Combination Treatment of TRPV4 Agonist with Cisplatin Promotes Vessel Normalization in an Animal Model of Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Administration of 4NQO
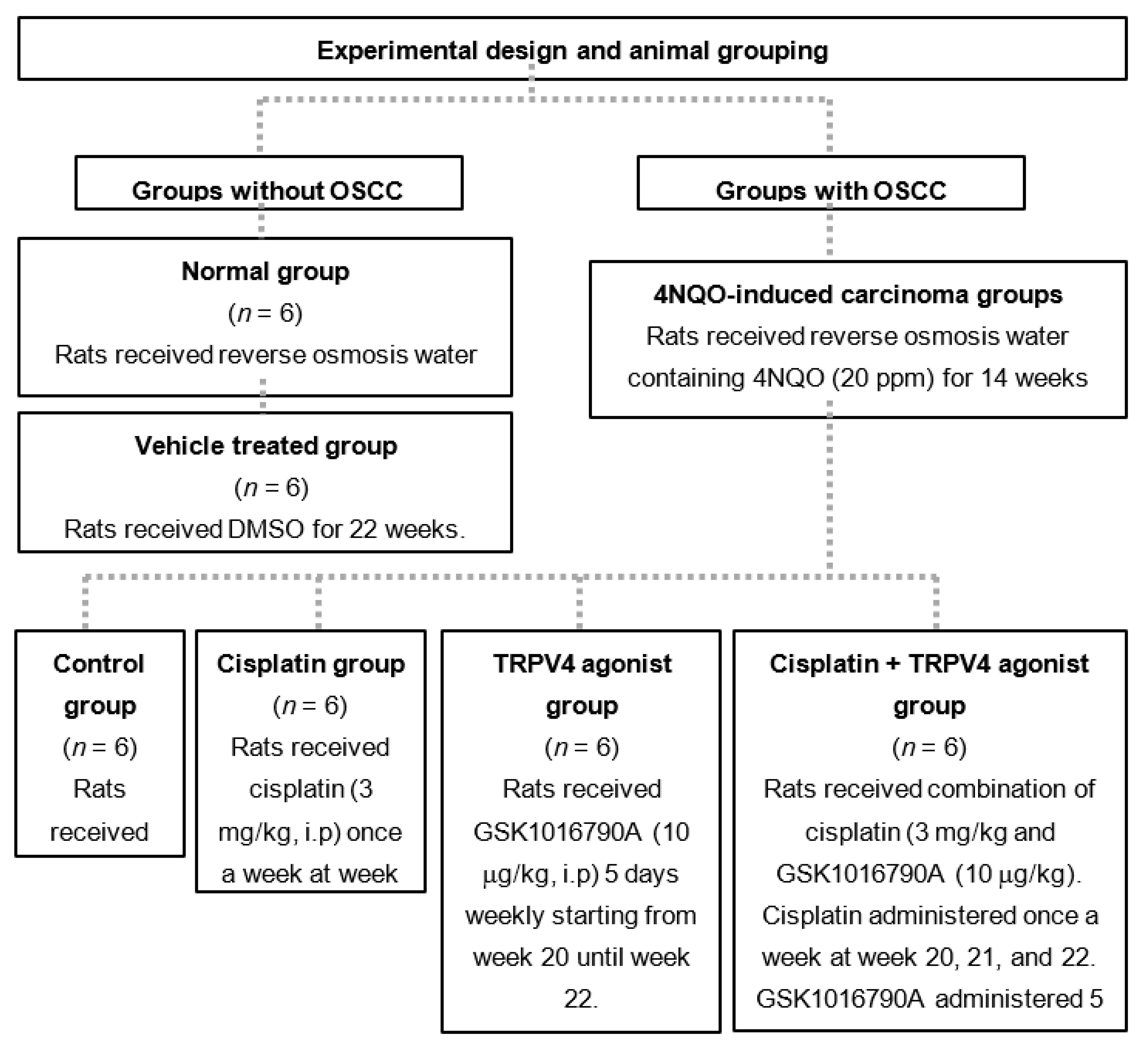
2.3. Experimental Design for Animal Grouping
2.4. Tumor Assessment and Histology
2.5. Administration of Cisplatin and GSK1016790A
2.6. RT2 Profiler Arrays
2.7. Single and Double Immunostaining
2.8. Immunoassay
2.9. Digital Histology Scoring
2.10. Microvessel Density and Microvessel-Pericyte-Coverage Index
2.11. Statistical Analysis
3. Results
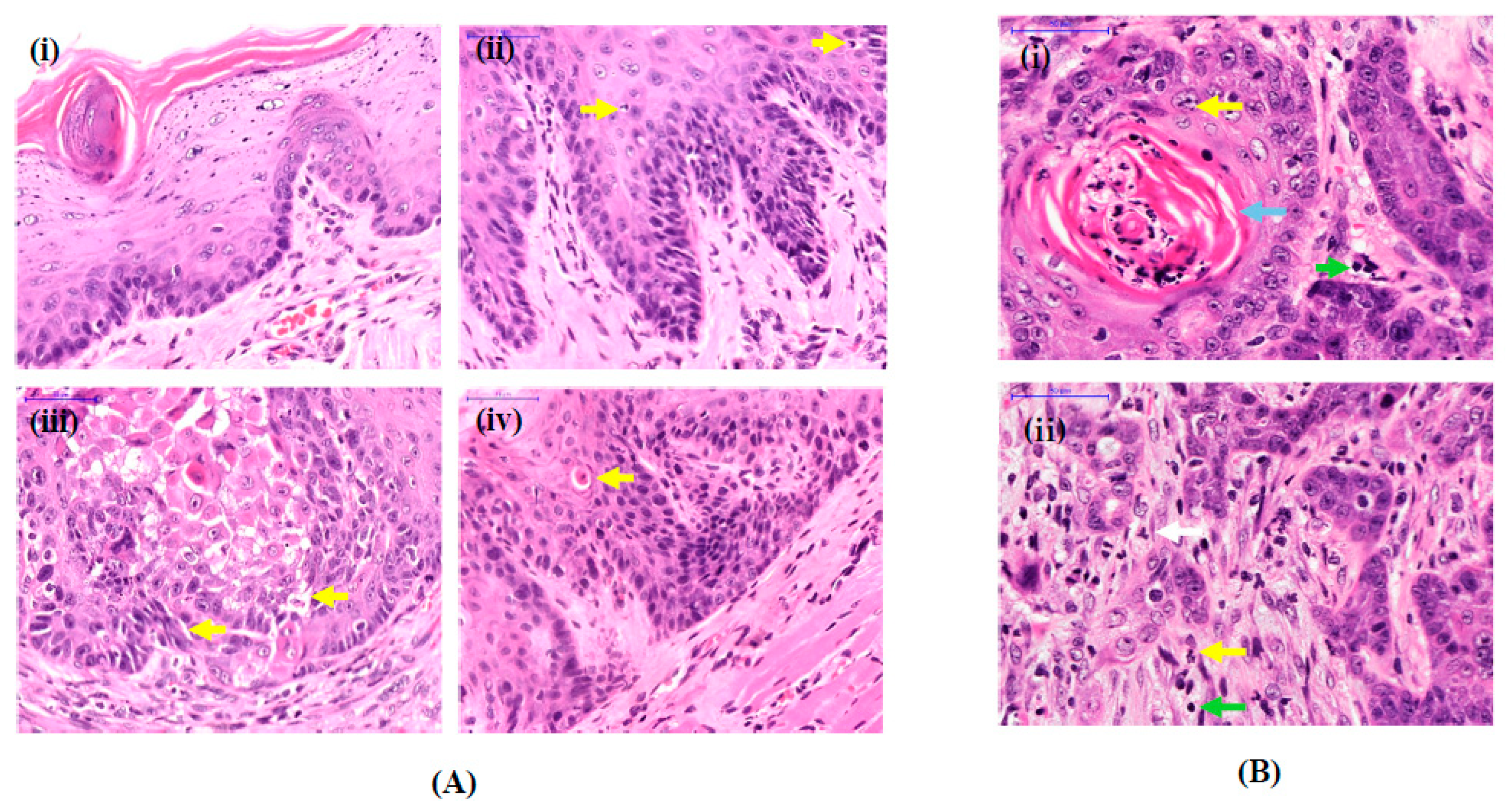
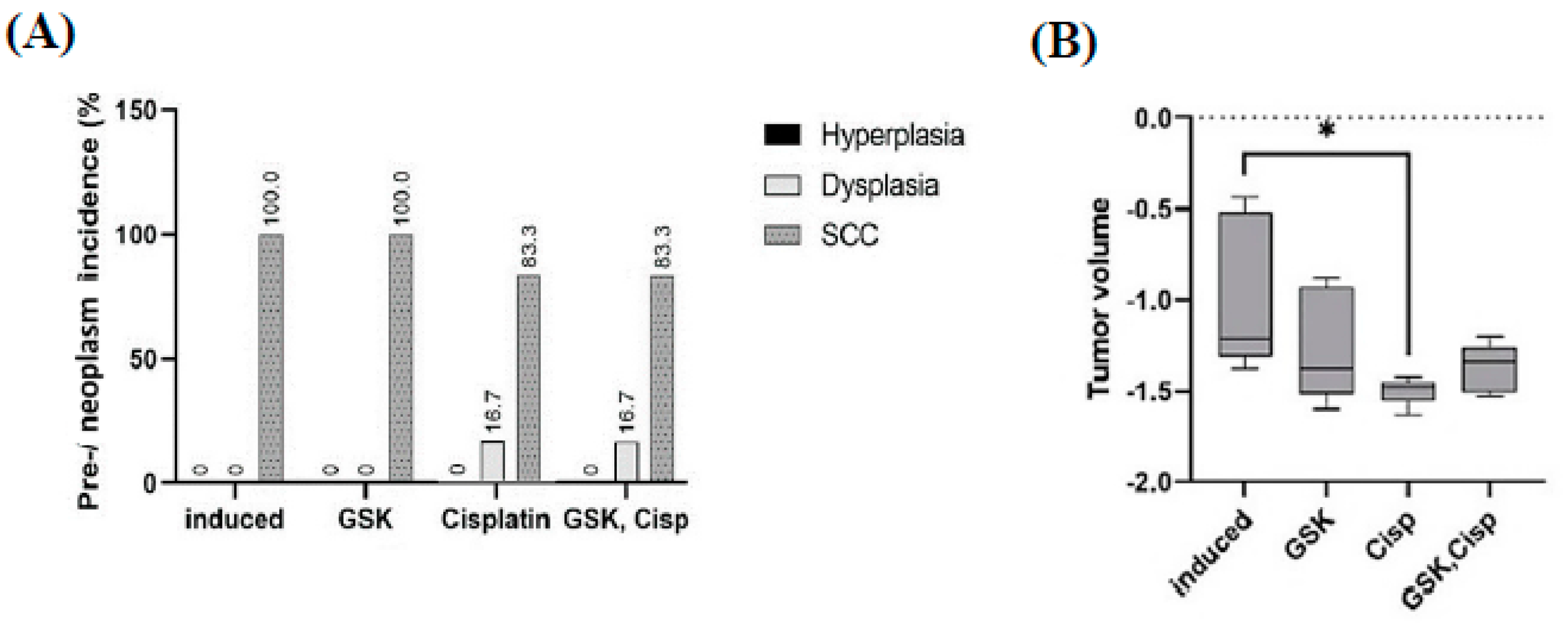
3.1. Histological Features of OSCC Lesions in Rats Induced by 4NQO
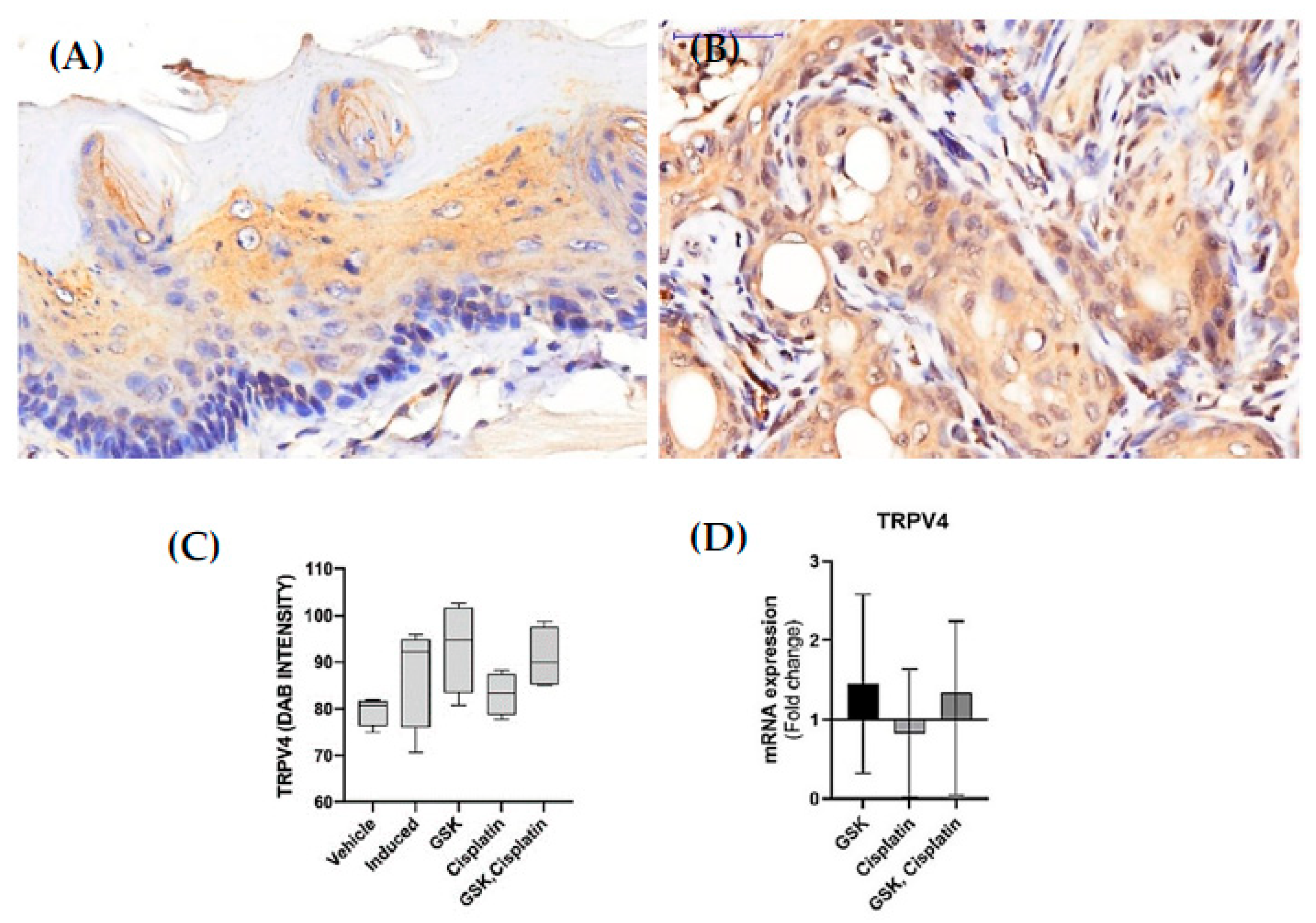
3.2. TRPV4 Expression in OSCC Lesions
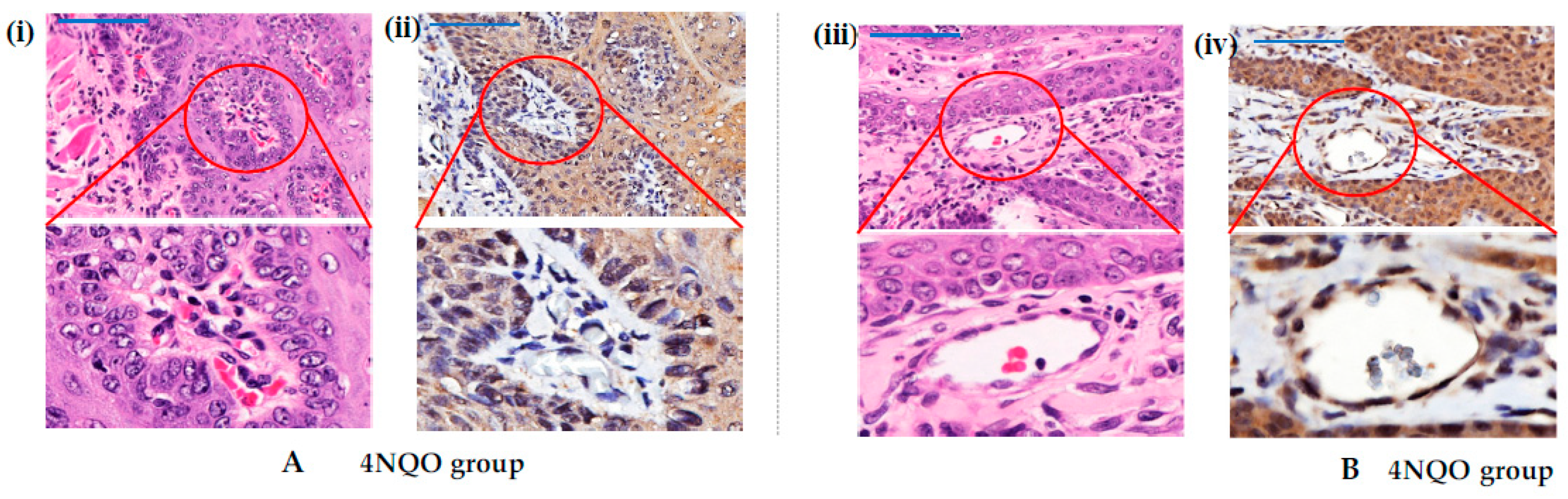
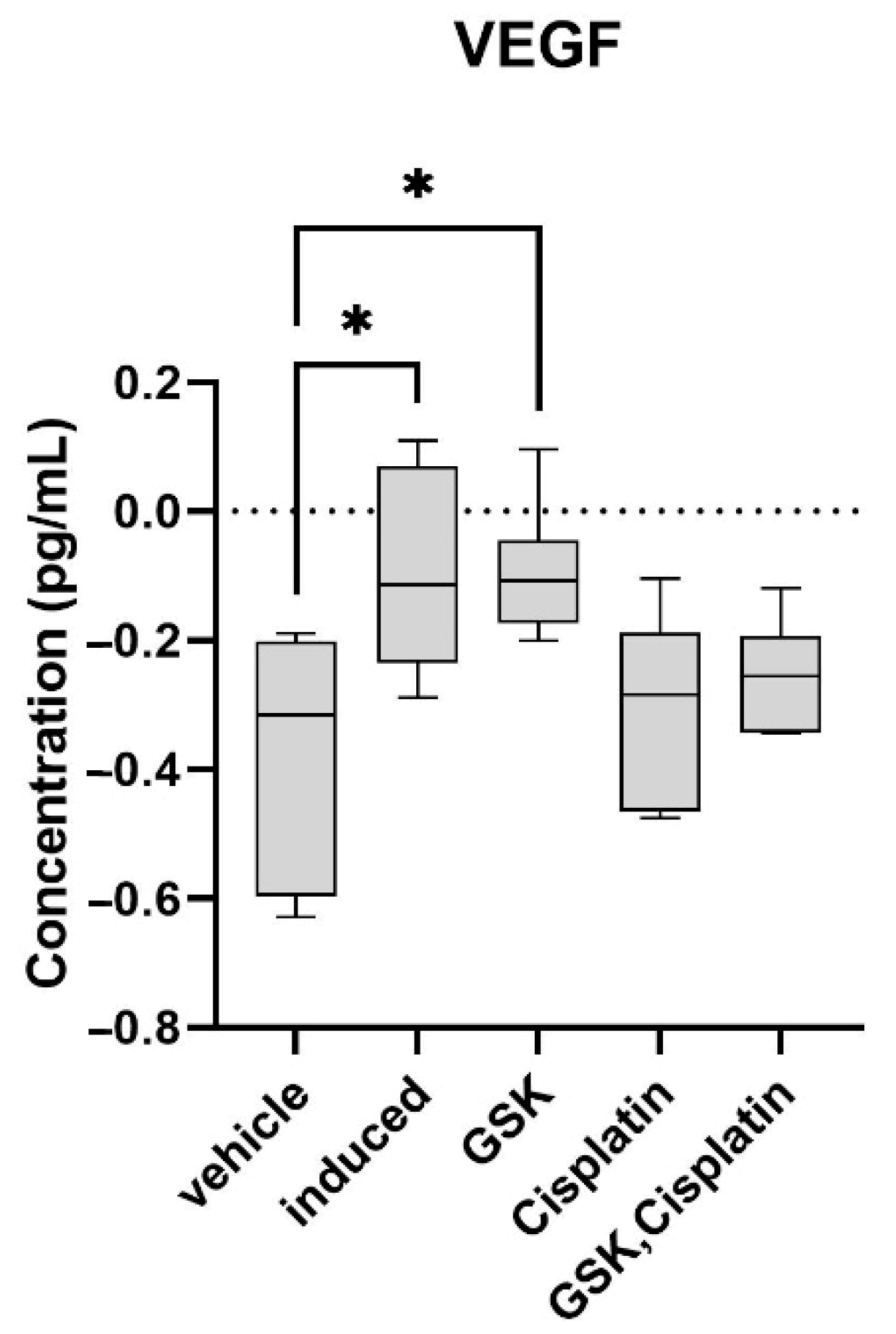
3.3. Vascular Endothelial Growth Factor (VEGF) in OSCC Lesions
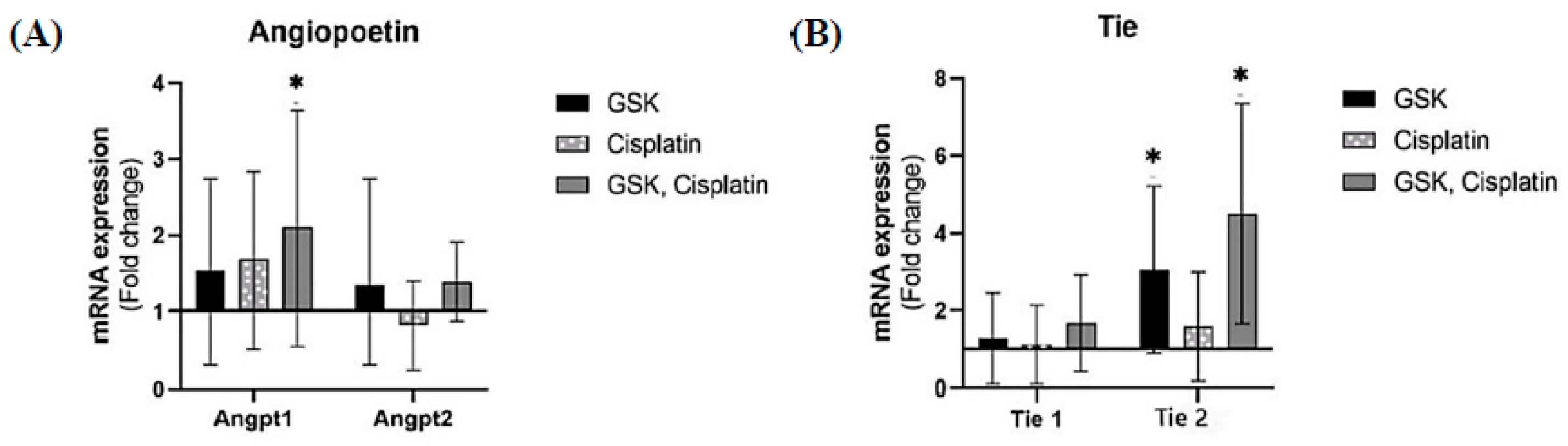
3.4. Angiopoietin/Tie Expression in OSCC Lesions
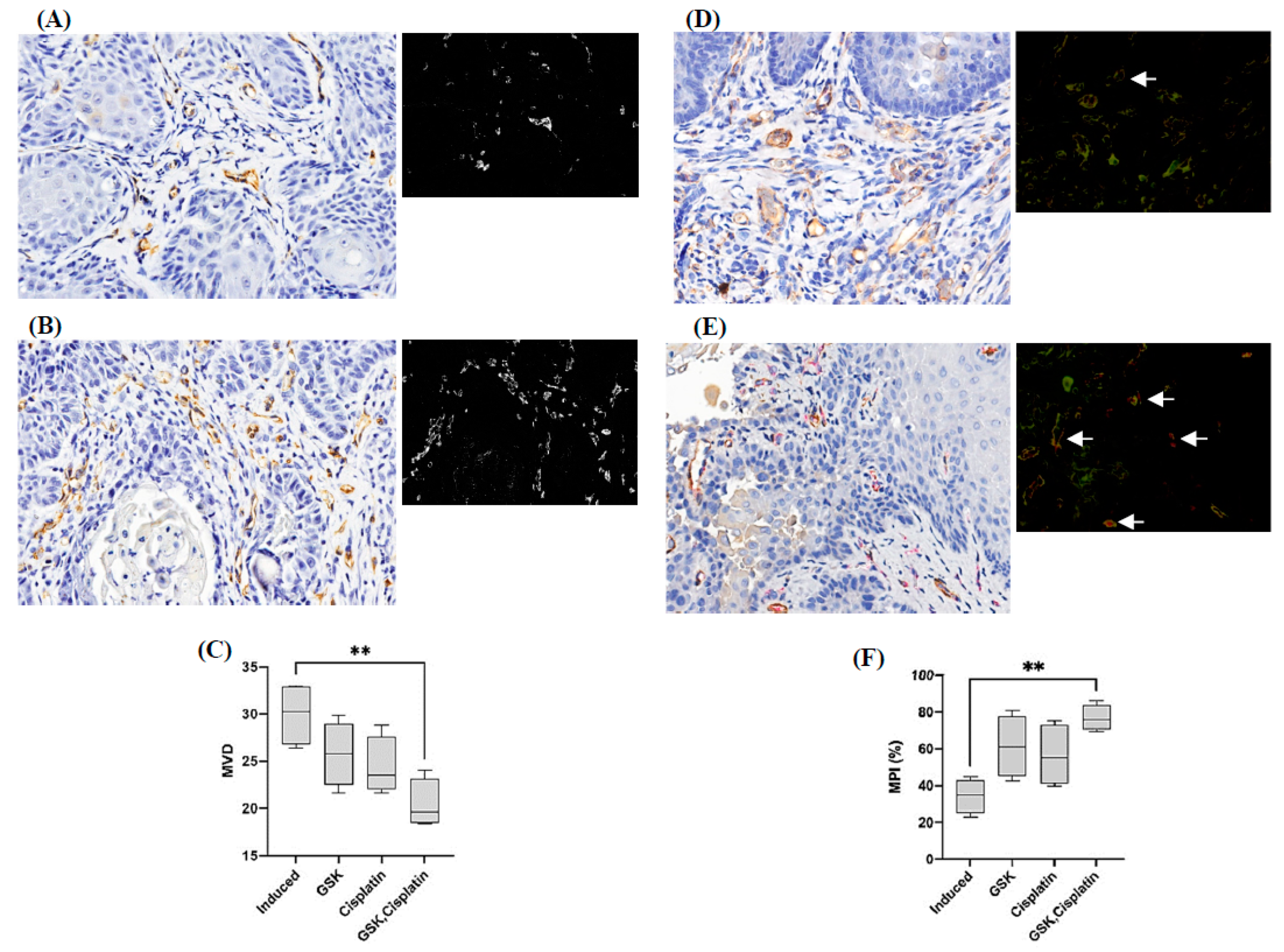
3.5. Microvessel Density and Microvessel-Pericyte-Coverage Index
3.6. Tumor Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omura, K. Current status of oral cancer treatment strategies: Surgical treatments for oral squamous cell carcinoma. Int. J. Clin. Oncol. 2014, 19, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 1884–1894. [Google Scholar]
- Bergers, G. The role of pericytes in blood-vessel formation and maintenance. Neuro. Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G. Pericytes, the mural cells of the microvascular system. In Angiogenesis: An Integrative Approach from Science to Medicine; Springer: Boston, MA, USA, 2008; pp. 45–53. [Google Scholar]
