Electrophysiological Features to Aid in the Construction of Predictive Models of Human–Agent Collaboration in Smart Environments
Abstract
1. Introduction
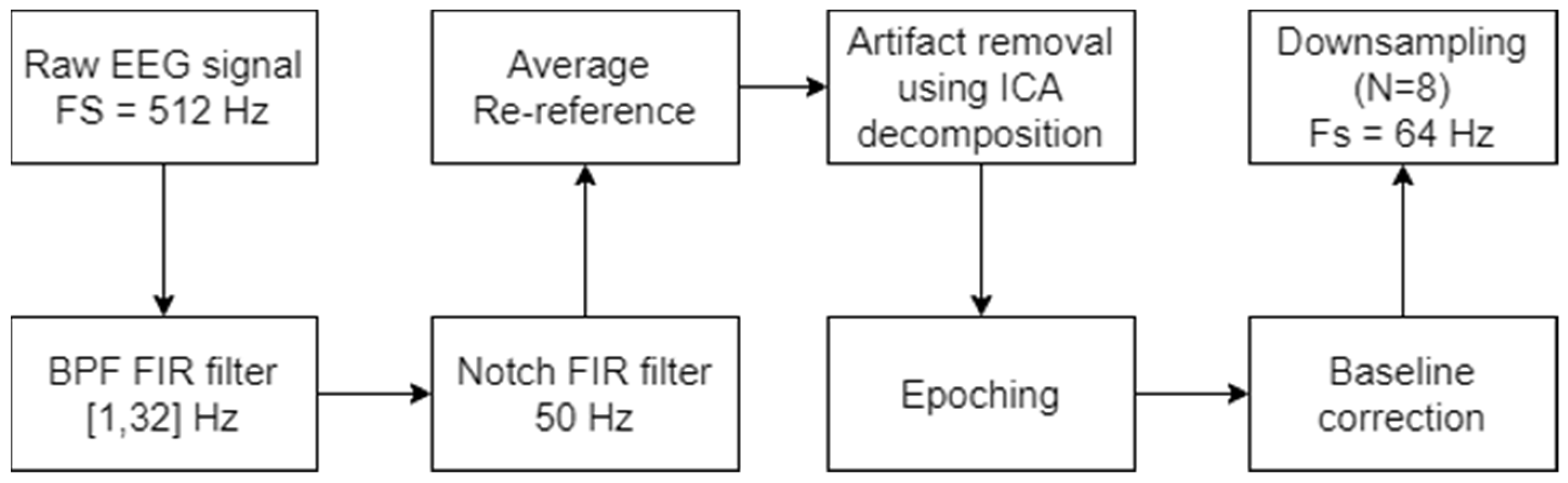
2. Materials and Methods
3. Results
3.1. EEG Frequency Bands’ Interactions as Function of the Experimental Condition
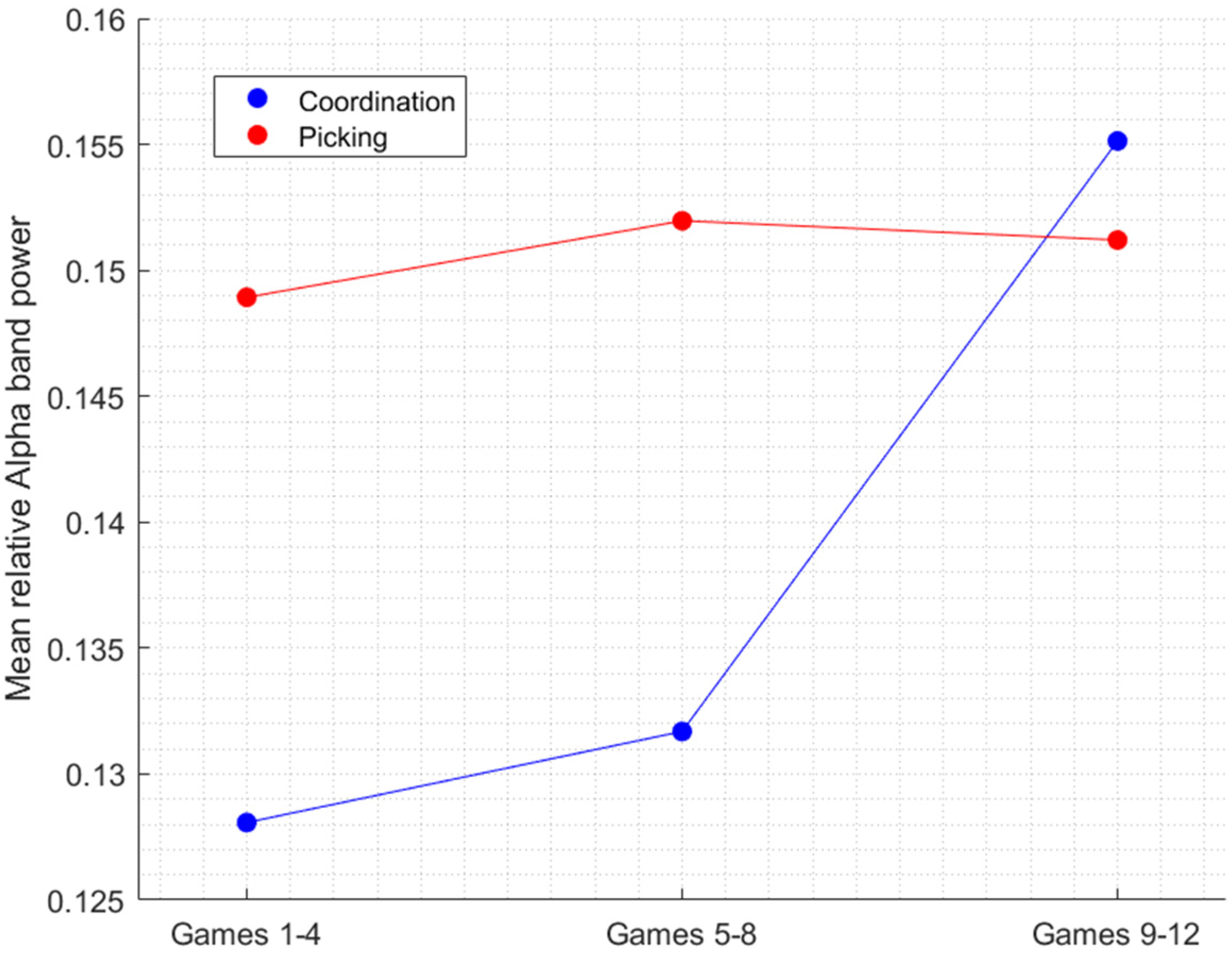
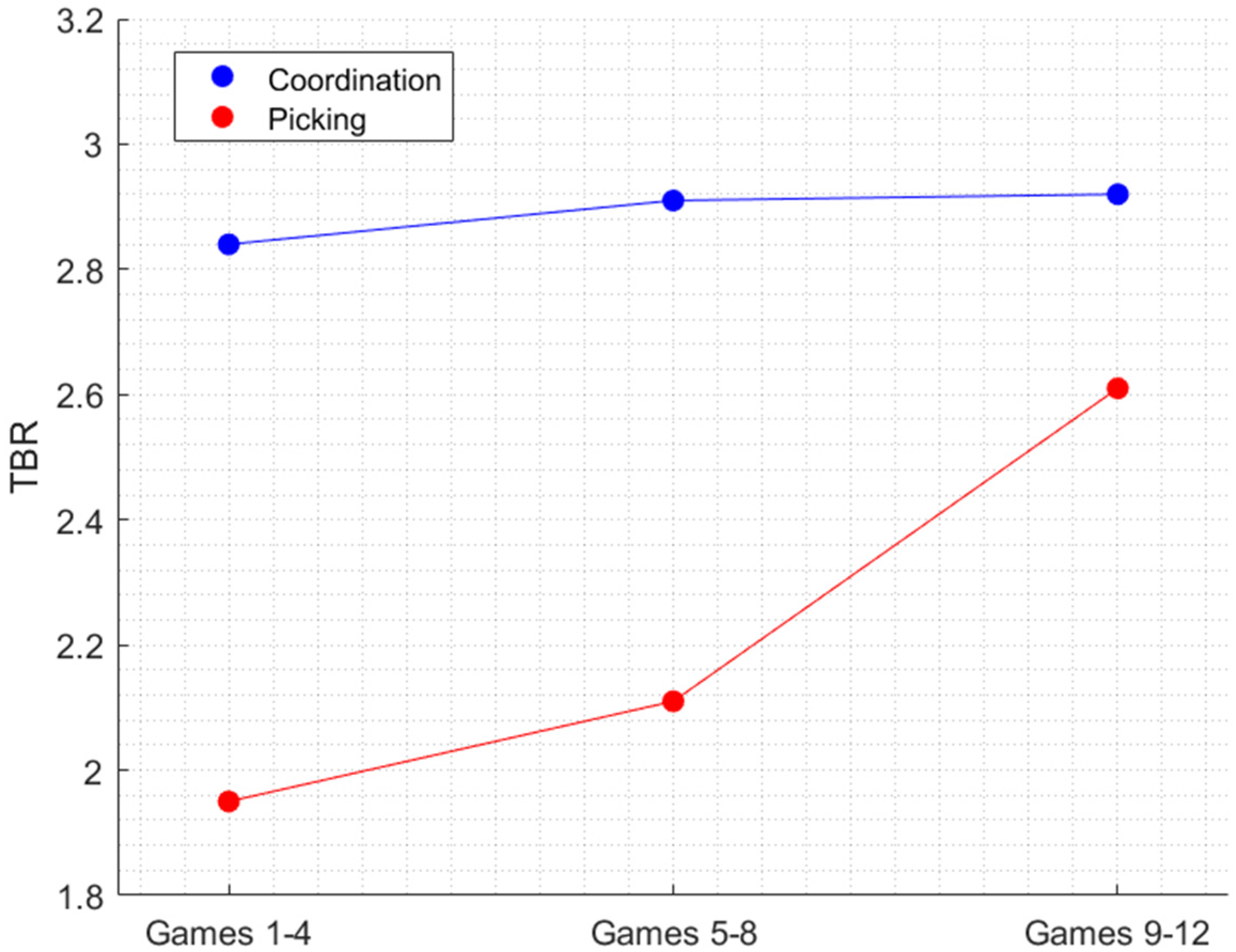
3.2. Changes in the Alpha Frequency Band and in the TBR across Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Tacit Coordination Game List
| Game Number | Option 1 | Option 2 | Option 3 | Option 4 |
|---|---|---|---|---|
| 1 | Water | Beer | Wine | Whisky |
| 2 | Tennis | Volleyball | Football | Chess |
| 3 | Blue | Gray | Green | Red |
| 4 | Iron | Steel | Plastic | Bronze |
| 5 | Ford | Ferrari | Jaguar | Porsche |
| 6 | 1 | 8 | 5 | 16 |
| 7 | Haifa | Tel-Aviv | Jerusalem | Netanya |
| 8 | Spinach | Carrot | Lettuce | Pear |
| 9 | London | Paris | Rome | Madrid |
| 10 | Hazel | Cashew | Almond | Peanut |
| 11 | Strawberry | Melon | Banana | Mango |
| 12 | Noodles | Pizza | Hamburger | Sushi |
Appendix B. Training Tasks Game List
| Game Number | Option 1 | Option 2 | Option 3 | Option 4 |
|---|---|---|---|---|
| 1 | Sapphire | Glass | Emerald | Diamond |
| 2 | Lion | Panther | Frog | Tiger |
| 3 | Boat | Helicopter | Bicycle | Plane |
| 4 | Thursday | Tuesday | Saturday | Sunday |
| 5 | 2019 | 2000 | 1995 | 1997 |
References
- Jan’t Hoen, P.; Tuyls, K.; Panait, L.; Luke, S.; La Poutré, J.A. An overview of cooperative and competitive multiagent learning. In Proceedings of the International Workshop on Learning and Adaption in Multi-Agent Systems, Utrecht, The Netherlands, 25 July 2005; pp. 1–46. [Google Scholar]
- Rosenfeld, A.; Zuckerman, I.; Azaria, A.; Kraus, S. Combining psychological models with machine learning to better predict people’s decisions. Synthese 2012, 189, 81–93. [Google Scholar] [CrossRef][Green Version]
