. 18: 5916. https://doi.org/10.3390/molecules27185916
APA StyleYamada, I., Shiba, K., Galindo, T. G. P., & Tagaya, M. (2022). Drug Molecular Immobilization and Photofunctionalization of Calcium Phosphates for Exploring Theranostic Functions. Molecules, 27(18), 5916. https://doi.org/10.3390/molecules27185916