- Kitahara, S.; Matsui, A.; Yoshii, A.; Kuwahara, Y.; Nishio, M.; Saeki, K.; Ezaki, T. Endothelial cell transplantation in tumors restores normal vasculature, reduces tumor hypoxia, and suppresses tumor outgrowth. J. Oral Biosci. 2016, 58, 150–157. [Google Scholar] [CrossRef]
- Duda, D.G. Antiangiogenesis and drug delivery to tumors: Bench to bedside and back. Cancer Res. 2006, 66, 3967–3970. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.; Stylianopoulos, T.; Duda, D.G.; Fukumura, D.; Jain, R.K. Benefits of vascular normalization are dose and time dependent—Letter. Cancer Res. 2013, 73, 7144–7146. [Google Scholar] [CrossRef]
- Lee, W.H.; Choong, L.; Mon, N.N.; Lu, S.Y.; Lin, Q.; Pang, B.; Yan, B.; Krishna, V.S.R.; Singh, H.; Tan, T.Z.; et al. TRPV4 regulates breast cancer cell extravasation, stiffness and actin cortex. Sci. Rep. 2016, 6, 27903. [Google Scholar] [CrossRef]
- Washiya, K.; Sasaki, R.; Nakanishi, Y.; Kitabatake, K.; Nishino, K.; Tanuma, S.; Kojima, S.; Tsukimoto, M. Involvement of TRPV1 and TRPV4 Channels in Enhancement of Metastatic Ability Induced by γ-Irradiation in Human Lung Cancer A549 Cells. BPB Rep. 2020, 3, 50–55. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Xie, C.; Sham, K.W.Y.; Ng, S.S.M.; Chen, Y.; Cheng, C.H.K. Activation of PTEN by inhibition of TRPV4 suppresses colon cancer development. Cell Death Dis. 2019, 10, 460. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, Z.; Zhou, J.; Jia, Y.; He, X.; Zhou, L.; Dong, Y.; Fan, Y.; Yang, X.; et al. Calcium and TRPV4 promote metastasis by regulating cytoskeleton through the RhoA/ROCK1 pathway in endometrial cancer. Cell Death Dis. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Tang, B.; Wu, J.; Zhu, M.X.; Sun, X.; Liu, J.; Xie, R.; Dong, T.X.; Xiao, Y.; Carethers, J.M.; Yang, S.; et al. VPAC1 couples with TRPV4 channel to promote calcium-dependent gastric cancer progression via a novel autocrine mechanism. Oncogene 2019, 38, 3946–3961. [Google Scholar] [CrossRef] [PubMed]
- Oghumu, S.; Casto, B.C.; Ahn-Jarvis, J.; Weghorst, L.C.; Maloney, J.; Geuy, P.; Horvath, K.Z.; Bollinger, C.E.; Warner, B.M.; Summersgill, K.F.; et al. Inhibition of pro-inflammatory and anti-apoptotic biomarkers during experimental oral cancer chemoprevention by dietary black raspberries. Front. Immunol. 2017, 8, 1325. [Google Scholar] [CrossRef] [PubMed]
- Arifin, W.N.; Zahiruddin, W.M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [PubMed]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef]
- Wali, R.K.; Kunte, D.P.; De La Cruz, M.; Tiwari, A.K.; Brasky, J.; Weber, C.R.; Wali, R.K.; Kunte, D.P.; De La Cruz, M.; Tiwari, A.K.; et al. Topical Polyethylene Glycol as a Novel Chemopreventive Agent for Oral Cancer via Targeting of Epidermal Growth Factor Response. PLoS ONE 2012, 7, e38047. [Google Scholar] [CrossRef]
- Adapala, R.K.; Thoppil, R.J.; Ghosh, K.; Cappelli, H.C.; Dudley, A.C.; Paruchuri, S.; Keshamouni, V.; Klagsbrun, M.; Meszaros, J.G.; Chilian, W.M.; et al. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene 2016, 35, 314–322. [Google Scholar] [CrossRef]
