MicroRNAs Mediated Plant Responses to Salt Stress
Abstract
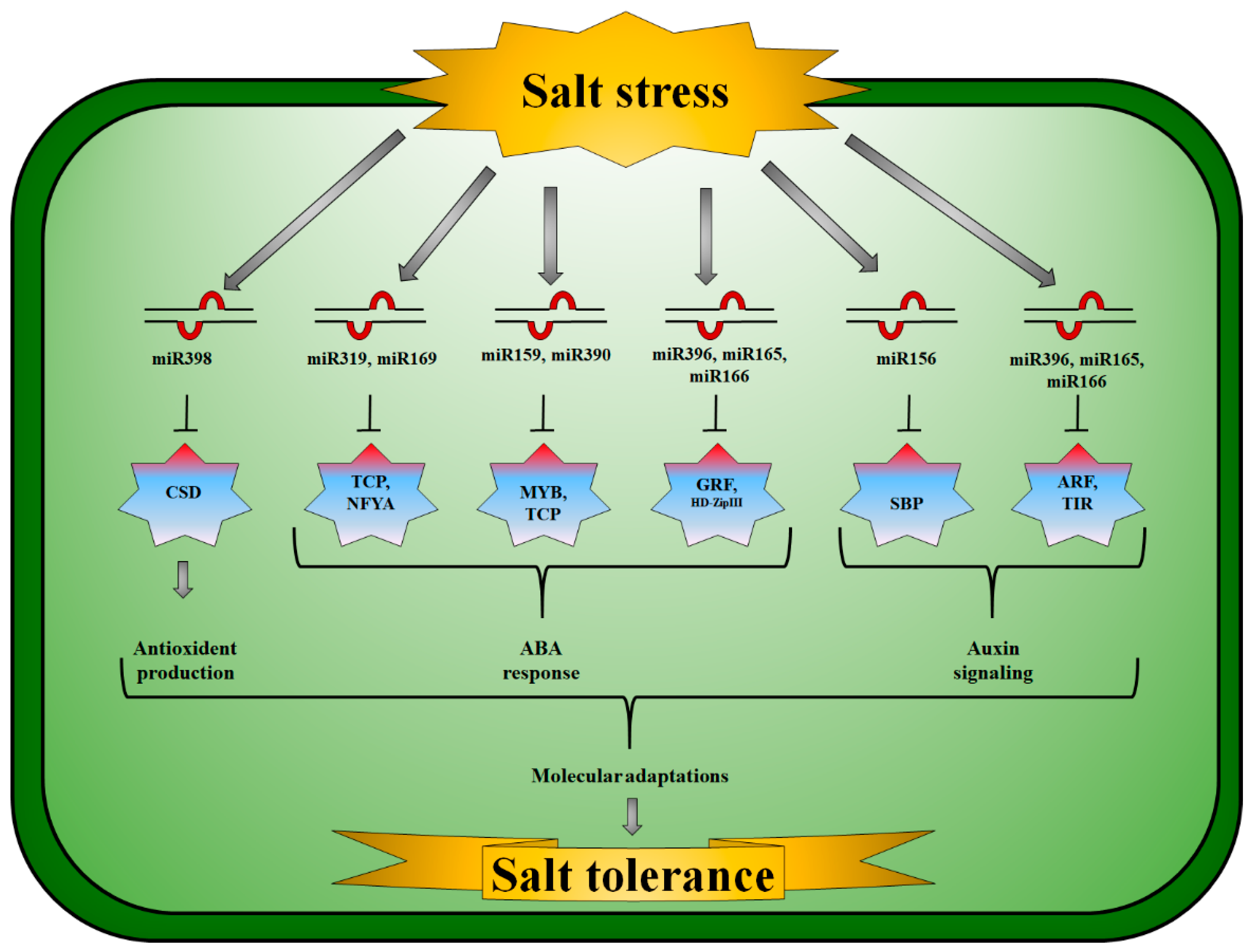
:1. Introduction
2. Abiotic Stress Responsive miRNAs in Plants
3. Plant miRNAs and Salt Stress
| microRNA | Target | Plant Species | miRNA/Target Module Function | Regulations | References | |
|---|---|---|---|---|---|---|
| Up | Down | |||||
| miR156 | Unknown | Gossypium raimondii | Abiotic stress tolerance | [81] | ||
| SPLs | Raphanus sativus | Delays flowering; regulates leaf development, fruit ripening, vegetative and reproductive stage transitions; tillering and branching; plays key roles in embryogenesis, morphogenesis, life cycle stage transformation, and flower formation. | [82] | |||
| SPLs | Panicum virgatum | [83] | ||||
| SPLs | Arabidopsis thaliana | [45] | ||||
| SPLs | Malus domestica | [84] | ||||
| POPTR_0007s01030 | Populus trichocarpa | Unknown | [85] | |||
| Unknown | Medicago truncatula | Abiotic stress tolerance | [86] | |||
| Unknown | Solanum lycopersicum | [87] | ||||
| UGTs | Hordeum spontaneum | Increases anthocyanin synthesis, leading to enhanced antioxidative capacity. | [88] | |||
| miR157 | SPLs | P. virgatum | Modulate leaf initiation rate | [83] | ||
| Unknown | G. raimondii | Regulation of biological processes | [81] | |||
| miR159 | MYBs | Oryza sativa | Growth and flowering, role in fruit development. | [89] | ||
| MYBs | P. virgatum | [83] | ||||
| MYBs | Nicotiana tabacum | [90] | ||||
| MYBs | M. truncatula | [91] | ||||
| miR160 | ARFs | G. raimondii | Regulating plant growth and development through auxin signaling pathways | [81] | ||
| ARFs | R. sativus | [82] | ||||
| ARFs | O. sativa | [89] | ||||
| ARFs | Setaria italica | [92] | ||||
| ARFs | Triticum aestivum | [93] | ||||
| miR161 | AGO | A. thaliana | Vital in salinity stress response | [94] | ||
| miR162 | DCLs | S. italica | miRNA biogenesis plays a vital role in saline and drought conditions | [92] | ||
| DCLs | P. virgatum | [83] | ||||
| miR164 | NAC | R. sativus | Critical role in regulating the response to salt and drought stress | [82] | ||
| NAC | A. thaliana | [95] | ||||
| NAC | P.euphratica | [96] | ||||
| Pavirv00056088m | P.virgatum | Despite regulating salt stress, involvement in any other regulatory mechanisms is still unknown. | [81] | |||
| POPTR_0007s08420 | P.trichocarpa | [97] | ||||
| GRMZM2G114850 | Zea mays | [98] | ||||
| miR165 | unknown | T. aestivum | Determining the positional fate of leaf tissues (adaxial or abaxial) and xylem differentiation in root stele tissues | [99] | ||
| HD-ZIP | A. thaliana | [45] | ||||
| miR166 | Unknown | G. raimondii | Plant development processes and abiotic stresses resistance | [81] | ||
| SPB-like | A. thaliana | [95] | ||||
| SPB-like | Glycine max | [100] | ||||
| SPB-like | Z. mays | [101] | ||||
| miR167 | Unknown | G. raimondii | Regulates some reproductive development processes, such as anther dehiscence, and ovule, embryonic, and seed development. | [81] | ||
| ARF | A. thaliana | [45] | ||||
| ARF | N. tabacum | [90] | ||||
| ARF | T. aestivum | [93] | ||||
| ARF | Z. mays | [102] | ||||
| miR168 | AGOs | Saccharum spp. | Facilitates plant adaptation to K+-deficiency stress, influences phase transition, leaf epinasty, and fruit development | [103] | ||
| AGOs | A. thaliana | [45] | ||||
| MYBs | P. euphratica | [84] | ||||
| AGOs | Z. mays | [102] | ||||
| Unknown | G. raimondii | [81] | ||||
| Unknown | Vigna unguiculata | [104] | ||||
| miR169 | NY-FA | Z. mays | Regulates tolerance to abiotic stresses in both monocots and dicots; plays a key role in nutrient uptake. | [78] | ||
| CCAAT-binding | A. thaliana | [45] | ||||
| CBF HAP2-like factor | N. tabacum | [90] | ||||
| CCAAT-binding TF | P. euphratica | [84] | ||||
| CBF HAP2-like factor | G. max | [105] | ||||
| CCAAT-binding TF | V. unguiculata | [104] | ||||
| miR171 | Scarecrow-like TFs | A. thaliana | Plant growth and development | [45] | ||
| AP2 | P. trichocarpa | [84] | ||||
| AP2 | S. italica | [92] | ||||
| Unknown | S. lycopersicum | [86] | ||||
| miR172 | AP2 | G. raimondii | Regulates the transitions between developmental stages and specifies floral organ identity | [81] | ||
| AP2 | N. tabacum | [90] | ||||
| AGOs | A. thaliana | [94] | ||||
| AP2 | H. spontaneum | [88] | ||||
| NNC1 | G. max | [106] | ||||
| MYBs | S. lycopersicum | [107] | ||||
| miR319 | TCPs | A. thaliana | Cooperatively regulates downstream genes, such as CUC genes, for cotyledon boundary, leaf serration formation, and other physiological responses. | [45] | ||
| MTR_3g011610 | M. truncatula | [108] | ||||
| PvPCF5 | A.s thaliana | [109] | ||||
| miR390 | ARFs | Populus spp. | Directs the production of tasiRNAs from Trans-acting siRNA3 (TAS3) transcripts to regulated ARF genes | [110] | ||
| TAS | Helianthus tuberosus | [111] | ||||
| miR393 | F-box | A. thaliana | Regulates the expression of different sets of TAAR genes following pathogen infection or nitrate treatment and regulates expression of the TIR1/AFB2 auxin receptor clade and auxin-related development | [45] | ||
| F-box | G. raimondii | [81] | ||||
| AFB2 | H. spontaneum | [88] | ||||
| AsTIR1 | Agrostis stolonifera | [64] | ||||
| miR394 | F-box | G. raimondii | Participates in the regulation of plant development and stress responses | [81] | ||
| F-box | A. thaliana | [45] | ||||
| F-box | G. max | [105] | ||||
| miR395 | Unknown | S. lycopersicum | An important regulator involved in sulfate transport and assimilation and a high-affinity sulfate transporter | [86] | ||
| ATP sulfurylase | P. virgatum | [83] | ||||
| miR396 | Unknown | G. raimondii | Control cell proliferation, margin, and vein pattern formation | [81] | ||
| GRFs | A. thaliana | [45] | ||||
| GRFs | N. tabacum | [90] | ||||
| GRFs | P. virgatum | [83] | ||||
| bHLH74 | R. sativus | [82] | ||||
| GRFs | A. stolonifera | [112] | ||||
| GRFs | A. thaliana | [113] | ||||
| miR397 | LACs | S. linnaeanum | Functioning in lignin synthesis and are involved in the development of plants under various conditions | [45] | ||