- Kraus, S. Predicting human decision-making: From prediction to action. In Proceedings of the 6th International Conference on Human-Agent Interaction, Southampton, UK, 15–18 December 2018; p. 1. [Google Scholar]
- Rosenfeld, A.; Bareket, Z.; Goldman, C.V.; Kraus, S.; LeBlanc, D.J.; Tsimoni, O. Learning Driver’s Behavior to Improve the Acceptance of Adaptive Cruise Control. In Proceedings of the IAAI, Toronto, ON, Canada, 22–26 July 2012. [Google Scholar]
- Azaria, A.; Rabinovich, Z.; Kraus, S.; Goldman, C.V.; Tsimoni, O. Giving Advice to People in Path Selection Problems. In Proceedings of the AAMAS, Valencia, Spain, 4–8 June 2012; pp. 459–466. [Google Scholar]
- Sun, Y.; Yang, Y.; Chen, D.; Wang, G.; Zhou, Y.; Wang, C.; Stoddart, J.F. Mechanized Silica Nanoparticles Based on Pillar[5]arenes for On-Command Cargo Release. Small 2013, 9, 3224–3229. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, D.; Zuckerman, I.; Laufer, I. Using a Stochastic Agent Model to Optimize Performance in Divergent Interest Tacit Coordination Games. Sensors 2020, 20, 7026. [Google Scholar] [CrossRef] [PubMed]
- Nahavandi, S. Industry 5.0—A human-centric solution. Sustainability 2019, 11, 4371. [Google Scholar] [CrossRef]
- Alsamhi, S.H.; Ma, O.; Ansari, M.S.; Gupta, S.K. Collaboration of Drone and Internet of Public Safety Things in Smart Cities: An Overview of QoS and Network Performance Optimization. Drones 2019, 3, 13. [Google Scholar] [CrossRef]
- Alsamhi, S.H.; Ma, O.; Ansari, M.S.; Almalki, F.A. Survey on collaborative smart drones and internet of things for improving smartness of smart cities. IEEE Access 2019, 7, 128125–128152. [Google Scholar] [CrossRef]
