Impacts of Parthenium hysterophorus L. on Plant Species Diversity in Ginir District, Southeastern Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Methods
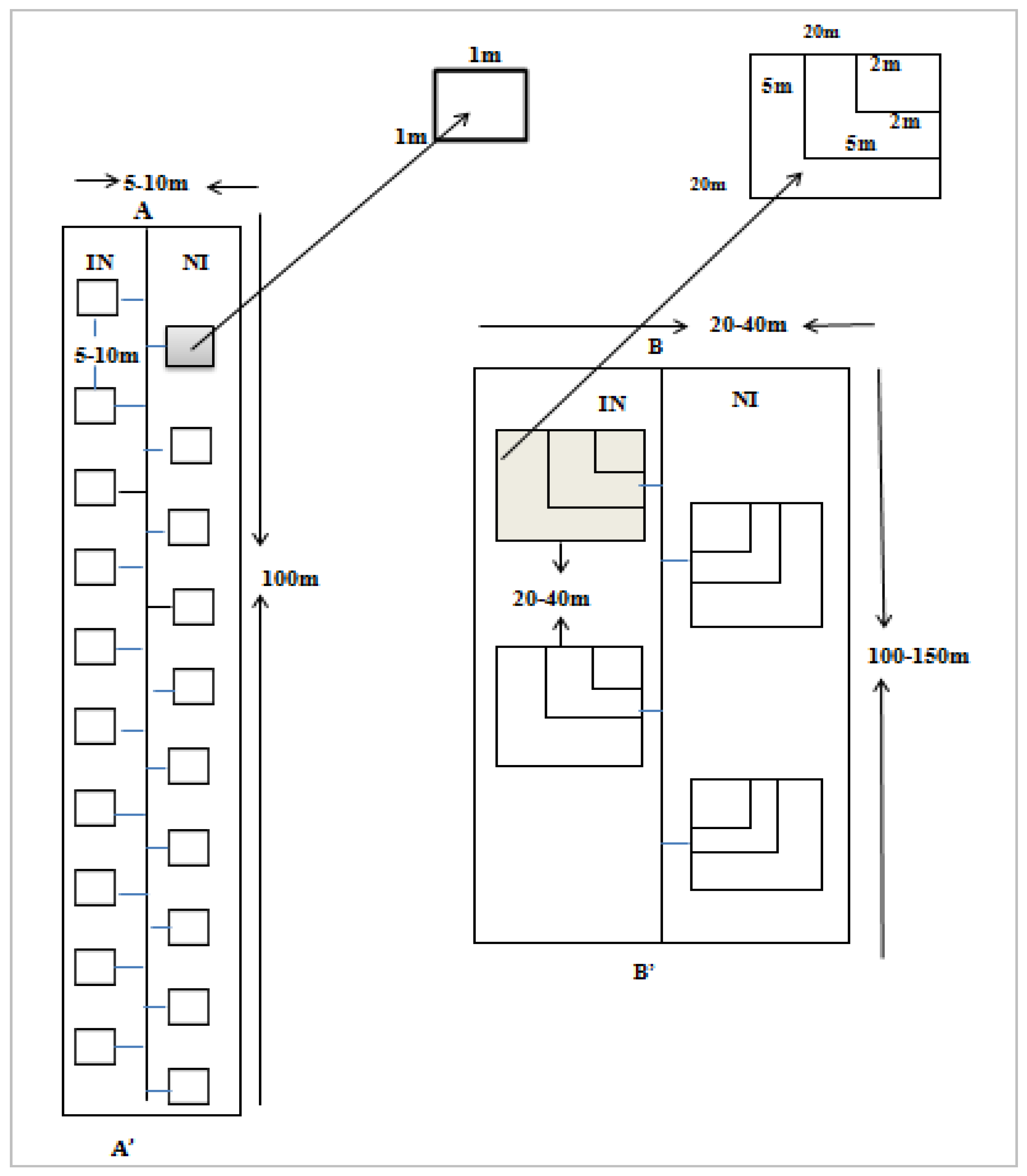
2.2.1. Sampling Design
2.2.2. Data Collection
2.2.3. Data Analysis
3. Results
3.1. Impacts of Parthenium on Herbaceous and Woody Species Diversity
3.1.1. Impacts of P. hysterophorus Invasion on Species Composition
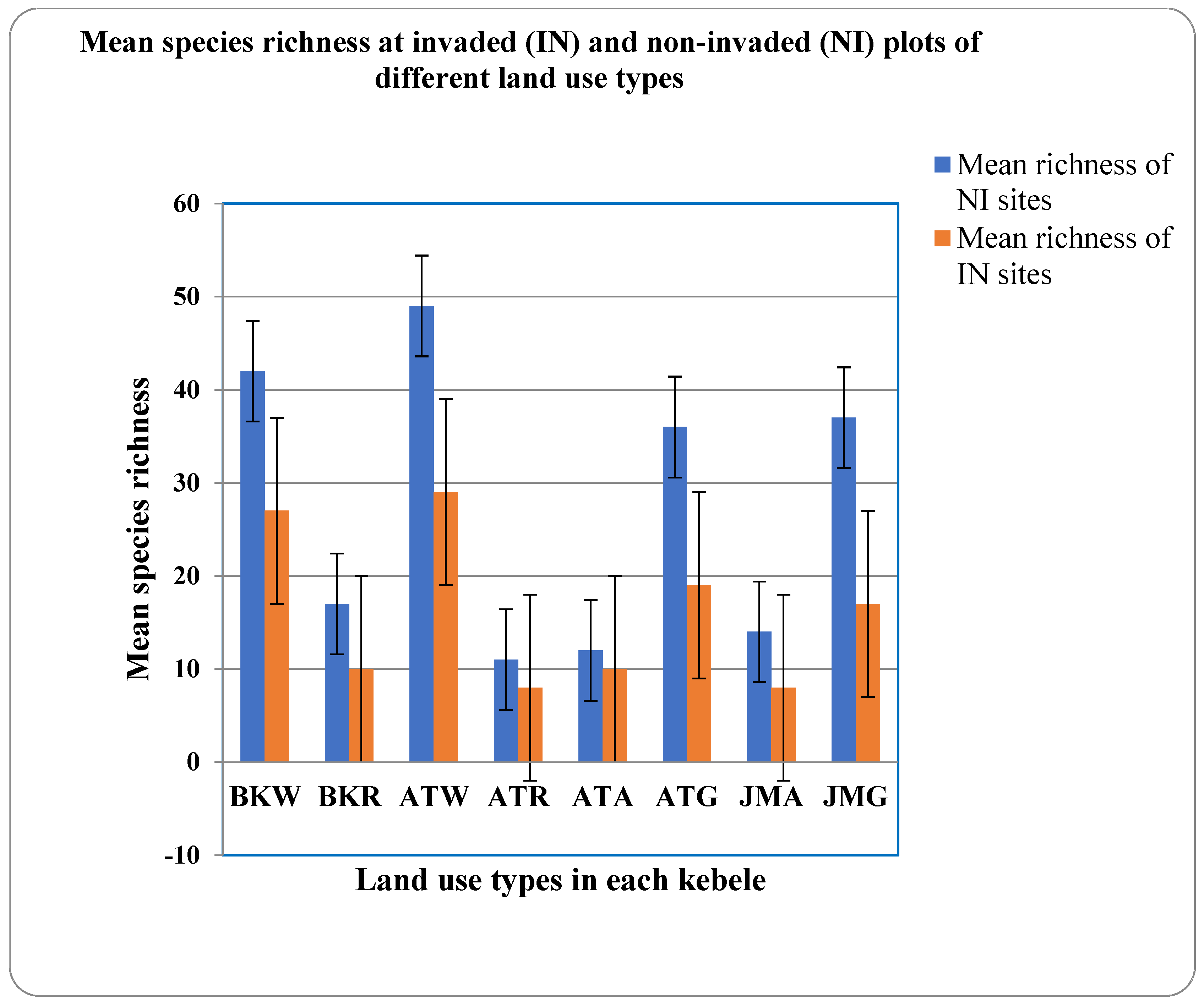
3.1.2. Species Similarity and Richness
3.1.3. Density, Percent Cover, and Height of P. hysterophorus across Different Land Use Types
3.1.4. Relationship between the Density of P. hysterophorus and Species Richness and Abundance
4. Discussion
4.1. Impacts of P. hysterophorus on Indigenous Plant Biodiversity
4.2. Impacts of P. hysterophorus on Species Similarity and Composition
4.3. Impacts of P. hysterophorus Density on Species Richness and Abundance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Number of Each Species in Each Land Use Types
| NO. | Botanical Name | Local Name | Family Name | Road side | Grazing Land | Acacia Woodland | Abandoned Agri. Land | No. of Individuals | |
| Oromic | Amharic | ||||||||
| 1. | Acacia albida Del. | Garbi | Fabaceae | 0 | 3 | 9 | 0 | 12 | |
| 2. | Acacia brevispica Harms | Hamaresa | Qwanta | Fabaceae | 6 | 4 | 12 | 0 | 22 |
| 3. | Acacia bussei Harms ex sjostede | Halo | Fabaceae | 0 | 0 | 3 | 0 | 3 | |
| 4. | Acacia etbaica Schweinf. | Derie; Qereta | Fabaceae | 0 | 2 | 5 | 0 | 7 | |
| 5. | Acacia gerrardii Benth. | Dodoti | Fabaceae | 0 | 2 | 4 | 0 | 6 | |
| 6. | Acacia mellifera (Vahl.) Benth. | Bilala; | Kontir; Atnkuy | Fabaceae | 0 | 3 | 7 | 0 | 10 |
| 7. | Acacia nilotica (L.) Willd. ex. Del. | Burquqe; Kasale | Burquqe | Fabaceae | 0 | 0 | 3 | 0 | 3 |
| 8. | Acacia oerfota (Forssk.) Schweinf. | Wangay | Fabaceae | 0 | 0 | 6 | 0 | 6 | |
| 9. | Acacia senegal (L.) Willd. | Gorsa | Kontir | Fabaceae | 0 | 2 | 5 | 0 | 7 |
