Canine B Cell Lymphoma- and Leukemia-Derived Extracellular Vesicles Moderate Differentiation and Cytokine Production of T and B Cells In Vitro
Abstract
1. Introduction
2. Results
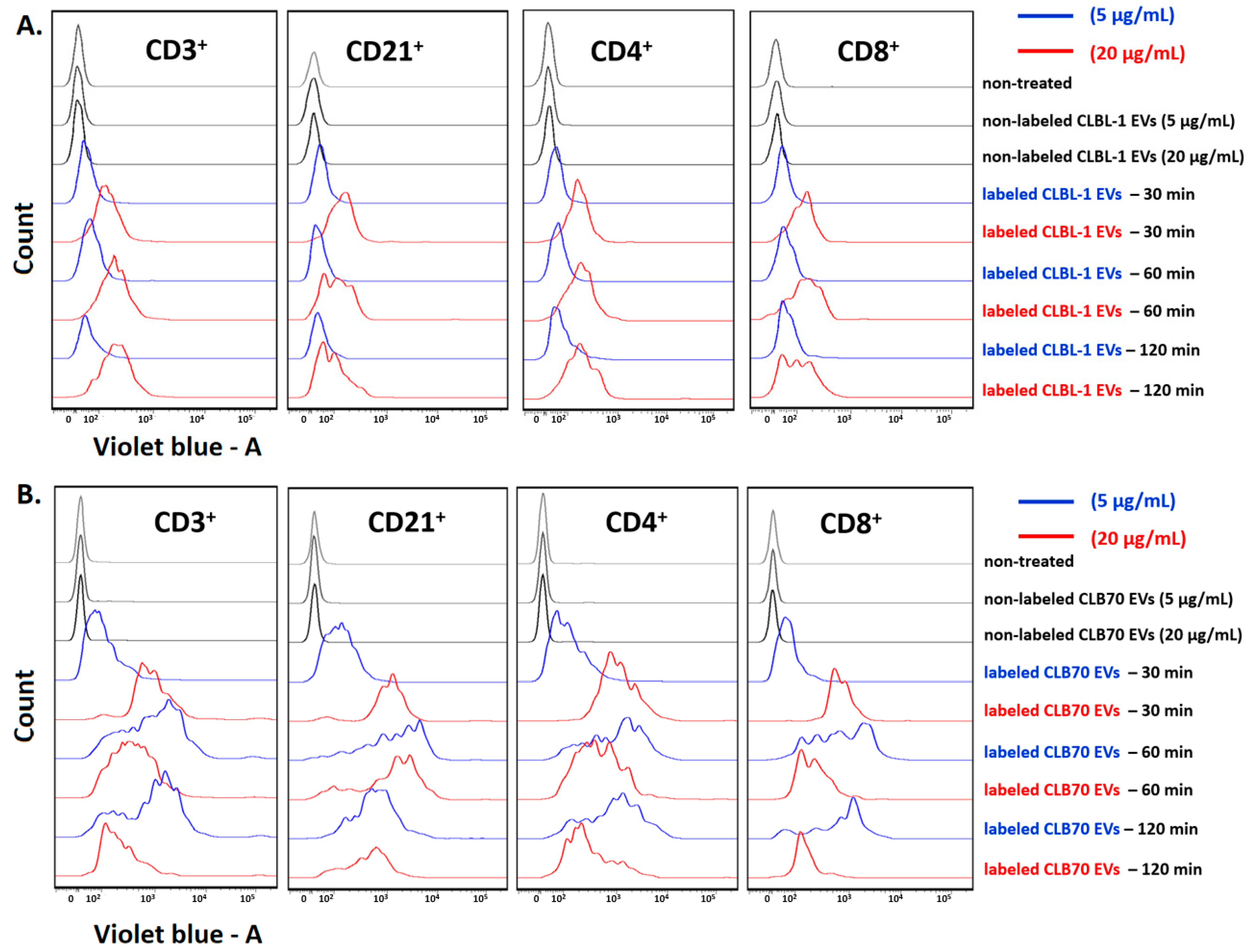
2.1. CLBL-1 EVs and CLB70 EVs Are Taken up by Lymphocytes
2.2. CLBL-1 EVs and CLB70 EVs Influence on Lymphocyte Subsets and Cytokine Production
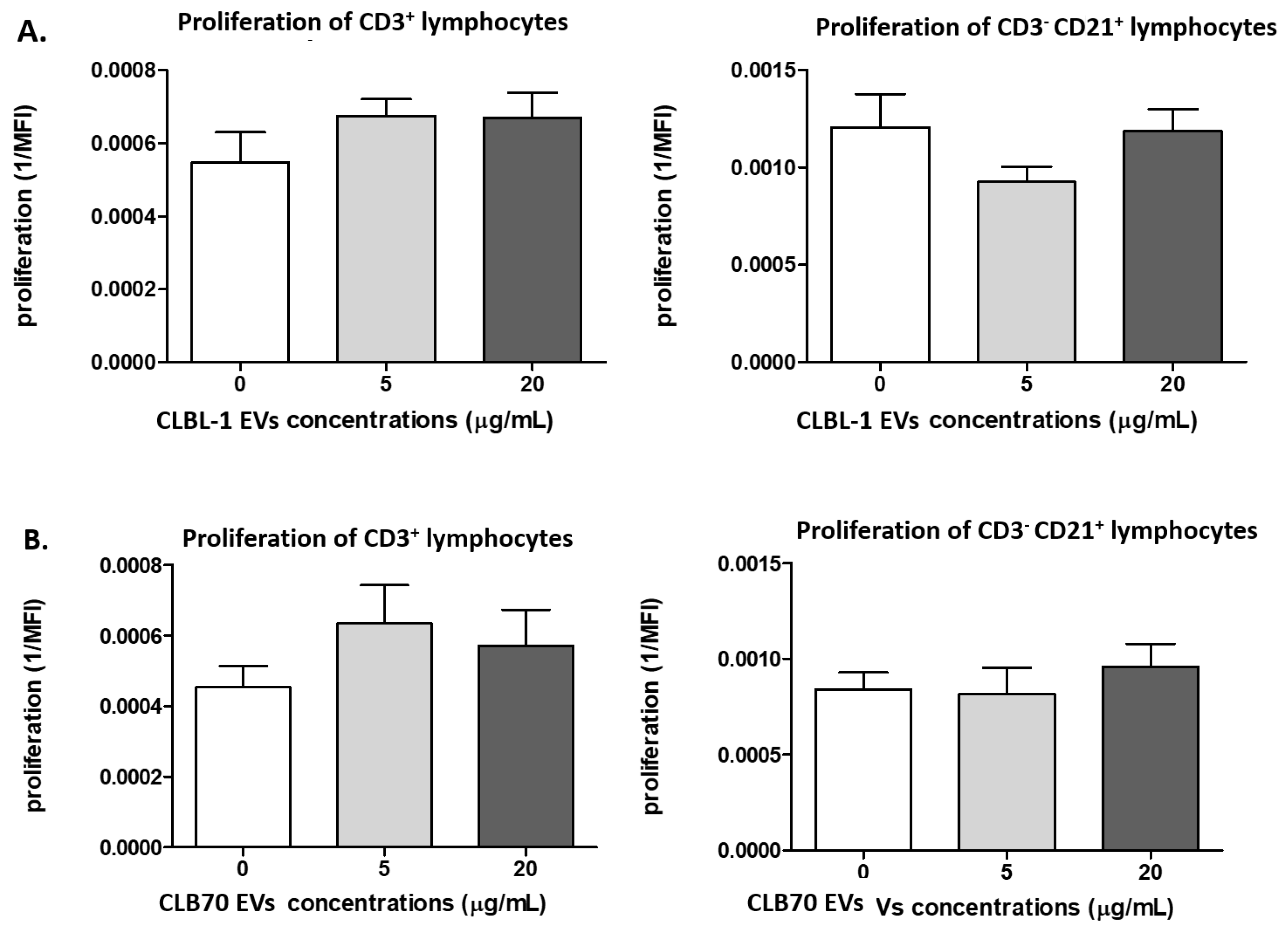
2.2.1. CLBL-1 EVs and CLB70 EVs Do Not Influence Lymphocyte Proliferative Capacity
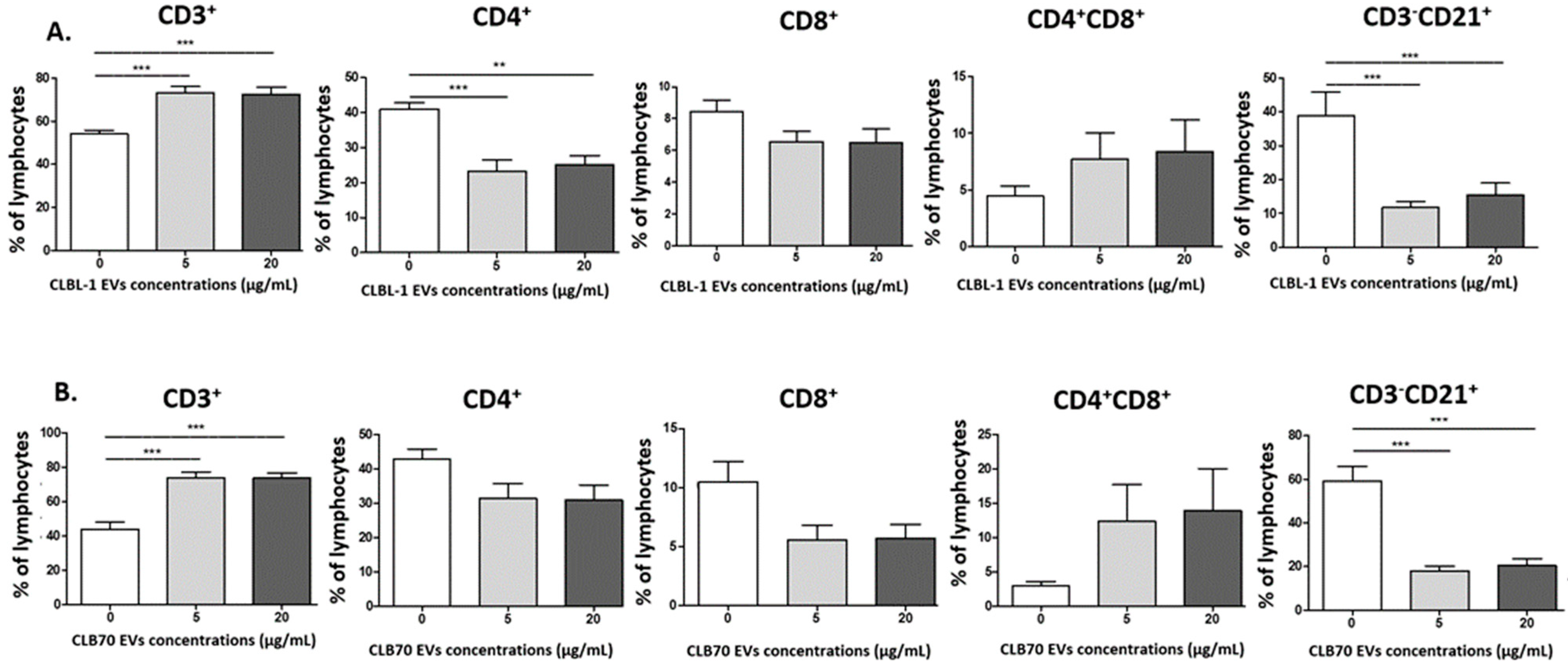
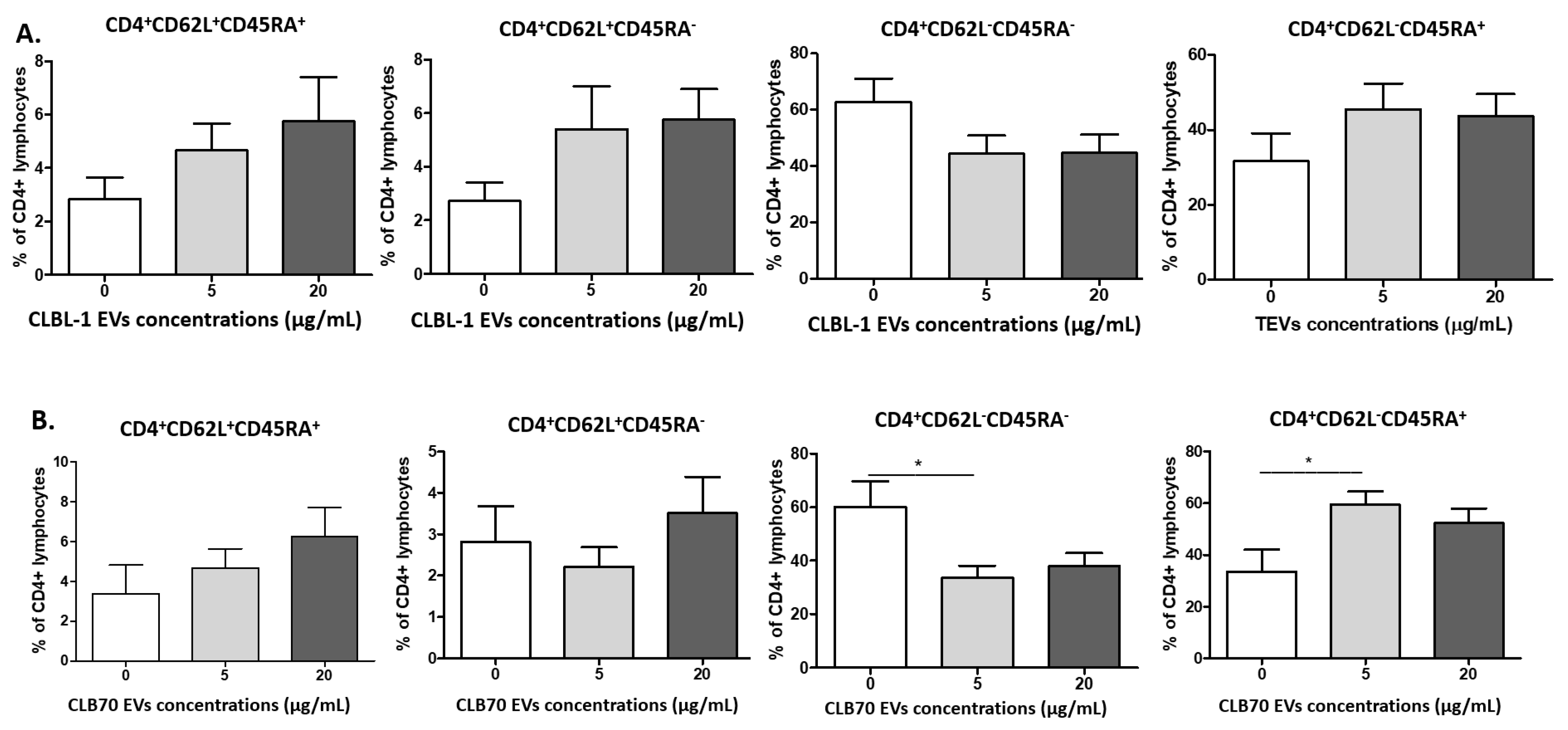
2.2.2. CLBL-1 EVs and CLB70 EVs Have an Impact on Lymphocyte Immunophenotype
2.2.3. CLB70 EVs Influence Lymphocytes’ T Cytokine Production
3. Discussion
4. Materials and Methods
4.1. Animals and Blood Samples
4.2. Isolation and Staining CLBL-1 EVs and CLB70 EVs
4.3. PBMC Isolation and Culture
4.4. Cell Staining
4.5. Flow Cytometry Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L.E. Platelets and extracellular vesicles and their cross talk with cancer. Blood 2021, 137, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Extracellular vesicles, news about their role in immune cells: Physiology, pathology and diseases. Clin. Exp. Immunol. 2019, 196, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 4, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef]
- Navarro-Tableros, V.; Gomez, Y.; Camussi, G.; Brizzi, M.F. Extracellular Vesicles: New Players in Lymphomas. Int. J. Mol. Sci. 2018, 20, 41. [Google Scholar] [CrossRef]
- Sedgwick, A.E.; D’Souza-Schorey, C. The biology of extracellular microvesicles. Traffic 2018, 19, 319–327. [Google Scholar] [CrossRef]