- Alsamhi, S.H.; Ma, O.; Ansari, M.S. Convergence of machine learning and robotics communication in collaborative assembly: Mobility, connectivity and future perspectives. J. Intell. Robot. Syst. 2020, 98, 541–566. [Google Scholar] [CrossRef]
- Jennings, N.R.; Corera, J.M.; Laresgoiti, I. Developing Industrial Multi-Agent Systems. In Proceedings of the ICMAS, San Francisco, CA, USA, 12–14 June 1995; pp. 423–430. [Google Scholar]
- Jennings, N.R.; Bussmann, S. Agent-based control systems. IEEE Control Syst. 2003, 23, 61–74. [Google Scholar]
- Hanga, K.M.; Kovalchuk, Y. Machine learning and multi-agent systems in oil and gas industry applications: A survey. Comput. Sci. Rev. 2019, 34, 100191. [Google Scholar] [CrossRef]
- Azaria, A.; Gal, Y.; Kraus, S.; Goldman, C.V. Strategic advice provision in repeated human-agent interactions. Auton. Agents Multi-Agent. Syst. 2016, 30, 4–29. [Google Scholar] [CrossRef]
- Rosenfeld, A.; Agmon, N.; Maksimov, O.; Kraus, S. Intelligent agent supporting human-multi-robot team collaboration. Artif. Intell. 2017, 252, 211–231. [Google Scholar] [CrossRef]
- Saikia, A.; Hazarika, S.M. cBDI: Towards an Architecture for Human–Machine Collaboration. Int. J. Soc. Robot. 2017, 9, 211–230. [Google Scholar] [CrossRef]
- Mohammed, Y.B.; Karagozlu, D. A Review of Human-Computer Interaction Design Approaches towards Information Systems Development. Broad Res. Artif. Intell. Neurosci. 2021, 12, 229–250. [Google Scholar] [CrossRef]
- Zander, T.O.; Kothe, C. Towards passive brain–computer interfaces: Applying brain-computer interface technology to human–machine systems in general. J. Neural Eng. 2011, 8, 025005. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, D.; Laufer, I.; Zuckerman, I. The Effect of Individual Coordination Ability on Cognitive-Load in Tacit Coordination Games. In Proceedings of the NeuroIS Retreat 2020, Vienna, Austria, 2–4 June 2020; Davis, F., Riedl, R., vom Brocke, J., Léger, P.