Advances in the Study of Magnesium Alloys and Their Use in Bone Implant Material
Abstract
1. Introduction
2. The Development of Magnesium Alloys and Preparation Processes
2.1. Magnesium Alloy Development
2.2. Magnesium Alloy Preparation Process
2.2.1. Reduced Material Manufacturing
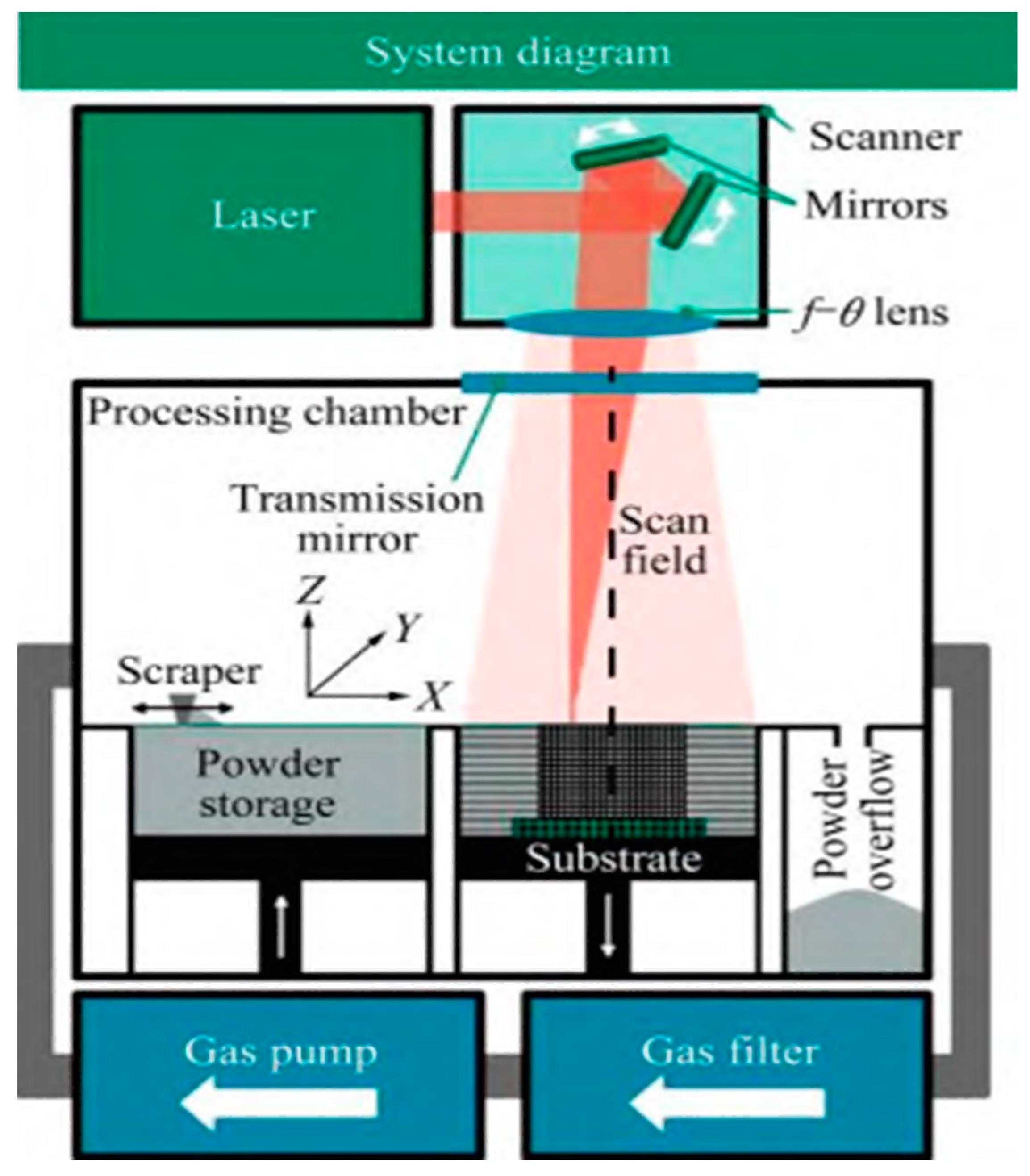
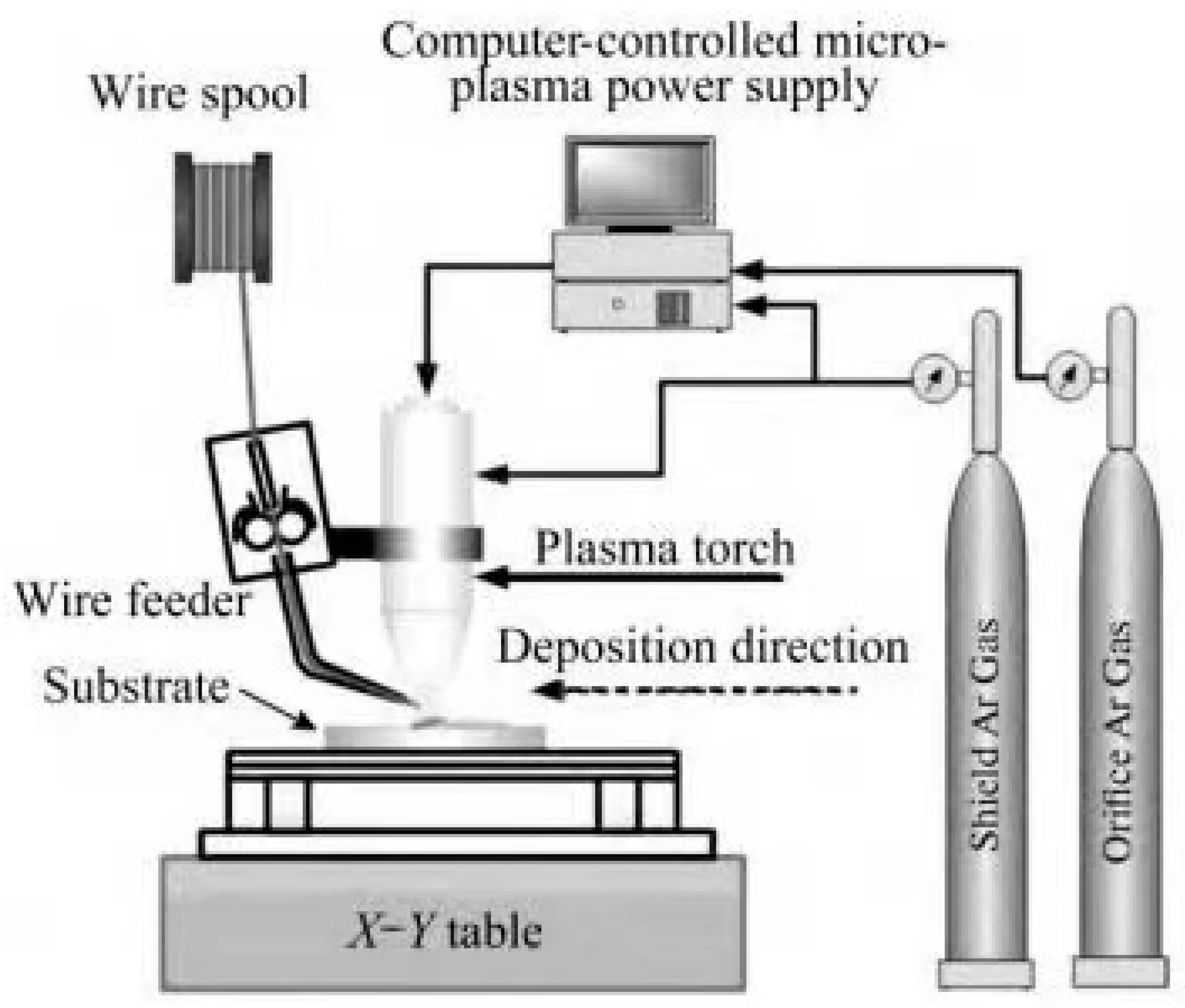
2.2.2. Additive Manufacturing
2.3. Magnesium Alloy Material Surface Modification Technology
2.3.1. Chemical Conversion Method
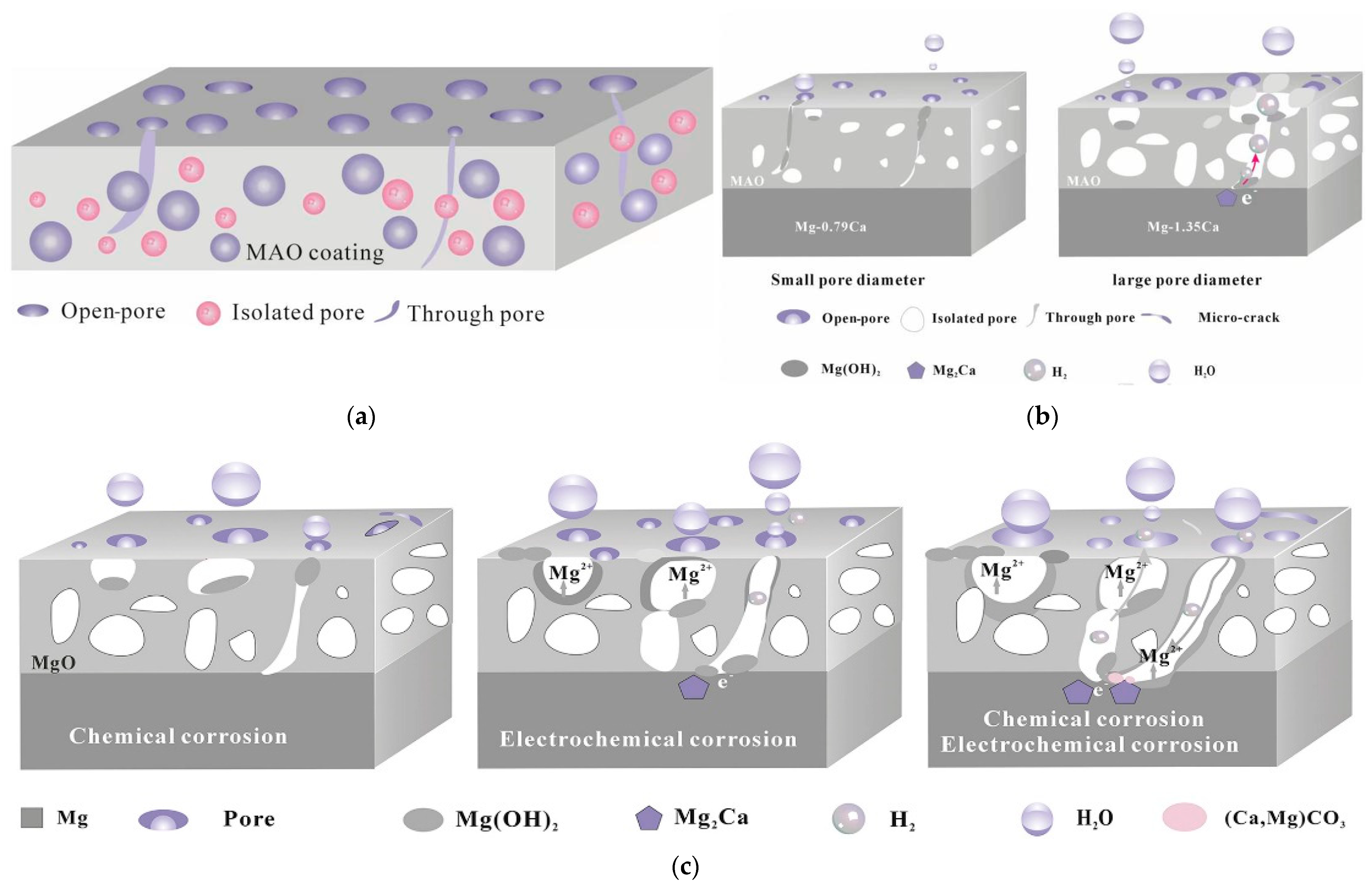
2.3.2. Anodic and Micro-Arc Oxidation Methods
2.3.3. Electrochemical Deposition Method
2.3.4. Bionic Deposition Method
2.3.5. Sol-Gel Method
2.3.6. Ion Injection Method
2.3.7. Polymer Coating
| Classification | Features | References | |
|---|---|---|---|
| Chemical conversion method | Fluoridation | Simple process, low cost, and good bonding of the coating to the substrate, but can increase localised corrosion. | [90] |
| Alkali heat treatment | Simple process, good biocompatibility of the coating, and a certain degree of protection of the magnesium alloy substrate. | ||
| Anodising and micro-arc oxidation | Anodising | High hardness, good bonding, and good biocompatibility. However, the method is complex, the coatings are mostly defective and brittle, and some of them do not degrade easily. | [95] |
| Micro-arc oxidation (MAO) | |||
| Electrochemical deposition | Good biocompatibility, but average coating adhesion. | [98] | |
| Bionic deposition method | It is a simple process with good biocompatibility but has poor binding power. | [101] | |
| Sol-gel method | Improves surface hardness, wear resistance, and corrosion resistance. | [104] | |
| Ion injection method | Improves surface hardness, wear resistance, and corrosion resistance. | [106] | |
| Polymer coatings | Dip and Lift Method | It is relatively homogeneous and dense, has a controlled composition thickness, and is available for subsequent drug loading. The disadvantage is that the degradation products are acidic and inflammatory, and the coating is less wear-resistant. | [108] |