- Fuhrich, D.; Lessey, B.; Savaris, R. Comparison of HSCORE assessment of endometrial β3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal. Quant. Cytopathol. Histopathol. 2013, 35, 210–216. [Google Scholar]
- Yonenaga, Y.; Mori, A.; Onodera, H.; Yasuda, S. Absence of Smooth Muscle Actin-Positive Pericyte Coverage of Tumor Vessels Correlates with Hematogenous Metastasis and Prognosis of Colorectal Cancer Patients. Oncology 2005, 69, 159–166. [Google Scholar] [CrossRef]
- Thorneloe, K.S.; Sulpizio, A.C.; Lin, Z.; Figueroa, D.J.; Clouse, A.K.; McCafferty, G.P.; Chendrimada, T.P.; Lashinger, E.S.R.; Gordon, E.; Evans, L.; et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2- carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I [S]. J. Pharmacol. Exp. Ther. 2008, 326, 432–442. [Google Scholar]
- Fujii, S.; Tajiri, Y.; Hasegawa, K.; Matsumoto, S.; Yoshimoto, R.U.; Wada, H.; Shosei Kishida, S.; Kido, M.A.; Yoshikawa, H.; Satoru Ozeki, S.; et al. The TRPV4-AKT axis promotes oral squamous cell carcinoma cell proliferation via CaMKII activation. Lab. Investig. 2020, 100, 311–323. [Google Scholar] [CrossRef]
- Sakakibara, A.; Sakakibara, S.; Kusumoto, J.; Takeda, D.; Hasegawa, T.; Akashi, M.; Minamikawa, T.; Hashikawa, K.; Terashi, H.; Komori, T. Upregulated Expression of Transient Receptor Potential Cation Channel Subfamily V Receptors in Mucosae of Patients with Oral Squamous Cell Carcinoma and Patients with a History of Alcohol Consumption or Smoking. PLoS ONE 2017, 12, e0169723. [Google Scholar]