| 10. | Acacia seyal Del. | Wacho | Fabaceae | 0 | 2 | 7 | 0 | 9 | |
| 11. | Acacia tortilis (Forssk.) Hayne. | Tadacha; Korera | Fabaceae | 0 | 1 | 6 | 0 | 7 | |
| 12. | Acalypha racemosa Baill. | Dhigri | Euphorbiaceae | 0 | 2 | 5 | 0 | 7 | |
| 13. | Acokanthera schimperi (A.DC) Schwein. | Qararu | Merenz | Apocynaceae | 0 | 0 | 4 | 0 | 4 |
| 14. | Agave sisalana Perro ex Eng. | Alge; Qaca | Agavaceae | 2 | 6 | 5 | 0 | 13 | |
| 15. | Aloe pirottae Berger. | Hargisa baru | Aloaceae | 2 | 0 | 6 | 0 | 8 | |
| 16. | Aloe retrospiciense Reynolds and Bally | Hargisa | Ret | Aloaceae | 3 | 0 | 7 | 0 | 10 |
| 17. | Andropogon gayanus Kunth. | Gaja | Poaceae | 0 | 274 | 0 | 0 | 274 | |
| 18. | Argemone mexicana L. | Qore adi | Nech Lebash | Papaveraceae | 36 | 23 | 11 | 52 | 122 |
| 19. | Asparagus falcatus L. | Seriti | Yeseit qest | Asparagaceae | 3 | 2 | 8 | 0 | 13 |
| 20. | Avena abyssinica Hochst. | Gaja gaca | Sinar | Poaceae | 0 | 0 | 0 | 23 | 23 |
| 21. | Balanites aegyptiaca (L.) Del. | Bedena | Jemo; Kudkuda | Balanitaceae | 0 | 0 | 4 | 0 | 4 |
| 22. | Barbeya oleoides Schweinf. | Adado | Barbeyaceae | 0 | 0 | 12 | 0 | 12 | |
| 23. | Barleria eranthemoides R. Br. | Balanwaranti | YesetAfe | Acanthaceae | 0 | 14 | 3 | 0 | 17 |
| 24. | Bothriochloa insculpta (Hochst. ex A.Rich.) | Suto | Poaceae | 0 | 286 | 0 | 0 | 286 | |
| 25. | Cadia purpurea (Picc.) Ait. | Tokeda; Hijire | Fabaceae | 2 | 0 | 6 | 0 | 8 | |
| 26. | Calpurina aurea (Ait.) Benth. | Cheketa | Digta | Fabaceae | 14 | 7 | 35 | 0 | 56 |
| 27. | Canthium pseudosetitflorum Bridson | Ladhana | Rubiaceae | 0 | 0 | 3 | 0 | 3 | |
| 28. | Caralluma speciosa (N. E.Br.) | Haleko aje | Asclpiadaceae | 0 | 0 | 4 | 0 | 4 | |
| 29. | Carissa spinarum L. | Hagamsa | Agam | Apocynaceae | 2 | 3 | 5 | 0 | 10 |
| 30. | Cissus cactiformis Gilg. | Gorsa; Matbot | Guraj | Vitaceae | 0 | 0 | 6 | 0 | 6 |
| 31. | Cissus quadrangularis L. | Chophi | Vitaceae | 0 | 4 | 3 | 0 | 7 | |