| Spin coating method |
3. Mechanical Properties of Magnesium Alloys
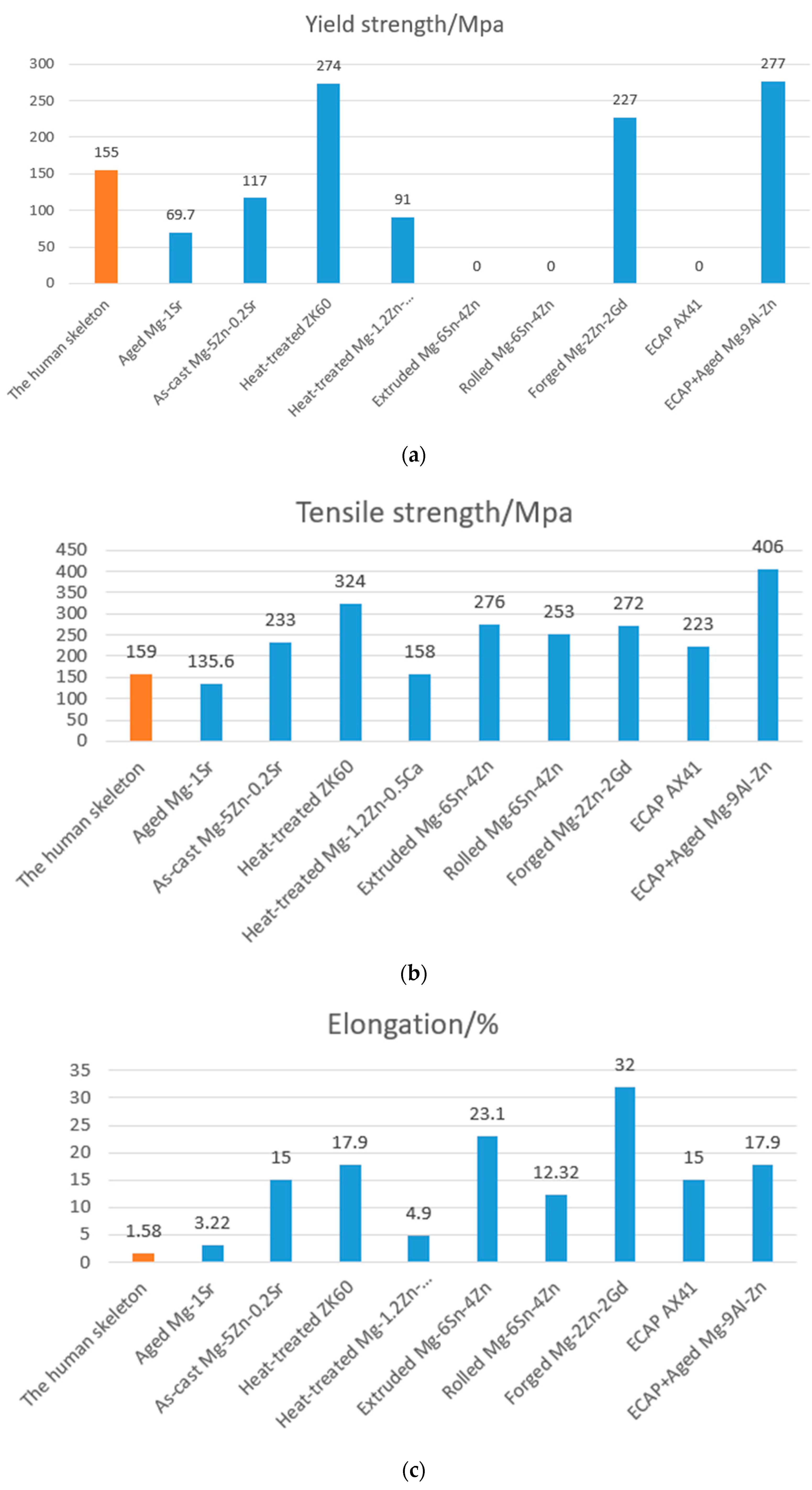
3.1. A Study of the Modulus of Elasticity
3.2. Frictional Wear Properties of Magnesium Alloys
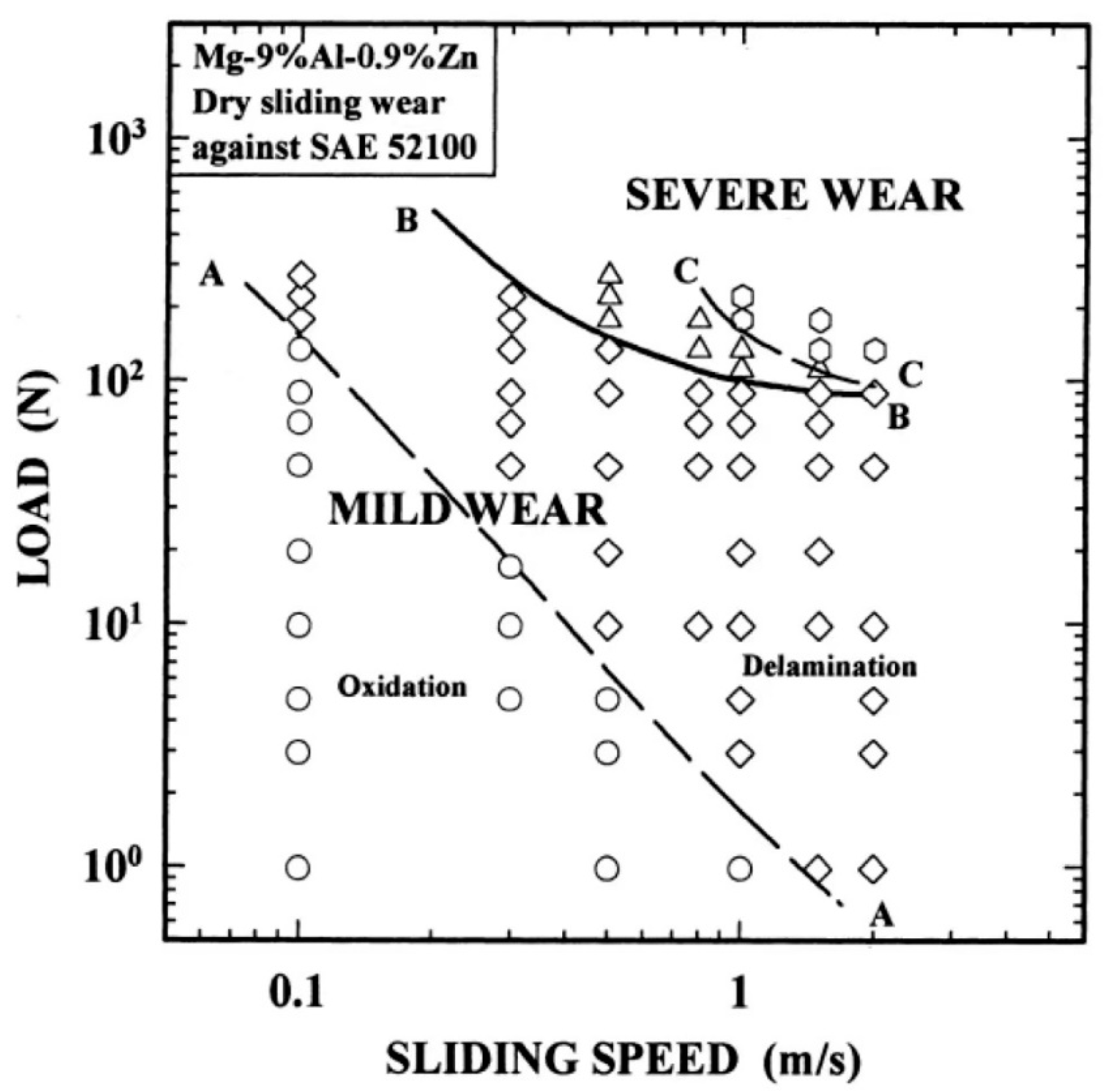
3.2.1. Frictional Wear of Mg-Al-Zn Alloys
3.2.2. Frictional Wear of Mg-Al-Si Alloys
3.2.3. Frictional Wear of Mg-Al-Ca and Mg-Zn-Zr Alloys
3.2.4. Frictional Wear of Rare Earth Magnesium Alloys
4. In Vitro, In Vivo, and Clinical Experimental Studies of Magnesium Alloys
4.1. Biocompatibility Studies In Vitro
4.1.1. Mg-Ca Series Alloys
4.1.2. Mg-Sr Series Alloys
4.1.3. Mg-Zn Series Alloys
4.1.4. Mg-Sn Series Alloys
4.1.5. Mg-Cu Series Alloys
4.1.6. Mg-Nd-Zn-Zr Alloys
4.2. In Vivo Biocompatibility Studies
4.3. Clinical Trial Studies
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kalfas, I.H. Principles of Bone Healing. Neurosurg. Focus 2001, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nuss, K.M.R.; von Rechenberg, B. Biocompatibility Issues with Modern Implants in Bone—A Review for Clinical Orthopedics. Open Orthop. J. 2008, 2, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Westerman, R.W.; Scammell, B.E. Principles of Bone and Joint Injuries and Their Healing. Surgery 2012, 30, 54–60. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Wan, P.; Tan, L.L.; Wang, K.; Yang, K. Preclinical Investigation of an Innovative Magnesium-Based Bone Graft Substitute for Potential Orthopaedic Applications. J. Orthop. Transl. 2014, 2, 139–148. [Google Scholar] [CrossRef]
- Lee, J.Y.; Han, G.; Kim, Y.C.; Byun, J.Y.; Jang, J.I.; Seok, H.K.; Yang, S.J. Effects of Impurities on the Biodegradation Behavior of Pure Magnesium. Met. Mater. Int. 2009, 15, 955–961. [Google Scholar] [CrossRef]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Stark, J.G. Use of Selective Estrogen Receptor Modulator for Joint Fusion and Other Repair or Healing of Connective Tissue. Available online: https://www.freepatentsonline.com/8933028.html (accessed on 7 November 2013).