| cDNA l-ascorbate oxidase precursor | P. virgatum | [83] | ||||
| miR398 | Cu/Zn Superoxide dismutase | A. thaliana | Regulates plant responses to oxidative stress, water deficit, salt stress, abscisic acid stress, ultraviolet stress, copper and phosphate deficiency | [45] | ||
| miR399 | ATP-dependent RNA helicase | M. truncatula | Regulates phosphate homeostasis | [108] | ||
| ATP-dependent RNA helicase | T. aestivum | [93] | ||||
| miR402 | DEMETER-LIKE protein 3 | A. thaliana | Regulator of seed germination and seedling growth | [109] | ||
| miR408 | DEAD-box helicases | O. sativa | Provide an important cross-link between plant growth, development, and stress response. | [114] | ||
| SnRK2 | T. aestivum | [69] | ||||
| Cu-binding proteins | N. benthamiana | [66] | ||||
| miR414 | GhFSD1 | A. thaliana | Critical role in regulating the growth and development of plants’ cell development and cell differentiation | [115] | ||
| miR474 | PPR | Populus cathayana | Plant nutrient homeostasis | [116] | ||
| miR482 | TIR-NBS-LRR | P. trichocarpa | Regulates defense mechanisms | [117] | ||
| GRAS | S. lycopersicum | [107] | ||||
| miR530 | F-box | P. trichocarpa | Plant resistance against multiple pathogens and nutrient homeostasis | [117] | ||
| miR1444 | POPTR_0001s39950 | P. trichocarpa | Regulates copper homeostasis | [117] | ||
| miR1445 | Unknown | P. trichocarpa | Unknown | [117] | ||
| miR1446 | GRM-like protein | P. euphratica | Nutrient homeostasis | [84] | ||
| miR1447 | ABC transport protein | P. euphratica | Abiotic stress tolerance | [84] | ||
| miR1448 | unknown | P. euphratica | Disease resistance against fungal pathogens | [84] | ||
| miR1507 | NBS-LRR | G. max | Activators of plant defense | [105] | ||
| miR1711 | unknown | P. trichocarpa | Unknown | [117] | ||
| miR2118 | APS-reductase | Phaseolus vulgaris | Involved in the production of 21-nt phasiRNAs | [118,119] | ||
| miRNVL5 | GhCHR | G. hirsutum | Vital in plant response to salinity | [120] | ||
4. The Target Genes and Related Pathways of Salt-Responsive miRNAs
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Goyal, V.; Jhanghel, D.; Mehrotra, S. Emerging warriors against salinity in plants: Nitric oxide and hydrogen sulphide. Physiol. Plant. 2021, 171, 896–908. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Mustafa, G.; Akhtar, M.S.; Abdullah, R. Global concern for salinity on various agro-ecosystems. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution: Volume 1; Springer: Singapore, 2019; pp. 1–19. ISBN 9789811388019. [Google Scholar]
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity Stress in Arid and Semi-Arid Climates: Effects and Management in Field Crops. In Climate Change and Agriculture; IntechOpen Limited: London, UK, 2019. [Google Scholar]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Ren, G. Plant microRNAs: Biogenesis, homeostasis, and degradation. Front. Plant Sci. 2019, 10, 360. [Google Scholar] [CrossRef]
- Tyagi, S.; Sharma, S.; Ganie, S.A.; Tahir, M.; Mir, R.R.; Pandey, R. Plant microRNAs: Biogenesis, gene silencing, web-based analysis tools and their use as molecular markers. 3 Biotech 2019, 9, 413. [Google Scholar] [CrossRef]
- Voinnet, O. Origin, Biogenesis, and Activity of Plant MicroRNAs. Cell 2009, 136, 669–687. [Google Scholar] [CrossRef]
- Rogers, K.; Chen, X. Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell 2013, 25, 2383–2399. [Google Scholar] [CrossRef]
- Naqvi, A.R.; Sarwat, M.; Hasan, S.; Roychodhury, N. Biogenesis, functions and fate of plant microRNAs. J. Cell. Physiol. 2012, 227, 3163–3168. [Google Scholar] [CrossRef]
- Wen-wen, K.; Hong-bo, W.; Jing, L. Biogenesis of Plant MicroRNAs. J. Northeast Agric. Univ. Engl. Ed. 2014, 21, 84–96. [Google Scholar] [CrossRef]
- Chen, X. microRNA biogenesis and function in plants. FEBS Lett. 2005, 579, 5923–5931. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Seo, P.J.; Park, C.M. MicroRNA biogenesis and function in higher plants. Plant Biotechnol. Rep. 2009, 3, 111–126. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.K.; Duan, C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J.; Westholm, J.O.; Lai, E.C. Vive la différence: Biogenesis and evolution of microRNAs in plants and animals. Genome Biol. 2011, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Pashkovskiy, P.P.; Ryazansky, S.S. Biogenesis, evolution, and functions of plant microRNAs. Biochem. 2013, 78, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Nie, J.; Wang, H. MicroRNA biogenesis in plant. Plant Growth Regul. 2021, 93, 1–12. [Google Scholar] [CrossRef]
- Chorostecki, U.; Moro, B.; Rojas, A.M.L.; Debernardi, J.M.; Schapire, A.L.; Notredame, C.; Palatnik, J.F. Evolutionary footprints reveal insights into plant microRNA biogenesis. Plant Cell 2017, 29, 1248–1261. [Google Scholar] [CrossRef]
- Zhu, J.K. Reconstituting plant miRNA biogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 9851–9852. [Google Scholar] [CrossRef]
- Islam, W.; Qasim, M.; Noman, A.; Adnan, M.; Tayyab, M.; Farooq, T.H.; Wei, H.; Wang, L. Plant microRNAs: Front line players against invading pathogens. Microb. Pathog. 2018, 118, 9–17. [Google Scholar] [CrossRef]
- Islam, W.; Noman, A.; Qasim, M.; Wang, L. Plant responses to pathogen attack: Small rnas in focus. Int. J. Mol. Sci. 2018, 19, 515. [Google Scholar] [CrossRef] [Green Version]
- Islam, W.; Adnan, M.; Huang, Z.; Lu, G.; Chen, H.Y.H. Small RNAs from seed to mature plant. CRC Crit. Rev. Plant Sci. 2019, 38, 117–139. [Google Scholar] [CrossRef]
- Islam, W.; Islam, S.U.; Qasim, M.; Wang, L. Host-Pathogen interactions modulated by small RNAs. RNA Biol. 2017, 14, 891–904. [Google Scholar] [CrossRef]
- Noman, A.; Sanaullah, T.; Khalid, N.; Islam, W.; Khan, S.; Irshad, M.K.; Aqeel, M. Crosstalk between plant miRNA and heavy metal toxicity. In Plant Metallomics and Functional Omics: A System-Wide Perspective; Springer: Cham, Denmark, 2019; pp. 145–168. ISBN 9783030191030. [Google Scholar]
- Paul, S.; Datta, S.K.; Datta, K. miRNA regulation of nutrient homeostasis in plants. Front. Plant Sci. 2015, 6, 232. [Google Scholar] [CrossRef]
