Microstructural Modification of TiAl6V4 Alloy to Avoid Detrimental Effects Due to Selective In Vivo Crevice Corrosion
Abstract
1. Introduction
- What influence do the microstructural constituents (e.g., phase, grain size, morphology) have on hardness, ductility and wear resistance?
- What effects do different physiologically relevant electrolyte compositions (PBS with H2O2 and HCl additives) have on the electrochemical properties of TiAl6V4?
- Are microstructural constituents of TiAl6V4 prone to dissolution under simulated inflammatory crevice conditions?
- Is cell adhesion affected by the present microstructure of TiAl6V4 alloy?
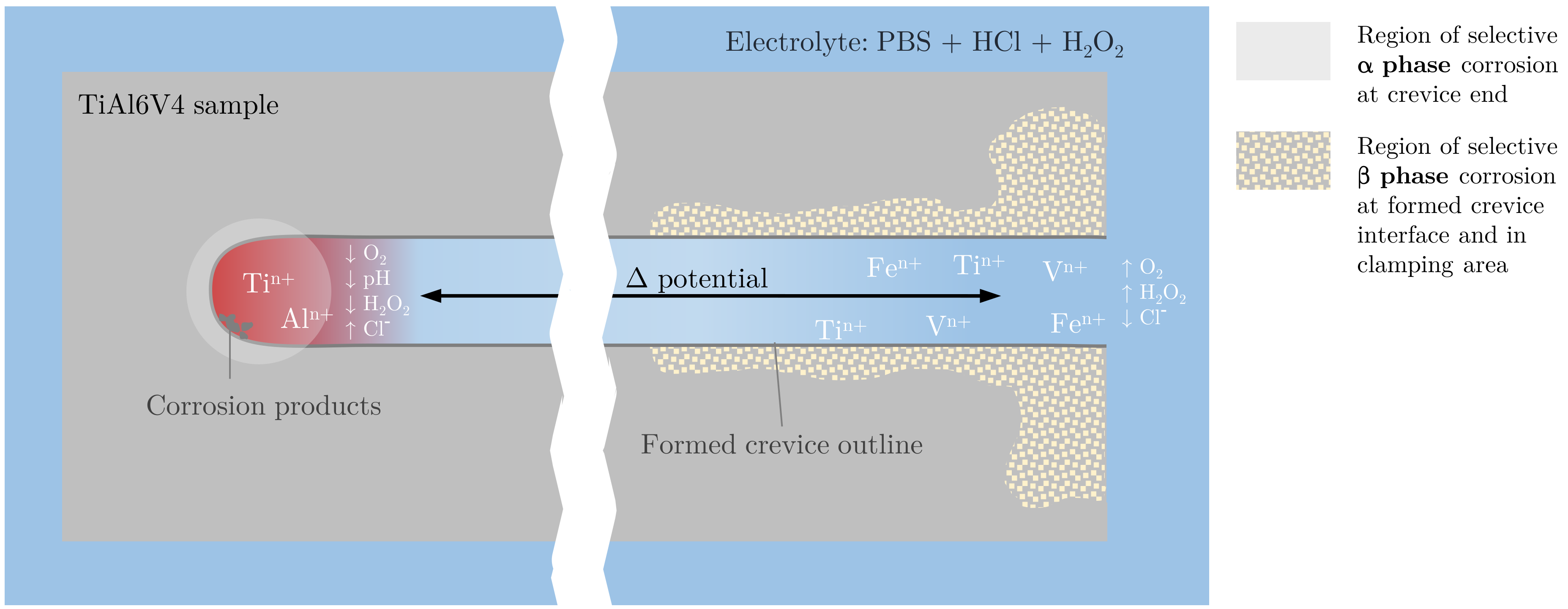
2. Materials and Methods
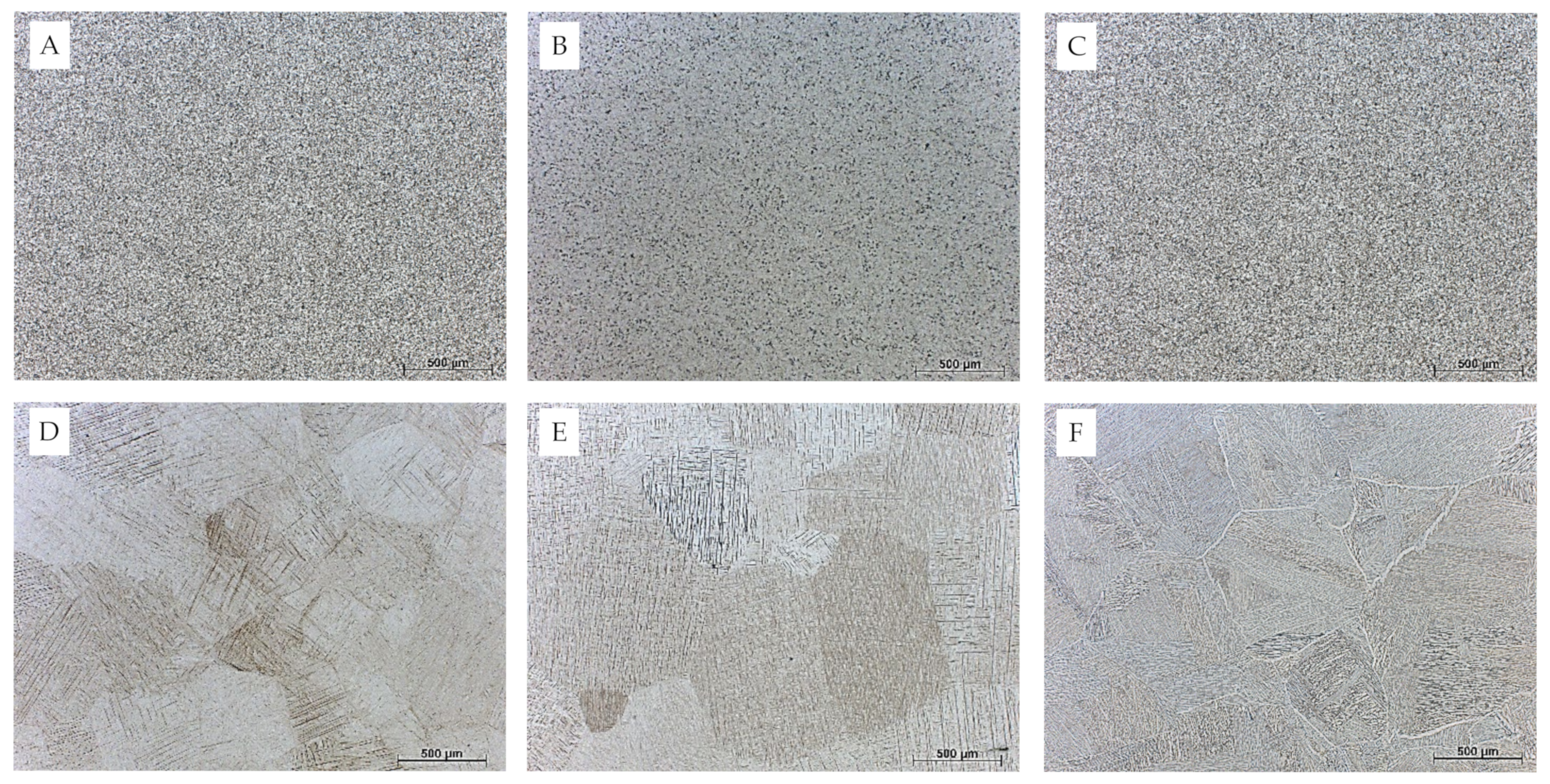
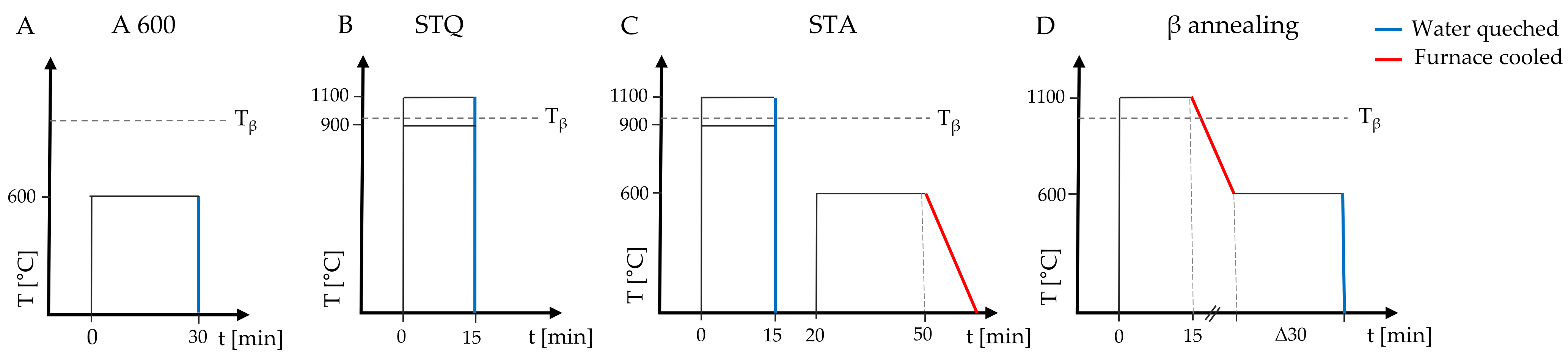
2.1. Sample Preparation and Heat Treatments
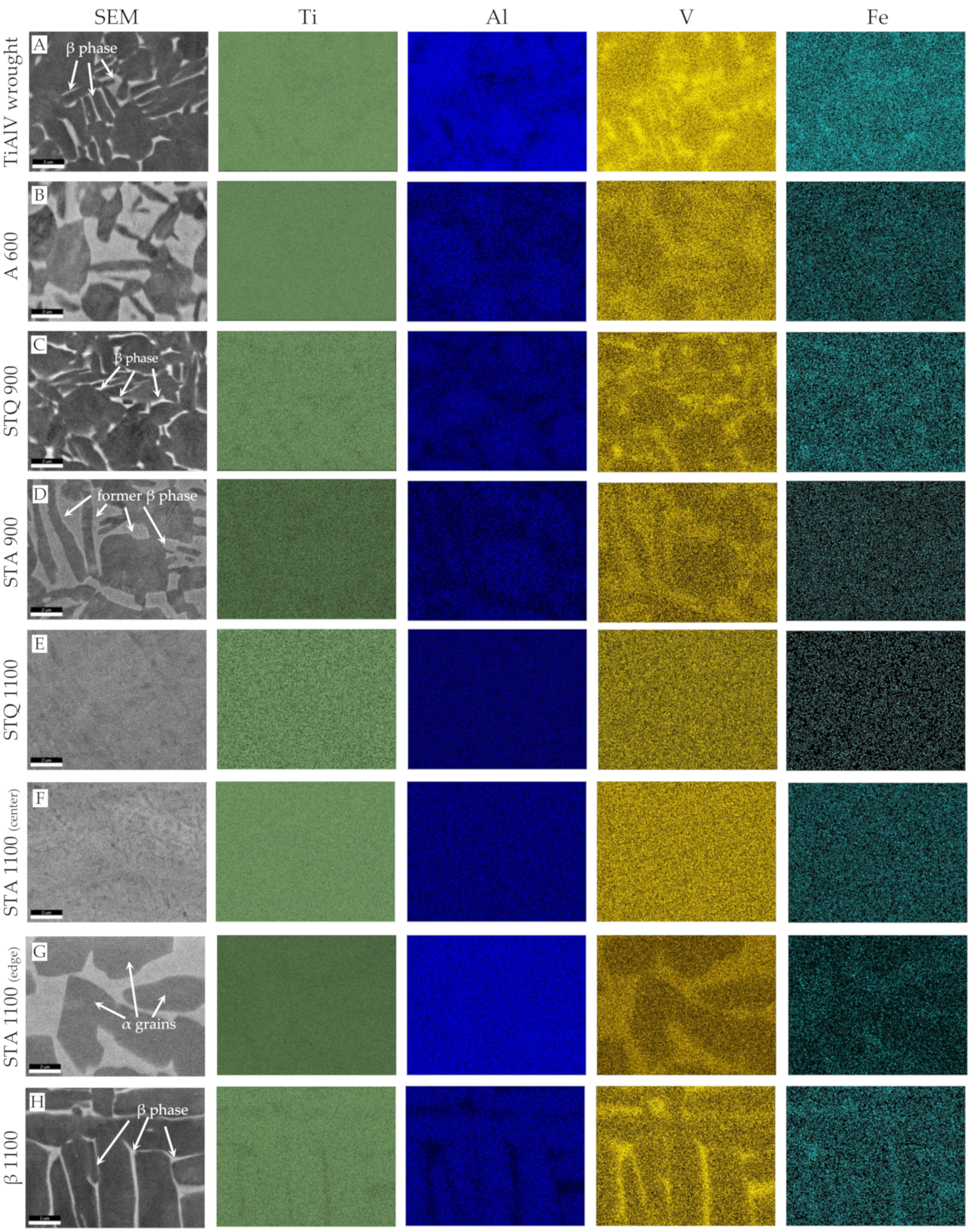
2.2. Metallographic Preparation and Microstructural Investigation
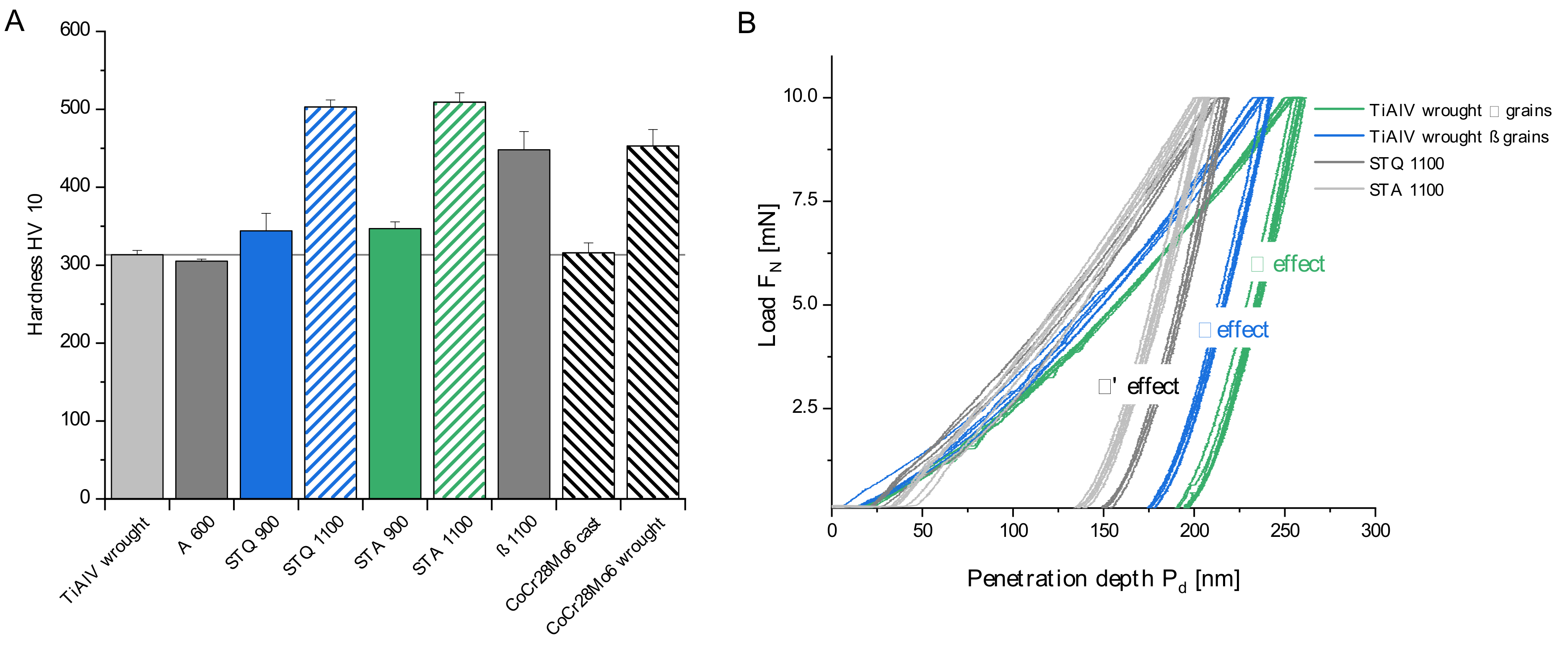
2.3. Mechanical Characterization
2.4. Tribological Tests
2.5. Corrosion Testing
2.5.1. Electrochemical Tests
2.5.2. Crevice Corrosion Tests
2.6. Cell Adhesion and Spreading
3. Results
3.1. Microstructure of Heat-Treated TiAl6V4 Alloy
3.2. Mechanical Characterization
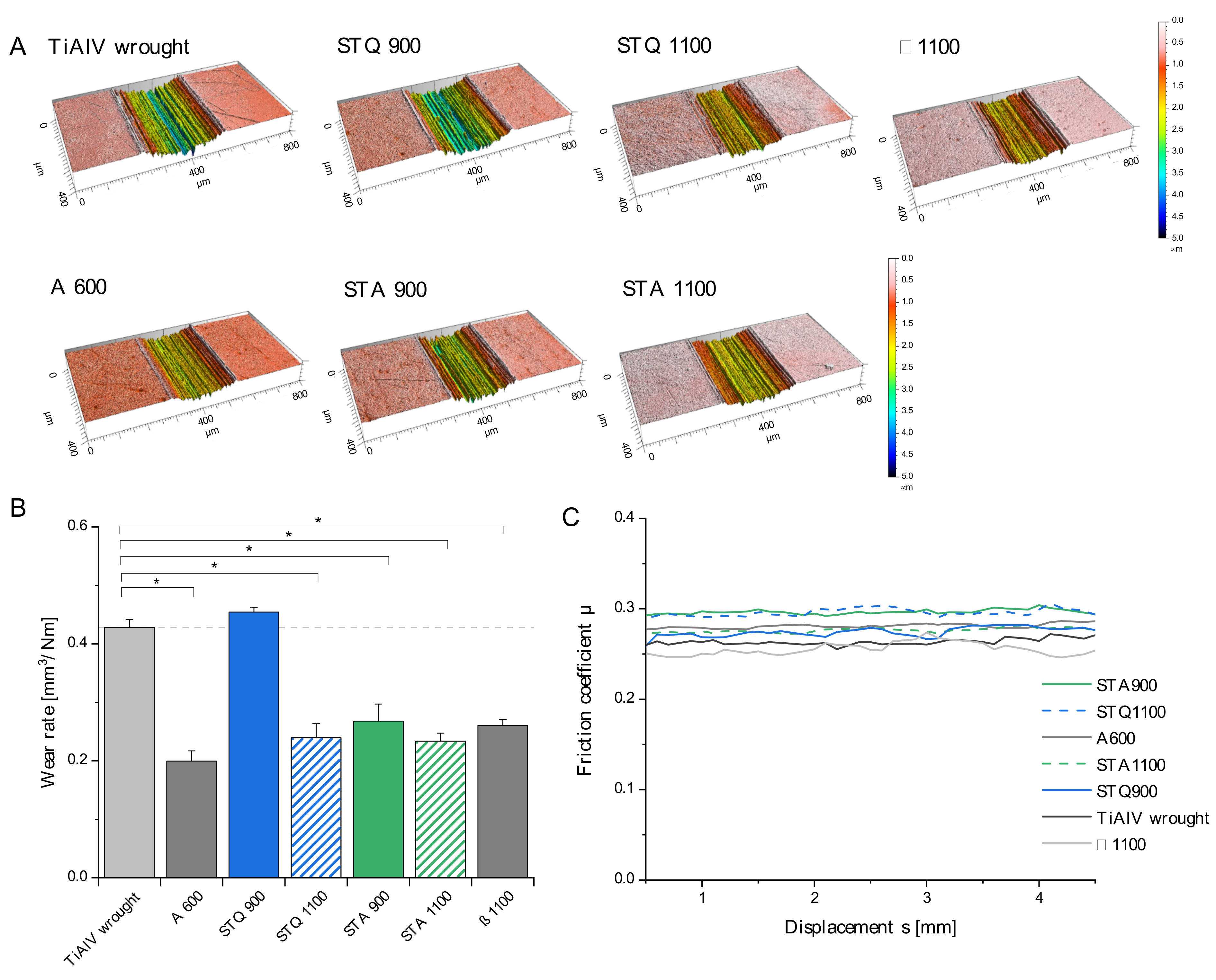
3.3. Tribological Properties
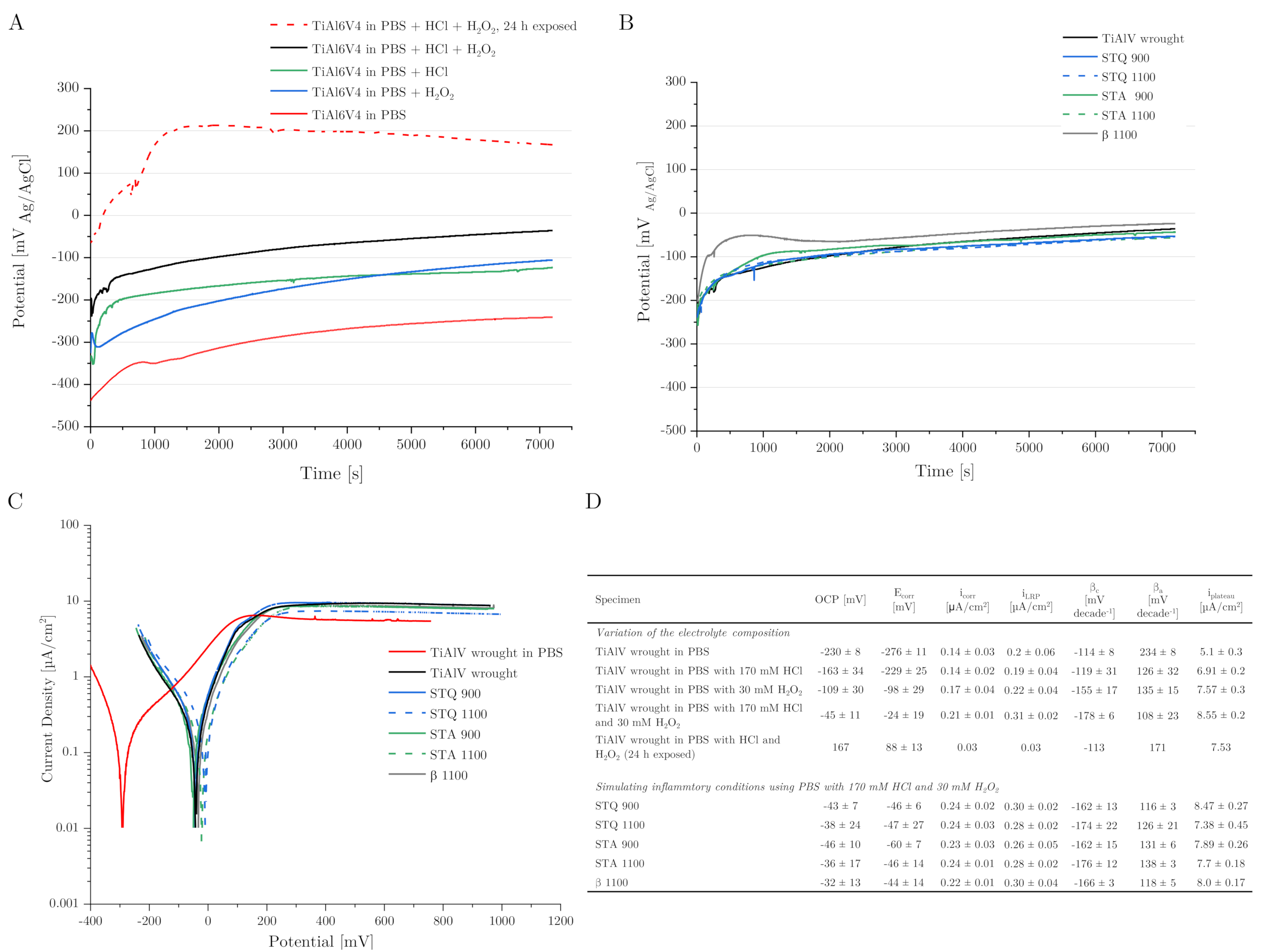