- Żmigrodzka, M.; Guzera, M.; Miśkiewicz, A.; Jagielski, D.; Winnicka, A. The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression. Tumor Biol. 2016, 37, 14391–14401. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Ortiz, A. Extracellular vesicles in cancer progression. Semin. Cancer Biol. 2021, 76, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Żmigrodzka, M.; Witkowska-Piłaszewicz, O.; Pingwara, R.; Winnicka, A. Platelet Extracellular Vesicles Are Taken up by Canine T Lymphocytes but Do Not Play a Role in Their Proliferation, Differentiation and Cytokine Production In Vitro. Int. J. Mol. Sci. 2022, 23, 5504. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Man, Q.; Gao, X.; Lin, H.; Wang, J.; Su, F.; Wang, H.; Bu, L.; Liu, B.; Chen, G. Tissue-derived extracellular vesicles in cancers and non-cancer diseases: Present and future. J. Extracell. Vesicles 2021, 10, e12175. [Google Scholar] [CrossRef]
- Matarredona, E.R.; Pastor, A.M. Extracellular Vesicle-Mediated Communication between the Glioblastoma and Its Microenvironment. Cells 2019, 9, 96. [Google Scholar] [CrossRef]
- Chang, W.-H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. Methods Mol. Biol. 2021, 2174, 143–170. [Google Scholar] [CrossRef]
- Pawlak, A.; Ziolo, E.; Kutkowska, J.; Blazejczyk, A.; Wietrzyk, J.; Krupa, A.; Hildebrand, W.; Dziegiel, P.; Dzimira, S.; Obminska-Mrukowicz, B.; et al. A novel canine B-cell leukaemia cell line. Establishment, characterisation and sensitivity to chemotherapeutics. Vet. Comp. Oncol. 2017, 15, 1218–1231. [Google Scholar] [CrossRef]
- Marconato, L.; Gelain, M.E.; Comazzi, S. The dog as a possible animal model for human non-Hodgkin lymphoma: A review. Hematol. Oncol. 2013, 31, 1–9. [Google Scholar] [CrossRef]