- Islam, W.; Tauqeer, A.; Waheed, A.; Zeng, F. MicroRNA Mediated Plant Responses to Nutrient Stress. Int. J. Mol. Sci. 2022, 23, 2562. [Google Scholar] [CrossRef]
- Liu, M.; Yu, H.; Zhao, G.; Huang, Q.; Lu, Y.; Ouyang, B. Identification of drought-responsive microRNAs in tomato using high-throughput sequencing. Funct. Integr. Genom. 2018, 18, 67–78. [Google Scholar] [CrossRef]
- Qiu, C.W.; Liu, L.; Feng, X.; Hao, P.F.; He, X.; Cao, F.; Wu, F. Genome-wide identification and characterization of drought stress responsive microRNAs in Tibetan wild barley. Int. J. Mol. Sci. 2020, 21, 2795. [Google Scholar] [CrossRef]
- Kuruvilla, L.; Sathik, M.; Luke, L.P.; Thomas, M. Identification and validation of drought-responsive microRNAs from Hevea brasiliensis. Acta Physiol. Plant. 2019, 41, 14. [Google Scholar] [CrossRef]
- Shinde, H.; Dudhate, A.; Anand, L.; Tsugama, D.; Gupta, S.K.; Liu, S.; Takano, T. Small RNA sequencing reveals the role of pearl millet miRNAs and their targets in salinity stress responses. S. Afr. J. Bot. 2020, 132, 395–402. [Google Scholar] [CrossRef]
- Sharma, A.; Ruiz-Manriquez, L.M.; Serrano-Cano, F.I.; Reyes-Pérez, P.R.; Alfaro, C.K.T.; Andrade, Y.E.B.; Aros, A.K.H.; Srivastava, A.; Paul, S. Identification of microRNAs and their expression in leaf tissues of guava (Psidium guajava L.) under salinity stress. Agronomy 2020, 10, 1920. [Google Scholar] [CrossRef]
- Parmar, S.; Gharat, S.A.; Tagirasa, R.; Chandra, T.; Behera, L.; Dash, S.K.; Shaw, B.P. Identification and expression analysis of miRNAs and elucidation of their role in salt tolerance in rice varieties susceptible and tolerant to salinity. PLoS ONE 2020, 15, e230958. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Xu, Y.; Jiang, J.; Zhang, F.; Ma, L.; Wu, D.; Wang, Y.; Sun, W. Identification of cold stress responsive microRNAs in two winter turnip rape (Brassica rapa L.) by high throughput sequencing. BMC Plant Biol. 2018, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheng, C.; Chen, F.; Ni, S.; Lin, Y.; Lai, Z. High-throughput sequencing of small RNAs revealed the diversified cold-responsive pathways during cold stress in the wild banana (Musa itinerans). BMC Plant Biol. 2018, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; He, J.; Jin, G.; Chen, Z.H.; Dai, F. Identification of novel microRNAs for cold deacclimation in barley. Plant Growth Regul. 2020, 92, 389–400. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, T.; Yang, D.; Luo, B.; Wang, W.P.; Yu, D.; He, F.L.; Wang, Q.M.; Rao, L.Q. Identification and characterization of heat-responsive micrornas at the booting stage in two rice varieties, 9311 and nagina 22. Genome 2021, 64, 969–984. [Google Scholar] [CrossRef]
- Vidya, S.M.; Ravishankar, K.V.; Laxman, R.H. Genome wide analysis of heat responsive microRNAs in banana during acquired thermo tolerance. J. Hortic. Sci. 2018, 13, 61–71. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Chen, Y.; Xu, Y.; Xu, J. Profiling of heat-responsive microRNAs in creeping bentgrass (Agrostis stolonifera L.). Curr. Bioinform. 2017, 12, 319–327. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Tong, Z.; Véronneau, P.Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Treatment Affected Senescence of Stored Strawberry Fruit with a Potential Role of MicroRNAs in the Activation of the Antioxidant System. J. Agric. Food Chem. 2018, 66, 12188–12197. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Cheng, J.; Jiang, Z.; Xu, N.; An, X.; Chen, Z.; Hao, J.; Yang, S.; Xu, Z.; et al. Identification of UV-B radiation responsive microRNAs and their target genes in chrysanthemum (Chrysanthemum morifolium Ramat) using high-throughput sequencing. Ind. Crops Prod. 2020, 151, 112484. [Google Scholar] [CrossRef]
- Dugas, D.V.; Bartel, B. Sucrose induction of Arabidopsis miR398 represses two Cu/Zn superoxide dismutases. Plant Mol. Biol. 2008, 67, 403–417. [Google Scholar] [CrossRef]
- Sunkar, R.; Kapoor, A.; Zhu, J.K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.H.; Tian, X.; Li, Y.J.; Wu, C.A.; Zheng, C.C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 2008, 14, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Cohu, C.M.; Abdel-Ghany, S.E.; Gogolin Reynolds, K.A.; Onofrio, A.M.; Bodecker, J.R.; Kimbrel, J.A.; Niyogi, K.K.; Pilon, M. Copper delivery by the copper chaperone for chloroplast and cytosolic copper/zinc-superoxide dismutases: Regulation and unexpected phenotypes in an arabidopsis mutant. Mol. Plant 2009, 2, 1336–1350. [Google Scholar] [CrossRef]
- Yuan, N.; Yuan, S.; Li, Z.; Li, D.; Hu, Q.; Luo, H. Heterologous expression of a rice miR395 gene in Nicotiana tabacum impairs sulfate homeostasis. Sci. Rep. 2016, 6, 28791. [Google Scholar] [CrossRef] [PubMed]
- Ai, Q.; Liang, G.; Zhang, H.; Yu, D. Control of sulfate concentration by miR395-targeted APS genes in Arabidopsis thaliana. Plant Divers. 2016, 38, 92–100. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Khare, T.; Tripathi, P.; Shah, T.; Ramakrishna, C.; Aglawe, S.; Mangrauthia, S.K. miRNA applications for engineering abiotic stress tolerance in plants. Biologia 2020, 75, 1063–1081. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Tang, R.; Qu, H.; Duan, X.; Jiang, Y. Banana sRNAome and degradome identify microRNAs functioning in differential responses to temperature stress 06 Biological Sciences 0604 Genetics 06 Biological Sciences 0601 Biochemistry and Cell Biology. BMC Genom. 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Bustamante, A.; Marques, M.C.; Sanz-Carbonell, A.; Mulet, J.M.; Gomez, G. Alternative processing of its precursor is related to miR319 decreasing in melon plants exposed to cold. Sci. Rep. 2018, 8, 15538. [Google Scholar] [CrossRef]
