Macrophage Activation Markers Predict Liver-Related Complications in Primary Biliary Cholangitis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Baseline Clinical Characteristics of Patients
2.2. Correlation of Histological Stage with IP Markers
2.3. Diagnostic Accuracy of IP Markers for Symptom Development in Patients with Primary Biliary Cholangitis
2.4. Predictive Factors for PBC-Related Complications
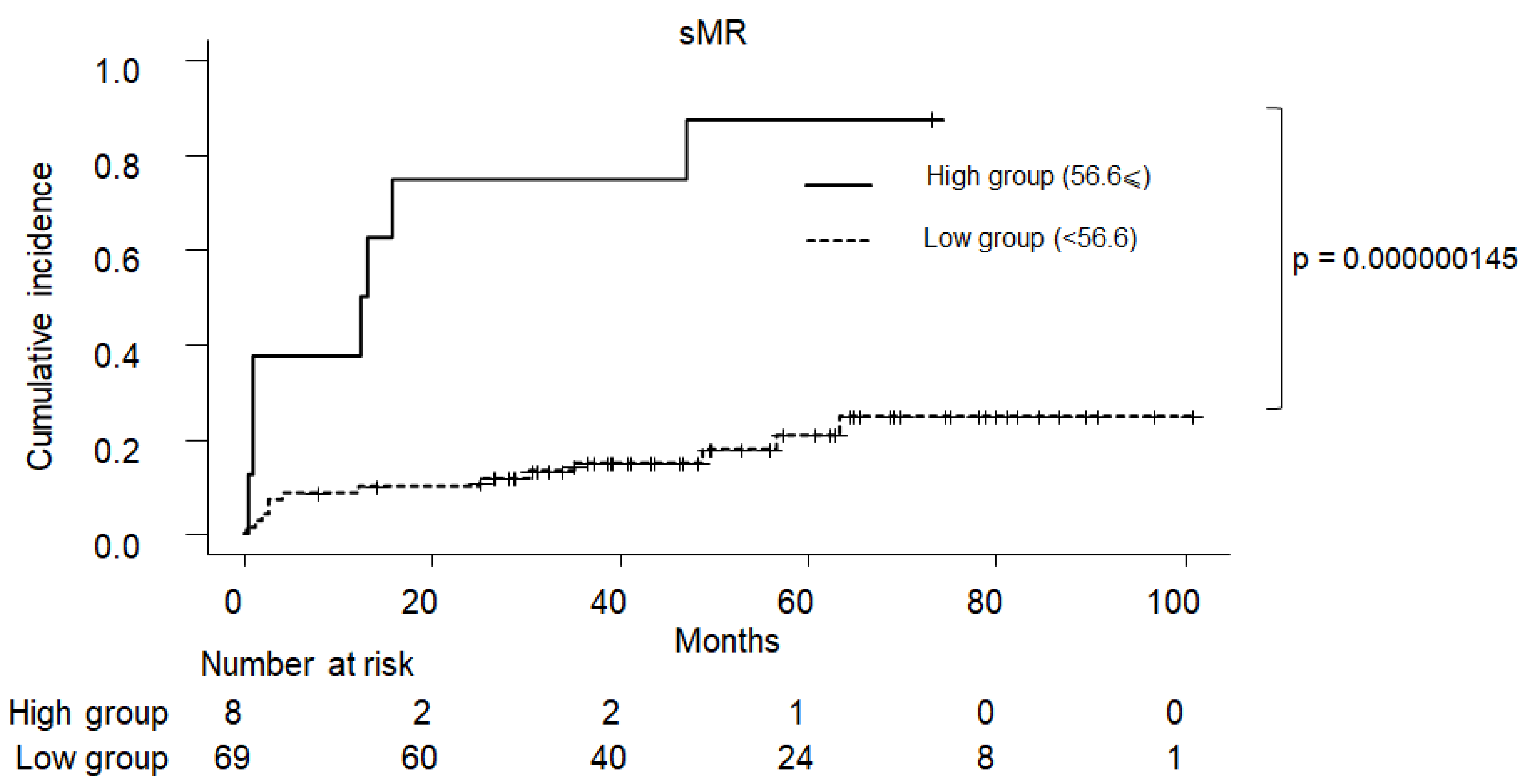
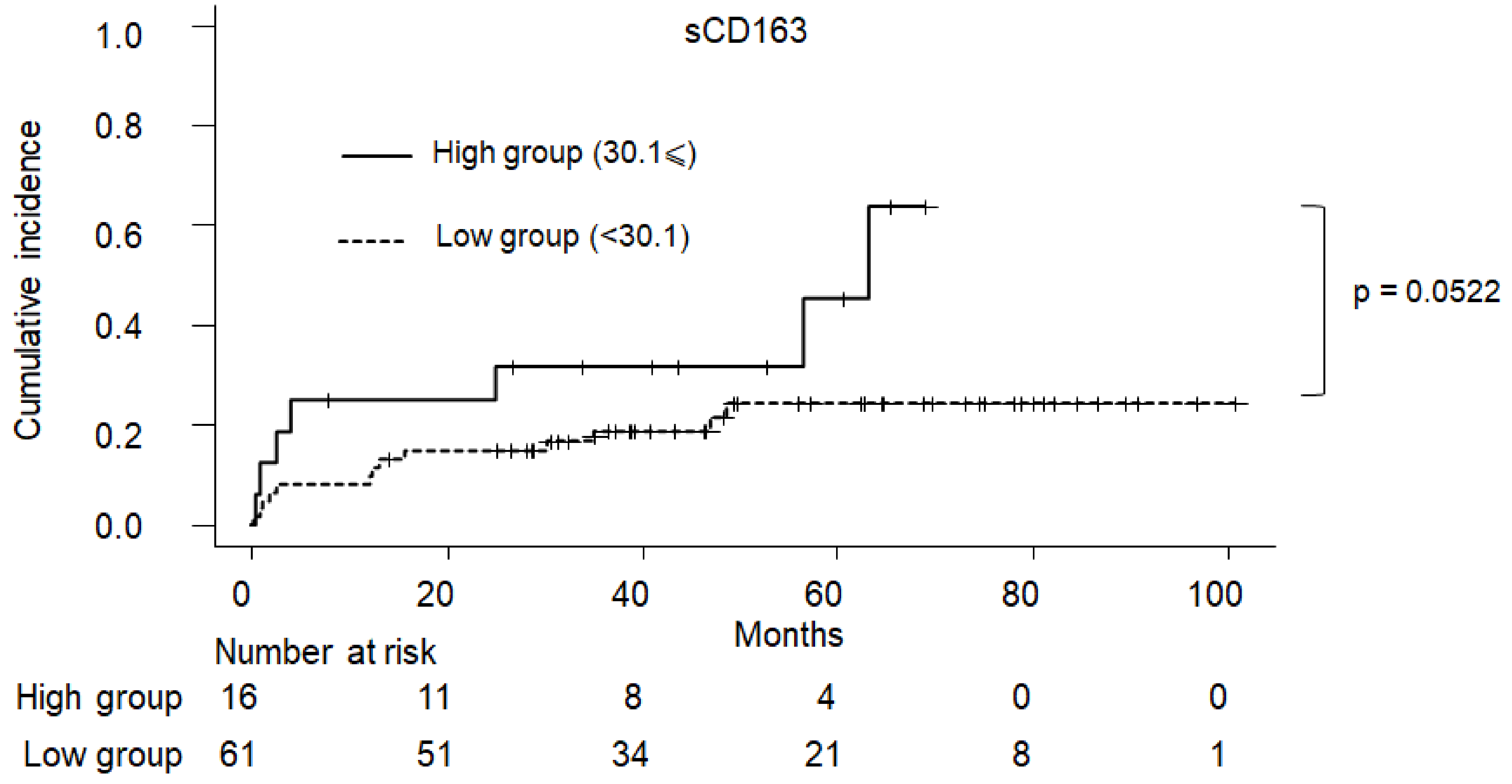
2.5. Cumulative Incidence of Clinical Symptom in PBC
3. Materials and Methods
3.1. Patient Population
3.2. Histological Evaluation of Liver Tissues According to the Scheuer and Nakanuma Classifications
3.3. IP Markers
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joshita, S.; Umemura, T.; Yamashita, Y.; Sugiura, A.; Yamazaki, T.; Fujimori, N.; Matsumoto, A.; Tanaka, E. Biochemical and plasma lipid responses to pemafibrate in patients with primary biliary cholangitis. Hepatol. Res. 2019, 49, 1236–1243. [Google Scholar] [CrossRef]
- Kawata, K.; Joshita, S.; Shimoda, S.; Yamashita, Y.; Yamashita, M.; Kitsugi, K.; Takatori, S.; Ohta, K.; Ito, J.; Shimoyama, S.; et al. The ursodeoxycholic acid response score predicts pathological features in primary biliary cholangitis. Hepatol. Res. 2021, 51, 80–89. [Google Scholar] [CrossRef]
- Tanaka, A.; Takikawa, H.; Mochida, S.; Koike, K.; Miwa, H.; Shimosegawa, T. Changing nomenclature for PBC from “primary biliary cirrhosis” to “primary biliary cholangitis”. J. Gastroenterol. 