- Pawlak, A.; Rapak, A.; Zbyryt, I.; Obmińska-Mrukowicz, B. The effect of common antineoplastic agents on induction of apoptosis in canine lymphoma and leukemia cell lines. In Vivo 2014, 28, 843–850. [Google Scholar]
- Avery, A.C. The Genetic and Molecular Basis for Canine Models of Human Leukemia and Lymphoma. Front. Oncol. 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Rout, E.D.; Labadie, J.D.; Yoshimoto, J.A.; Avery, P.R.; Curran, K.M.; Avery, A.C. Clinical outcome and prognostic factors in dogs with B-cell chronic lymphocytic leukemia: A retrospective study. J. Vet. Intern. Med. 2021, 35, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Henklewska, M.; Pawlak, A.; Li, R.-F.; Yi, J.; Zbyryt, I.; Obmińska-Mrukowicz, B. Benzyl Isothiocyanate, a Vegetable-Derived Compound, Induces Apoptosis via ROS Accumulation and DNA Damage in Canine Lymphoma and Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 11772. [Google Scholar] [CrossRef] [PubMed]
- Żmigrodzka, M.; Witkowska-Piłaszewicz, O.; Rzepecka, A.; Cywińska, A.; Jagielski, D.; Winnicka, A. Extracellular Vesicles in the Blood of Dogs with Cancer—A Preliminary Study. Animals 2019, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Friend, C.; Marovitz, W.; Henie, G.; Henie, W.; Tsuei, D.; Hirschhorn, K.; Holland, J.G.; Cuttner, J. Observations on cell lines derived from a patient with Hodgkin’s disease. Cancer Res. 1978, 38, 2581–2591. [Google Scholar]
- Nehrbas, J.; Butler, J.T.; Chen, D.-W.; Kurre, P. Extracellular Vesicles and Chemotherapy Resistance in the AML Microenvironment. Front. Oncol. 2020, 10, 90. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Corrado, C.; Fontana, S.; Flugy, A.; Rizzo, A.; De Leo, G.; Alessandro, R. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun. Signal. 2015, 13, 8. [Google Scholar] [CrossRef]