-M., Randolph, A., Fischer, T., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Belloc, M.; Bilancini, E.; Boncinelli, L.; D’Alessandro, S. Intuition and Deliberation in the Stag Hunt Game. Sci. Rep. 2019, 9, 14833. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, J.; Thomas, K.; DeScioli, P.; Pinker, S. Common knowledge, coordination, and strategic mentalizing in human social life. Proc. Natl. Acad. Sci. USA 2019, 116, 13751–13758. [Google Scholar] [CrossRef]
- Poulsen, A.; Sonntag, A. Focality Is Intuitive—Experimental Evidence on the Effects of Time Pressure in Coordination Games; University of East Anglia: Norwich, UK, 2019. [Google Scholar]
- Picken, C.; Clarke, A.R.; Barry, R.J. The Theta/Beta Ratio as an Index of Cognitive Processing in Adults with the Combined Type of Attention Deficit Hyperactivity Disorder. Clin. EEG Neurosci. 2020, 51, 167–173. [Google Scholar] [CrossRef]
- Laufer, I.; Mizrahi, D.; Zuckerman, I. An electrophysiological model for assessing cognitive load in tacit coordination games. Sensors 2022, 22, 477. [Google Scholar] [CrossRef]
- Renard, Y.; Lotte, F.; Gibert, G.; Congedo, M.; Maby, E.; Delannoy, V.; Bertrand, O.; Le’cuyer, A. Openvibe: An open-source software platform to design, test, and use brain–computer interfaces in real and virtual environments. Presence Teleoperators Virtual Environ. 2010, 19, 35–53. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Shensa, M.J. The Discrete Wavelet Transform: Wedding the a Trous and Mallat Algorithms. IEEE Trans. Signal Process. 1992, 40, 2464–2482. [Google Scholar] [CrossRef]
- Jensen, A.; la Cour-Harbo, A. Ripples in Mathematics: The Discrete Wavelet Transform; Springer Science & Business Media: Berlin, Germany, 2001. [Google Scholar]