2016, 51, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.G.; Brunt, E.M.; Heathcote, J.; Gores, G.J.; Lindor, K.D.; Mayo, M.J. American Association for the Study of Liver Diseases endpoints conference: Design and endpoints for clinical trials in primary biliary cirrhosis. Hepatology 2010, 52, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Abe, K.; Fujita, M.; Okai, K.; Takahashi, A.; Ohira, H. Changes in serum levels of leucine-rich alpha2-glycoprotein predict prognosis in primary biliary cholangitis. Hepatol. Res. 2019, 49, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.I.; Chetwynd, A.; Craig, W.L.; Metcalf, J.V.; James, O.F. Asymptomatic primary biliary cirrhosis: Clinical features, prognosis, and symptom progression in a large population based cohort. Gut 2004, 53, 865–870. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Asano, T.; Arisaka, T.; Mashima, H.; Irisawa, A.; Tamano, M. Effects of pemafibrate on primary biliary cholangitis with dyslipidemia. Hepatol. Res. 2022, 52, 522–531. [Google Scholar] [CrossRef]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2019, 69, 394–419. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Recent advances in the management of pruritus in chronic liver diseases. World J. Gastroenterol. 2017, 23, 3418–3426. [Google Scholar] [CrossRef]
- Schwenger, K.J.; Clermont-Dejean, N.; Allard, J.P. The role of the gut microbiome in chronic liver disease: The clinical evidence revised. JHEP rep. JHEP rep. JHEP Rep. 2019, 1, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Abe, K.; Fujita, M.; Takahashi, A.; Hashimoto, Y.; Ohira, H. Serum Gas6 and Axl as non-invasive biomarkers of advanced histological stage in primary biliary cholangitis. Hepatol. Res. 2020, 50, 1337–1346. [Google Scholar] [CrossRef]