- Pando, A.; Reagan, J.L.; Quesenberry, P.; Fast, L.D. Extracellular vesicles in leukemia. Leuk. Res. 2018, 64, 52–60. [Google Scholar] [CrossRef]
- Menck, K.; Bleckmann, A.; Wachter, A.; Hennies, B.; Ries, L.; Schulz, M.; Balkenhol, M.; Pukrop, T.; Schatlo, B.; Rost, U.; et al. Characterisation of tumour-derived microvesicles in cancer patients’ blood and correlation with clinical outcome. J. Extracell. Vesicles 2017, 6, 1340745. [Google Scholar] [CrossRef]
- Menck, K.; Sivaloganathan, S.; Bleckmann, A.; Binder, C. Microvesicles in Cancer: Small Size, Large Potential. Int. J. Mol. Sci. 2020, 21, 5373. [Google Scholar] [CrossRef]
- Noonin, C.; Thongboonkerd, V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics 2021, 11, 4436–4451. [Google Scholar] [CrossRef] [PubMed]
- Fortis, S.P.; Papageorgiou, E.G.; Antonelou, M.H.; Kriebardis, A.G. Blood Cell-Derived Microvesicles in Hematological Diseases and beyond. Biomolecules 2022, 12, 803. [Google Scholar] [CrossRef]
- Swatler, J.; Turos-Korgul, L.; Brewinska-Olchowik, M.; De Biasi, S.; Dudka, W.; Le, B.V.; Kominek, A.; Cyranowski, S.; Pilanc, P.; Mohammadi, E.; et al. 4-1BBL–containing leukemic extracellular vesicles promote immunosuppressive effector regulatory T cells. Blood Adv. 2022, 6, 1879–1894. [Google Scholar] [CrossRef]