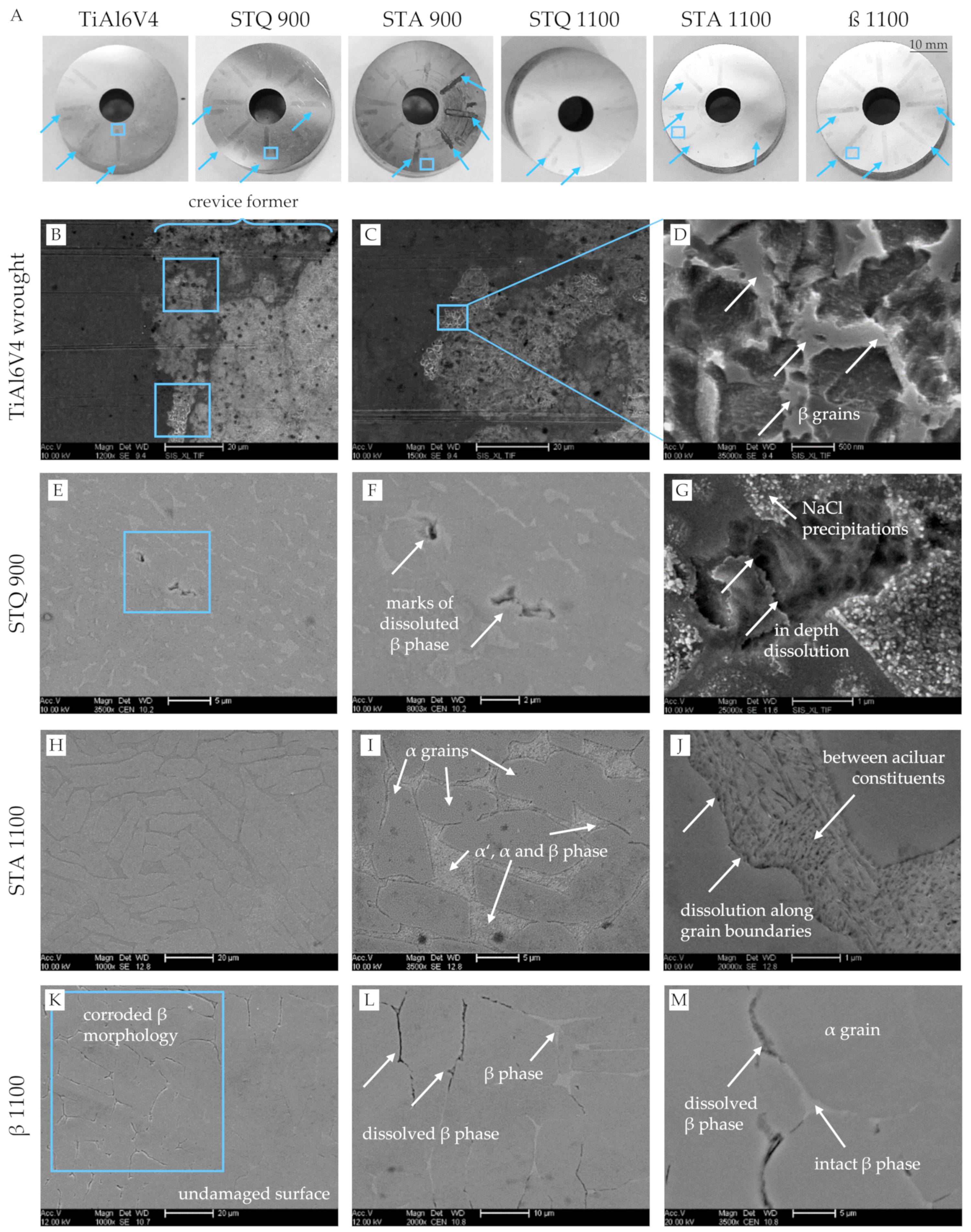
3.4. Corrosion Behavior
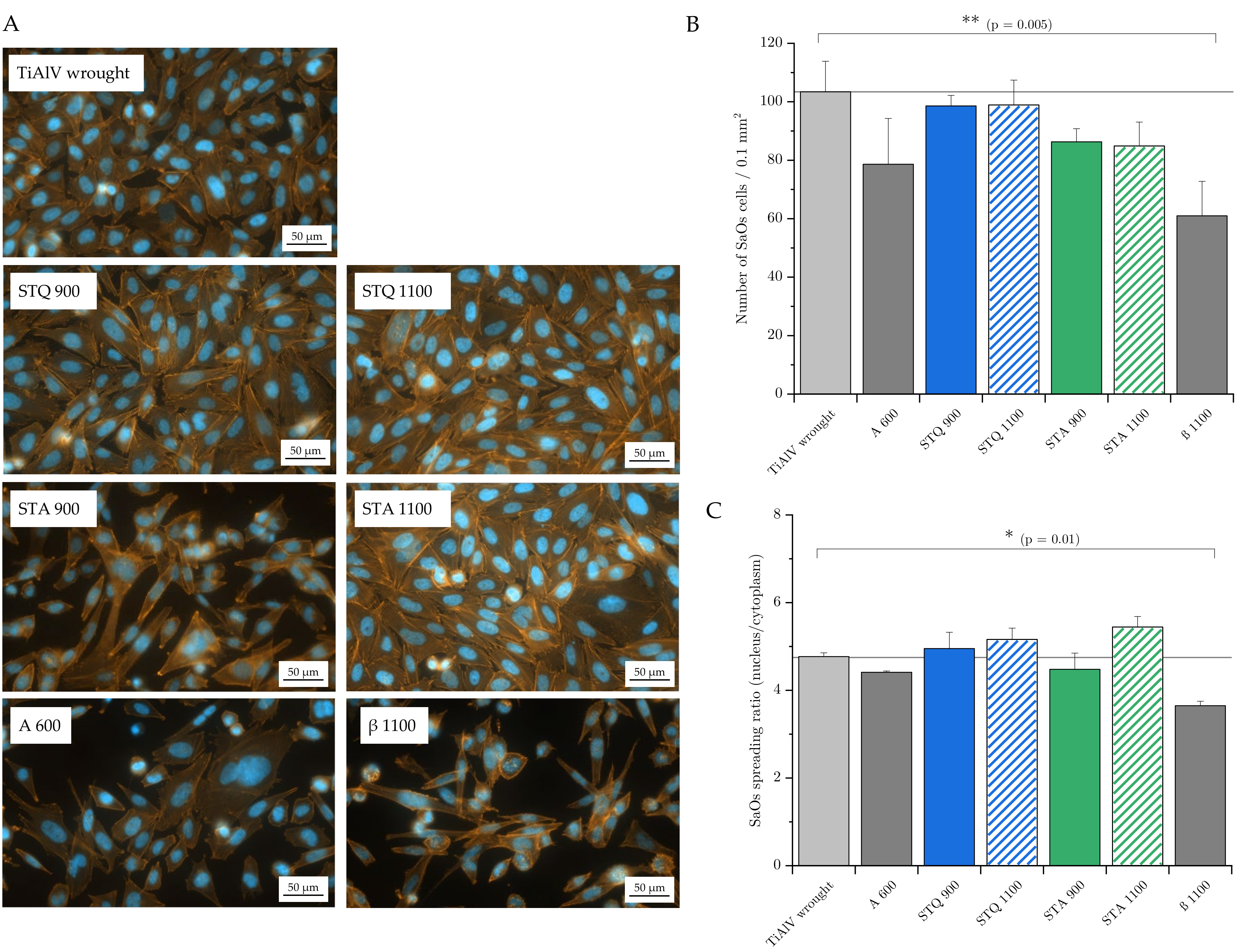
3.5. Cell Adhesion and Spreading
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Grimberg, A.; Jansson, V.; Lützner, J.; Melsheimer, O.; Morlock, M.; Steinbrück, A. EPRD Jahresbericht 2021; EPRD Endoprothesenregister Deutschland gGmbH: Berlin, Germany, 2021. [Google Scholar]
- ISO 5832-3 DIN EN ISO 5832-3:2017-03; Implants for Surgery Metallic Materials. Part 3: Wrought Titanium 6-Aluminium 4-Vanadium Alloy. Beuth Verlag GmbH: Berlin, Germany, 2017; pp. 65–97. [CrossRef]
- ASTM International, F04 Committee. Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant Applications (UNS R56401): ASTM F136-13(2021)e1(F136); ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar] [CrossRef]
- ASTM International, F04 Committee. ASTM F1472-20a: Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications (UNS R56400)(F1472); ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Urban, R.M.; Jacobs, J.J.; Gilbert, J.L. In vivo severe corrosion and hydrogen embrittlement of retrieved modular body titanium alloy hip-implants. J. Biomed. Mater. Res. 2009, 88, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.L.; Mali, S.; Urban, R.M.; Silverton, C.D.; Jacobs, J.J. In vivo oxide-induced stress corrosion cracking of Ti-6Al-4V in a neck-stem modular taper: Emergent behavior in a new mechanism of in vivo corrosion. J. Biomed. Mater. Res. 2012, 100, 584–594. [Google Scholar] [CrossRef]