- Mizrahi, D.; Laufer, I.; Zuckerman, I. Topographic Analysis of Cognitive Load in Tacit Coordination Games Based on Electrophysiological Measurements. In Proceedings of the NeuroIS Retreat 2021, Vienna, Austria, 1–3 June 2021. [Google Scholar]
- Mizrahi, D.; Laufer, I.; Zuckerman, I. Level-K Classification from EEG Signals Using Transfer Learning. Sensors 2021, 21, 7908. [Google Scholar] [CrossRef] [PubMed]
- Gartner, M.; Grimm, S.; Bajbouj, M. Frontal midline theta oscillations during mental arithmetic: Effects of stress. Front. Behav. Neurosci. 2015, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- De Vico Fallani, F.; Nicosia, V.; Sinatra, R.; Astolfi, L.; Cincotti, F.; Mattia, D.; Wilke, C.; Doud, A.; Latora, V.; He, B.; et al. Defecting or not defecting: How to “read” human behavior during cooperative games by EEG measurements. PLoS ONE 2010, 5, e14187. [Google Scholar] [CrossRef]
- Boudewyn, M.; Roberts, B.M.; Mizrak, E.; Ranganath, C.; Carter, C.S. Prefrontal transcranial direct current stimulation (tDCS) enhances behavioral and EEG markers of proactive control. Cogn. Neurosci. 2019, 10, 57–65. [Google Scholar] [CrossRef]
- Moliadze, V.; Sierau, L.; Lyzhko, E.; Stenner, T.; Werchowski, M.; Siniatchkin, M.; Hartwigsen, G. After-effects of 10 Hz tACS over the prefrontal cortex on phonological word decisions. Brain Stimul. 2019, 12, 1464–1474. [Google Scholar] [CrossRef]
- Raufi, B.; Longo, L. An Evaluation of the EEG alpha-to-theta and theta-to-alpha band Ratios as Indexes of Mental Workload. Front. Neuroinform. 2022, 16, 861967. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Sterman, M.B.; Mann, C.A. Concepts and applications of EEG analysis in aviation performance evaluation. Biol. Psychol. 1995, 40, 115–130. [Google Scholar] [CrossRef]
- So, W.K.Y.; Wong, S.W.H.; Mak, J.N.; Chan, R.H.M. An evaluation of mental workload with frontal EEG. PLoS ONE 2017, 12, e0174949. [Google Scholar]