- Takamura, M.; Matsuda, Y.; Kimura, N.; Takatsuna, M.; Setsu, T.; Tsuchiya, A.; Osaki, A.; Waguri, N.; Yanagi, M.; Takahashi, T.; et al. Changes in disease characteristics of primary biliary cholangitis: An observational retrospective study from 1982 to 2016. Hepatol. Res. 2021, 51, 166–175. [Google Scholar] [CrossRef]
- Rainer, F.; Horvath, A.; Sandahl, T.D.; Leber, B.; Schmerboeck, B.; Blesl, A.; Groselj-Strele, A.; Stauber, R.E.; Fickert, P.; Stiegler, P.; et al. Soluble CD163 and soluble mannose receptor predict survival and decompensation in patients with liver cirrhosis, and correlate with gut permeability and bacterial translocation. Aliment. Pharmacol. Ther. 2018, 47, 657–664. [Google Scholar] [CrossRef]
- Bossen, L.; Rebora, P.; Bernuzzi, F.; Jepsen, P.; Gerussi, A.; Andreone, P.; Galli, A.; Terziroli, B.; Alvaro, D.; Labbadia, G.; et al. Soluble CD163 and mannose receptor as markers of liver disease severity and prognosis in patients with primary biliary cholangitis. Liver Int. 2020, 40, 1408–1414. [Google Scholar] [CrossRef]
- Working Subgroup, English Version for Clinical Practice Guidelines for Primary Biliary Cirrhosis. Guidelines for the management of primary biliary cirrhosis: The Intractable Hepatobiliary Disease Study Group supported by the Ministry of Health, Labour and Welfare of Japan. Hepatol. Res. 2014, 44 (Suppl. S1), 71–90. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Namisaki, T.; Moriya, K.; Kitade, M.; Kawaratani, H.; Shimozato, N.; Kaji, K.; Takaya, H.; Sawada, Y.; Seki, K.; et al. Identification of clinical risk factors for histological progression of primary biliary cholangitis. Hepatol. Res. 2019, 49, 1015–1025. [Google Scholar] [CrossRef]