- Hall, D.J.; Pourzal, R.; Della Valle, C.J.; Galante, J.O.; Jacobs, J.J.; Urban, R.M. Corrosion of Modular Junctions in Femoral and Acetabular Components for Hip Arthroplasty and Its Local and Systemic Effects. In Modularity and Tapers in Total Joint Replacement Devices; Greenwald, A.S., Kurtz, S.M., Lemons, J.E., Mihalko, W.M., Eds.; ASTM International: West Conshohocken, PA, USA, 2015; pp. 410–427. [Google Scholar]
- Luqman, M.; Seikh, A.H.; Sarkar, A.; Ragab, S.A.; Mohammed, J.A.; Ijaz, M.F.; Abdo, H.S. A Comparative Study of the Electrochemical Behavior of α and β Phase Ti6Al4V Alloy in Ringer’s Solution. Crystals 2020, 10, 190. [Google Scholar] [CrossRef]
- Prestat, M.; Thierry, D. Corrosion of titanium under simulated inflammation conditions: Clinical context and in vitro investigations. Acta Biomater. 2021, 136, 72–87. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Kobayashi, T.; Sumita, M.; Yamamoto, A.; Hanawa, T. Metal ion release from titanium with active oxygen species generated by rat macrophagesin vitro. J. Biomed. Mater. Res. 2000, 49, 238–243. [Google Scholar] [CrossRef]