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The Use of Bone-Graft Substitutes in Large Bone Defects: Any Specific Needs? Injury 2011, 42, S56–S63. [Google Scholar] [CrossRef]
- Van der Stok, J.; van Lieshout, E.M.M.; El-Massoudi, Y.; van Kralingen, G.H.; Patka, P. Bone Substitutes in the Netherlands—A Systematic Literature Review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [CrossRef]
- Auer, J.A.; von Rechenberg, B.; Bohner, M.; Hofmann-Amtenbrink, M. Bone Grafts and Bone Replacements; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1081–1095. [Google Scholar] [CrossRef]
- Kim, H.M. Ceramic Bioactivity and Related Biomimetic Strategy. Curr. Opin. Solid State Mater. Sci. 2003, 7, 289–299. [Google Scholar] [CrossRef]
- Dias, A.G.; Lopes, M.A.; Gibson, I.R.; Santos, J.D. In Vitro Degradation Studies of Calcium Phosphate Glass Ceramics Prepared by Controlled Crystallization. J. Non-Cryst. Solids 2003, 330, 81–89. [Google Scholar] [CrossRef]
- Aoki, S.; Yamaguchi, S.; Nakahira, A.; Suganuma, K. A New Approach to an Artificial Joint Based on Bio-Cartilage/Porous β-Tricalcium Phosphate System. J. Eur. Ceram. Soc. 2003, 23, 2939–2946. [Google Scholar] [CrossRef]
- Mohamed, K.R.; Beherei, H.H.; El-Rashidy, Z.M. In Vitro Study of Nano-Hydroxyapatite/Chitosan–Gelatin Composites for Bio-Applications. J. Adv. Res. 2014, 5, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yoganand, C.P.; Selvarajan, V.; Cannillo, V.; Sola, A.; Roumeli, E.; Goudouri, O.M.; Paraskevopoulos, K.M.; Rouabhia, M. Characterization and in Vitro-Bioactivity of Natural Hydroxyapatite Based Bio-Glass–Ceramics Synthesized by Thermal Plasma Processing. Ceram. Int. 2010, 36, 1757–1766. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone Substitutes: An Update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Böstman, O.; Pihlajamäki, H. Clinical Biocompatibility of Biodegradable Orthopaedic Implants for Internal Fixation: A Review. Biomaterials 2000, 21, 2615–2621. [Google Scholar] [CrossRef]
- Chai, H.; Guo, L.; Wang, X.; Fu, Y.; Guan, J.; Tan, L.; Ren, L.; Yang, K. Antibacterial Effect of 317L Stainless Steel Contained Copper in Prevention of Implant-Related Infection in Vitro and in Vivo. J. Mater. Sci. Mater. Med. 2011, 22, 2525–2535. [Google Scholar] [CrossRef]
- Nan, L.; Yang, K. Cu Ions Dissolution from Cu-Bearing Antibacterial Stainless Steel. J. Mater. Sci. Technol. 2010, 26, 941–944. [Google Scholar] [CrossRef]
- Shirai, T.; Tsuchiya, H.; Shimizu, T.; Ohtani, K.; Zen, Y.; Tomita, K. Prevention of Pin Tract Infection with Titanium-Copper Alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91B, 373–380. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Ishii, D. Mechanical and Biodegradable Properties of Porous Titanium Filled with Poly-L-Lactic Acid by Modified in Situ Polymerization Technique. J. Mech. Behav. Biomed. Mater. 2011, 4, 1206–1218. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical Testing and Degradation Analysis of MgCa0.8 Alloy Screws: A Comparative in Vivo Study in Rabbits. Acta Biomater. 2011, 7, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Tschernitschek, H.; Borchers, L.; Geurtsen, W. Nonalloyed Titanium as a Bioinert Metal—A Review. J. Prosthet. Dent. 2006, 96, 12. [Google Scholar] [CrossRef]
- Hong, D.; Saha, P.; Chou, D.T.; Lee, B.; Collins, B.E.; Tan, Z.; Dong, Z.; Kumta, P.N. In Vitro Degradation and Cytotoxicity Response of Mg–4% Zn–0.5% Zr (ZK40) Alloy as a Potential Biodegradable Material. Acta Biomater. 2013, 9, 8534–8547. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.W.K.; Wong, K.H.M. Biodegradable Metallic Materials for Orthopaedic Implantations: A Review. Technol. Health Care 2012, 20, 345–362. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium Phosphate Coatings on Magnesium Alloys for Biomedical Applications: A Review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Yang, H.; Yan, X.; Ling, M.; Xiong, Z.; Ou, C.; Lu, W. In Vitro Corrosion and Cytocompatibility Properties of Nano-Whisker Hydroxyapatite Coating on Magnesium Alloy for Bone Tissue Engineering Applications. Int. J. Mol. Sci. 2015, 16, 6113–6123. [Google Scholar] [CrossRef]
- Lin, X.; Tan, L.; Zhang, Q.; Yang, K.; Hu, Z.; Qiu, J.; Cai, Y. The in Vitro Degradation Process and Biocompatibility of a ZK60 Magnesium Alloy with a Forsterite-Containing Micro-Arc Oxidation Coating. Acta Biomater. 2013, 9, 8631–8642. [Google Scholar] [CrossRef]
- Persaud-Sharma, D.; Mcgoron, A. Biodegradable Magnesium Alloys: A Review of Material Development and Applications. J. Biomim. Biomater. Tissue Eng. 2011, 12, 25–39. [Google Scholar] [CrossRef]
- Musso, C.G. Magnesium Metabolism in Health and Disease. Int. Urol. Nephrol. 2009, 41, 357–362. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and Metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
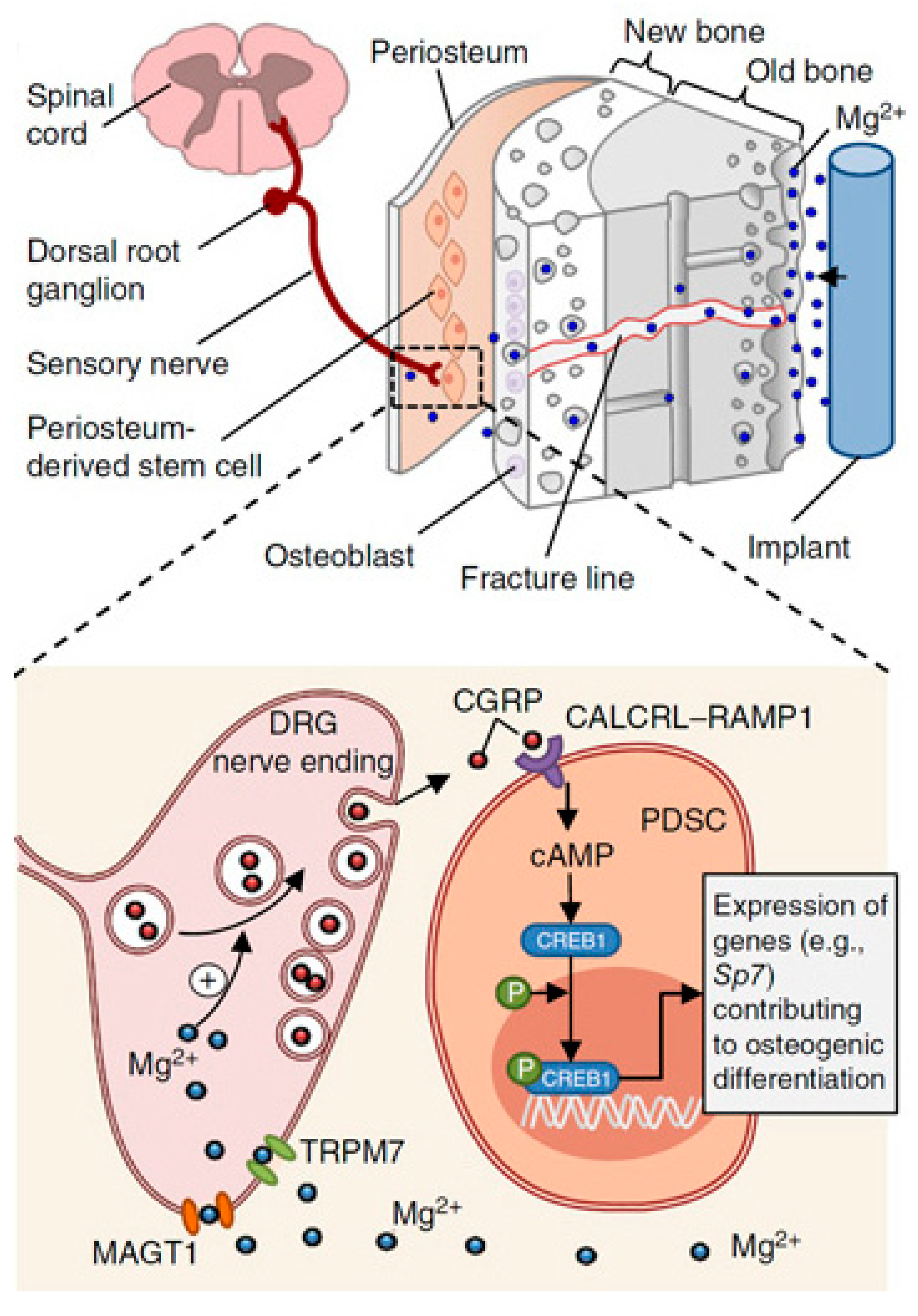
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-Derived Magnesium Induces Local Neuronal Production of CGRP to Improve Bone-Fracture Healing in Rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Waizy, H.; Seitz, J.M.; Reifenrath, J.; Weizbauer, A.; Bach, F.W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable Magnesium Implants for Orthopedic Applications. J. Mater. Sci. 2012, 48, 39–50. [Google Scholar] [CrossRef]
- Bornapour, M.; Celikin, M.; Cerruti, M.; Pekguleryuz, M. Magnesium Implant Alloy with Low Levels of Strontium and Calcium: The Third Element Effect and Phase Selection Improve Bio-Corrosion Resistance and Mechanical Performance. Mater. Sci. Eng. C 2014, 35, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Yun, Y.; Schulz, M.J.; Shanov, V. Corrosion Protection of Biodegradable Magnesium Implants Using Anodization. Mater. Sci. Eng. C 2011, 31, 215–223. [Google Scholar] [CrossRef]
- Seal, C.K.; Vince, K.; Hodgson, M.A. Biodegradable Surgical Implants Based on Magnesium Alloys—A Review of Current Research. IOP Conf. Ser. Mater. Sci. Eng. 2009, 4, 012011. [Google Scholar] [CrossRef]
- Brar, H.S.; Wong, J.; Manuel, M.V. Investigation of the Mechanical and Degradation Properties of Mg–Sr and Mg–Zn–Sr Alloys for Use as Potential Biodegradable Implant Materials. J. Mech. Behav. Biomed. Mater. 2012, 7, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent Advances on the Development of Magnesium Alloys for Biodegradable Implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Yun, Y.H.; Dong, Z.; Yang, D.; Schulz, M.J.; Shanov, V.N.; Yarmolenko, S.; Xu, Z.; Kumta, P.; Sfeir, C. Biodegradable Mg Corrosion and Osteoblast Cell Culture Studies. Mater. Sci. Eng. C 2009, 29, 1814–1821. [Google Scholar] [CrossRef]
- Song, G.; Song, S. A Possible Biodegradable Magnesium Implant Material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.N.; Zheng, W.; Cheng, Y.; Zheng, Y.F. A Study on Alkaline Heat Treated Mg–Ca Alloy for the Control of the Biocorrosion Rate. Acta Biomater. 2009, 5, 2790–2799. [Google Scholar] [CrossRef]
- Lloyd, A.W. Interfacial Bioengineering to Enhance Surface Biocompatibility. Med. Device Technol. 2002, 13, 18–21. [Google Scholar] [PubMed]
- Jebson, P.J.L.; Hayden, R.J. AO Principles of Fracture Management. JAMA 2008, 300, 2432–2433. [Google Scholar] [CrossRef]