- Namisaki, T.; Fujinaga, Y.; Moriya, K.; Yoshiji, H. The association of histological progression with biochemical response to ursodeoxycholic acid in primary biliary cholangitis. Hepatol. Res. 2021, 51, 31–38. [Google Scholar] [CrossRef]
- Chazouillères, O.; Wendum, D.; Serfaty, L.; Montembault, S.; Rosmorduc, O.; Poupon, R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: Clinical features and response to therapy. Hepatology 1998, 28, 296–301. [Google Scholar] [CrossRef]
- Scheuer, P. Primary biliary cirrhosis. Proc. R. Soc. Med. 1967, 60, 1257–1260. [Google Scholar]
- Nakanuma, Y.; Zen, Y.; Harada, K.; Sasaki, M.; Nonomura, A.; Uehara, T.; Sano, K.; Kondo, F.; Fukusato, T.; Tsuneyama, K.; et al. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: Interobserver agreement. Pathol. Int. 2010, 60, 167–174. [Google Scholar] [CrossRef]
- Cholongitas, E.; Senzolo, M.; Standish, R.; Marelli, L.; Quaglia, A.; Patch, D.; Dhillon, A.P.; Burroughs, A.K. A systematic review of the quality of liver biopsy specimens. Am. J. Clin. Pathol. 2006, 125, 710–721. [Google Scholar] [CrossRef]
- Ruiz-Briseño, M.D.R.; De Arcos-Jiménez, J.C.; Ratkovich-González, S.; Sánchez-Reyes, K.; González-Hernández, L.A.; Andrade-Villanueva, J.F.; Alvarez-Zavala, M. Association of intestinal and systemic inflammatory biomarkers with immune reconstitution in HIV+ patients on ART. J. Inflamm. 2020, 17, 32. [Google Scholar] [CrossRef]