- Pan, J.; Liao, H.; Leygraf, C.; Thierry, D.; Li, J. Variation of oxide films on titanium induced by osteoblast-like cell culture and the influence of an H2O2 pretreatment. J. Biomed. Mater. Res. 1998, 40, 244–256. [Google Scholar] [CrossRef]
- Berbel, L.O.; Banczek, E.d.P.; Karoussis, I.K.; Kotsakis, G.A.; Costa, I. Determinants of corrosion resistance of Ti-6Al-4V alloy dental implants in an In Vitro model of peri-implant inflammation. PLoS ONE 2019, 14, e0210530. [Google Scholar] [CrossRef]
- Prestat, M.; Vucko, F.; Holzer, L.; Thierry, D. Microstructural aspects of Ti6Al4V degradation in H2O2-containing phosphate buffered saline. Corros. Sci. 2021, 190, 109640. [Google Scholar] [CrossRef]
- Yu, F.; Addison, O.; Davenport, A.J. A synergistic effect of albumin and H₂O₂ accelerates corrosion of Ti6Al4V. Acta Biomater. 2015, 26, 355–365. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany; John Wiley & Sons: Chichester, UK, 2003. [Google Scholar]
- El-Hadad, S.; Nady, M.; Khalifa, W.; Shash, A. Influence of heat treatment conditions on the mechanical properties of Ti–6Al–4V alloy. Can. Metall. Q. 2018, 57, 186–193. [Google Scholar] [CrossRef]
- Meyer, L.W.; Krüger, L.; Sommer, K.; Halle, T.; Hockauf, M. Dynamic strength and failure behavior of titanium alloy Ti-6Al-4V for a variation of heat treatments. Mech. Time Depend. Mater. 2008, 12, 237–247. [Google Scholar] [CrossRef]