- Kuhlmann, J.; Bartsch, I.; Willbold, E.; Schuchardt, S.; Holz, O.; Hort, N.; Höche, D.; Heineman, W.R.; Witte, F. Fast Escape of Hydrogen from Gas Cavities around Corroding Magnesium Implants. Acta Biomater. 2013, 9, 8714–8721. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, Z. In Vitro Biodegradation Behavior of Magnesium and Magnesium Alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98B, 203–209. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable Biomaterials Based on Magnesium Corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Gill, P.; Munroe, N.; Dua, R.; Ramaswamy, S. Corrosion and Biocompatibility Assessment of Magnesium Alloys. J. Biomater. Nanobiotechnol. 2012, 3, 10–13. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical Coatings on Magnesium Alloys—A Review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Yang, K. Recent Advances on the Development of Biodegradable Magnesium Alloys: A Review. Mater. Technol. 2016, 31, 681–688. [Google Scholar] [CrossRef]
- Paramsothy, M.; Ramakrishna, S. Biodegradable Materials for Clinical Applications: A Review. Rev. Adv. Sci. Eng. 2016, 4, 221–238. [Google Scholar] [CrossRef]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-Based Biodegradable Alloys: Degradation, Application, and Alloying Elements. Interv. Med. Appl. Sci. 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Payr, E. Beiträge zur Technik der Blutgefäss- und Nervennaht Nebst Mittheilungen Über Die Verwendung Eines Resorbirbaren Metalles in Der Chirurgie. Arch. Klin. Chir. 1900, 62, 67–93. [Google Scholar]
- Lambotte, A. Technique et Indications de La Prothèse Perdue Dans Le Traitement des Fractures. Press Med Belge. 1909, 17, 321–323. [Google Scholar]
- Verbrugge, J. Le Matériel Métallique Résorbable En Chirurgie Osseuse. Presse Med. 1934, 23, 460–465. [Google Scholar]
- Mcbride, E.D. Absorbable metal in bone surgery: A further report on the use of magnesium alloys. J. Am. Med. Assoc. 1938, 111, 2464–2467. [Google Scholar] [CrossRef]
- Maier, O. Über Die Verwendbarkeit von Leichtmetallen in Der Chirurgie (Metallisches Magnesium Als Reizmittel Zur Knochenneubildung). Dtsch. Z. Chir. 1940, 253, 552–556. [Google Scholar] [CrossRef]
- Troitskii, V.V.; Tsitrin, D.N. The Resorbing Metallic Alloy “OSteosinthezit” as Material for Fastening Broken Bone. Khirurgiia. 1944, 8, 41–44. [Google Scholar]
- Witte, F. The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Witte, F.; Ulrich, H.; Palm, C.; Willbold, E. Biodegradable Magnesium Scaffolds: Part II: Peri-Implant Bone Remodeling. J. Biomed. Mater. Res. A 2007, 81A, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Kolbeinsson, I.; Kirkland, N.T.; Nguyen, T.; Dias, G.; Woodfield, T.B.F. Synthesis of Topologically-Ordered Open-Cell Porous Magnesium. Mater. Lett. 2010, 64, 2572–2574. [Google Scholar] [CrossRef]
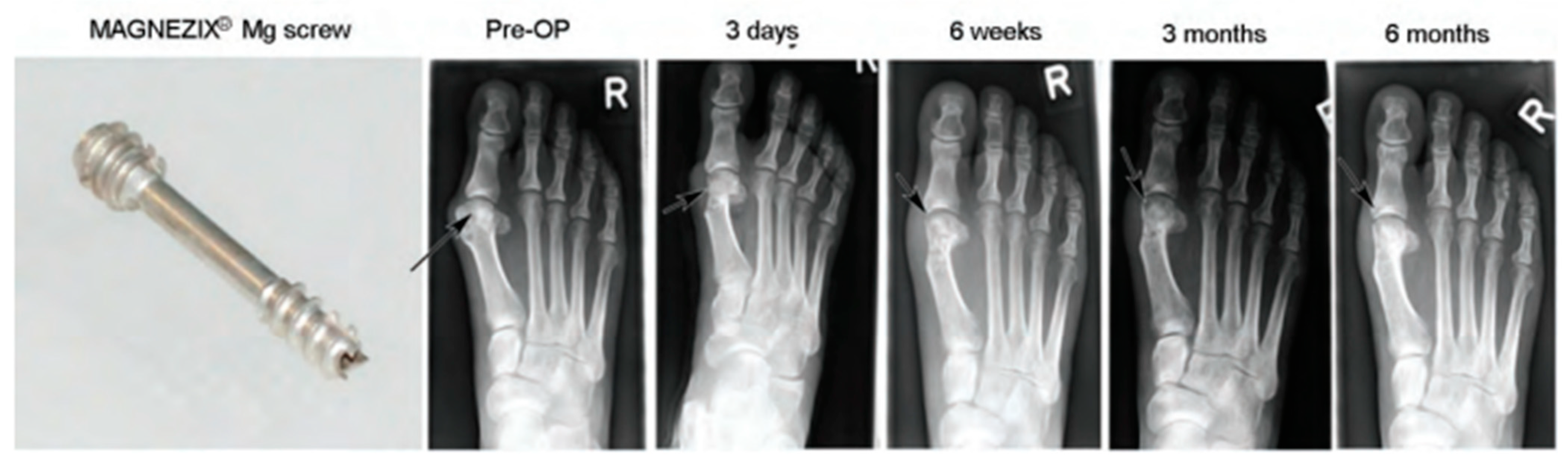
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable Magnesium-Based Screw Clinically Equivalent to Titanium Screw in Hallux Valgus Surgery: Short Term Results of the First Prospective, Randomized, Controlled Clinical Pilot Study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Plaass, C.; Ettinger, S.; Sonnow, L.; Koenneker, S.; Noll, Y.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Daniilidis, K.; Stukenborg-Colsman, C.; et al. Early Results Using a Biodegradable Magnesium Screw for Modified Chevron Osteotomies. J. Orthop. Res. 2016, 34, 2207–2214. [Google Scholar] [CrossRef]
- Seitz, J.M.; Lucas, A.; Kirschner, M. Magnesium-Based Compression Screws: A Novelty in the Clinical Use of Implants. JOM 2016, 68, 1177–1182. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, H.S.; Han, K.J.; Park, J.; Jeon, H.; Ok, M.R.; Seok, H.K.; Ahn, J.P.; Lee, K.E.; Lee, D.H.; et al. Long-Term Clinical Study and Multiscale Analysis of in Vivo Biodegradation Mechanism of Mg Alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized Bone Grafting Fixed by Biodegradable Magnesium Screw for Treating Osteonecrosis of the Femoral Head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef]
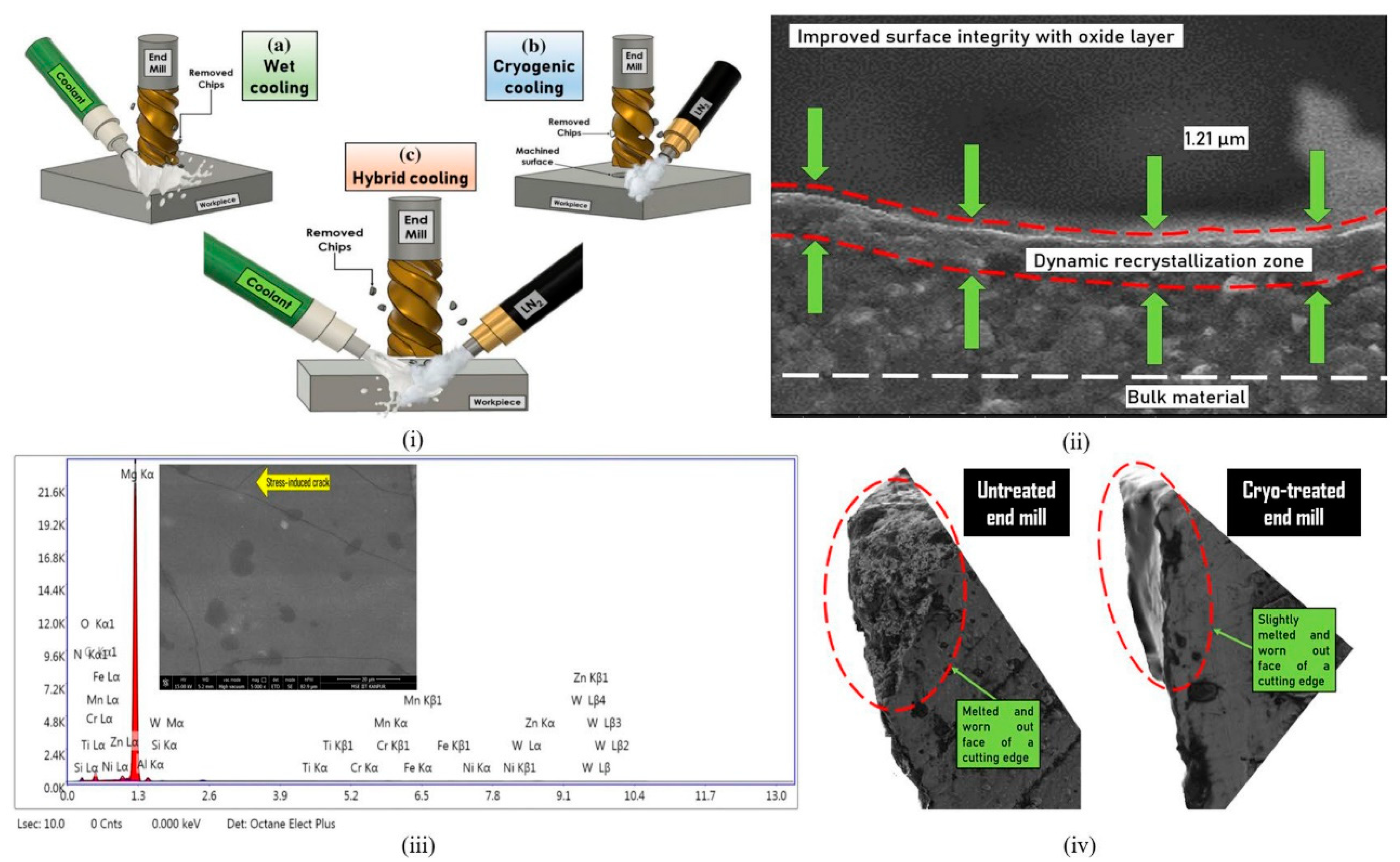
- Danish, M.; Ginta, T.L.; Habib, K.; Carou, D.; Rani, A.M.A.; Saha, B.B. Thermal Analysis during Turning of AZ31 Magnesium Alloy under Dry and Cryogenic Conditions. Int. J. Adv. Manuf. Technol. 2017, 91, 2855–2868. [Google Scholar] [CrossRef]
- Carou, D.; Rubio, E.M.; Davim, J.P. Analysis of Ignition Risk in Intermittent Turning of UNS M11917 Magnesium Alloy at Low Cutting Speeds Based on the Chip Morphology. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2014, 229, 365–371. [Google Scholar] [CrossRef]
- Bertolini, R.; Bruschi, S.; Ghiotti, A.; Pezzato, L.; Dabalà, M. The Effect of Cooling Strategies and Machining Feed Rate on the Corrosion Behavior and Wettability of AZ31 Alloy for Biomedical Applications. Procedia CIRP 2017, 65, 7–12. [Google Scholar] [CrossRef]
- Davis, R.; Singh, A. Performance Study of Cryo-Treated End Mill Via Wet, Cryogenic, and Hybrid Lubri-Coolant-Milling Induced Surface Integrity of Biocompatible Mg Alloy AZ91D. Proc. Inst. Mech Eng. C J. Mech Eng. Sci 2021, 235, 7045–7061. [Google Scholar] [CrossRef]
- Davis, R.; Singh, A. Tailoring Surface Integrity of Biomedical Mg Alloy AZ31B Using Distinct End Mill Treatment Conditions and Machining Environments. J. Mater. Eng. Perform. 2020, 29, 7617–7635. [Google Scholar] [CrossRef]
- Wen, P.; Qin, Y.; Chen, Y.; Voshage, M.; Jauer, L.; Poprawe, R.; Schleifenbaum, J.H. Laser Additive Manufacturing of Zn Porous Scaffolds: Shielding Gas Flow, Surface Quality and Densification. J. Mater. Sci. Technol. 2019, 35, 368–376. [Google Scholar] [CrossRef]
- Al-Kazzaz, H.; Medraj, M.; Cao, X.; Jahazi, M. Nd:YAG Laser Welding of Aerospace Grade ZE41A Magnesium Alloy: Modeling and Experimental Investigations. Mater. Chem. Phys. 2008, 109, 61–76. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of Energy Input on Formability, Microstructure and Mechanical Properties of Selective Laser Melted AZ91D Magnesium Alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, F.; Kovacevic, R. Laser Hot-Wire Cladding of Co-Cr-W Metal Cored Wire. Opt. Lasers Eng. 2020, 128, 105998. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Zhang, G.; Qin, R.; Liu, W.; Xiong, H.; Shi, G.; Blackburn, J. Progress in Additive Manufacturing on New Materials: A Review. J. Mater. Sci. Technol. 2019, 35, 242–269. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Konovalov, S.V. Additive Manufacturing Based on Welding Arc: A Low-Cost Method. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2018, 11, 1317–1328. [Google Scholar] [CrossRef]
- Li, J.L.Z.; Alkahari, M.R.; Rosli, N.A.B.; Hasan, R.; Sudin, M.N.; Ramli, F.R. Review of Wire Arc Additive Manufacturing for 3d Metal Printing. Int. J. Autom. Technol. 2019, 13, 346–353. [Google Scholar] [CrossRef]
- Wu, B.; Pan, Z.; Ding, D.; Cuiuri, D.; Li, H.; Xu, J.; Norrish, J. A Review of the Wire Arc Additive Manufacturing of Metals: Properties, Defects and Quality Improvement. J. Manuf. Process. 2018, 35, 127–139. [Google Scholar] [CrossRef]
- Takagi, H.; Sasahara, H.; Abe, T.; Sannomiya, H.; Nishiyama, S.; Ohta, S.; Nakamura, K. Material-Property Evaluation of Magnesium Alloys Fabricated Using Wire-and-Arc-Based Additive Manufacturing. Addit. Manuf. 2018, 24, 498–507. [Google Scholar] [CrossRef]
- Unocic, R.R.; DuPont, J.N. Process Efficiency Measurements in the Laser Engineered Net Shaping Process. Metall. Mater. Trans. B 2004, 35, 143–152. [Google Scholar] [CrossRef]
- Pierron, N.; Sallamand, P.; Matteï, S. Study of Magnesium and Aluminum Alloys Absorption Coefficient during Nd:YAG Laser Interaction. Appl. Surf. Sci. 2007, 253, 3208–3214. [Google Scholar] [CrossRef]
- Ding, D.; Pan, Z.; Cuiuri, D.; Li, H. Wire-Feed Additive Manufacturing of Metal Components: Technologies, Developments and Future Interests. Int. J. Adv. Manuf. Technol. 2015, 81, 465–481. [Google Scholar] [CrossRef]
- Braszczyńska-Malik, K.N.; Mróz, M. Gas-Tungsten Arc Welding of AZ91 Magnesium Alloy. J. Alloys Compd. 2011, 509, 9951–9958. [Google Scholar] [CrossRef]