- Bennit, H.R.F.; Gonda, A.; Oppegard, L.J.; Chi, D.P.; Khan, S.; Wall, N.R. Uptake of lymphoma-derived exosomes by peripheral blood leukocytes. Blood Lymphat. Cancer: Targets Ther. 2017, 7, 9–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hazan-Halevy, I.; Rosenblum, D.; Weinstein, S.; Bairey, O.; Raanani, P.; Peer, D. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett. 2015, 364, 59–69. [Google Scholar] [CrossRef]
- O’Brien, K.; Ughetto, S.; Mahjoum, S.; Nair, A.V.; Breakefield, X.O. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep. 2022, 39, 110651. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Zucchetti, A.E.; Enserink, L.; Jouve, M.; Lankar, D.; Saitakis, M.; Martin-Jaular, L.; Théry, C. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017, 36, 3012–3028. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Whiteside, T.L. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem. Soc. Trans. 2013, 41, 245–251. [Google Scholar] [CrossRef]
- Shinohara, H.; Kuranaga, Y.; Kumazaki, M.; Sugito, N.; Yoshikawa, Y.; Takai, T.; Taniguchi, K.; Ito, Y.; Akao, Y. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer–Derived Extracellular Vesicles. J. Immunol. 2017, 199, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J.; Whiteside, T.L. Tumor-Derived Microvesicles Promote Regulatory T Cell Expansion and Induce Apoptosis in Tumor-Reactive Activated CD8+ T Lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef] [PubMed]
- Szajnik, M.; Czystowska, M.; Szczepanski, M.J.; Mandapathil, M.; Whiteside, T.L. Tumor-Derived Microvesicles Induce, Expand and Up-Regulate Biological Activities of Human Regulatory T Cells (Treg). PLoS ONE 2010, 5, e11469. [Google Scholar] [CrossRef]
- Hong, C.-S.; Muller, L.; Whiteside, T.L.; Boyiadzis, M. Plasma Exosomes as Markers of Therapeutic Response in Patients with Acute Myeloid Leukemia. Front. Immunol. 2014, 5, 160. [Google Scholar] [CrossRef]