- Oh, S.-T.; Woo, K.-D.; Kim, J.-H.; Kwak, S.-M. The Effect of Al and V on Microstructure and Transformation of β Phase during Solution Treatments of Cast Ti-6Al-4V Alloy. Korean J. Met. Mater. 2017, 55, 150–155. [Google Scholar] [CrossRef]
- Wanying, L.; Yuanhua, L.; Yuhai, C.; Taihe, S.; Singh, A. Effect of Different Heat Treatments on Microstructure and Mechanical Properties of Ti6Al4V Titanium Alloy. Rare Met. Mater. Eng. 2017, 46, 634–639. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Guastaldi, A.C. Electrochemical stability and corrosion resistance of Ti-Mo alloys for biomedical applications. Acta Biomater. 2009, 5, 399–405. [Google Scholar] [CrossRef]
- Oliveira, N.T.; Aleixo, G.; Caram, R.; Guastaldi, A.C. Development of Ti–Mo alloys for biomedical applications: Microstructure and electrochemical characterization. Mater. Sci. Eng. A 2007, 452, 727–731. [Google Scholar] [CrossRef]
- Lütjering, G.; Williams, J.C. Titanium: With 51 Tables, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Kolli, R.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Yankov, E.H. Corrosion Study of Ti5Al4V and Ti6Al4V in Different Simulated Body Fluids. In Materials Design and Applications II; Da Silva, L.F.M., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Dull, D.L.; Raymond, L. Thermal and Mechanical Effects on the Corrosion Behavior of Ti-6Al-4V Alloy. J. Electrochem. Soc. 1973, 120, 1632. [Google Scholar] [CrossRef]