- Kaji, K.; Saikawa, S.; Takaya, H.; Fujinaga, Y.; Furukawa, M.; Kitagawa, K.; Ozutsumi, T.; Kaya, D.; Tsuji, Y.; Sawada, Y.; et al. Rifaximin alleviates endotoxemia with decreased serum levels of soluble CD163 and mannose receptor and partial modification of gut microbiota in cirrhotic patients. Antibiotics 2020, 9, 145. [Google Scholar] [CrossRef]
- Esnafoglu, E.; Cırrık, S.; Ayyıldız, S.N.; Erdil, A.; Ertürk, E.Y.; Daglı, A.; Noyan, T. Increased serum zonulin levels as an intestinal permeability marker in autistic subjects. J. Pediatr. 2017, 188, 240–244. [Google Scholar] [CrossRef]
- Carbone, M.; Sharp, S.J.; Flack, S.; Paximadas, D.; Spiess, K.; Adgey, C.; Griffiths, L.; Lim, R.; Trembling, P.; Williamson, K.; et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 2016, 63, 930–950. [Google Scholar] [CrossRef]
- Grønbaek, H.; Sandahl, T.D.; Mortensen, C.; Vilstrup, H.; Møller, H.J.; Møller, S. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment. Pharmacol. Ther. 2012, 36, 173–180. [Google Scholar] [CrossRef]
- Sandahl, T.D.; Støy, S.H.; Laursen, T.L.; Rødgaard-Hansen, S.; Møller, H.J.; Møller, S.; Vilstrup, H.; Grønbæk, H. The soluble mannose receptor (sMR) is elevated in alcoholic liver disease and associated with disease severity, portal hypertension, and mortality in cirrhosis patients. PLoS ONE 2017, 12, e0189345. [Google Scholar] [CrossRef]
- Kazankov, K.; Barrera, F.; Møller, H.J.; Bibby, B.M.; Vilstrup, H.; George, J.; Grønbaek, H. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology 2014, 60, 521–530. [Google Scholar] [CrossRef]