- Ng, C.C.; Savalani, M.M.; Man, H.C.; Gibson, I. Layer Manufacturing of Magnesium and Its Alloy Structures for Future Applications. Virtual Phys. Prototyp. 2010, 5, 13–19. [Google Scholar] [CrossRef]
- Shen, X.; Ma, G.; Chen, P. Effect of Welding Process Parameters on Hybrid GMAW-GTAW Welding Process of AZ31B Magnesium Alloy. Int. J. Adv. Manuf. Technol. 2017, 94, 2811–2819. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Wong, M.H.; Cheng, F.T.; Man, H.C. Characterization and Corrosion Studies of Fluoride Conversion Coating on Degradable Mg Implants. Surf. Coat. Technol. 2007, 202, 590–598. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Vogt, C.; Vogt, J.; Donath, T.; Beckmann, F. In Vivo Corrosion and Corrosion Protection of Magnesium Alloy LAE442. Acta Biomater. 2010, 6, 1792–1799. [Google Scholar] [CrossRef]
- Seitz, J.M.; Collier, K.; Wulf, E.; Bormann, D.; Bach, F.W. Comparison of the Corrosion Behavior of Coated and Uncoated Magnesium Alloys in an In Vitro Corrosion Environment. Adv. Eng. Mater. 2011, 13, B313–B323. [Google Scholar] [CrossRef]
- Maurya, R.; Siddiqui, A.R.; Balani, K. An Environment-Friendly Phosphate Chemical Conversion Coating on Novel Mg-9Li-7Al-1Sn and Mg-9Li-5Al-3Sn-1Zn Alloys with Remarkable Corrosion Protection. Appl. Surf. Sci. 2018, 443, 429–440. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Strategies to Improve the Corrosion Resistance of Microarc Oxidation (MAO) Coated Magnesium Alloys for Degradable Implants: Prospects and Challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Gu, X.N.; Li, N.; Zhou, W.R.; Zheng, Y.F.; Zhao, X.; Cai, Q.Z.; Ruan, L. Corrosion Resistance and Surface Biocompatibility of a Microarc Oxidation Coating on a Mg–Ca Alloy. Acta Biomater. 2011, 7, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Guan, S.K.; Chen, J.; Wang, L.G.; Zhu, S.J.; Hu, J.H.; Ren, Z.W. Fabrication and Characterization of Rod-like Nano-Hydroxyapatite on MAO Coating Supported on Mg–Zn–Ca Alloy. Appl. Surf. Sci. 2011, 257, 2231–2237. [Google Scholar] [CrossRef]
- Cui, L.Y.; Zeng, R.C.; Guan, S.K.; Qi, W.C.; Zhang, F.; Li, S.Q.; Han, E.H. Degradation Mechanism of Micro-Arc Oxidation Coatings on Biodegradable Mg-Ca Alloys: The Influence of Porosity. J. Alloys Compd. 2017, 695, 2464–2476. [Google Scholar] [CrossRef]
- Wang, H.X.; Guan, S.K.; Wang, X.; Ren, C.X.; Wang, L.G. In Vitro Degradation and Mechanical Integrity of Mg–Zn–Ca Alloy Coated with Ca-Deficient Hydroxyapatite by the Pulse Electrodeposition Process. Acta Biomater. 2010, 6, 1743–1748. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Electrodeposition of Ca–P Coatings on Biodegradable Mg Alloy: In Vitro Biomineralization Behavior. Acta Biomater. 2010, 6, 1736–1742. [Google Scholar] [CrossRef]
- Keim, S.; Brunner, J.G.; Fabry, B.; Virtanen, S. Control of Magnesium Corrosion and Biocompatibility with Biomimetic Coatings. J. Biomed. Mater. Res. B Appl Biomater. 2011, 96B, 84–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Wei, M. Controlling the Biodegradation Rate of Magnesium Using Biomimetic Apatite Coating. J. Biomed. Mater. Res. B Appl Biomater. 2009, 89B, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Gray-Munro, J.E.; Strong, M. The Mechanism of Deposition of Calcium Phosphate Coatings from Solution onto Magnesium Alloy AZ31. J. Biomed. Mater. Res. A 2009, 90A, 339–350. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, L.; Xu, W.; Zhang, B.; Yang, K. Dynamic Behaviors of a Ca–P Coated AZ31B Magnesium Alloy during in Vitro and in Vivo Degradations. Mater. Sci. Eng. B 2011, 176, 1718–1726. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Cui, B.; Bai, K.; Guan, S.; Wang, L.; Zhu, S. In Vitro Degradation of AZ31 Magnesium Alloy Coated with Nano TiO2 Film by Sol–Gel Method. Appl. Surf. Sci. 2011, 257, 8772–8777. [Google Scholar] [CrossRef]
- Roy, A.; Singh, S.S.; Datta, M.K.; Lee, B.; Ohodnicki, J.; Kumta, P.N. Novel Sol–Gel Derived Calcium Phosphate Coatings on Mg4Y Alloy. Mater. Sci. Eng. B 2011, 176, 1679–1689. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Xiong, G.Y.; Luo, H.L.; He, F.; Huang, Y.; Wang, Y.L. Influence of Zinc Ion Implantation on Surface Nanomechanical Performance and Corrosion Resistance of Biomedical Magnesium–Calcium Alloys. Appl. Surf. Sci. 2008, 254, 5514–5516. [Google Scholar] [CrossRef]
- Wu, G.S.; Zeng, X.Q.; Yao, S.S.; Han, H.B. Ion Implanted AZ31 Magnesium Alloy. Mater. Sci. Forum 2007, 546–549, 551–554. [Google Scholar] [CrossRef]
- Li, J.N.; Cao, P.; Zhang, X.N.; Zhang, S.X.; He, Y.H. In Vitro Degradation and Cell Attachment of a PLGA Coated Biodegradable Mg–6Zn Based Alloy. J. Mater. Sci. 2010, 45, 6038–6045. [Google Scholar] [CrossRef]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K.; Cheung, K.M.C. A Biodegradable Polymer-Based Coating to Control the Performance of Magnesium Alloy Orthopaedic Implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tie, D.; Guan, R.; Wang, N.; Shang, Y.; Cui, T.; Li, J. Microstructures, Mechanical Properties, and Degradation Behaviors of Heat-Treated Mg-Sr Alloys as Potential Biodegradable Implant Materials. J. Mech. Behav. Biomed. Mater. 2017, 77, 47–57. [Google Scholar] [CrossRef]
- Ibrahim, H.; Klarner, A.D.; Poorganji, B.; Dean, D.; Luo, A.A.; Elahinia, M. Microstructural, Mechanical and Corrosion Characteristics of Heat-Treated Mg-1.2Zn-0.5Ca (Wt%) Alloy for Use as Resorbable Bone Fixation Material. J. Mech. Behav. Biomed. Mater. 2017, 69, 203–212. [Google Scholar] [CrossRef]
- Maier, P.; Peters, R.; Mendis, C.L.; Müller, S.; Hort, N. Influence of Precipitation Hardening in Mg-Y-Nd on Mechanical and Corrosion Properties. JOM 2015, 68, 1183–1190. [Google Scholar] [CrossRef]
- Tan, L.; Dong, J.; Chen, J.; Yang, K. Development of Magnesium Alloys for Biomedical Applications: Structure, Process to Property Relationship. Mater. Technol. 2017, 33, 235–243. [Google Scholar] [CrossRef]
- el Mahallawy, N.; Ahmed Diaa, A.; Akdesir, M.; Palkowski, H. Effect of Zn Addition on the Microstructure and Mechanical Properties of Cast, Rolled and Extruded Mg-6Sn-XZn Alloys. Mater. Sci. Eng. A 2017, 680, 47–53. [Google Scholar] [CrossRef]
- Li, K.; Injeti, V.S.Y.; Trivedi, P.; Murr, L.E.; Misra, R.D.K. Nanoscale Deformation of Multiaxially Forged Ultrafine-Grained Mg-2Zn-2Gd Alloy with High Strength-High Ductility Combination and Comparison with the Coarse-Grained Counterpart. J. Mater. Sci. Technol. 2018, 34, 311–316. [Google Scholar] [CrossRef]
- Krajňák, T.; Minárik, P.; Stráská, J.; Gubicza, J.; Máthis, K.; Janeček, M. Influence of the Initial State on the Microstructure and Mechanical Properties of AX41 Alloy Processed by ECAP. J. Mater. Sci. 2018, 54, 3469–3484. [Google Scholar] [CrossRef]
- Habibnejad-Korayem, M.; Mahmudi, R.; Ghasemi, H.M.; Poole, W.J. Tribological Behavior of Pure Mg and AZ31 Magnesium Alloy Strengthened by Al2O3 Nano-Particles. Wear 2010, 268, 405–412. [Google Scholar] [CrossRef]
- Das, S.; Morales, A.T.; Alpas, A.T. Microstructural Evolution during High Temperature Sliding Wear of Mg–3% Al–1% Zn (AZ31) Alloy. Wear 2010, 268, 94–103. [Google Scholar] [CrossRef]
- Zafari, A.; Ghasemi, H.M.; Mahmudi, R. Effect of Rare Earth Elements Addition on the Tribological Behavior of AZ91D Magnesium Alloy at Elevated Temperatures. Wear 2013, 303, 98–108. [Google Scholar] [CrossRef]
- Asl, K.M.; Tari, A.; Khomamizadeh, F. Effect of Deep Cryogenic Treatment on Microstructure, Creep and Wear Behaviors of AZ91 Magnesium Alloy. Mater. Sci. Eng. A 2009, 523, 27–31. [Google Scholar] [CrossRef]
- Chen, H.; Alpas, A.T. Sliding Wear Map for the Magnesium Alloy Mg-9Al-0.9 Zn (AZ91). Wear 2000, 246, 106–116. [Google Scholar] [CrossRef]
- Wang, S.Q.; Yang, Z.R.; Zhao, Y.T.; Wei, M.X. Sliding Wear Characteristics of AZ91D Alloy at Ambient Temperatures of 25–200 °C. Tribol. Lett. 2010, 38, 39–45. [Google Scholar] [CrossRef]