| 32. | Clematis sp. | Gadila | Ranunculaceae | 22 | 6 | 0 | 0 | 28 | |
| 33. | Combretum molle R. Br. ex G. Don | Biresa; | Abalo; Weyba | Combertaceae | 0 | 0 | 7 | 0 | 7 |
| 34. | Commiphora africana (A. Rich) Engl. | Hammesa | Anquwa | Burseraceae | 0 | 0 | 5 | 0 | 5 |
| 35. | Commiphora confusa Vollesen | Chacho; Hamesa | Burseraceae | 0 | 0 | 2 | 0 | 2 | |
| 36. | Commiphora erythraea (Ehrenb.) Engl. | Hagarsu | Bursseraceae | 0 | 0 | 11 | 0 | 11 | |
| 37. | Commiphora sp. | Dhiga | Bursseraceae | 0 | 0 | 2 | 0 | 2 | |
| 38. | Conomitra linearis Fenzl | Hanchagire | Asclepiadaceae | 0 | 0 | 9 | 0 | 9 | |
| 39. | Cordia africana Lam. | Wadesda | Wanza | Boraginaceae | 0 | 0 | 5 | 0 | 5 |
| 40. | Crepis rueppellii Sch. Bip. | Aanano | Yefyel Wotet | Asteraceae | 0 | 5 | 0 | 9 | 14 |
| 41. | Croton macrostachyus Del. | Bakanisa | Bissana | Euphorbiaceae | 3 | 4 | 6 | 0 | 13 |
| 42. | Cucumis prophetarum L. | Yemdere embway | Cucurbitaceae | 0 | 2 | 0 | 0 | 2 | |
| 43. | Cymbopogon commutatus (Steud.) Stapf | Sembelet | Poaceae | 0 | 258 | 0 | 0 | 258 | |
| 44. | Cynodon dactylon (L.) Pers. | Sardo | Poaceae | 0 | 315 | 0 | 18 | 333 | |
| 45. | Cynodon aethiopicus Clayton and Harlan | Poaceae | 0 | 276 | 0 | 0 | 276 | ||
| 46. | Cynoglossum geometricum Hochst. ex A.DC. | Matane-chati | Chigogot | Boraginaceae | 8 | 0 | 0 | 26 | 34 |
| 47. | Datura stramonium L. | Banji | Atse Faris | Solanaceae | 46 | 0 | 0 | 23 | 69 |
| 48. | Dichrostachys cinerea L. | Adesa; Jirme | Ader | Fabaceae | 0 | 0 | 6 | 0 | 6 |
| 49. | Digitaria abyssinica (Hochst ex. A.Rich.) Stapf | Wariat | Poaceae | 0 | 252 | 0 | 0 | 252 | |
| 50. | Dodonaea angustifolia L.f. | Etecha | Kitkita | Sapindaceae | 30 | 12 | 48 | 0 | 90 |
| 51. | Dombeya torrida (J. F. Gmel.) P. Bamps | Danisa | Wulkfa | Sterculiaceae | 0 | 0 | 5 | 0 | 5 |
| 52. | Dracaena ellenbeckiana Engler | Metti; Yabelo | Deracenaceae | 0 | 0 | 3 | 0 | 3 | |
| 53. | Ehretia cymosa Thonn. | Ulaga; Mukereba | Boraginaceae | 0 | 0 | 2 | 0 | 2 | |