- Sun, Z.; Shu, L.; Zhang, W.; Wang, Z. Cca-miR398 increases copper sulfate stress sensitivity via the regulation of CSD mRNA transcription levels in transgenic Arabidopsis thaliana. PeerJ 2020, 2020, e9105. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, L.; Zhang, Y.; Kang, X.; Zhang, Z.; Wang, Y. Identification and characterization of salt-responsive microRNAs in Populus tomentosa by high-throughput sequencing. Biochimie 2013, 95, 743–750. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Shen, H.; Zhu, X.; Zhai, L.; Xu, L.; Wang, R.; Gong, Y.; Limera, C.; Liu, L. Identification of Radish (Raphanus sativus L.) miRNAs and Their Target Genes to Explore miRNA-Mediated Regulatory Networks in Lead (Pb) Stress Responses by High-Throughput Sequencing and Degradome Analysis. Plant Mol. Biol. Rep. 2015, 33, 358–376. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Hao, Q.; Sha, A.; Zhou, R.; Zhou, X.; Yuan, L. Elucidation of miRNAs-Mediated Responses to Low Nitrogen Stress by Deep Sequencing of Two Soybean Genotypes. PLoS ONE 2013, 8, e67423. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Chen, X.; Song, C.; Zou, Z.; Wang, Y.; Wang, M.; Fang, W.; Li, X. Identification and characterization of coldresponsive microRNAs in tea plant (Camellia sinensis) and their targets using high-throughput sequencing and degradome analysis. BMC Plant Biol. 2014, 14, 271. [Google Scholar] [CrossRef]
- Shaw, B.P. Salt stress tolerance in plants: The role of miRNAs. Adv. Plants Agric. Res. 2018, 8, 1. [Google Scholar] [CrossRef]
- Singroha, G.; Sharma, P.; Sunkur, R. Current status of microRNA-mediated regulation of drought stress responses in cereals. Physiol. Plant. 2021, 172, 1808–1821. [Google Scholar] [CrossRef] [PubMed]
- Candar-Cakir, B.; Arican, E.; Zhang, B. Small RNA and degradome deep sequencing reveals drought-and tissue-specific micrornas and their important roles in drought-sensitive and drought-tolerant tomato genotypes. Plant Biotechnol. J. 2016, 14, 1727–1746. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, A.; Gitau, M.M.; Huang, X.; Chen, L.; Fu, J. Insights into the MicroRNA-regulated response of bermudagrass to cold and salt stress. Environ. Exp. Bot. 2018, 145, 64–74. [Google Scholar] [CrossRef]
- Ning, L.H.; Du, W.K.; Song, H.N.; Shao, H.B.; Qi, W.C.; Sheteiwy, M.S.A.; Yu, D.-Y. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Environ. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- Gao, P.; Bai, X.; Yang, L.; Lv, D.; Pan, X.; Li, Y.; Cai, H.; Ji, W.; Chen, Q.; Zhu, Y. Osa-MIR393: A salinity- and alkaline stress-related microRNA gene. Mol. Biol. Rep. 2011, 38, 237–242. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, L.; Han, N.; Hu, J.; Yang, Y.; Xiang, T.; Zhang, X.; Wang, L. Overexpression of a miR393-resistant form of transport inhibitor response protein 1 (mTIR1) enhances salt tolerance by increased osmoregulation and Na+ exclusion in arabidopsis thaliana. Plant Cell Physiol. 2015, 56, 73–83. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, S.; Zhou, M.; Yuan, N.; Li, Z.; Hu, Q.; Bethea, F.G.; Liu, H.; Li, S.; Luo, H. Transgenic creeping bentgrass overexpressing Osa-miR393a exhibits altered plant development and improved multiple stress tolerance. Plant Biotechnol. J. 2019, 17, 233–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Burd, S.; Lers, A. MiR408 is involved in abiotic stress responses in Arabidopsis. Plant J. 2015, 84, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Niu, J.; Cao, X. Heterologous expression of salvia miltiorrhiza microRNA408 enhances tolerance to salt stress in nicotiana benthamiana. Int. J. Mol. Sci. 2018, 19, 3985. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Feng, J.; Wang, R.; Zhang, H.; Huang, J. Comparative analysis of microRNAs and their targets in the roots of two cultivars with contrasting salt tolerance in rice (Oryza sativa L.). Plant Growth Regul. 2019, 87, 139–148. [Google Scholar] [CrossRef]
- Hajyzadeh, M.; Turktas, M.; Khawar, K.M.; Unver, T. MiR408 overexpression causes increased drought tolerance in chickpea. Gene 2015, 555, 186–193. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, X.; Chen, X.; Shi, G.; Liu, Z.; Guo, C.; Xiao, K. Wheat miRNA taemir408 acts as an essential mediator in plant tolerance to pi deprivation and salt stress via modulating stress-associated physiological processes. Front. Plant Sci. 2018, 9, 499. [Google Scholar] [CrossRef]
- Fang, Y.; Zheng, Y.; Lu, W.; Li, J.; Duan, Y.; Zhang, S.; Wang, Y. Roles of miR319-regulated TCPs in plant development and response to abiotic stress. Crop J. 2021, 9, 17–28. [Google Scholar] [CrossRef]
- Zhou, M.; Li, D.; Li, Z.; Hu, Q.; Yang, C.; Zhu, L.; Luo, H. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass. Plant Physiol. 2013, 161, 1375–1391. [Google Scholar] [CrossRef]
- Pieczynski, M.; Marczewski, W.; Hennig, J.; Dolata, J.; Bielewicz, D.; Piontek, P.; Wyrzykowska, A.; Krusiewicz, D.; Strzelczyk-Zyta, D.; Konopka-Postupolska, D.; et al. Down-regulation of CBP80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnol. J. 2013, 11, 459–469. [Google Scholar] [CrossRef]
- Yang, C.; Li, D.; Mao, D.; Liu, X.; Ji, C.; Li, X.; Zhao, X.; Cheng, Z.; Chen, C.; Zhu, L. Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice (Oryza sativa L.). Plant Cell Environ. 2013, 36, 2207–2218. [Google Scholar] [CrossRef]
- Kang, T.; Yu, C.Y.; Liu, Y.; Song, W.M.; Bao, Y.; Guo, X.T.; Li, B.; Zhang, H.X. Subtly Manipulated Expression of ZmmiR156 in Tobacco Improves Drought and Salt Tolerance Without Changing the Architecture of Transgenic Plants. Front. Plant Sci. 2020, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Zhou, T.; Bo, W.; Xu, F.; Wu, R. Genome-wide analysis of salt-responsive and novel microRNAs in Populus euphratica by deep sequencing. BMC Genet. 2014, 15, S6. [Google Scholar] [CrossRef]
- Shuai, P.; Liang, D.; Zhang, Z.; Yin, W.; Xia, X. Identification of drought-responsive and novel Populus trichocarpa microRNAs by high-throughput sequencing and their targets using degradome analysis. BMC Genom. 2013, 14, 233. [Google Scholar] [CrossRef]