- Huang, W.J.; Lin, Q.; Liu, C.L. Tribological Behaviour of AZ71E Alloy at High Temperatures. Trans. Nonferrous Met. Soc. China 2012, 22, 2057–2065. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W.; Lim, L.E.N. Wear Behaviour of AZ91D Alloy at Low Sliding Speeds. Wear 2008, 265, 780–786. [Google Scholar] [CrossRef]
- El-Morsy, A.W. Dry Sliding Wear Behavior of Hot Deformed Magnesium AZ61 Alloy as Influenced by the Sliding Conditions. Mater. Sci. Eng. A 2008, 473, 330–335. [Google Scholar] [CrossRef]
- Zafari, A.; Ghasemi, H.M.; Mahmudi, R. Tribological Behavior of AZ91D Magnesium Alloy at Elevated Temperatures. Wear 2012, 292–293, 33–40. [Google Scholar] [CrossRef]
- Yang, Z.R.; Wei, M.X.; Zhao, Y.T.; Wang, S.Q. Dry Sliding Wear Behavior and Mechanism of AM60B Alloy at 25–200 °C. Trans. Nonferrous Met. Soc. China 2011, 21, 2584–2591. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Q.; Ye, B.; Zhou, H. Enhanced Microstructure Homogeneity and Mechanical Properties of AZ31–Si Composite by Cyclic Closed-Die Forging. J. Alloys Compd. 2013, 552, 409–417. [Google Scholar] [CrossRef]
- Ajith Kumar, K.K.; Pillai, U.T.S.; Pai, B.C.; Chakraborty, M. Dry Sliding Wear Behaviour of Mg–Si Alloys. Wear 2013, 303, 56–64. [Google Scholar] [CrossRef]
- Mehta, D.S.; Masood, S.H.; Song, W.Q. Investigation of Wear Properties of Magnesium and Aluminum Alloys for Automotive Applications. J. Mater. Process. Technol. 2004, 155–156, 1526–1531. [Google Scholar] [CrossRef]
- Hu, B.; Peng, L.; Yang, Y.; Ding, W. Effect of Solidification Conditions on Microstructure, Mechanical and Wear Properties of Mg–5Al–3Ca–0.12Sr Magnesium Alloy. Mater. Des. 2010, 31, 3901–3907. [Google Scholar] [CrossRef]
- Matsuoka, T.; Sakaguthi, K.; Mukai, T.; Matsuyama, M.; Yoshioka, R. Effects of the Grain Size on Friction and Wear Properties of ZK60 Magnesium Alloy. Zair. J. Soc. Mater. Sci. Jpn. 2002, 51, 1154–1159. [Google Scholar] [CrossRef]
- Meshinchi Asl, K.; Masoudi, A.; Khomamizadeh, F. The Effect of Different Rare Earth Elements Content on Microstructure, Mechanical and Wear Behavior of Mg–Al–Zn Alloy. Mater. Sci. Eng. A 2010, 527, 2027–2035. [Google Scholar] [CrossRef]
- Somekawa, H.; Maeda, S.; Hirayama, T.; Matsuoka, T.; Inoue, T.; Mukai, T. Microstructural Evolution during Dry Wear Test in Magnesium and Mg–Y Alloy. Mater. Sci. Eng. A 2013, 561, 371–377. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, S.; Xu, C.; Yang, X.; Lu, D. Enhancing Wear Resistance of Mg–Zn–Gd Alloy by Cryogenic Treatment. Mater. Lett. 2012, 76, 201–204. [Google Scholar] [CrossRef]
- Selvan, S.A.; Ramanathan, S. Dry Sliding Wear Behavior of As-Cast ZE41A Magnesium Alloy. Mater. Des. 2010, 31, 1930–1936. [Google Scholar] [CrossRef]
- López, A.J.; Rodrigo, P.; Torres, B.; Rams, J. Dry Sliding Wear Behaviour of ZE41A Magnesium Alloy. Wear 2011, 271, 2836–2844. [Google Scholar] [CrossRef]
- Hu, M.L.; Wang, Q.D.; Li, C.; Ding, W.J. Dry Sliding Wear Behavior of Cast Mg–11Y–5Gd–2Zn Magnesium Alloy. Trans. Nonferrous Met. Soc. China 2012, 22, 1918–1923. [Google Scholar] [CrossRef]
- Hu, M.; Wang, Q.; Chen, C.; Yin, D.; Ding, W.; Ji, Z. Dry Sliding Wear Behaviour of Mg-10Gd-3Y-0.4Zr Alloy. Mater. Des. 2012, 42, 223–229. [Google Scholar] [CrossRef]
- He, S.M.; Zeng, X.Q.; Peng, L.M.; Gao, X.; Nie, J.F.; Ding, W.J. Precipitation in a Mg–10Gd–3Y–0.4Zr (Wt.%) Alloy during Isothermal Ageing at 250 °C. J. Alloys Compd. 2006, 421, 309–313. [Google Scholar] [CrossRef]
- Yin, D.D.; Wang, Q.D.; Gao, Y.; Chen, C.J.; Zheng, J. Effects of Heat Treatments on Microstructure and Mechanical Properties of Mg–11Y–5Gd–2Zn–0.5Zr (Wt.%) Alloy. J. Alloys Compd. 2011, 509, 1696–1704. [Google Scholar] [CrossRef]
- An, J.; Li, R.G.; Lu, Y.; Chen, C.M.; Xu, Y.; Chen, X.; Wang, L.M. Dry Sliding Wear Behavior of Magnesium Alloys. Wear 2008, 265, 97–104. [Google Scholar] [CrossRef]
- Itoi, T.; Gonda, K.; Hirohashi, M. Relationship of Wear Properties to Basal-Plane Texture of Worn Surface of Mg Alloys. Wear 2011, 270, 606–612. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of Alloying Elements on the Corrosion Behavior and Biocompatibility of Biodegradable Magnesium Alloys: A Review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Design of Magnesium Alloys with Controllable Degradation for Biomedical Implants: From Bulk to Surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.C.; Kim, J.G.; Lee, J.Y.; Seok, H.K. Influence of Ca on the Corrosion Properties of Magnesium for Biomaterials. Mater. Lett. 2008, 62, 4146–4148. [Google Scholar] [CrossRef]
- Wan, Y.; Xiong, G.; Luo, H.; He, F.; Huang, Y.; Zhou, X. Preparation and Characterization of a New Biomedical Magnesium–Calcium Alloy. Mater. Des. 2008, 29, 2034–2037. [Google Scholar] [CrossRef]
- Rad, H.R.B.; Idris, M.H.; Kadir, M.R.A.; Farahany, S. Microstructure Analysis and Corrosion Behavior of Biodegradable Mg–Ca Implant Alloys. Mater. Des. 2012, 33, 88–97. [Google Scholar] [CrossRef]
- Li, Y.; Hodgson, P.D.; Wen, C. The Effects of Calcium and Yttrium Additions on the Microstructure, Mechanical Properties and Biocompatibility of Biodegradable Magnesium Alloys. J. Mater. Sci. 2010, 46, 365–371. [Google Scholar] [CrossRef]
- Gu, X.N.; Li, X.L.; Zhou, W.R.; Cheng, Y.; Zheng, Y.F. Microstructure, Biocorrosion and Cytotoxicity Evaluations of Rapid Solidified Mg–3Ca Alloy Ribbons as a Biodegradable Material. Biomed. Mater. 2010, 5, 35013. [Google Scholar] [CrossRef]
- Yin, P.; Li, N.F.; Lei, T.; Liu, L.; Ouyang, C. Effects of Ca on Microstructure, Mechanical and Corrosion Properties and Biocompatibility of Mg–Zn–Ca Alloys. J. Mater. Sci. Mater. Med. 2013, 24, 1365–1373. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomater. Guildford. 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Bornapour, M.; Muja, N.; Shum-Tim, D.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and Biodegradability of Mg–Sr Alloys: The Formation of Sr-Substituted Hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wu, G.H.; Zhai, C.Q. Effect of Strontium on Mechanical Properties and Corrosion Resistance of AZ91D. Mater. Sci. Forum 2007, 546–549, 567–570. [Google Scholar] [CrossRef]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In Vitro and in Vivo Studies on a Mg–Sr Binary Alloy System Developed as a New Kind of Biodegradable Metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.G.; Cipriano, A.F.; Zhao, Z.Y.; Lock, J.; Tie, D.; Zhao, T.; Cui, T.; Liu, H. Development and Evaluation of a Magnesium–Zinc–Strontium Alloy for Biomedical Applications—Alloy Processing, Microstructure, Mechanical Properties, and Biodegradation. Mater. Sci. Eng. C 2013, 33, 3661–3669. [Google Scholar] [CrossRef]
- Tie, D.; Guan, R.; Liu, H.; Cipriano, A.; Liu, Y.; Wang, Q.; Huang, Y.; Hort, N. An in Vivo Study on the Metabolism and Osteogenic Activity of Bioabsorbable Mg–1Sr Alloy. Acta Biomater. 2016, 29, 455–467. [Google Scholar] [CrossRef]
- Li, H.; Peng, Q.; Li, X.; Li, K.; Han, Z.; Fang, D. Microstructures, Mechanical and Cytocompatibility of Degradable Mg–Zn Based Orthopedic Biomaterials. Mater. Des. 2014, 58, 43–51. [Google Scholar] [CrossRef]
- Wang, W.; Han, J.; Yang, X.; Li, M.; Wan, P.; Tan, L.; Zhang, Y.; Yang, K. Novel Biocompatible Magnesium Alloys Design with Nutrient Alloying Elements Si, Ca and Sr: Structure and Properties Characterization. Mater. Sci. Eng. B 2016, 214, 26–36. [Google Scholar] [CrossRef]
- Wang, C.; Lin, K.; Chang, J.; Sun, J. Osteogenesis and Angiogenesis Induced by Porous β-CaSiO3/PDLGA Composite Scaffold via Activation of AMPK/ERK1/2 and PI3K/Akt Pathways. Biomaterials 2013, 34, 64–77. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic Effects of Dual Zn/Ag Ion Implantation in Osteogenic Activity and Antibacterial Ability of Titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef]