- Møller, H.J.; Grønbaek, H.; Schiødt, F.V.; Holland-Fischer, P.; Schilsky, M.; Munoz, S.; Hassanein, T.; Lee, W.M.; U.S. Acute Liver Failure Study Group. Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J. Hepatol. 2007, 47, 671–676. [Google Scholar] [CrossRef]
- Lleo, A.; Bowlus, C.L.; Yang, G.X.; Invernizzi, P.; Podda, M.; Van de Water, J.; Ansari, A.A.; Coppel, R.L.; Worman, H.J.; Gores, G.J.; et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology 2010, 52, 987–998. [Google Scholar] [CrossRef]
- Ramachandran, P.; Iredale, J.P. Macrophages: Central regulators of hepatic fibrogenesis and fibrosis resolution. J. Hepatol. 2012, 56, 1417–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Andersen, M.N.; Rittig, N.; Rødgaard-Hansen, S.; Grønbaek, H.; Moestrup, S.K.; Møller, H.J.; Etzerodt, A. The macrophage-related biomarkers sCD163 and sCD206 are released by different shedding mechanisms. J. Leukoc. Biol. 2019, 106, 1129–1138. [Google Scholar] [CrossRef]
- Rødgaard-Hansen, S.; Rafique, A.; Christensen, P.A.; Maniecki, M.B.; Sandahl, T.D.; Nexø, E.; Møller, H.J. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin. Chem. Lab. Med. 2014, 52, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Floreani, A.; Baragiotta, A.; Pizzuti, D.; Martines, D.; Cecchetto, A.; Chiarelli, S. Mucosal IgA defect in primary biliary cirrhosis. Am. J. Gastroenterol. 2002, 97, 508–510. [Google Scholar] [CrossRef] [PubMed]

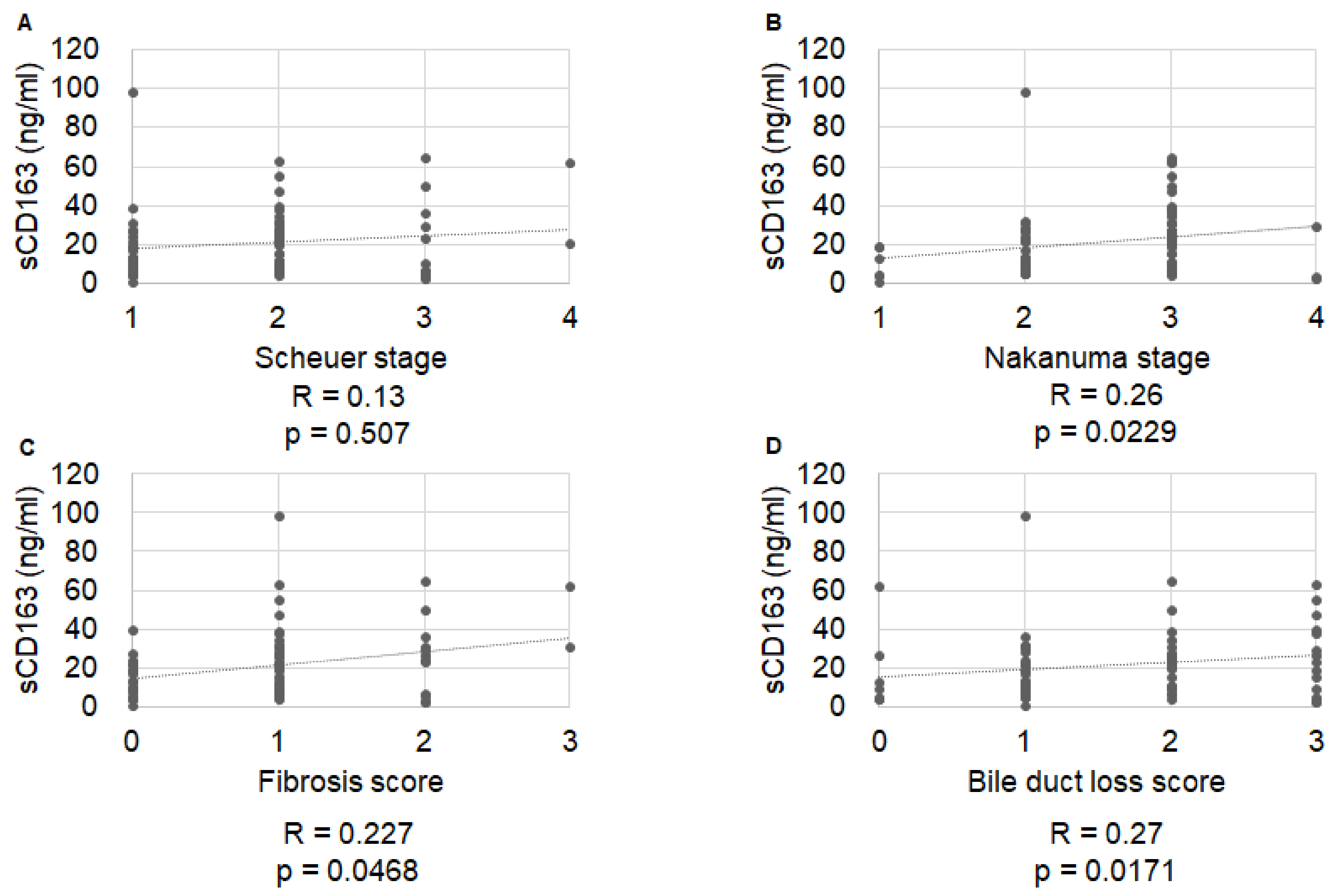
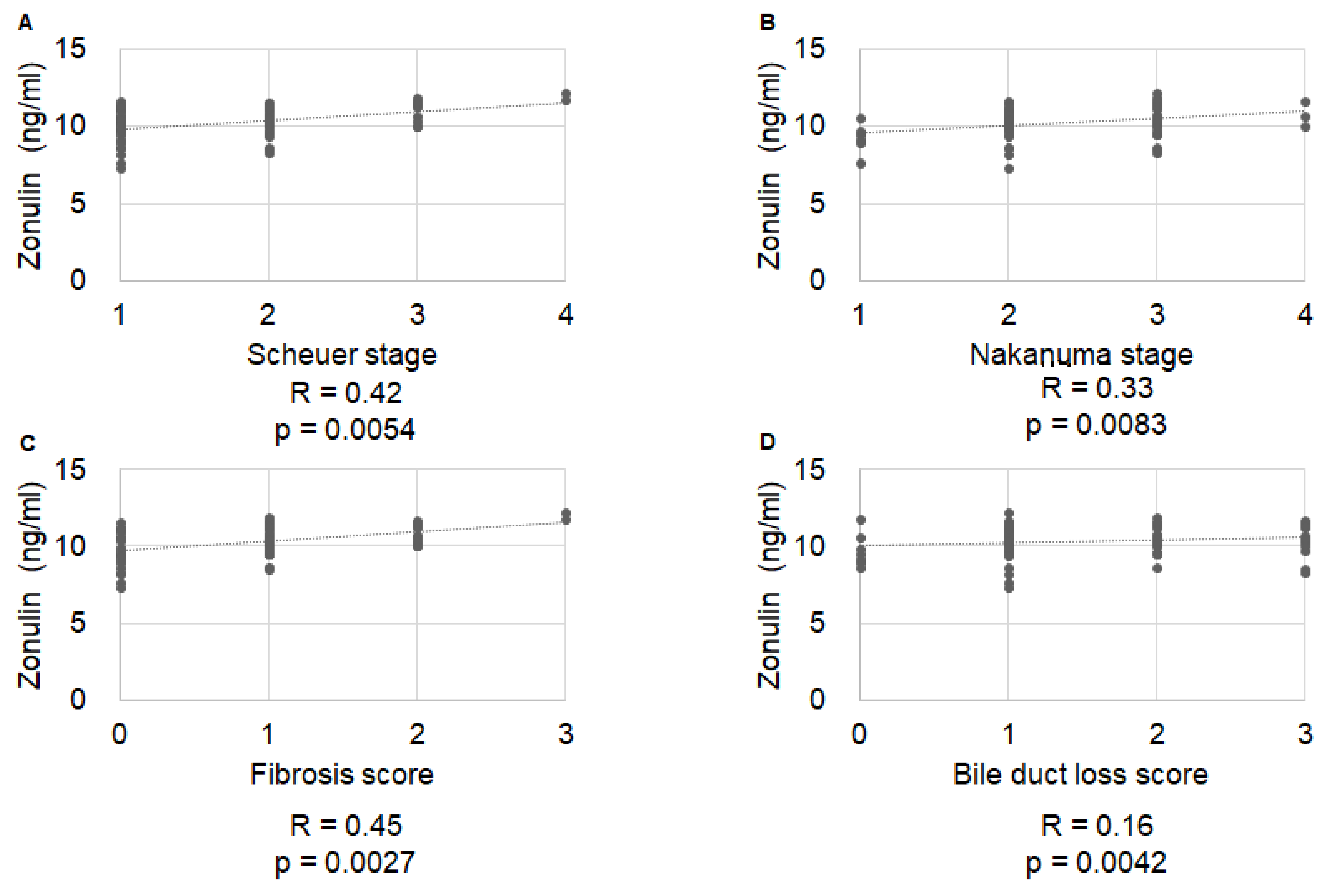

| Variable | Total (n = 77) | No Symptom Development (n = 57) | Symptom Development (n = 20) | p Value |
|---|---|---|---|---|
| Gender (M/F) | 11/66 | 9/48 | 2/18 | 0.718 |
| Age (years) † | 63.5 ± 9.8 | 62.6 ± 9.5 | 66.1 ± 10.4 | 0.166 |