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; Wang, Q.; Baffert, F.; Rudge, J.; Papadopoulos, N.; Jean-Guillaume, D.; Wiegand, S.; Yancopoulos, G.D.; McDonald, D.M. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development 2005, 132, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; Rudge, J.S.; Ioffe, E.; Zhou, H.; Ross, L.; Croll, S.D.; Glazer, N.; Holash, J.; McDonald, D.M.; Yancopoulos, G.D. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 2000, 6, 460–463. [Google Scholar] [CrossRef]
- Hawighorst, T.; Skobe, M.; Streit, M.; Hong, Y.K.; Velasco, P.; Brown, L.F.; Riccardi, L.; Lange-Asschenfeldt, B.; Detmar, M. Activation of the Tie2 receptor by angiopoietin-1 enhances tumor vessel maturation and impairs sauamous cell carcinoma growth. Am. J. Pathol. 2002, 160, 1381–1392. [Google Scholar] [CrossRef]
- Thurston, G.; Daly, C. The complex role of angiopoietin-2 in the angiopoietin-Tie signaling pathway. Cold Spring Harb. Perspect. Med. 2012, 2, a006650. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef]
- Hanahan, D. Signaling vascular morphogenesis and maintenance. Science 1997, 277, 48–50. [Google Scholar] [CrossRef]
- Holash, J.; Wiegand, S.J.; Yancopoulos, G.D. New model of tumor angiogenesis: Dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 1999, 18, 5356–5362. [Google Scholar] [CrossRef]
- Brown, J.L.; Cao, Z.A.; Pinzon-Ortiz, M.; Kendrew, J.; Reimer, C.; Wen, S.; Zhou, J.Q.; Tabrizi, M.; Emery, S.; McDermott, B.; et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol. Cancer Ther. 2010, 9, 145–156. [Google Scholar] [CrossRef]