- Yan, Y.; Cao, H.; Kang, Y.; Yu, K.; Xiao, T.; Luo, J.; Deng, Y.; Fang, H.; Xiong, H.; Dai, Y. Effects of Zn Concentration and Heat Treatment on the Microstructure, Mechanical Properties and Corrosion Behavior of as-Extruded Mg-Zn Alloys Produced by Powder Metallurgy. J. Alloys Compd. 2017, 693, 1277–1289. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.H.; Chen, Y.G.; Zheng, Q.; Zhang, X.N.; Zhao, C.L.; Zhang, S.X.; Yan, J. In Vitro and in Vivo Evaluation of Effects of Mg–6Zn Alloy on Tight Junction of Intestinal Epithelial Cell. Trans. Nonferrous Met. Soc. China 2015, 25, 3760–3766. [Google Scholar] [CrossRef]
- He, G.; Wu, Y.; Zhang, Y.; Zhu, Y.; Liu, Y.; Li, N.; Li, M.; Zheng, G.; He, B.; Yin, Q.; et al. Addition of Zn to the Ternary Mg–Ca–Sr Alloys Significantly Improves Their Antibacterial Properties. J. Mater. Chem. B 2015, 3, 6676–6689. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, C.W.; Wu, M.M.; Kuo, T.L. Cancer Potential in Liver, Lung, Bladder and Kidney Due to Ingested Inorganic Arsenic in Drinking Water. Br. J. Cancer 1992, 66, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, Z.; Zhao, H.; Pan, Y.; Pavlinich, S.; Liu, X.; Li, X.; Zheng, Y.; Li, L. Microstructure, Mechanical Properties, Corrosion Behavior and Biocompatibility of As-Extruded Biodegradable Mg–3Sn–1Zn–0.5Mn Alloy. J. Mater. Sci. Technol. 2016, 32, 874–882. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, F.; Zhao, S.; Pan, H.; Song, K.; Tang, A. Microstructure, Corrosion Behavior and Cytotoxicity of Biodegradable Mg–Sn Implant Alloys Prepared by Sub-Rapid Solidification. Mater. Sci. Eng. C 2015, 54, 245–251. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, F.; Zhao, S.; Pan, H.; Song, K.; Tang, A. Preparation and Characterization of As-Extruded Mg–Sn Alloys for Orthopedic Applications. Mater. Des. 2015, 70, 60–67. [Google Scholar] [CrossRef]
- Harris, E.D. A Requirement for Copper in Angiogenesis. Nutr. Rev. 2004, 62, 60–64. [Google Scholar] [CrossRef]
- Harris, E. Basic and Clinical Aspects of Copper. Crit. Rev. Clin. Lab. Sci. 2003, 40, 547–586. [Google Scholar] [CrossRef]
- Wilson, T.; Katz, J.M.; Gray, D.H. Inhibition of Active Bone Resorption by Copper. Calcif. Tissue Int. 1981, 33, 35–39. [Google Scholar] [CrossRef]
- Gérard, C.; Bordeleau, L.J.; Barralet, J.; Doillon, C.J. The Stimulation of Angiogenesis and Collagen Deposition by Copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef]
- Strause, L.; Saltman, P.; Glowacki, J. The Effect of Deficiencies of Manganese and Copper on Osteoinduction and on Resorption of Bone Particles in Rats. Calcif. Tissue Int. 1987, 41, 145–150. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Christopher Ellison, E.; Hunt, T.K.; Roy, S. Copper-Induced Vascular Endothelial Growth Factor Expression and Wound Healing. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.F. Copper Stimulates Proliferation of Human Endothelial Cells under Culture. J. Cell. Biochem. 1998, 69, 326–335. [Google Scholar] [CrossRef]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg-Cu Alloys with Enhanced Osteogenesis, Angiogenesis, and Long-Lasting Antibacterial Effects. Sci. Rep. 2016, 6, 27374. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Wan, P.; Zhai, Z.; Mao, Z.; Ouyang, Z.; Yu, D.; Sun, Q.; Tan, L.; Ren, L.; et al. Biodegradable Mg-Cu Alloy Implants with Antibacterial Activity for the Treatment of Osteomyelitis: In Vitro and in Vivo Evaluations. Biomaterials 2016, 106, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, G.; Mao, L.; Niu, J.; Ding, W. Biocorrosion Properties of As-Extruded Mg–Nd–Zn–Zr Alloy Compared with Commercial AZ31 and WE43 Alloys. Mater. Lett. 2012, 66, 209–211. [Google Scholar] [CrossRef]
- Zong, Y.; Yuan, G.; Zhang, X.; Mao, L.; Niu, J.; Ding, W. Comparison of Biodegradable Behaviors of AZ31 and Mg–Nd–Zn–Zr Alloys in Hank’s Physiological Solution. Mater. Sci. Eng. B 2012, 177, 395–401. [Google Scholar] [CrossRef]
- Wang, Y.P.; He, Y.H.; Zhu, Z.J.; Jiang, Y.; Zhang, J.; Niu, J.L.; Mao, L.; Yuan, G.Y. In Vitro Degradation and Biocompatibility of Mg-Nd-Zn-Zr Alloy. Chin. Sci. Bull. 2012, 57, 2163–2170. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; He, Y.; Jiang, Y.; Zhang, J.; Niu, J.; Mao, L.; Yuan, G. In Vivo Degradation Behavior and Biocompatibility of Mg-Nd-Zn-Zr Alloy at Early Stage. Int. J. Mol. Med. 2012, 29, 178–184. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, Y.; Peng, X.; Mao, L.; Yuan, G.; Jiang, Y.; He, Y. Effects of Degradable MG-ND-ZN-ZR Alloy on Osteoblastic Cell Function. Int. J. Immunopathol. Pharmacol. 2012, 25, 597–606. [Google Scholar] [CrossRef]
- Liao, Y.; Ouyang, Y.; Niu, J.; Zhang, J.; Wang, Y.; Zhu, Z.; Yuan, G.; He, Y.; Jiang, Y. In Vitro Response of Chondrocytes to a Biodegradable Mg–Nd–Zn–Zr Alloy. Mater. Lett. 2012, 83, 206–208. [Google Scholar] [CrossRef]
- Wang, Y.P.; Ouyang, Y.M.; He, Y.H.; Chen, D.Y.; Jiang, Y.; Mao, L.; Niu, J.L.; Zhang, J.; Yuan, G.Y. Biocompatibility of Mg-Nd-Zn-Zr Alloy with Rabbit Blood. Chin. Sci. Bull. 2013, 58, 2903–2908. [Google Scholar] [CrossRef]
- Yu, W.; Chen, D.; Ding, Z.; Qiu, M.; Zhang, Z.; Shen, J.; Zhang, X.; Zhang, S.; He, Y.; Shi, Z. Synergistic Effect of a Biodegradable Mg–Zn Alloy on Osteogenic Activity and Anti-Biofilm Ability: An in Vitro and in Vivo Study. RSC Adv. 2016, 6, 45219–45230. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, H.; Ding, Z.; Zhang, Z.; Sun, B.; Shen, J.; Chen, S.; Zhang, B.; Yang, K.; Liu, M.; et al. In Vitro and in Vivo Evaluation of MgF2 Coated AZ31 Magnesium Alloy Porous Scaffolds for Bone Regeneration. Colloids Surf. B Biointerfaces 2017, 149, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Miura, C.; Shimizu, Y.; Imai, Y.; Mukai, T.; Yamamoto, A.; Sano, Y.; Ikeo, N.; Isozaki, S.; Takahashi, T.; Oikawa, M.; et al. In Vivo Corrosion Behaviour of Magnesium Alloy in Association with Surrounding Tissue Response in Rats. Biomed. Mater. 2016, 11, 025001. [Google Scholar] [CrossRef]
- Sato, T.; Shimizu, Y.; Odashima, K.; Sano, Y.; Yamamoto, A.; Mukai, T.; Ikeo, N.; Takahashi, T.; Kumamoto, H. In Vitro and in Vivo Analysis of the Biodegradable Behavior of a Magnesium Alloy for Biomedical Applications. Dent. Mater. J. 2019, 38, 11–21. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Chen, M.; Liu, D. Biological Activity Evaluation of Magnesium Fluoride Coated Mg-Zn-Zr Alloy in Vivo. Mater. Sci. Eng. C 2017, 75, 1068–1074. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, N.; Sun, C.; Zhu, J.; Wang, D.; Dai, Y.; Wu, Y.; Wang, Y.; Li, J.; Zhao, D.; et al. In Vivo Study of a Novel Micro-Arc Oxidation Coated Magnesium-Zinc-Calcium Alloy Scaffold/Autologous Bone Particles Repairing Critical Size Bone Defect in Rabbit. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 298–305. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Wang, L.; Yuan, G.; Dai, K.; Pei, J.; Hao, Y. Dual Modulation of Bone Formation and Resorption with Zoledronic Acid-Loaded Biodegradable Magnesium Alloy Implants Improves Osteoporotic Fracture Healing: An in Vitro and in Vivo Study. Acta Biomater. 2018, 65, 486–500. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Wang, H.; Geng, F.; Shao, M.; Xu, S.; Xia, X.; Ma, X.; Lu, F.; Jiang, J. Evaluation of a Porous Bioabsorbable Interbody Mg-Zn Alloy Cage in a Goat Cervical Spine Model. BioMed Res. Int. 2018, 2018, 7961509. [Google Scholar] [CrossRef]