- Ryba, J.; Kawalec, M.; Tyrala, E.; Krawiec, H. Effect of Thermal Treatment of Biomedical Ti-6Al-4V Alloy on Corrosion Resistance in Simulated Physiological Solutions. Arch. Metall. Mater. 2019, 65, 39–45. [Google Scholar]
- Atapour, M.; Pilchak, A.; Frankel, G.S.; Williams, J.C.; Fathi, M.H.; Shamanian, M. Corrosion Behavior of Ti-6Al-4V with Different Thermomechanical Treatments and Microstructures. CORROSION 2010, 66, 065004-1–065004-9. [Google Scholar] [CrossRef]
- Herbster, M.; Rosemann, P.; Michael, O.; Harnisch, K.; Ecke, M.; Heyn, A.; Lohmann, C.H.; Bertrand, J.; Halle, T. Microstructure-dependent crevice corrosion damage of implant materials CoCr28Mo6 TiAl6V4 and REX 734 under severe inflammatory conditions. J. Biomed. Mater. Res. 2022, 110, 1687–1704. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 14577-1:2015-11; Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters—Part 1: Test Method (ISO 14577-1:2015); German Version EN ISO 14577-1:2015 (DIN EN ISO 14577-1:2015-11). Deutsches Institut für Normung e.V. DIN: Berlin, Germany, 2015.
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- ISO 14242-1; Implants for Surgery—Wear of Total Hip-Joint Prostheses (14242-1). BSI Standards Publication: London, UK, 2016.