- Falcón, B.L.; Hashizume, H.; Koumoutsakos, P.; Chou, J.; Bready, J.V.; Coxon, A.; Oliner, J.D.; McDonald, D.M. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am. J. Pathol. 2009, 175, 2159–2170. [Google Scholar] [CrossRef]
- Hashizume, H.; Falcón, B.L.; Kuroda, T.; Baluk, P.; Coxon, A.; Yu, D.; Bready, J.V.; Oliner, J.D.; McDonald, D.M. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010, 70, 2213–2223. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Miettinen, J.; Wirkkala, R.; Anisimov, A.; Winderlich, M.; Nottebaum, A.; Vestweber, D.; Deutsch, U.; Young Koh, G.; et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol. 2008, 10, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, A.; Kahlert, S.; Goede, V.; Hemmerlein, B.; Plate, K.H.; Augustin, H.G. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: Implications for antiangiogenic tumor therapies. Cancer Res. 2000, 60, 1388–1393. [Google Scholar] [PubMed]
- Welén, K.; Jennbacken, K.; Tešan, T.; Damber, J.E. Pericyte coverage decreases invasion of tumour cells into blood vessels in prostate cancer xenografts. Prostate Cancer Prostatic Dis. 2009, 12, 41–46. [Google Scholar] [CrossRef]
- Xian, X.; Håkansson, J.; Ståhlberg, A.; Lindblom, P.; Betsholtz, C.; Gerhardt, H.; Semb, H. Pericytes limit tumor cell metastasis. J. Clin. Investig. 2006, 116, 642–651. [Google Scholar] [CrossRef]
- Mcdonald, D.M. Angiogenesis and Vascular Remodeling in Inflammation and Cancer: Biology and Architecture of the Vasculature. In Angiogenesis: An Integrative Approach from Science to Medicine; Springer: Boston, MA, USA, 2008; pp. 33–49. [Google Scholar]