| Pruritus/ascites/esophageal varices/HCC/jaundice | 12/2/6/2/2 | - | 12/2/6/2/2 | - |
| Scheuer stage (I/II/III/IV) | 25/39/11/2 | 20/28/9/0 | 5/11/2/2 | 0.085 |
| Nakanuma stage (1/2/3/4) | 6/28/40/3 | 5/22/27/3 | 1/6/13/0 | 0.474 |
| Fibrosis score (0/1/2/3) | 19/43/13/2 | 16/32/9/0 | 3/11/4/2 | 0.076 |
| Bile duct loss score (0/1/2/3) | 7/32/24/14 | 5/23/17/12 | 2/9/7/2 | 0.747 |
| Observation period (years) † | 4.4 ± 1.8 | 4.3 ± 1.8 | 4.5 ± 1.8 | 0.717 |
| PLT (×104/mL) † | 21.2 ± 6.9 | 22.1 ± 6.0 | 19.1 ± 7.8 | 0.087 |
| ALB (g/dL) † | 4.1 ± 0.5 | 4.2 ± 0.4 | 4.0 ± 0.5 | 0.262 |
| AST (IU/L) ‡ | 47.0 [32.0–77.0] | 46.0 [31.0–63.0] | 61.0 [35.0–83.5] | 0.149 |
| ALT (IU/L) ‡ | 41.0 [27.0–73.0] | 40.0 [27.0–67.0] | 53.0 [28.5–75.0] | 0.414 |
| ALP (IU/L) † | 534.0 ± 369.3 | 501.2 ± 372.5 | 627.4 ± 352.6 | 0.191 |
| γ-GTP (IU/l) ‡ | 131.0 [86.0–261.0] | 119.0 [79.0–236.0] | 176.0 [117.3–330.5] | 0.384 |
| T-Bil (mg/dL) † | 1.0 ± 0.7 | 0.9 ± 0.4 | 1.3 ± 1.2 | 0.103 |
| Smr (ng/mL) † | 39.5 ± 19.0 | 36.3 ± 10.9 | 48.7 ± 31.2 | 0.011 |
| sCD163 (ng/mL) † | 21.2 ± 17.6 | 18.8 ± 14.9 | 27.8 ± 22.8 | 0.048 |
| Zonulin (ng/mL) † | 10.3 ± 1.0 | 10.1 ± 1.3 | 10.2 ± 1.2 | 0.759 |
| Variable | Cutoff | Sensitivity | Specificity | PPV | NPV | AUC (95% Confidence Interval) |
|---|---|---|---|---|---|---|
| sCD163 | 30.1 | 0.35 | 0.842 | 0.438 | 0.787 | 0.596 (0.479–0.713) |
| sMR | 56.6 | 0.35 | 0.982 | 0.875 | 0.812 | 0.666 (0.566–0.775) |
| sMR + sCD163 | 0.254 | 0.60 | 0.825 | 0.545 | 0.855 | 0.739 (0.609–0.861) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value |