| 54. | Eleusine floccifolia (Forssk.) Spreng. | Dagoo | Akirma | Poaceae | 0 | 216 | 0 | 0 | 216 |
| 55. | Enteropogon macrostachyus (Hochst ex A.Rich.) Benth. | Poaceae | 0 | 235 | 0 | 0 | 235 | ||
| 56. | Eragrostis papposa (Roem. and Schult.) Steud. | Poaceae | 0 | 292 | 0 | 0 | 292 | ||
| 57. | Erythrina brucei Schweinf. | Walena | Korch | Fabaceae | 0 | 0 | 1 | 0 | 1 |
| 58. | Euclea racemosa subsp. schimperi | Mieasa | Dedeho | Ebenaceae | 12 | 24 | 62 | 0 | 98 |
| 59. | Euphorbia dumalis S. Carter | Guri | Anterfa | Euphorbiaceae | 0 | 0 | 6 | 0 | 6 |
| 60. | Euphorbia sp. | Qulqwalit | Euphorbiaceae | 0 | 6 | 0 | 0 | 6 | |
| 61. | Euphorbia tirucalli L. | Kinchib | Euphorbiaceae | 2 | 0 | 6 | 0 | 8 | |
| 62. | Ficus sycomorus L. | Oda | Moraceae | 0 | 0 | 2 | 0 | 2 | |
| 63. | Ficus vasta Forssk. | Qiltu | Warka | Moraceae | 0 | 0 | 3 | 0 | 3 |
| 64. | Grewia mollis A. Juss. | Haroresa | Betre Musie | Tiliaceae | 0 | 0 | 4 | 0 | 4 |
| 65. | Guizotia scabra (Vis.) Chiov. | Hadaa; Tufo | Gime | Asteraceae | 0 | 0 | 0 | 42 | 42 |
| 66. | Guizotia schimperi Sch. Bip. ex Walp. | Hadaa; | Asteraceae | 0 | 0 | 0 | 34 | 34 | |
| 67. | Haplocoelum foliolosum (Hiem) Bullock | Chena | Adey Abeba | Sapindaceae | 0 | 0 | 11 | 0 | 11 |
| 68. | Hibiscus macranthus Hochst. ex A. Rich | Sukumeta; | Nacha | Malvaceae | 4 | 0 | 0 | 0 | 4 |
| 69. | Hyparrhenia hirta (L.) Stapf | Sembeliet | Poaceae | 0 | 296 | 0 | 0 | 296 | |
| 70. | Ipomoea kituiensis Vatke | Gale; kossole | Convolvulaceae | 0 | 0 | 5 | 0 | 5 | |
| 71. | Juniperus procera Hochst. Ex Endl. | Hindhesa | Tid | Cupressaceae | 0 | 0 | 10 | 0 | 10 |
| 72. | Justicia schimperiana (Hochst. ex Nees) T. Anders. | Dumoga | Sensel | Acanthaceae | 14 | 0 | 6 | 0 | 20 |
| 73. | Kalanchoe petitiana A.Rich. | Hancura | Crassulaceae | 5 | 0 | 7 | 0 | 12 | |
| 74. | Lactuca inermis Forssk. | Mech Algu | Asteraceae | 3 | 4 | 0 | 9 | 16 | |