- Büyük, İ.; İlhan, E.; Şener, D.; Özsoy, A.U.; Aras, S. Genome-wide identification of CAMTA gene family members in Phaseolus vulgaris L. and their expression profiling during salt stress. Mol. Biol. Rep. 2019, 46, 2721–2732. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Xu, M.; Lu, Y.; Zhang, L.; Fan, Y.; Wang, L. Expression of zma-miR169 miRNAs and their target ZmNF-YA genes in response to abiotic stress in maize leaves. Gene 2015, 555, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ge, L.; Liang, R.; Li, W.; Ruan, K.; Lin, H.; Jin, Y. Members of miR-169 family are induced by high salinity and transiently inhibit the NF-YA transcription factor. BMC Mol. Biol. 2009, 10, 29. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, M.; Zhao, Y.; He, X.; Ding, C.; Wang, S.; Feng, Y.; Song, X.; Li, P.; Wang, B. Identification of salt tolerance-related microRNAs and their targets in maize (Zea mays L.) using high-throughput sequencing and degradome analysis. Front. Plant Sci. 2017, 8, 864. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Q.; Sun, R.; Zhang, B. Deep sequencing reveals important roles of microRNAs in response to drought and salinity stress in cotton. J. Exp. Bot. 2015, 66, 789–804. [Google Scholar] [CrossRef]
- Sun, X.; Xu, L.; Wang, Y.; Yu, R.; Zhu, X.; Luo, X.; Gong, Y.; Wang, R.; Limera, C.; Zhang, K.; et al. Identification of novel and salt-responsive miRNAs to explore miRNA-mediated regulatory network of salt stress response in radish (Raphanus sativus L.). BMC Genom. 2015, 16, 1–16. [Google Scholar] [CrossRef]
- Sun, G.; Stewart, C.N.; Xiao, P.; Zhang, B. MicroRNA expression analysis in the cellulosic biofuel crop switchgrass (panicum virgatum) under abiotic stress. PLoS ONE 2012, 7, e32017. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Duan, Z.; Xia, X.; Yin, W. Expression profiles of precursor and mature microRNAs under dehydration and high salinity shock in Populus euphratica. Plant Cell Rep. 2011, 30, 1893–1907. [Google Scholar] [CrossRef]
- Cao, C.; Long, R.; Zhang, T.; Kang, J.; Wang, Z.; Wang, P.; Sun, H.; Yu, J.; Yang, Q. Genome-wide identification of microRNAs in response to salt/alkali stress in medicago truncatula through high-throughput sequencing. Int. J. Mol. Sci. 2018, 19, 4076. [Google Scholar] [CrossRef]
- Çakır, Ö.; Arıkan, B.; Karpuz, B.; Turgut-Kara, N. Expression analysis of miRNAs and their targets related to salt stress in Solanum lycopersicum H-2274. Biotechnol. Biotechnol. Equip. 2021, 35, 283–290. [Google Scholar] [CrossRef]
- Kuang, L.; Shen, Q.; Wu, L.; Yu, J.; Fu, L.; Wu, D.; Zhang, G. Identification of microRNAs responding to salt stress in barley by high-throughput sequencing and degradome analysis. Environ. Exp. Bot. 2019, 160, 59–70. [Google Scholar] [CrossRef]
- Barrera-Figueroa, B.E.; Gao, L.; Wu, Z.; Zhou, X.; Zhu, J.; Jin, H.; Liu, R.; Zhu, J.K. High throughput sequencing reveals novel and abiotic stress-regulated microRNAs in the inflorescences of rice. BMC Plant Biol. 2012, 12, 132. [Google Scholar] [CrossRef]
- Frazier, T.P.; Sun, G.; Burklew, C.E.; Zhang, B. Salt and drought stresses induce the aberrant expression of microRNA genes in tobacco. Mol. Biotechnol. 2011, 49, 159–165. [Google Scholar] [CrossRef]
- Chen, L.; Wang, T.; Zhao, M.; Tian, Q.; Zhang, W.H. Identification of aluminum-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. Planta 2012, 235, 375–386. [Google Scholar] [CrossRef]
- Khan, Y.; Yadav, A.; Bonthala, V.S.; Muthamilarasan, M.; Yadav, C.B.; Prasad, M. Comprehensive genome-wide identification and expression profiling of foxtail millet [Setaria italica (L.)] miRNAs in response to abiotic stress and development of miRNA database. Plant Cell. Tissue Organ Cult. 2014, 118, 279–292. [Google Scholar] [CrossRef]
- Lu, W.; Li, J.; Liu, F.; Gu, J.; Guo, C.; Xu, L.; Zhang, H.; Xiao, K. Expression pattern of wheat miRNAs under salinity stress and prediction of salt-inducible miRNAs targets. Front. Agric. China 2011, 5, 413–422. [Google Scholar] [CrossRef]
- Dolata, J.; Bajczyk, M.; Bielewicz, D.; Niedojadlo, K.; Niedojadlo, J.; Pietrykowska, H.; Walczak, W.; Szweykowska-Kulinska, Z.; Jarmolowski, A. Salt stress reveals a new role for ARGONAUTE1 in miRNA biogenesis at the transcriptional and posttranscriptional levels. Plant Physiol. 2016, 172, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amor, B.B.; Wirth, S.; Merchan, F.; Laporte, P.; D’Aubenton-Carafa, Y.; Hirsch, J.; Maizel, A.; Mallory, A.; Lucas, A.; Deragon, J.M.; et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009, 19, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Dun, H.; Lian, C.; Zhang, X.; Yin, W.; Xia, X. The role of peu-miR164 and its target PeNAC genes in response to abiotic stress in Populus euphratica. Plant Physiol. Biochem. 2017, 115, 418–438. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Duan, H.; Li, J.; Deng, X.W.; Yin, W.; Xia, X. Global identification of miRNAs and targets in Populus euphratica under salt stress. Plant Mol. Biol. 2013, 81, 525–539. [Google Scholar] [CrossRef]
- Shan, T.; Fu, R.; Xie, Y.; Chen, Q.; Wang, Y.; Li, Z.; Song, X.; Li, P.; Wang, B. Regulatory Mechanism of Maize (Zea mays L.) miR164 in Salt Stress Response. Russ. J. Genet. 2020, 56, 835–842. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.F.; Song, N.; Wei, J.P.; Wang, X.J.; Feng, H.; Yin, Z.Y.; Kang, Z.S. MicroRNAs involving in cold, wounding and salt stresses in Triticum aestivum L. Plant Physiol. Biochem. 2014, 80, 90–96. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Zhang, Y.X.; Liu, J.Y. Identification and analysis of seven H2O2-responsive miRNAs and 32 new miRNAs in the seedlings of rice (Oryza sativa L. ssp. indica). Nucleic Acids Res. 2011, 39, 2821–2833. [Google Scholar] [CrossRef]
- Kong, Y.; Elling, A.A.; Chen, B.; Deng, X. Differential Expression of microRNAs in Maize Inbred and Hybrid Lines during Salt and Drought Stress. Am. J. Plant Sci. 2010, 1, 69–76. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, L.; Wang, H.; Liu, Z.; Zhang, Z.; Zheng, Y. Differential expression of miRNAs in response to salt stress in maize roots. Ann. Bot. 2009, 103, 29–38. [Google Scholar] [CrossRef]
