Investigating the Efficacy of Tasmannia lanceolata Extract in Inactivating Fungi and Prolonging the Shelf Life of Date Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extract
2.2. Preparation of Fungal Spore Suspensions
2.3. Disc Diffusion Assay
2.4. Serial/Micro Dilution Assay
2.5. Treatment of Date Fruits (In Vivo)
2.6. Statistical Analysis
3. Results and Discussion
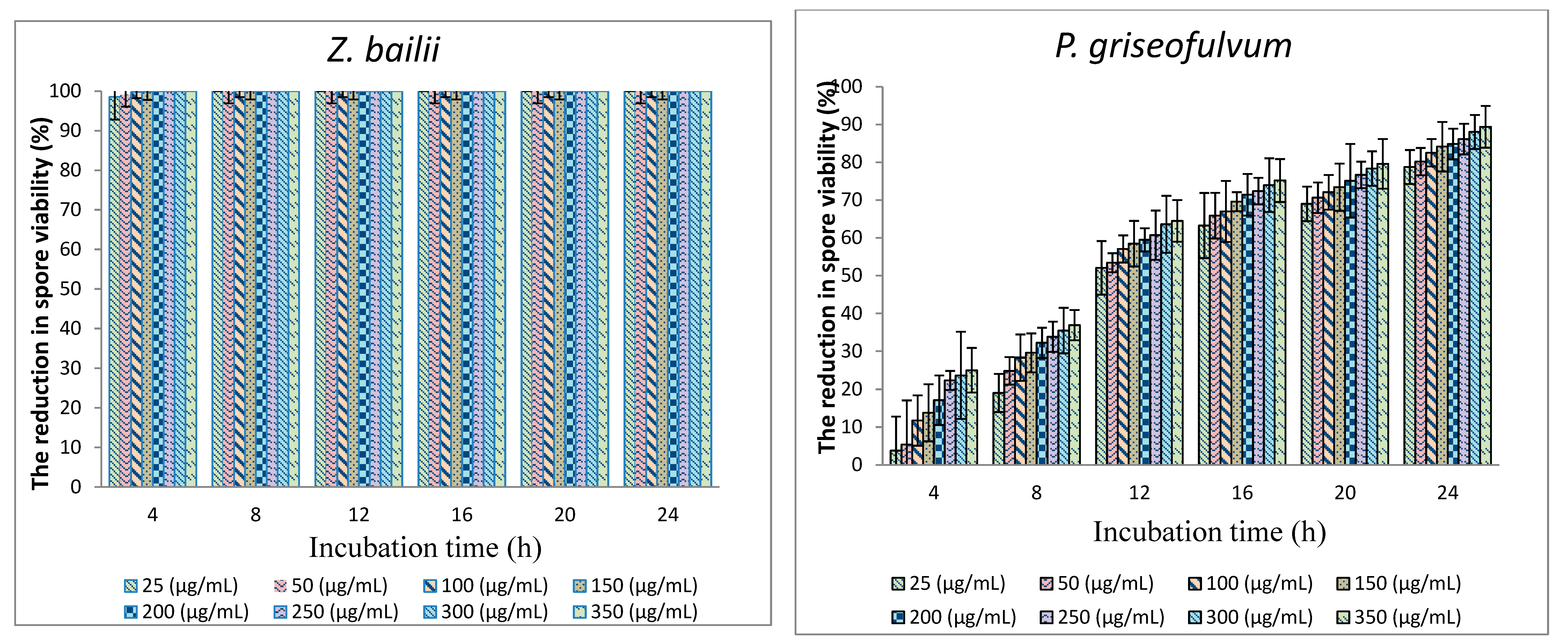
3.1. Antifungal Activity of TPL Extract (In Vitro)
3.2. Shelf Life Extension of Fresh Dates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarraf, M.; Jemni, M.; Kahramanoğlu, I.; Artés, F.; Shahkoomahally, S.; Namsi, A.; Ihtisham, M.; Brestic, M.; Mohammadi, M.; Rastogi, A. Commercial techniques for preserving date palm (Phoenix dactylifera) fruit quality and safety: A review. Saudi J. Biol. Sci. 2021, 28, 4408–4420. [Google Scholar] [CrossRef]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef]
- Atia, M. Efficiency of physical treatments and essential oils in controlling fungi associated with some stored date palm fruits. Aust. J. Basic Appl. Sci. 2011, 5, 1572. [Google Scholar]
- Al Hazzani, A.A.; Shehata, A.I.; Rizwana, H.; Moubayed, N.M.; Alshatwi, A.A.; Munshi, A.; Elgaaly, G. Postharvest fruit spoilage bacteria and fungi associated with date palm (Phoenix dactylifera L) from Saudi Arabia. Afr. J. Microbiol. Res. 2014, 8, 1228–1236. [Google Scholar]
- Anjili, S.; Channya, F.; Chimbekujwo, I. Fungi Associated with Post-harvest Spoilage of Date Palm (Phoenix dactylifera L.) in Yola, Adamawa State. Int. J. Res. Agric. For. 2015, 2, 14–22. [Google Scholar]
- Gherbawy, Y.A.; Elhariry, H.M.; Bahobial, A.A.S. Mycobiota and Mycotoxins (Aflatoxins and Ochratoxin) Associated with Some Saudi Date Palm Fruits. Foodborne Pathog. Dis. 2012, 9, 561–567. [Google Scholar] [CrossRef]
- Sultanbawa, Y. Food Preservation and the Antimicrobial Activity of Australian Native Plants. In Australian Native Plants: Cultivation and Uses in the Health and Food Industries; CRC Press: Boca Raton, FL, USA, 2016; pp. 251–263. [Google Scholar]
- Zhao, J.; Agboola, S.O. Functional Properties of Australian Bushfoods: A Report for the Rural Industries Research and Development Corporation; Rural Industries Research and Development Corporation: Barton, ACT, Australia, 2007.
- Sultanbawa, Y. Tasmanian pepper leaf (Tasmannia lanceolata) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 819–823. [Google Scholar]