| 75. | Lannea schimperi (A. Rich) Engl. | Ruku; Rukesa | Anacardiaceae | 0 | 0 | 4 | 0 | 4 | |
| 76. | Launaea intybacea (Jacq.) Beauv. | Hoola-gabbisa, | Asteraceae | 0 | 8 | 0 | 26 | 34 | |
| 77. | Leucas martinicensis (Jacq.) R. Br. | Yeferes Zeng | Lamiaceae | 0 | 3 | 0 | 17 | 20 | |
| 78. | Lippia adoensis Hochst. ex Walp. | Kassie | Kassie | Verbenaceae | 0 | 6 | 0 | 0 | 6 |
| 79. | Maerua aethiopica (Fenzl) Oliv. | Kontr | Capparidaceae | 0 | 3 | 0 | 0 | 3 | |
| 80. | Olea europaea L. subsp. cuspidata | Ejersa | Wieyra | Oleaceae | 0 | 2 | 10 | 0 | 12 |
| 81. | Opuntia ficus-indica (L.) Miller. | Shonka | Qulqual | Cactaceae | 16 | 0 | 24 | 0 | 40 |
| 82. | Osteospermum vailliantii (Decne) T. Norl. | Gurbi halooftu | Asteraceae | 0 | 0 | 0 | 31 | 31 | |
| 83. | Ozoroa insignis Del. | Garri | Anacardiaceae | 0 | 0 | 3 | 0 | 3 | |
| 84. | Pappea capensis Eckl. and Zeyh. | Biqa | Sapindaceae | 0 | 0 | 3 | 0 | 3 | |
| 85. | Parthenium hysterophorus L. | Anamale; Faramsis | Asteraceae | ||||||
| 86. | Pennisetum sphacelatum (Nees) Th. Dur. and Schinz | Geta | Sendedo | Poaceae | 0 | 244 | 0 | 0 | 244 |
| 87. | Psydrax schimperiana (A. Rich) Bridson | Galoo; Seged | Rubiaceae | 0 | 0 | 8 | 0 | 8 | |
| 88. | Rhoicissus revoilii Planch. | Aremo Saged | Vitaceae | 0 | 0 | 5 | 0 | 5 | |
| 89. | Rhus natalensis Krauss. | Gongoma | mst-aybelash | Anacardiaceae | 0 | 0 | 6 | 0 | 6 |
| 90. | Rhus vulgaris Meikle | Tatesa | Embs | Anacardiaceae | 0 | 0 | 4 | 0 | 4 |
| 91. | Ricinus communis L. | Kobo | Gulo | Euphorbiaceae | 38 | 0 | 0 | 0 | 38 |
| 92. | Rosa abyssinica Lindley | Gora | Kega | Rosaceae | 6 | 0 | 15 | 0 | 21 |
| 93. | Rumex nepalensis Spreng. | Tult | Polygonaceae | 0 | 24 | 0 | 46 | 70 | |
| 94. | Secamone Parvifolia (Olive.) Bullock | sari | Asclepiadaceae | 0 | 0 | 6 | 0 | 6 | |
| 95. | Senna didymobotrya (Fresen.)Irwin and Barneby | Fabaceae | 28 | 0 | 0 | 0 | 28 | ||
| 96. | Senra incana Cav. | Nechilo | Malvaceae | 0 | 5 | 10 | 0 | 15 | |