- Carnavale Bottino, M.; Rosario, S.; Grativol, C.; Thiebaut, F.; Rojas, C.A.; Farrineli, L.; Hemerly, A.S.; Ferreira, P.C.G. High-Throughput Sequencing of Small RNA Transcriptome Reveals Salt Stress Regulated MicroRNAs in Sugarcane. PLoS ONE 2013, 8, e59423. [Google Scholar] [CrossRef]
- Paul, S.; Kundu, A.; Pal, A. Identification and validation of conserved microRNAs along with their differential expression in roots of Vigna unguiculata grown under salt stress. Plant Cell. Tissue Organ Cult. 2011, 105, 233–242. [Google Scholar] [CrossRef]
- Li, H.; Dong, Y.; Yin, H.; Wang, N.; Yang, J.; Liu, X.; Wang, Y.; Wu, J.; Li, X. Characterization of the stress associated microRNAs in Glycine max by deep sequencing. BMC Plant Biol. 2011, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Sahito, Z.A.; Wang, L.; Sun, Z.; Yan, Q.; Zhang, X.; Jiang, Q.; Ullah, I.; Tong, Y.; Li, X. The miR172c-NNC1 module modulates root plastic development in response to salt in soybean. BMC Plant Biol. 2017, 17, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yu, H.; Liu, M.; Lu, Y.; Ouyang, B. Identification of salt-stress responsive microRNAs from Solanum lycopersicum and Solanum pimpinellifolium. Plant Growth Regul. 2017, 83, 129–140. [Google Scholar] [CrossRef]
- Lelandais-Brière, C.; Naya, L.; Sallet, E.; Calenge, F.; Frugier, F.; Hartmann, C.; Gouzy, J.; Crespi, M. Genome-wide medicago truncatula small RNA analysis revealed novel microRNAs and isoforms differentially regulated in roots and nodules. Plant Cell 2009, 21, 2780–2796. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwak, K.J.; Jung, H.J.; Lee, H.J.; Kang, H. MicroRNA402 affects seed germination of arabidopsis thaliana under stress conditions via targeting DEMETER-LIKE Protein3 mRNA. Plant Cell Physiol. 2010, 51, 1079–1083. [Google Scholar] [CrossRef]
- He, F.; Xu, C.; Fu, X.; Shen, Y.; Guo, L.; Leng, M.; Luo, K. The microRNA390/TRANS-ACTING SHORT INTERFERING RNA3 module mediates lateral root growth under salt stress via the auxin pathway. Plant Physiol. 2018, 177, 775–791. [Google Scholar] [CrossRef]
- Wen, F.L.; Yue, Y.; He, T.F.; Gao, X.M.; Zhou, Z.S.; Long, X.H. Identification of miR390-TAS3-ARF pathway in response to salt stress in Helianthus tuberosus L. Gene 2020, 738, 144460. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, J.; Li, Z.; Hu, Q.; Yuan, N.; Zhou, M.; Xia, X.; Noorai, R.; Saski, C.; Li, S.; et al. MicroRNA396-mediated alteration in plant development and salinity stress response in creeping bentgrass. Hortic. Res. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Pegler, J.L.; Nguyen, D.Q.; Oultram, J.M.J.; Grof, C.P.L.; Eamens, A.L. Molecular manipulation of the mir396/grf expression module alters the salt stress response of arabidopsis thaliana. Agronomy 2021, 11, 1751. [Google Scholar] [CrossRef]
- Macovei, A.; Tuteja, N. MicroRNAs targeting DEAD-box helicases are involved in salinity stress response in rice (Oryza sativa L.). BMC Plant Biol. 2012, 12, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Liu, D.; Chen, D.; Cheng, Y.; Zhang, X.; Song, L.; Hu, M.; Dong, J.; Shen, F. MicroRNA414c affects salt tolerance of cotton by regulating reactive oxygen species metabolism under salinity stress. RNA Biol. 2019, 16, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, M.; Jiang, J.; Qiao, G.; Lin, S.; Li, H.; Xie, L.; Zhuo, R. Expression profile of miRNAs in Populus cathayana L. and Salix matsudana Koidz under salt stress. Mol. Biol. Rep. 2012, 39, 8645–8654. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.H.; Chiang, V.L. Stress-responsive microRNAs in Populus. Plant J. 2008, 55, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Huertero, C.; Pérez, B.; Rabanal, F.; Blanco-Melo, D.; De La Rosa, C.; Estrada-Navarrete, G.; Sanchez, F.; Covarrubias, A.A.; Reyes, J.L. Conserved and novel miRNAs in the legume Phaseolus vulgaris in response to stress. Plant Mol. Biol. 2009, 70, 385–401. [Google Scholar] [CrossRef]
- Han, J.; Xie, H.; Kong, M.L.; Sun, Q.P.; Li, R.Z.; Pan, J.B. Computational identification of miRNAs and their targets in Phaseolus vulgaris. Genet. Mol. Res. 2014, 13, 310–322. [Google Scholar] [CrossRef]
- Gao, S.; Yang, L.; Zeng, H.Q.; Zhou, Z.S.; Yang, Z.M.; Li, H.; Sun, D.; Xie, F.; Zhang, B. A cotton miRNA is involved in regulation of plant response to salt stress. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.J.; Jung, H.J.; Maruyama, K.; Suzuki, N.; Kang, H. Overexpression of microRNA395c or 395e affects differently the seed germination of arabidopsis thaliana under stress conditions. Planta 2010, 232, 1447–1454. [Google Scholar] [CrossRef]
- Dong, Z.; Shi, L.; Wang, Y.; Chen, L.; Cai, Z.; Wang, Y.; Jin, J.; Li, X. Identification and dynamic regulation of microRNAs involved in salt stress responses in functional soybean nodules by high-throughput sequencing. Int. J. Mol. Sci. 2013, 14, 2717–2738. [Google Scholar] [CrossRef]
- De Paola, D.; Cattonaro, F.; Finetti Sialer, M.M.; Catalano, D.; Fracchiolla, A.; Morgese, A.; Pignone, D.; Sonnante, G. MicroRNAs in the globe artichoke: Detection and analysis. Acta Hortic. 2013, 983, 187–192. [Google Scholar] [CrossRef]
- Pokoo, R.; Ren, S.; Wang, Q.; Motes, C.M.; Hernandez, T.D.; Ahmadi, S.; Monteros, M.J.; Zheng, Y.; Sunkar, R. Genotype- and tissue-specific miRNA profiles and their targets in three alfalfa (Medicago sativa L.) genotypes. BMC Genom. 2018, 19, 115–131. [Google Scholar] [CrossRef]