- Kamzanova, A.T.; Kustubayeva, A.M.; Matthews, G. Use of EEG workload indices for diagnostic monitoring of vigilance decrement. Hum. Factors 2014, 56, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- MacLean, M.H.; Arnell, K.M.; Cote, K.A. Resting EEG in alpha and beta bands predicts individual differences in attentional blink magnitude. Brain Cogn. 2012, 78, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Rojas, R.; Debie, E.; Fidock, J.; Barlow, M.; Kasmarik, K.; Anavatti, S.; Garratt, M.; Abbass, H. Electroencephalographic Workload Indicators During Teleoperation of an Unmanned Aerial Vehicle Shepherding a Swarm of Unmanned Ground Vehicles in Contested Environments. Front. Neurosci. 2020, 14, 40. [Google Scholar] [CrossRef]
- Schwab, D.; Benedek, M.; Papousek, I.; Weiss, E.M.; Fink, A. The time-course of EEG alpha power changes in creative ideation. Front. Hum. Neurosci. 2014, 8, 310. [Google Scholar] [CrossRef]
- Benwell, C.S.Y.; London, R.E.; Tagliabue, C.F.; Veniero, D.; Gross, J.; Keitel, C.; Thutb, G. Frequency and power of human alpha oscillations drift systematically with time-on-task. Neuroimage 2019, 192, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Jap, B.T.; Lal, S.; Fischer, P.; Bekiaris, E. Using EEG spectral components to assess algorithms for detecting fatigue. Expert Syst. Appl. 2009, 36, 2352–2359. [Google Scholar] [CrossRef]
- Trejo, L.J.; Kubitz, K.; Rosipal, R.; Kochavi, R.L.; Montgomery, L.D. EEG-based estimation and classification of mental fatigue. Psychology 2015, 6, 572–589. [Google Scholar] [CrossRef]
- Bühler, M.; Weisswange, T. Theory of Mind based Communication for Human Agent Cooperation. In Proceedings of the IEEE International Conference on Human-Machine Systems, Rome, Italy, 7–9 September 2020. [Google Scholar]