| 97. | Snowdenia polystachya (Fresen.) Pilg. | Muja | Poaceae | 0 | 32 | 0 | 36 | 68 | |
| 98. | Solanum incanum L. | Hidi | Embwa`y | Solanaceae | 54 | 37 | 0 | 28 | 119 |
| 99. | Sporobolus africanus (Poir.) Robyns and Tournay | Murie; Migra | Poaceae | 0 | 192 | 0 | 0 | 192 | |
| 100. | Tagetes minuta L. | Aje | Gime;Yahiya Ariti | Asteraceae | 0 | 0 | 0 | 74 | 74 |
| 101. | Tamarandus indica L. | Roqa | Fabaceae | 0 | 0 | 2 | 0 | 2 | |
| 102. | Terminalia brownii Fresen. | Biresa; Weyba | Combretaceae | 0 | 0 | 7 | 0 | 7 | |
| 103. | Woodfordia uniflora (A. Rich) Koehne. | Dambitto; Mar Mate | Lythraceae | 0 | 0 | 2 | 0 | 2 | |
| 104. | Xanthium strumarium L. | Bandoo Abdulhakim | Asteraceae | 17 | 9 | 0 | 23 | 49 | |
| 105. | Ziziphus mauritiana Lam. | Kurkura | Rhamnaceae | 0 | 0 | 4 | 0 | 4 | |
| 378 | 3408 | 541 | 517 | 4844 | |||||
References
- Kumar, N.H.; Murali, M.; Girish, H.V.; Chandrashekar, S.; Amruthesh, K.N.; Sreenivasa, M.Y.; Jagannath, S. Impact of climate change on biodiversity and shift in major biomes. In Global Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–44. [Google Scholar]
- Sonwa, D.J.; Dieye, A.; El Mzouri, E.H.; Majule, A.; Mugabe, F.T.; Omolo, N.; Wouapi, H.; Obando, J.; Brooks, N. Drivers of climate risk in African agriculture. Clim. Dev. 2017, 9, 383–398. [Google Scholar] [CrossRef]
- Afrin, S.; Sharmin, S.; Mowla, Q.A. The environmental impact of alien invasive plant species in Bangladesh. In Proceedings of the International Conference on Environmental Aspects of Bangladesh (ICEAB10), Kitakyushu, Japan, 4 September 2010; pp. 62–64. [Google Scholar]
- Tola, J.; Tessema, T. Abundance and Distribution of Invasive Alien Plant Species in Illu Ababora Zone of Oromia National Regional State, Ethiopia. J. Agric. Sci. Food Technol. 2015, 1, 94–100. [Google Scholar]
- Convention on Biological Diversity. What Are Invasive Alien Species? Available online: https://www.cbd.int/idb/2009/about/what/ (accessed on 22 July 2022).