- Schroeder, J.C.; Puntigam, L.; Hofmann, L.; Jeske, S.S.; Beccard, I.J.; Doescher, J.; Laban, S.; Hoffmann, T.K.; Brunner, C.; Theodoraki, M.-N.; et al. Circulating Exosomes Inhibit B Cell Proliferation and Activity. Cancers 2020, 12, 2110. [Google Scholar] [CrossRef]
- Patel, S.J.; Darie, C.C.; Clarkson, B.D. Exosome mediated growth efect on the non-growing pre-B acute lymphoblastic leukemia cells at low starting cell density. Am. J. Transl Res. 2016, 8, 3614–3629. [Google Scholar]
- Haque, S.; Vaiselbuh, S.R. Leukemia-derived exosomes induce paracrine and autocrine cell proliferation in pediatric ALL. Blood 2016, 128, 4080. [Google Scholar]
- Kuen, D.S.; Kim, B.S.; Chung, Y. IL-17-Producing Cells in Tumor Immunity: Friends or Foes? Immune Netw. 2020, 1, e6. [Google Scholar]
- Shao, Q.; Deng, L.; Liu, H.; Liu, Z.; Chen, J.; Jiang, F.; Yan, S.; Fu, R. Involvement of MM cell-derived exosomes in T lymphocytes immune responses. Oncol. Lett. 2020, 4, 31. [Google Scholar] [CrossRef]
- Zhu, S.; Li, S.; Yi, M.; Li, N.; Wu, K. Roles of Microvesicles in Tumor Progression and Clinical Applications. Int. J. Nanomed. 2021, 16, 7071–7090. [Google Scholar] [CrossRef]
- Marshall, N.A.; Christie, L.E.; Munro, L.R.; Culligan, D.J.; Johnston, P.W.; Barker, R.N.; Vickers, M. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 2004, 103, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar]