- Gonçalves, G.E.G.; Morais, T.R.; Lago, J.H.G.; Caseli, L. Incorporation of polygodial in Langmuir films of selected lipids. Thin Solid Films 2019, 669, 19–28. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.R.; Lunde, C.S.; Kubo, I. In vitro antifungal susceptibilities of Candida albicans and other fungal pathogens to polygodial, a sesquiterpene dialdehyde. Planta Med. 1999, 65, 204–208. [Google Scholar] [CrossRef]
- Himejima, M.; Kubo, I. Fungicidal activity of polygodial in combination with anethole and indole against candida-albicans. J. Agric. Food Chem. 1993, 41, 1776–1779. [Google Scholar] [CrossRef]
- Kubo, I.; Lee, S.H.; Shimizu, K. Combination effect of miconazole with polygodial against Candida albicans. Open J. Med. Microbiol. 2011, 1, 7. [Google Scholar] [CrossRef]
- Lunde, C.S.; Kubo, I. Effect of polygodial on the mitochondrial ATPase of Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2000, 44, 1943–1953. [Google Scholar] [CrossRef]
- Yano, Y.; Taniguchi, M.; Tada, E.; Tanaka, T.; Oi, S.; Haraguchi, H.; Hashimoto, K.; Kubo, I. Potentiation by polygodial of the respiration inhibiting activity of maesanin in candida-utilis. Agric. Biol. Chem. 1989, 53, 1525–1530. [Google Scholar] [CrossRef]
- Zacchino, S.; Santecchia, C.; López, S.; Gattuso, S.; Muñoz, J.D.D.; Cruañes, A.; Vivot, E.; Cruañes, M.D.C.; Salinas, A.; De Ruiz, R. In vitro antifungal evaluation and studies on mode of action of eight selected species from the Argentine flora. Phytomedicine 1998, 5, 389–395. [Google Scholar] [CrossRef]
- Fujita, K.; Kubo, I. Multifunctional action of antifungal polygodial against Saccharomyces cerevisiae: Involvement of pyrrole formation on cell surface in antifungal action. Bioorganic Med. Chem. 2005, 13, 6742–6747. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.I.; Lee, S.H. Antifungal mechanism of polygodial. J. Agric. Food Chem. 2001, 49, 1607–1611. [Google Scholar] [CrossRef]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Kubo, I.; Lee, S.H.; Ha, T.J. Effect of EDTA alone and in combination with polygodial on the growth of Saccharomyces cerevisiae. J. Agric. Food Chem. 2005, 53, 1818–1822. [Google Scholar] [CrossRef]
- Kubo, I.; Himejima, M. Potentiation of antifungal activity of sesquiterpene dialdehydes against Candida albicans and two other fungi. Experientia 1992, 48, 1162–1164. [Google Scholar] [CrossRef]
- Taniguchi, M.; Adachi, T.; Oi, S.; Kimura, A.; Katsumura, S.; Isoe, S.; Kubo, I. Structure-activity relationship of the Warburgia sesquiterpene dialdehydes. Agric. Biol. Chem. 1984, 48, 73–78. [Google Scholar] [CrossRef]