- English, R.; Ashkanfar, A.; Rothwell, G. The effect of different assembly loads on taper junction fretting wear in total hip replacements. Tribol. Int. 2016, 95, 199–210. [Google Scholar] [CrossRef]
- Stern, M.; Geaby, A.L. Electrochemical Polarization. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Viswanathan, G.B.; Lee, E.; Maher, D.M.; Banerjee, S.; Fraser, H.L. Direct observations and analyses of dislocation substructures in the α phase of an α/β Ti-alloy formed by nanoindentation. Acta Mater. 2005, 53, 5101–5115. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; He, B.; Zou, Y. High-speed nanoindentation mapping of a near-alpha titanium alloy made by additive manufacturing. J. Mater. Res. 2021, 36, 2223–2234. [Google Scholar] [CrossRef]
- Hanada, S.; Ozaki, T.; Takahashi, E.; Watanabe, S.; Yoshimi, K.; Abumiya, T. Composition Dependence of Young’s Modulus in Beta Titanium Binary Alloys. Mater. Sci. Forum. 2003, 426, 3103–3108. [Google Scholar] [CrossRef]
- Fedotov, S.G. Peculiarities of Changes in Elastic Properties of Titanium Martensite. In Titanium Science and Technology; Jaffee, R.I., Burte, H.M., Eds.; Springer: Boston, MA, USA, 1973. [Google Scholar]
- Cai, J.; Li, F.; Liu, T.; Chen, B. Investigation of mechanical behavior of quenched Ti–6Al–4V alloy by microindentation. Mater. Charact. 2011, 62, 287–293. [Google Scholar] [CrossRef]
- Cvijovic-Alagic, I.; Mitrovic, S.; Cvijovic, Z.; Veljovic, D.; Babic, M.; Rakin, M. Influence of the Heat Treatment on the Tribological Characteristics of the Ti-based Alloy for Biomedical Applications. Tribol. Ind. 2009, 31, 17–22. [Google Scholar]
- Fan, Y.; Tian, W.; Guo, Y.; Sun, Z.; Xu, J. Relationships among the Microstructure, Mechanical Properties, and Fatigue Behavior in Thin Ti6Al4V. Adv. Mater. Sci. Eng. 2016, 2016, 7278267. [Google Scholar] [CrossRef]
- Youssef, S.S.S.; Ibrahim, K.M.; Abdel-Karim, M. Effect of heat treatment process on tribological behavior of Ti-6Al-4V Alloy. Int. J. Mech. Eng. Robot. Res. 2013, 2, 385–394. [Google Scholar] [CrossRef]
- Reda, R.; Nofal, A.; Hussein, A.-H.; El-Banna, E.-S. Tailoring of microstructure of Ti-6Al-4V implant castings for abrasive wear resistance. Int. J. Metall. Mater. Sci. Eng. 2014, 4, 29–44. [Google Scholar]
- Ganesh, B.K.C.; Ramanaih, N.; Chandrasekhar Rao, P.V. Dry Sliding Wear Behavior of Ti–6Al–4V Implant Alloy Subjected to Various Surface Treatments. Trans. Indian Inst. Met. 2012, 65, 425–434. [Google Scholar] [CrossRef]
- Ganesh, B.K.C. Effect of heat treatment on dry sliding wear of titanium-aluminum- vanadium (Ti-6Al-4V) implant alloy. J. Mech. Eng. Res. 2012, 4, 67–74. [Google Scholar] [CrossRef]
- Carsten, K. Einfluss der Wärmebehandlung auf Gefüge, Korrosionsbeständigkeit und Biokompatiblität von CoCrMo-Legierungen. Master’s Thesis, Otto-von-Guericke Unviersity, Magdeburg, Germany, 2018. [Google Scholar]
- Noguchi, T.; Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Discoloration and dissolution of titanium and titanium alloys with immersion in peroxide- or fluoride-containing solutions. Dent. Mater. J. 2008, 27, 117–123. [Google Scholar] [CrossRef]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Discoloration of titanium alloy in acidic saline solutions with peroxide. Dent. Mater. J. 2013, 32, 19–24. [Google Scholar] [CrossRef][Green Version]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical and XPS studies of titanium for biomaterial applications with respect to the effect of hydrogen peroxide. J. Biomed. Mater. Res. 1994, 28, 113–122. [Google Scholar] [CrossRef]
- Yu, F.; Addison, O.; Davenport, A. Temperature-Dependence Corrosion Behavior of Ti6Al4V in the Presence of HCl. Front. Mater. 2022, 9, 196. [Google Scholar] [CrossRef]
- Geetha, M.; Kamachi Mudali, U.; Gogia, A.; Asokamani, R.; Raj, B. Influence of microstructure and alloying elements on corrosion behavior of Ti–13Nb–13Zr alloy. Corros. Sci. 2004, 46, 877–892. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Kwokal, A.; Piljac, J. The influence of niobium and vanadium on passivity of titanium-based implants in physiological solution. Biomaterials 2003, 24, 3765–3775. [Google Scholar] [CrossRef]
- Kurtz, M.A.; Khullar, P.; Gilbert, J.L. Cathodic Activation and Inflammatory Species Are Critical to Simulating In Vivo Ti-6Al-4V Selective Dissolution. Acta Biomater. 2022, 149, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Ruzickova, M.; Hildebrand, H.; Virtanen, S. On the Stability of Passivity of Ti-Al Alloys in Acidic Environment. Z. Für Phys. Chem. 2005, 219, 1447–1459. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Peter, L.M.; Williams, D.E. Stability and open circuit breakdown of the passive oxide film on titanium. Electrochim. Acta 1988, 33, 1143–1149. [Google Scholar] [CrossRef]
- Smith, D.W. Ionic hydration enthalpies. J. Chem. Educ. 1977, 54, 540. [Google Scholar] [CrossRef]
- Challa, V.S.A.; Mali, S.; Misra, R.D.K. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti-6Al-4V. J. Biomed. Mater. Res. A 2013, 101, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Rao, S.; Asao, S.; Tateishi, T.; Katsuda, S.; Furuki, Y. Effects of Ti, Al and V Concentrations on Cell Viability. Mater. Trans. JIM 1998, 39, 1053–1062. [Google Scholar] [CrossRef]