- Withers, S.S.; Moore, P.F.; Chang, H.; Choi, J.W.; McSorley, S.J.; Kent, M.S.; Monjazeb, A.M.; Canter, R.J.; Murphy, W.J.; Sparger, E.E.; et al. Multi-color flow cytometry for evaluating age-related changes in memory lymphocyte subsets in dogs. Dev. Comp. Immunol. 2018, 87, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, K.; Nelson, M.H.; Bailey, S.R.; Bowers, J.S.; Yu, X.-Z.; Rubinstein, M.P.; Himes, R.A.; Paulos, C.M. Exploiting IL-17-producing CD4+ and CD8+ T cells to improve cancer immunotherapy in the clinic. Cancer Immunol. Immunother. 2016, 65, 247–259. [Google Scholar] [CrossRef]
- Rütgen, B.C.; Hammer, S.E.; Gerner, W.; Christian, M.; de Arespacochaga, A.G.; Willmann, M.; Kleiter, M.; Schwendenwein, I.; Saalmüller, A. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk. Res. 2010, 34, 932–938. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Paone, S.; Zanker, D.J.; Duan, M.; Phan, T.K.; Chen, W.; Hulett, M.; Poon, I.K.H. Isolation of cell type-specific apoptotic bodies by fluorescence-activated cell sorting. Sci. Rep. 2017, 7, 39846. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef]
- Czernek, L.; Chworos, A.; Duechler, M. The Uptake of Extracellular Vesicles is Affected by the Differentiation Status of Myeloid Cells. Scand. J. Immunol. 2015, 82, 506–514. [Google Scholar] [CrossRef]
- Rhys, H.I.; Dell’Accio, F.; Pitzalis, C.; Moore, A.; Norling, L.V.; Perretti, M. Neutrophil Microvesicles from Healthy Control and Rheumatoid Arthritis Patients Prevent the Inflammatory Activation of Macrophages. eBioMedicine 2018, 29, 60–69. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Clone | Isotype | Host Species | Fluorochrome | Dilution |
|---|---|---|---|---|---|
| CD4:CD8 | YKIX302.9/YCATE55.9 | IgG2a | rat | FITC:PE | 1:5 |
| CD4 | YKIX302.9 | IgG2a | rat | AF647 | 1:5 |
| CD8 | YCATE55.9 | IgG1 | rat | FITC | 1:5 |
| CD3:CD8 | CA17.2A12/YCATE55.9 | IgG1 | mouse | FITC:PE | 1:5 |