| 56.6 ⩽ Soluble MR | 30.20 (3.410–267.0) | 0.00220 | 33.70 (3.6600–311.0) | 0.0019 |
| 30.1 ⩽ sCD163 | 2.870 (0.898–9.18) | 0.07530 | 3.410 (0.9310–12.50) | 0.0640 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujinaga, Y.; Namisaki, T.; Tsuji, Y.; Suzuki, J.; Murata, K.; Takeda, S.; Takaya, H.; Inoue, T.; Noguchi, R.; Fujimoto, Y.; et al. Macrophage Activation Markers Predict Liver-Related Complications in Primary Biliary Cholangitis. Int. J. Mol. Sci. 2022, 23, 9814. https://doi.org/10.3390/ijms23179814
Fujinaga Y, Namisaki T, Tsuji Y, Suzuki J, Murata K, Takeda S, Takaya H, Inoue T, Noguchi R, Fujimoto Y, et al. Macrophage Activation Markers Predict Liver-Related Complications in Primary Biliary Cholangitis. International Journal of Molecular Sciences. 2022; 23(17):9814. https://doi.org/10.3390/ijms23179814
Chicago/Turabian StyleFujinaga, Yukihisa, Tadashi Namisaki, Yuki Tsuji, Junya Suzuki, Koji Murata, Soichi Takeda, Hiroaki Takaya, Takashi Inoue, Ryuichi Noguchi, Yuki Fujimoto, and et al. 2022. "Macrophage Activation Markers Predict Liver-Related Complications in Primary Biliary Cholangitis" International Journal of Molecular Sciences 23, no. 17: 9814. https://doi.org/10.3390/ijms23179814
APA StyleFujinaga, Y., Namisaki, T., Tsuji, Y., Suzuki, J., Murata, K., Takeda, S., Takaya, H., Inoue, T., Noguchi, R., Fujimoto, Y., Enomoto, M., Nishimura, N., Kitagawa, K., Kaji, K., Kawaratani, H., Akahane, T., Mitoro, A., & Yoshiji, H. (2022). Macrophage Activation Markers Predict Liver-Related Complications in Primary Biliary Cholangitis. International Journal of Molecular Sciences, 23(17), 9814. https://doi.org/10.3390/ijms23179814