- Kao, Y.L.; Tu, G.C.; Huang, C.A.; Liu, T.T. A study on the hardness variation of α- and β-pure titanium with different grain sizes. Mater. Sci. Eng. A 2005, 398, 93–98. [Google Scholar] [CrossRef]
- Okazaki, Y.; Rao, S.; Ito, Y.; Tateishi, T. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials 1998, 19, 1197–1215. [Google Scholar] [CrossRef]
- Molinuevo, M.S.; Cortizo, A.M.; Etcheverry, S.B. Vanadium (IV) complexes inhibit adhesion, migration and colony formation of UMR106 osteosarcoma cells. Cancer Chemother Pharm. 2008, 61, 767–773. [Google Scholar] [CrossRef]
- Hosseini, M.-J.; Seyedrazi, N.; Shahraki, J.; Pourahmad, J. Vanadium induces liver toxicity through reductive activation by glutathione and mitochondrial dysfunction. Adv. Biosci. Biotechnol. 2012, 3, 1096–1103. [Google Scholar] [CrossRef]
- ASTM International, F04 Committee. ASTM FF2129-19a Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]

| Implant Material | Standard | Ti | Al | V | Fe | O | N | C | H |
|---|---|---|---|---|---|---|---|---|---|
| TiAl6V4 wrought alloy, ELI | ISO 5832-3, ASTM F136 | Balance | 5.5–6.5 | 3.5–4.5 | ≤0.25 | ≤0.13 | ≤0.05 | ≤0.08 | ≤0.0125 |
| TiAl6V4 ELI (certificate) | Balance | 6.06 | 4.14 | 0.2 | 0.11 | 0.01 | <0.01 | 0.01 |
| Sample | Grain Size (µm2) | Area Fraction (A %) | Ti (m %) | Al (m %) | V (m %) | Fe (m %) | Total Alloying Elements (m%) | N (m%) | O (m%) |
|---|---|---|---|---|---|---|---|---|---|
| ISO 5832-3 * | balance | 5.5–6.75 | 3.5–4.5 | ≤0.3 | 9–11.6 | <0.05 | <0.2 | ||
| TiAl6V4 wrought (integral) untreated control condition | 88.9 ± 0.4 | 7.0 ± 0.1 | 3.8 ± 0.3 | 0.3 ± 0.1 | 11.1 ± 0.4 | 0.002 ± 0.001 | 0.003 ± 0.001 | ||
| 12.5 ± 6.2 | 85.1 ± 0.8 | 86.5 ± 0.2 | 8.0 ± 0.1 | 4.7 ± 0.2 | 0.7 ± 0.2 | 13.4 ± 0.5 | ||
| 0.9 ± 0.3 | 14.9 ± 0.2 | 77.7 ± 1.6 | 4.7 ± 0.2 | 15.7 ± 1.5 | 1.8 ± 0.1 | 22.2 ± 1.8 | ||
| A 600 (integral) | 42 ± 21 | 88.8 ± 0.4 | 7.0 ± 0.1 | 3.8 ± 0.1 | 0.3 ± 0.1 | 11.1 ± 0.3 | 0.004 ± 0.002 | 0.003 ± 0.001 | |
| 57.8 ± 1.3 | 86.6 ± 0.5 | 7.7 ± 0.1 | 4.7 ± 0.2 | 0.7 ± 0.1 | 13.1 ± 0.3 | |||
| 42.2 ± 1.4 | 84.3 ± 0.1 | 6.1 ± 0.1 | 8.5 ± 0.1 | 1.0 ± 0.1 | 15.6 ± 0.3 | |||
| STQ 900 (integral) | 58 ± 21 | 88.4 ± 0.3 | 7.0 ± 0.1 | 4.2 ± 0.3 | 0.3 ± 0.1 | 11.5 ± 0.5 | 0.005 ± 0.001 | 0.003 ± 0.001 | |
| 87.3 ± 0.5 | 86.5 ± 0.2 | 8.0 ± 0.1 | 4.7 ± 0.2 | 0.4 ± 0.2 | 13.1 ± 0.5 | |||
| 12.7 ± 0.3 | 77.7 ± 1.6 | 4.7 ± 0.2 | 15.7 ± 1.6 | 1.9 ± 0.2 | 22.3 ± 2 | |||
| STA 900 (integral) | 35 ± 28 | 89.3 ± 0.3 | 6.6 ± 0.1 | 3.8 ± 0.1 | 0.3 ± 0.1 | 10.7 ± 0.3 | 0.005 ± 0.001 | 0.003 ± 0.001 | |
| 70.1 ± 1.1 | 87.8 ± 0.1 | 7.3 ± 0.2 | 4.2 ± 0.2 | 0.6 ± 0.1 | 12.1 ± 0.4 | |||
| 29.9 ± 0.6 | 85.3 ± 0.2 | 5.8 ± 0.1 | 8.0 ± 0.1 | 0.9 ± 0.1 | 14.7 ± 0.3 | |||
| STQ 1100 (integral) | 245,000 ± 160,000 | 89.8 ± 0.3 | 6.8 ± 0.2 | 3.4 ± 0.3 | 0.3 ± 0.1 | 10.5 ± 0.6 | 0.002 ± 0.001 | 0.013 ± 0.002 | |
| STA 1100 (integral) | 475,000 ± 350,000 | 89.3 ± 0.1 | 6.8 ± 0.1 | 3.6 ± 0.1 | 0.3 ± 0.1 | 10.7 ± 0.3 | 0.002 ± 0.001 | 0.013 ± 0.003 | |
| 88.4 ± 0.2 | 7.7 ± 0.1 | 3.2 ± 0.1 | 0.6 ± 0.1 | 11.5 ± 0.3 | ||||
| ß 1100 (integral) | 454,000 ± 357,500 | 89.3 ± 0.2 | 6.9 ± 0.1 | 3.7 ± 0.2 | 0.3 ± 0.1 | 10.9 ± 0.4 | 0.005 ± 0.003 | 0.013 ± 0.002 | |
| 88.2 ± 0.8 | 87.7 ± 0.1 | 7.8 ± 0.1 | 3.8 ± 0.2 | 0.6 ± 0.1 | 12.2 ± 0.4 | |||
| 11.8 ± 0.3 | 79.1 ± 0.9 | 4.4 ± 0.4 | 14.9 ± 1.1 | 1.7 ± 0.2 | 21.0 ± 1.7 | |||
| Sample with Phase Effects | HIT (GPa) | EIT (GPa) |
|---|---|---|
| TiAl6V4 wrought | ||
| 5.9 ± 0.6 | 144.6 ± 6.9 |
| 4.9 ± 0.1 | 135.8 ± 5.7 |
| A 600 | ||
| 6.1 ± 0.1 | 141.6 ± 1.2 |
| 4.9 ± 0.1 | 133.1 ± 6.91 |
| STQ 900 | ||
| 6.1 ± 0.4 | 143.0 ± 8.2 |
| 5.5 ± 0.2 | 136.6 ± 7.1 |
| STA 900 | ||
| 6.0 ± 0.3 | 147.1 ± 6.5 |
| 5.0 ± 0.1 | 138.1 ± 6.1 |
| STQ 1100 (martensite) | 6.6 ± 0.4 | 164.8 ± 9.9 |
| STA 1100 (martensite) | 7.3 ± 0.5 | 148.8 ± 9.8 |
| β 1100 (air) | ||
| 8.5 ± 0.5 | 161.3 ± 7.2 |
| 6.2 ± 0.3 | 135.9 ± 7.1 |
| β 1100 (argon atmosphere) | ||
| 5.8 ± 0.1 | 149.7 ± 6.5 |
| 4.6 ± 0.2 | 140.8 ± 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbster, M.; Harnisch, K.; Kriegel, P.; Heyn, A.; Krüger, M.; Lohmann, C.H.; Bertrand, J.; Halle, T. Microstructural Modification of TiAl6V4 Alloy to Avoid Detrimental Effects Due to Selective In Vivo Crevice Corrosion. Materials 2022, 15, 5733. https://doi.org/10.3390/ma15165733
Herbster M, Harnisch K, Kriegel P, Heyn A, Krüger M, Lohmann CH, Bertrand J, Halle T. Microstructural Modification of TiAl6V4 Alloy to Avoid Detrimental Effects Due to Selective In Vivo Crevice Corrosion. Materials. 2022; 15(16):5733. https://doi.org/10.3390/ma15165733
Chicago/Turabian StyleHerbster, Maria, Karsten Harnisch, Paulina Kriegel, Andreas Heyn, Manja Krüger, Christoph H. Lohmann, Jessica Bertrand, and Thorsten Halle. 2022. "Microstructural Modification of TiAl6V4 Alloy to Avoid Detrimental Effects Due to Selective In Vivo Crevice Corrosion" Materials 15, no. 16: 5733. https://doi.org/10.3390/ma15165733
APA StyleHerbster, M., Harnisch, K., Kriegel, P., Heyn, A., Krüger, M., Lohmann, C. H., Bertrand, J., & Halle, T. (2022). Microstructural Modification of TiAl6V4 Alloy to Avoid Detrimental Effects Due to Selective In Vivo Crevice Corrosion. Materials, 15(16), 5733. https://doi.org/10.3390/ma15165733