- Cock, I.E. The phytochemistry and chemotherapeutic potential of Tasmannia lanceolata (Tasmanian pepper): A review. Pharmacogn. Commun. 2013, 3, 13. [Google Scholar]
- Castelli, M.V.; Lodeyro, A.F.; Malheiros, A.; Zacchino, S.A.; Roveri, O.A. Inhibition of the mitochondrial ATP synthesis by polygodial, a naturally occurring dialdehyde unsaturated sesquiterpene. Biochem. Pharmacol. 2005, 70, 82–89. [Google Scholar] [CrossRef]
- Hsieh, P.-C.; Mau, J.-L.; Huang, S.-H. Antimicrobial effect of various combinations of plant extracts. Food Microbiol. 2001, 18, 35–43. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Sultanbawa, Y. Plant antimicrobials in food applications: Minireview. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 2, 1084–1099. [Google Scholar]
- Anke, H.; Sterner, O. Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms. Planta Med. 1991, 57, 344–346. [Google Scholar] [CrossRef] [PubMed]




| Degree of Fungal Infection | Date Fruits | Consideration of Infection | ||
|---|---|---|---|---|
| No mycelia growth |  |  |  | Not Infected |
| Slight mycelia growth |  |  |  | Infected |
| Moderate mycelia growth |  |  |  |
Infected |
| Severe mycelia growth |  |  |  | Infected |
| Polygodial (µg/mL) | ||
|---|---|---|
| Fungi | MIC | MFC90 |
| A. niger | <25 | <25 |
| A. flavus | <25 | <25 |
| P. griseofulvum | >350 | 350 |
| P. chrysogenum | <25 | <25 |
| F. oxysporum | <25 | <25 |
| C. albicans | <25 | <25 |
| Z. bailii | <25 | <25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asmari, F.; Akter, S.; Mereddy, R.; Sultanbawa, Y. Investigating the Efficacy of Tasmannia lanceolata Extract in Inactivating Fungi and Prolonging the Shelf Life of Date Fruit. Foods 2022, 11, 2631. https://doi.org/10.3390/foods11172631
Al-Asmari F, Akter S, Mereddy R, Sultanbawa Y. Investigating the Efficacy of Tasmannia lanceolata Extract in Inactivating Fungi and Prolonging the Shelf Life of Date Fruit. Foods. 2022; 11(17):2631. https://doi.org/10.3390/foods11172631
Chicago/Turabian StyleAl-Asmari, Fahad, Saleha Akter, Ram Mereddy, and Yasmina Sultanbawa. 2022. "Investigating the Efficacy of Tasmannia lanceolata Extract in Inactivating Fungi and Prolonging the Shelf Life of Date Fruit" Foods 11, no. 17: 2631. https://doi.org/10.3390/foods11172631
APA StyleAl-Asmari, F., Akter, S., Mereddy, R., & Sultanbawa, Y. (2022). Investigating the Efficacy of Tasmannia lanceolata Extract in Inactivating Fungi and Prolonging the Shelf Life of Date Fruit. Foods, 11(17), 2631. https://doi.org/10.3390/foods11172631