- Otto, A.R.; Skatova, A.; Madlon-Kay, S.; Daw, N.D. Cognitive control predicts use of model-based reinforcement learning. J. Cogn. Neurosci. 2014, 27, 319–333. [Google Scholar] [CrossRef]
- Nowé, A.; Vrancx, P.; Hauwere, Y.-M. De Game Theory and Multi-agent Reinforcement Learning. Reinf. Learn. 2012, 50, 441–470. [Google Scholar]
- Haukipuro, E.-S.; Kolehmainen, V.; Myllarinen, J.; Remander, S.; Salo, J.; Takko, T.; Nguyen, L.N.; Sigg, S.; Findling, R.D. Mobile brainwaves: On the interchangeability of simple authentication tasks with low-cost, single-electrode EEG devices. IEICE Trans. Commun. 2019, 102, 760–767. [Google Scholar] [CrossRef]
- Nagar, P.; Sethia, D. Brain mapping based stress identification using portable eeg based device. In Proceedings of the 11th International Conference on Communication Systems & Networks (COMSNETS), Bengaluru, India, 7–11 January 2019; pp. 601–606. [Google Scholar]
- Murphy, R.O.; Ackermann, K.A.; Handgraaf, M.J.J. Measuring Social Value Orientation. Judgm. Decis. Mak. 2011, 6, 771–781. [Google Scholar] [CrossRef]
- Liebrand, W.B.; Mccllntock, C.G. The ring measure of social values: A computerized procedure for assessing individual differences in information processing and social value orientation. Eur. J. Pers. 1988, 2, 217–230. [Google Scholar] [CrossRef]
- Mizrahi, D.; Laufer, I.; Zuckerman, I. The Effect of Loss-Aversion on Strategic Behaviour of Players in Divergent Interest Tacit Coordination Games. In Proceedings of the International Conference on Brain Informatics, Padova, Italy, 19 September 2020; pp. 41–49. [Google Scholar]
- Mizrahi, D.; Laufer, I.; Zuckerman, I. The Effect of Expected Revenue Proportion and Social Value Orientation Index on Players’ Behavior in Divergent Interest Tacit Coordination Games. In Proceedings of the International Conference on Brain Informatics, Virtual Event, 17–19 September 2021; pp. 25–34. [Google Scholar]