- Rapidis, A.D.; Gullane, P.; Langdon, J.D.; Lefebvre, J.L.; Scully, C.; Shah, J.P. Major advances in the knowledge and understanding of the epidemiology, aetiopathogenesis, diagnosis, management and prognosis of oral cancer. Oral Oncol. 2009, 45, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Mazeron, R.; Tao, Y.; Lusinchi, A.; Bourhis, J. Current concepts of management in radiotherapy for head and neck squamous-cell cancer. Oral Oncol. 2009, 45, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Lemmer, J. Oral Squamous Cell Carcinoma: Epidemiology, Clinical Presentation and Treatment. J. Cancer Ther. 2012, 3, 263–268. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Lorch, J.H.; Posner, M.R.; Wirth, L.J.; Haddad, R.I. Seeking alternative biological therapies: The future of targeted molecular treatment. Oral Oncol. 2009, 45, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.K.; Ku, M.; Yang, J. Beyond EGFR inhibition: Multilateral combat strategies to stop the progression of head and neck cancer. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
| Symbols | Description | Refseq |
|---|---|---|
| Ang1 | Angiopoietin 1 | NM_053546 |
| Ang2 | Angiopoietin 2 | NM_134454 |
| Tie1 | Tyrosine kinase with immunoglobulin-like and EGF-like domains 1 | NM_053545 |
| TRPV4 | Transient receptor potential cation channel, subfamily vanilloid, member 4 | NM_023970 |
| Tek/Tie2 | TEK tyrosine kinase, endothelial | NM_001105737 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | NM_017008 |
| 18SrRNA | Rat 18’s rRNA sequence | X01117 |
| Actb | Beta-actin | NM_031144 |
| Antibody | Dilution Ratio | Incubation Time (Minutes) | Company |