- Bi, F.; Meng, X.; Ma, C.; Yi, G. Identification of miRNAs involved in fruit ripening in Cavendish bananas by deep sequencing. BMC Genom. 2015, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, J.; Cai, Y.; Gong, X.; Xiong, X.; Qi, W.; Pang, Q.; Wang, X.; Wang, Y. Genome-wide identification and characterization of Eutrema salsugineum microRNAs for salt tolerance. Physiol. Plant. 2016, 157, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Nie, X.; Cui, L.; Deng, P.; Wang, M.; Song, W. Genome-wide identification and characterization of salinity stress-responsive miRNAS in wild emmer wheat (Triticum turgidum ssp. dicoccoides). Genes 2017, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.K.; Panda, A.K.; Rawal, H.C.; Sharma, T.R. Discovery of microRNA-target modules of African rice (Oryza glaberrima) under salinity stress. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ai, B.; Chen, Y.; Zhao, M.; Ding, G.; Xie, J.; Zhang, F. Overexpression of miR1861h increases tolerance to salt stress in rice (Oryza sativa L.). Genet. Resour. Crop Evol. 2021, 68, 87–92. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, X.; Zhou, Y.; Xie, J. Genome-wide identification of conserved microRNA and their response to drought stress in Dongxiang wild rice (Oryza rufipogon Griff.). Biotechnol. Lett. 2016, 38, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.M.; Alaraidh, I.A.; Khan, M.A.; Migdadi, H.M.; Alghamdi, S.S.; Alsahli, A.A. Identification and characterization of salt-responsive micrornas in vicia faba by high-throughput sequencing. Genes 2019, 10, 303. [Google Scholar] [CrossRef]
- Naik, H.K.; Varadahalli, R.D. Genomic identification of salt induced microRNAs in niger (Guizotia abyssinica Cass.). Plant Gene 2020, 23, 100242. [Google Scholar] [CrossRef]
- Nguyen, D.Q.; Brown, C.W.; Pegler, J.L.; Eamens, A.L.; Grof, C.P.L. Molecular manipulation of microRNA397 abundance influences the development and salt stress response of arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 7879. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Li, J. Global identification and analysis of micrornas involved in salt stress responses in two alfalfa (Medicago sativa millennium) Lines. Can. J. Plant Sci. 2020, 100, 445–455. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, P.; Kang, H.; Liu, L.; Cao, Q.; Sun, J.; Dong, T.; Zhu, M.; Li, Z.; Xu, T. High-throughput deep sequencing reveals the important role that microRNAs play in the salt response in sweet potato (Ipomoea batatas L.). BMC Genom. 2020, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pegler, J.L.; Nguyen, D.Q.; Grof, C.P.L.; Eamens, A.L. Profiling of the salt stress responsive MicroRNA landscape of C4 genetic model species Setaria viridis (L.) beauv. Agronomy 2020, 10, 837. [Google Scholar] [CrossRef]
- Zeeshan, M.; Qiu, C.W.; Naz, S.; Cao, F.; Wu, F. Genome-wide discovery of mirnas with differential expression patterns in responses to salinity in the two contrasting wheat cultivars. Int. J. Mol. Sci. 2021, 22, 2556. [Google Scholar] [CrossRef] [PubMed]
- Baldrich, P.; Beric, A.; Meyers, B.C. Despacito: The slow evolutionary changes in plant microRNAs. Curr. Opin. Plant Biol. 2018, 42, 16–22. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Khalid, N.; Islam, W.; Sanaullah, T.; Anwar, M.; Khan, S.; Ye, W.; Lou, Y. Zinc finger protein transcription factors: Integrated line of action for plant antimicrobial activity. Microb. Pathog. 2019, 132, 141–149. [Google Scholar] [CrossRef]
- Jagannadham, P.T.K.; Muthusamy, S.K.; Chidambaranathan, P. Micromics: A Novel Approach to Understand the Molecular Mechanisms in Plant Stress Tolerance. In Recent Approaches in Omics for Plant Resilience to Climate Change; Springer: Cham, Denmark, 2019; pp. 93–108. [Google Scholar]
- Ganie, S.A.; Molla, K.A.; Henry, R.J.; Bhat, K.V.; Mondal, T.K. Advances in understanding salt tolerance in rice. Theor. Appl. Genet. 2019, 132, 851–870. [Google Scholar] [CrossRef]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 2016, 124, 39–63. [Google Scholar] [CrossRef]
- Liu, S.; Wu, L.; Qi, H.; Xu, M. LncRNA/circRNA–miRNA–mRNA networks regulate the development of root and shoot meristems of Populus. Ind. Crops Prod. 2019, 133, 333–347. [Google Scholar] [CrossRef]
- Mittal, D.; Sharma, N.; Sharma, V.; Sopory, S.K.; Sanan-Mishra, N. Role of microRNAs in rice plant under salt stress. Ann. Appl. Biol. 2016, 168, 2–18. [Google Scholar] [CrossRef]
- Pegler, J.L.; Oultram, J.M.J.; Grof, C.P.L.; Eamens, A.L. Molecular manipulation of the miR399/PHO2 expression module alters the salt stress response of arabidopsis thaliana. Plants 2021, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Finet, C.; Berne-Dedieu, A.; Scutt, C.P.; Marlétaz, F. Evolution of the ARF gene family in land plants: Old domains, new tricks. Mol. Biol. Evol. 2013, 30, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; Xie, Z.Z.; Hu, C.G.; Zhang, J.Z. A review of auxin response factors (ARFs) in plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Roosjen, M.; Paque, S.; Weijers, D. Auxin Response Factors: Output control in auxin biology. J. Exp. Bot. 2018, 69, 179–188. [Google Scholar] [CrossRef]
- Tang, Y.; Du, G.; Xiang, J.; Hu, C.; Li, X.; Wang, W.; Zhu, H.; Qiao, L.; Zhao, C.; Wang, J.; et al. Genome-wide identification of auxin response factor (ARF) gene family and the miR160-ARF18-mediated response to salt stress in peanut (Arachis hypogaea L.). Genomics 2022, 114, 171–184. [Google Scholar] [CrossRef]
- Kim, J.K.; Cao, J.; Wu, R. Regulation and interaction of multiple protein factors with the proximal promoter regions of a rice high pl α-amylase gene. MGG Mol. Gen. Genet. 1992, 232, 383–393. [Google Scholar] [CrossRef]
- Pastuglia, M.; Roby, D.; Dumas, C.; Cock, J.M. Rapid induction by wounding and bacterial infection of an S gene family receptor-like kinase gene in Brassica oleracea. Plant Cell 1997, 9, 49–60. [Google Scholar] [CrossRef]
- Simpson, S.D.; Nakashima, K.; Narusaka, Y.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 2003, 33, 259–270. [Google Scholar] [CrossRef]
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 2003, 15, 1591–1604. [Google Scholar] [CrossRef]
- Li, Z.X.; Li, S.G.; Zhang, L.F.; Han, S.Y.; Li, W.F.; Xu, H.Y.; Yang, W.H.; Liu, Y.L.; Fan, Y.R.; Qi, L.W. Over-expression of miR166a inhibits cotyledon formation in somatic embryos and promotes lateral root development in seedlings of Larix leptolepis. Plant Cell. Tissue Organ Cult. 2016, 127, 461–473. [Google Scholar] [CrossRef]