- Cihova, M.; Martinelli, E.; Schmutz, P.; Myrissa, A.; Schäublin, R.; Weinberg, A.M.; Uggowitzer, P.J.; Löffler, J.F. The Role of Zinc in the Biocorrosion Behavior of Resorbable Mg-Zn-Ca Alloys. Acta Biomater. 2019, 100, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B.; Saulacic, N.; Beck, S.; Imwinkelried, T.; Liu, E.W.Y.; Nakahara, K.; Hofstetter, W.; Iizuka, T. Osteosynthesis of Partial Rib Osteotomy in a Miniature Pig Model Using Human Standard-Sized Magnesium Plate/Screw Systems: Effect of Cyclic Deformation on Implant Integrity and Bone Healing. J. Cranio-Maxillofac. Surg. 2017, 45, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhao, Y.; Chu, P.K.; Wang, L.; Pan, H.; Zheng, Y.; Wu, S.; Liu, X.; Cheung, K.M.C.; Wong, T.; et al. A Functionalized TiO2/Mg2TiO4 Nano-Layer on Biodegradable Magnesium Implant Enables Superior Bone-Implant Integration and Bacterial Disinfection. Biomaterials 2019, 219, 119372. [Google Scholar] [CrossRef]
- Marukawa, E.; Tamai, M.; Takahashi, Y.; Hatakeyama, I.; Sato, M.; Higuchi, Y.; Kakidachi, H.; Taniguchi, H.; Sakamoto, T.; Honda, J.; et al. Comparison of Magnesium Alloys and Poly-l-Lactide Screws as Degradable Implants in a Canine Fracture Model. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1282–1289. [Google Scholar] [CrossRef]
- Oshibe, N.; Marukawa, E.; Yoda, T.; Harada, H. Degradation and Interaction with Bone of Magnesium Alloy WE43 Implants: A Long-Term Follow-up in Vivo Rat Tibia Study. J. Biomater. Appl. 2019, 33, 1157–1167. [Google Scholar] [CrossRef]
- Torroni, A.; Xiang, C.; Witek, L.; Rodriguez, E.D.; Flores, R.L.; Gupta, N.; Coelho, P.G. Histo-Morphologic Characteristics of Intra-Osseous Implants of WE43 Mg Alloys with and without Heat Treatment in an in Vivo Cranial Bone Sheep Model. J. Cranio-Maxillofac. Surg. 2018, 46, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Torroni, A.; Xiang, C.; Witek, L.; Rodriguez, E.D.; Coelho, P.G.; Gupta, N. Biocompatibility and Degradation Properties of WE43 Mg Alloys with and without Heat Treatment: In Vivo Evaluation and Comparison in a Cranial Bone Sheep Model. J. Cranio-Maxillofac. Surg. 2017, 45, 2075–2083. [Google Scholar] [CrossRef]
- Niu, J.; Xiong, M.; Guan, X.; Zhang, J.; Huang, H.; Pei, J.; Yuan, G. The in Vivo Degradation and Bone-Implant Interface of Mg-Nd-Zn-Zr Alloy Screws: 18 Months Post-Operation Results. Corros. Sci. 2016, 113, 183–187. [Google Scholar] [CrossRef]
- Bai, C.Y.; Li, J.W.; Ta, W.B.; Li, B.; Han, Y. In Vivo Study on the Corrosion Behavior of Magnesium Alloy Surface Treated with Micro-Arc Oxidation and Hydrothermal Deposition. Orthop. Surg. 2017, 9, 296–303. [Google Scholar] [CrossRef]
- Plaass, C.; von Falck, C.; Ettinger, S.; Sonnow, L.; Calderone, F.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Waizy, H.; Daniilidis, K.; et al. Bioabsorbable Magnesium versus Standard Titanium Compression Screws for Fixation of Distal Metatarsal Osteotomies—3 Year Results of a Randomized Clinical Trial. J. Orthop. Sci. 2018, 23, 321–327. [Google Scholar] [CrossRef]
- Helmecke, P.; Ezechieli, M.; Becher, C.; Köhler, J.; Denkena, B. Resorbable Interference Screws Made of Magnesium Based Alloy. Biomed. Eng. Biomed. Tech. 2013, 58, 000010151520134074. [Google Scholar] [CrossRef] [PubMed]


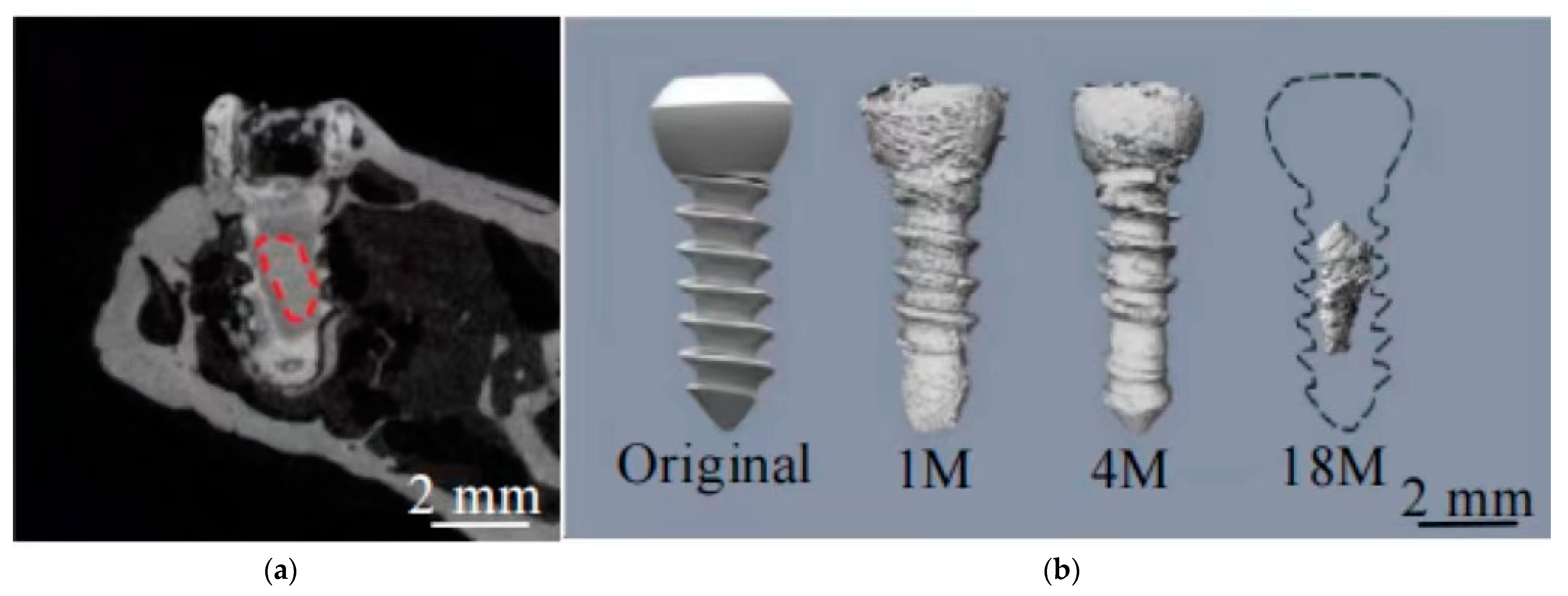
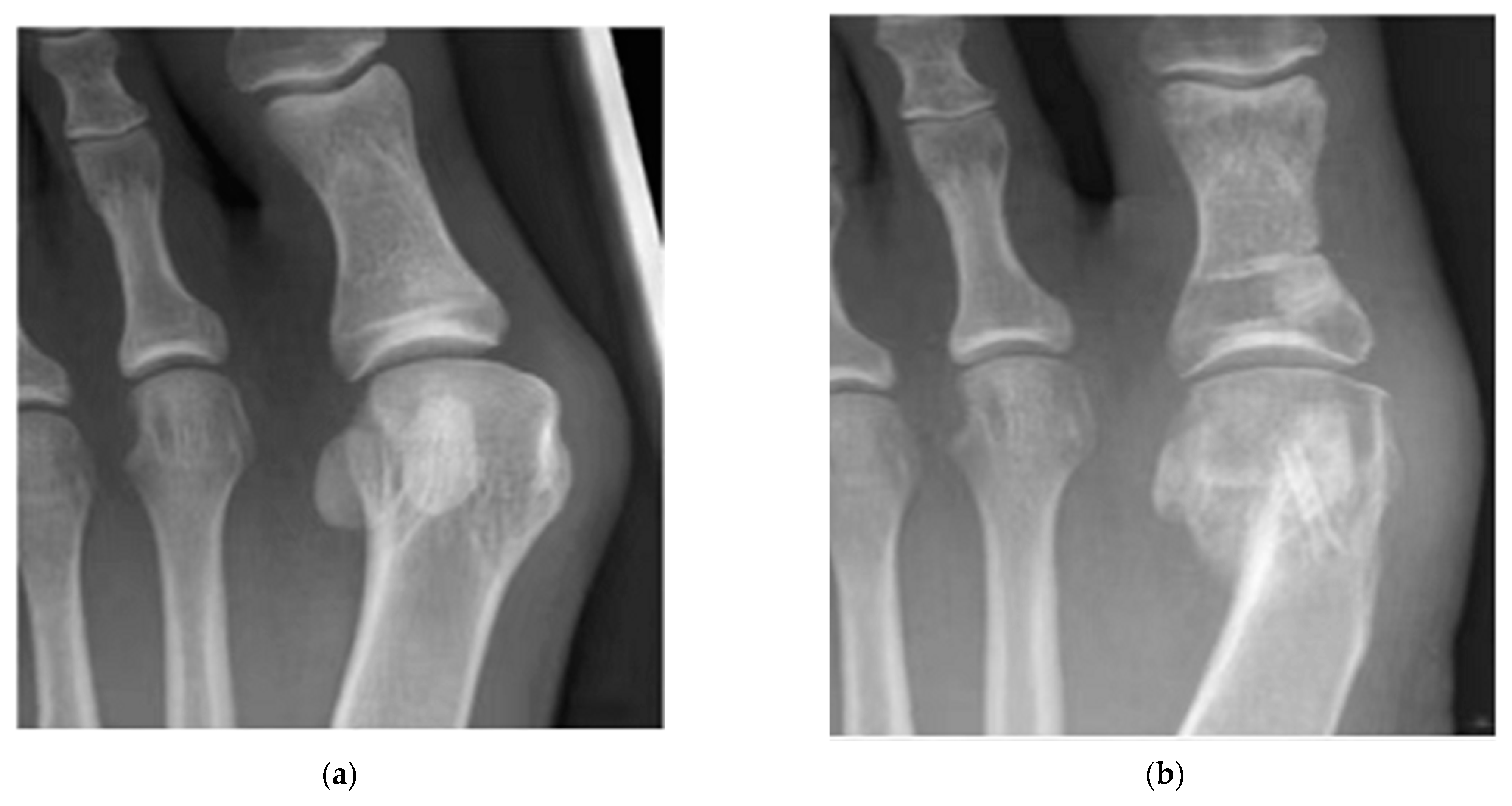
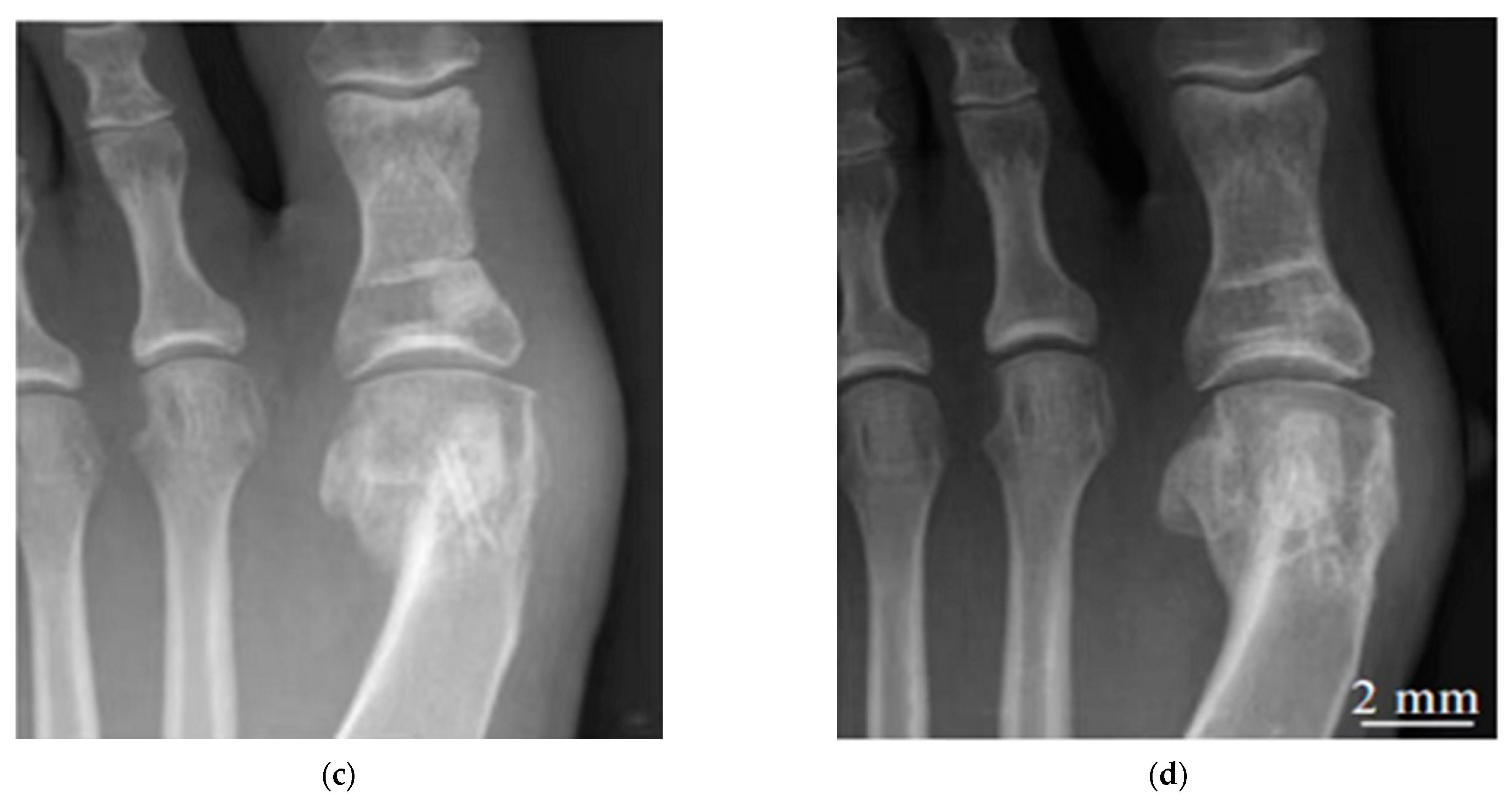
| Alloy | Processing | Test Cells | Cell Viability Assay | Cell Culture Time (Day) | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| One | Two | Three | Four | Five | Six | Seven | |||||
| Mg-3Ca | Rapid solidified | L-929 | MTT | >90 | >90 | >90 | [150] | ||||
| Mg-5Zn-1Ca | As-cast | L-929 | MTT | >100 | >90 | >80 | [151] | ||||
| Mg-1Ca | As-extruded | L-929 | MTT | >90 | >90 | >90 | [152] | ||||
| Mg-0.5Sr | As-cast | HUVECs | Alamar blue | >95 | >100 | >110 | [153] | ||||
| Mg-1Sr | As-cast | MG63 | MTT | >70 | >90 | >80 | [155] | ||||
| pure Mg | As-rolled | MG63 | MTT | >60 | >70 | >70 | [155] | ||||
| Mg-1Zn-0.8Sr | Backward extruded | L-929 | MTT | >80 | >90 | >100 | [158] | ||||
| Mg-1.38Si-0.5Sr-0.6Ca | As-cast | MC3T3-E1 | CCK-8 | >60 | >100 | >100 | [159] | ||||
| Mg-6%Zn | As-extruded | L-929 | MTT | >80 | >80 | >80 | [162] | ||||
| Mg-Zn | As-cast | rBMSCs | CCK-8 | >50 | >70 | >90 | [185] | ||||
| Mg-1Sn | Sub-rapid solidified | MG63 | MTT | >100 | >100 | >100 | [167] | ||||
| Mg-3Sn | Sub-rapid solidified | MG63 | MTT | >100 | >100 | >100 | [167] | ||||
| Mg-0.03Cu | As-cast | MC3T3-E1 | MTT | >100 | >100 | >100 | [176] | ||||
| HUVECs | >100 | >100 | >100 | ||||||||
| Mg-Nd-Zn-Zr | MC3T3-E1 | MTT | >70 | >90 | >80 | [180] | |||||
| Alloy Types and Author | Implant Form and Site | Duration of Implantation (Weeks) | Degradation | References |
|---|---|---|---|---|
| Magnesium and aluminium alloy Miura et al. | Metal plates; rat ventral, head, dorsum, and femur | 4 | Abdomen > Head > Back > Femur Bone | [187] |
| Magnesium and aluminium alloy Sato et al. | Metal plates; rat ventral, head, back, tibia, femur | 4 | Abdomen > Head, Back > Tibia Bone and femur | [188] |
| Magnesium and aluminium alloy Yu et al. | Porous scaffold, rabbit femur | 18 | Reduce | [186] |
| Magnesium-zinc-zirconium alloy Jiang et al. | Screws, rabbit femur | 24 | Reduce | [189] |
| Magnesi-um-zinc-zirconium alloy Li et al. | Intramedullary nail, rat fe-mur | 12 | Reduce | [191] |
| Magnesium, zinc and calcium alloys Zhang et al. | Stents, rabbit ulna | 12 | Reduce | [190] |
| Magnesium rare earth alloys Bai et al. | Intramedullary nail, rabbit femoral medullary cavity | 12 | Reduce | [201] |
| Magnesium rare earth alloys Torroni et al. | Plate nail fixation system, sheep forehead, nasal bone | 6 | Reduce | [199] |
| Magnesium rare earth alloys Marukawa et al. | Bone screws, canine tibia | 4 | Not described | [196] |
| Magnesium rare earth alloys Oshibe et al. | Intramedullary nail, rat tibia | 48 | Volume loss after 48 weeks 27.7% | [197] |
| Magnesium rare earth alloys Lin et al. | Screws, rat femur | 12 | Reduce | [195] |
| Calcium magnesium alloyCihova et al. | Intramedullary nail, rat femur | 4 | Reduce | [193] |
| Magnesium Alloy Material Types | Part | Sample Numbers | Country | Degree of Healing | References |
|---|---|---|---|---|---|
| Mg-Y-RE-Zr screws | Bunion correction | 13 | Germany | All healed | [202] |
| Mg-5 wt%Ca-1 wt%Zn | Internal fixation of fractures of the metacarpal and carpal bones | 53 | Korea | All healed | [68] |
| Mg-Y-RE-Zr screws | Bunion orthopaedics | 40 | Germany | 79% healing after 6 weeks, 90% healing after 12 weeks | [202] |
| Pure magnesium screws | Femoral head ischaemic necrosis graft tape; vascular bone flap fixation | 48 | China | No displacement or collapse of bone flap after operation | [69] |
| Mg-Y-RE-Zr screws | Bunion orthopaedics | 100 | Germany | All healed | [202] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, P.; Liu, L.; Chang, J.; Liu, C.; Zhang, Q.; Zhou, J.; Liu, Z.; Fan, Y. Advances in the Study of Magnesium Alloys and Their Use in Bone Implant Material. Metals 2022, 12, 1500. https://doi.org/10.3390/met12091500
Zhi P, Liu L, Chang J, Liu C, Zhang Q, Zhou J, Liu Z, Fan Y. Advances in the Study of Magnesium Alloys and Their Use in Bone Implant Material. Metals. 2022; 12(9):1500. https://doi.org/10.3390/met12091500
Chicago/Turabian StyleZhi, Peixuan, Leixin Liu, Jinke Chang, Chaozong Liu, Qiliang Zhang, Jian Zhou, Ziyu Liu, and Yubo Fan. 2022. "Advances in the Study of Magnesium Alloys and Their Use in Bone Implant Material" Metals 12, no. 9: 1500. https://doi.org/10.3390/met12091500
APA StyleZhi, P., Liu, L., Chang, J., Liu, C., Zhang, Q., Zhou, J., Liu, Z., & Fan, Y. (2022). Advances in the Study of Magnesium Alloys and Their Use in Bone Implant Material. Metals, 12(9), 1500. https://doi.org/10.3390/met12091500