|---|---|---|---|
| TRPV4 (NB110-74960) | 1:3500 | 30 | Novus Biologicals, Littleton, CO, USA |
| CD 31 [EPR17259] (ab182981) | 1:2000 | 30 | Abcam, Cambridge, UK |
| Alpha smooth muscle actin (α-SMA) [EPR5368] (ab124964) | 1:2000 | 30 | Abcam, Cambridge, UK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahya, F.; Mohd Bakri, M.; Hossain, M.Z.; Syed Abdul Rahman, S.N.; Mohammed Alabsi, A.; Ramanathan, A. Combination Treatment of TRPV4 Agonist with Cisplatin Promotes Vessel Normalization in an Animal Model of Oral Squamous Cell Carcinoma. Medicina 2022, 58, 1229. https://doi.org/10.3390/medicina58091229
Yahya F, Mohd Bakri M, Hossain MZ, Syed Abdul Rahman SN, Mohammed Alabsi A, Ramanathan A. Combination Treatment of TRPV4 Agonist with Cisplatin Promotes Vessel Normalization in an Animal Model of Oral Squamous Cell Carcinoma. Medicina. 2022; 58(9):1229. https://doi.org/10.3390/medicina58091229
Chicago/Turabian StyleYahya, Farhana, Marina Mohd Bakri, Mohammad Zakir Hossain, Syarifah Nur Syed Abdul Rahman, Aied Mohammed Alabsi, and Anand Ramanathan. 2022. "Combination Treatment of TRPV4 Agonist with Cisplatin Promotes Vessel Normalization in an Animal Model of Oral Squamous Cell Carcinoma" Medicina 58, no. 9: 1229. https://doi.org/10.3390/medicina58091229
APA StyleYahya, F., Mohd Bakri, M., Hossain, M. Z., Syed Abdul Rahman, S. N., Mohammed Alabsi, A., & Ramanathan, A. (2022). Combination Treatment of TRPV4 Agonist with Cisplatin Promotes Vessel Normalization in an Animal Model of Oral Squamous Cell Carcinoma. Medicina, 58(9), 1229. https://doi.org/10.3390/medicina58091229