- Mizrahi, D.; Laufer, I.; Zuckerman, I.; Zhang, T. The effect of culture and social orientation on Player’s performances in tacit coordination games. In Proceedings of the International Conference on Brain Informatics, Arlington, TX, USA, 7–9 December 2018; pp. 437–447. [Google Scholar]
- Mizrahi, D.; Laufer, I.; Zuckerman, I. Collectivism-individualism: Strategic behavior in tacit coordination games. PLoS ONE 2020, 15, e0226929. [Google Scholar] [CrossRef] [PubMed]
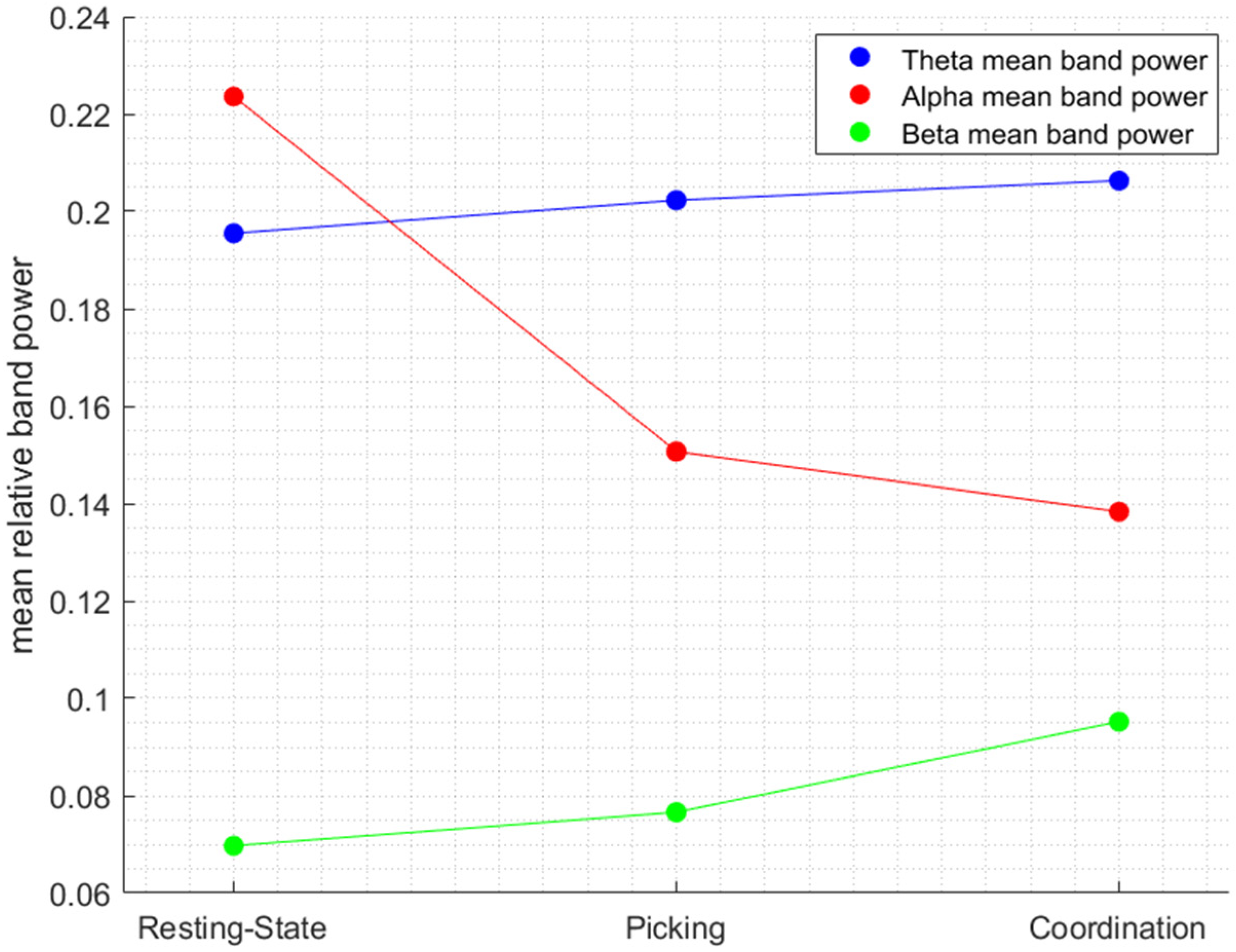
| Picking–Coordination | Resting State–Picking | Resting State–Coordination | |
|---|---|---|---|
| ✗ | ✗ | ✓ | Theta |
| p = 0.7675 | p = 0.2561 | p < 0.05 | [4,8] (Hz) |
| ✗ | ✓ | ✓ | Alpha |
| p = 0.1095 | p < 0.001 | p < 0.001 | [8,16] (Hz) |
| ✓ | ✓ | ✓ | Beta |
| p < 0.001 | p < 0.001 | p < 0.001 | [16,32] (Hz) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizrahi, D.; Zuckerman, I.; Laufer, I. Electrophysiological Features to Aid in the Construction of Predictive Models of Human–Agent Collaboration in Smart Environments. Sensors 2022, 22, 6526. https://doi.org/10.3390/s22176526
Mizrahi D, Zuckerman I, Laufer I. Electrophysiological Features to Aid in the Construction of Predictive Models of Human–Agent Collaboration in Smart Environments. Sensors. 2022; 22(17):6526. https://doi.org/10.3390/s22176526
Chicago/Turabian StyleMizrahi, Dor, Inon Zuckerman, and Ilan Laufer. 2022. "Electrophysiological Features to Aid in the Construction of Predictive Models of Human–Agent Collaboration in Smart Environments" Sensors 22, no. 17: 6526. https://doi.org/10.3390/s22176526
APA StyleMizrahi, D., Zuckerman, I., & Laufer, I. (2022). Electrophysiological Features to Aid in the Construction of Predictive Models of Human–Agent Collaboration in Smart Environments. Sensors, 22(17), 6526. https://doi.org/10.3390/s22176526