- Xi, D.; Chen, X.; Wang, Y.; Zhong, R.; He, J.; Shen, J.; Ming, F. Arabidopsis ANAC092 regulates auxin-mediated root development by binding to the ARF8 and PIN4 promoters. J. Integr. Plant Biol. 2019, 61, 1015–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauclair, L.; Yu, A.; Bouché, N. MicroRNA-directed cleavage and translational repression of the copper chaperone for superoxide dismutase mRNA in Arabidopsis. Plant J. 2010, 62, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Shazwan, S.; Muhammad Aliff, M.; Asral Wirda, A.A.; Hayati, A.R.; Maizatul Azma, M.; Nur Syahrina, A.R.; Nazefah, A.H.; Jameela, S.; Nur Fariha, M.M. Microrna expression in antiphospholipid syndrome: A systematic review and microrna target genes analysis. Malays. J. Pathol. 2016, 38, 273–283. [Google Scholar]
- Liebsch, D.; Palatnik, J.F. MicroRNA miR396, GRF transcription factors and GIF co-regulators: A conserved plant growth regulatory module with potential for breeding and biotechnology. Curr. Opin. Plant Biol. 2020, 53, 31–42. [Google Scholar] [CrossRef]
- Dalmadi, Á.; Miloro, F.; Bálint, J.; Várallyay, É.; Havelda, Z. Controlled RISC loading efficiency of miR168 defined by miRNA duplex structure adjusts ARGONAUTE1 homeostasis. Nucleic Acids Res. 2021, 49, 12912–12928. [Google Scholar] [CrossRef] [PubMed]
- Shriram, V.; Kumar, V.; Devarumath, R.M.; Khare, T.S.; Wani, S.H. Micrornas as potential targets for abiotic stress tolerance in plants. Front. Plant Sci. 2016, 7, 817. [Google Scholar] [CrossRef]
- Sabbione, A.; Daurelio, L.; Vegetti, A.; Talón, M.; Tadeo, F.; Dotto, M. Genome-wide analysis of AGO, DCL and RDR gene families reveals RNA-directed DNA methylation is involved in fruit abscission in Citrus sinensis. BMC Plant Biol. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Dutta, T.; Neelapu, N.R.R.; Wani, S.H.; Surekha, C. Salt stress tolerance and small RNA. In Plant Small RNA; Elsevier: Amsterdam, The Netherlands, 2020; pp. 191–207. [Google Scholar]
- Sunkar, R.; Li, Y.F.; Jagadeeswaran, G. Functions of microRNAs in plant stress responses. Trends Plant Sci. 2012, 17, 196–203. [Google Scholar] [CrossRef]
- Windels, D.; Bielewicz, D.; Ebneter, M.; Jarmolowski, A.; Szweykowska-Kulinska, Z.; Vazquez, F. miR393 is required for production of proper Auxin signalling outputs. PLoS ONE 2014, 9, e95972. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, Z.; Song, G.; Yao, H.; Han, L. Antioxidant enzyme activity and microRNA are associated with growth of Poa pratensis callus under salt stress. Plant Biotechnol. Rep. 2020, 14, 429–438. [Google Scholar] [CrossRef]
- Makkar, H.; Arora, S.; Khuman, A.K.; Chaudhary, B. Target-Mimicry-Based miR167 Diminution Confers Salt-Stress Tolerance During In Vitro Organogenesis of Tobacco (Nicotiana tabacum L.). J. Plant Growth Regul. 2022, 41, 1462–1480. [Google Scholar] [CrossRef]
- Hobecker, K.V.; Reynoso, M.A.; Bustos-Sanmamed, P.; Wen, J.; Mysore, K.S.; Crespi, M.; Blanco, F.A.; Zanetti, M.E. The microRNA390/TAS3 pathway mediates symbiotic nodulation and lateral root growth. Plant Physiol. 2017, 174, 2469–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, D.; Chun, H.J.; Kang, S.; Shin, G.; Park, S.J.; Hong, H.; Kim, C.; Kim, D.H.; Lee, S.Y.; Kim, M.C.; et al. A role for arabidopsis miR399f in salt, drought, and ABA signaling. Mol. Cells 2016, 39, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.G.; Shan, J.X.; Shi, M.; Gao, J.P.; Lin, H.X. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014, 80, 1108–1117. [Google Scholar] [CrossRef]
- Li, W.; Wang, T.; Zhang, Y.; Li, Y. Overexpression of soybean miR172c confers tolerance to water deficit and salt stress, but increases ABA sensitivity in transgenic Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 175–194. [Google Scholar] [CrossRef]
- Long, R.; Li, M.; Li, X.; Gao, Y.; Zhang, T.; Sun, Y.; Kang, J.; Wang, T.; Cong, L.; Yang, Q. A Novel miRNA Sponge Form Efficiently Inhibits the Activity of miR393 and Enhances the Salt Tolerance and ABA Insensitivity in Arabidopsis thaliana. Plant Mol. Biol. Report. 2017, 35, 409–415. [Google Scholar] [CrossRef]
- Song, J.B.; Gao, S.; Sun, D.; Li, H.; Shu, X.X.; Yang, Z.M. MiR394 and LCR are involved in Arabidopsis salt and drought stress responses in an abscisic acid-dependent manner. BMC Plant Biol. 2013, 13, 210. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Yan, J.; Wang, K.; Luo, H.; Zhang, W. MiR319 mediated salt tolerance by ethylene. Plant Biotechnol. J. 2019, 17, 2370–2383. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Xi, L.; Huang, W.D.; Liang, J.; Chen, J.G. RACK1 is a negative regulator of ABA responses in arabidopsis. J. Exp. Bot. 2009, 60, 3819–3833. [Google Scholar] [CrossRef]
- Denver, J.B.; Ullah, H. miR393s regulate salt stress response pathway in Arabidopsis thaliana through scaffold protein RACK1A mediated ABA signaling pathways. Plant Signal. Behav. 2019, 14, 1600394. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Y.F.; Randlett, M.D.; Zhao, X.C.; Findell, J.L.; Kieber, J.J.; Schaller, G.E. Localization of the Raf-like Kinase CTR1 to the Endoplasmic Reticulum of Arabidopsis through Participation in Ethylene Receptor Signaling Complexes. J. Biol. Chem. 2003, 278, 34725–34732. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhou, L.; Chen, W.; Ye, N.; Xia, J.; Zhuang, C. Overexpression of a microRNA-targeted NAC transcription factor improves drought and salt tolerance in Rice via ABA-mediated pathways. Rice 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; He, Q.; Tang, S.; Wang, H.; Zhang, X.; Lv, M.; Liu, H.; Gao, Q.; Zhou, Y.; Wang, Q.; et al. The miR172/IDS1 signaling module confers salt tolerance through maintaining ROS homeostasis in cereal crops. New Phytol. 2021, 230, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, W.; Waheed, A.; Naveed, H.; Zeng, F. MicroRNAs Mediated Plant Responses to Salt Stress. Cells 2022, 11, 2806. https://doi.org/10.3390/cells11182806
Islam W, Waheed A, Naveed H, Zeng F. MicroRNAs Mediated Plant Responses to Salt Stress. Cells. 2022; 11(18):2806. https://doi.org/10.3390/cells11182806
Chicago/Turabian StyleIslam, Waqar, Abdul Waheed, Hassan Naveed, and Fanjiang Zeng. 2022. "MicroRNAs Mediated Plant Responses to Salt Stress" Cells 11, no. 18: 2806. https://doi.org/10.3390/cells11182806