| CD21 | CA2.1D6 | IgG1 | mouse | AF647 | 1:5 |
| CD45RA | CA4.1D3 | IgG1 | mouse | - | 1:5 |
| CD62L | FMC46 | IgG2b | mouse | PB | 1:5 |
| IL-17A | eBio17B7 | IgG2a,κ | rat | PE | 1:20 |
| IFNγ | CC302 | IgG1 | mouse | AF647 | 1:5 |
| IgG1 | M1-14D12 | IgG1 | rat | PE | 1:5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zmigrodzka, M.; Witkowska-Pilaszewicz, O.; Pingwara, R.; Pawlak, A.; Winnicka, A. Canine B Cell Lymphoma- and Leukemia-Derived Extracellular Vesicles Moderate Differentiation and Cytokine Production of T and B Cells In Vitro. Int. J. Mol. Sci. 2022, 23, 9831. https://doi.org/10.3390/ijms23179831
Zmigrodzka M, Witkowska-Pilaszewicz O, Pingwara R, Pawlak A, Winnicka A. Canine B Cell Lymphoma- and Leukemia-Derived Extracellular Vesicles Moderate Differentiation and Cytokine Production of T and B Cells In Vitro. International Journal of Molecular Sciences. 2022; 23(17):9831. https://doi.org/10.3390/ijms23179831
Chicago/Turabian StyleZmigrodzka, Magdalena, Olga Witkowska-Pilaszewicz, Rafał Pingwara, Aleksandra Pawlak, and Anna Winnicka. 2022. "Canine B Cell Lymphoma- and Leukemia-Derived Extracellular Vesicles Moderate Differentiation and Cytokine Production of T and B Cells In Vitro" International Journal of Molecular Sciences 23, no. 17: 9831. https://doi.org/10.3390/ijms23179831
APA StyleZmigrodzka, M., Witkowska-Pilaszewicz, O., Pingwara, R., Pawlak, A., & Winnicka, A. (2022). Canine B Cell Lymphoma- and Leukemia-Derived Extracellular Vesicles Moderate Differentiation and Cytokine Production of T and B Cells In Vitro. International Journal of Molecular Sciences, 23(17), 9831. https://doi.org/10.3390/ijms23179831