- Zenni, R.D.; Essl, F.; García-Berthou, E.; McDermott, S.M. The economic costs of biological invasions around the world. NeoBiota 2021, 67, 1. [Google Scholar] [CrossRef]
- Stern, N. The Economics of Climate Change; HM Treasury: London, UK, 2006. [Google Scholar]
- IBC. Report on the Celebrations of the International Day for Biological Diversity; Invasion Management: Addis Ababa, Ethiopia, 2009. [Google Scholar]
- Crystal-Ornelas, R.; Hudgins, E.J.; Cuthbert, R.N.; Haubrock, P.J.; Fantle-Lepczyk, J.; Angulo, E.; Kramer, A.M.; Ballesteros-Mejia, L.; Leroy, B.; Leung, B.; et al. Economic costs of biological invasions within North America. NeoBiota 2021, 67, 485–510. [Google Scholar] [CrossRef]
- Bang, A.; Cuthbert, R.N.; Haubrock, P.J.; Fernandez, R.D.; Moodley, D.; Diagne, C.; Turbelin, A.J.; Renault, D.; Dalu, T.; Courchamp, F. Massive economic costs of biological invasions despite widespread knowledge gaps: A dual setback for India. Biol. Invasions 2022, 24, 2017–2039. [Google Scholar] [CrossRef]
- Nghiem, L.T.; Soliman, T.; Yeo, D.C.; Tan, H.T.; Evans, T.A.; Mumford, J.D.; Keller, R.P.; Baker, R.H.; Corlett, R.T.; Carrasco, L.R. Economic and environmental impacts of harmful non-indigenous species in Southeast Asia. PLoS ONE 2013, 8, e71255. [Google Scholar] [CrossRef]
- Rosa, W. Goal 2. End Hunger, Achieve Food Security and Improved Nutrition, and Promote Sustainable Agriculture. In A New Era in Global Health: Nursing and the United Nations 2030 Agenda for Sustainable Development; United Nations: Rome, Italy, 2017; p. 257. [Google Scholar]
- Genovesi, P.; Carboneras, C.; Vilà, M.; Walton, P. EU adopts innovative legislation on invasive species: A step towards a global response to biological invasions? Biol. Invasions 2015, 17, 1307–1311. [Google Scholar] [CrossRef]
- Sintayehu, D.W. Impact of climate change on biodiversity and associated key ecosystem services in Africa: A systematic review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- Boy, G.; Witt, A. Invasive Alien Plants and Their Management in Africa; Synthesis Report of the UNEP/GEF Removing Barriers to Invasive Plant Management in Africa (RBIPMA) Project, Implemented in Four African Countries (Ethiopia, Ghana, Uganda and Zambia) between 2005 and 2010; Gutenberg Press Limited: Iż-Żejtun, Malta, 2013. [Google Scholar]
- Tamiru, G. Invasive alien weed species distribution, impacts on agriculture, challenge and reaction in Ethiopia: A review. J. Biol. Agric. Healthc. 2017, 7, 136–146. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization). Parthenium hysterophorus L. Asteraceae—Parthenium weed. Data sheets on invasive alien plants. OEPP Bull. 2014, 44, 474–478. [Google Scholar]
- Bhowmik, P.C.; Sarkar, D.; Yaduraju, N.T. The status of Parthenium hysterophorus and its potential management. Ecoprint Int. J. Ecol. 2007, 14, 1–17. [Google Scholar] [CrossRef]
- Adkins, S.; Shabbir, A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag. Sci. 2014, 70, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Nigatu, L.; Hassen, A.; Sharma, J.; Adkins, S.W. Impact of Parthenium hysterophorus on grazing land communities in north-eastern Ethiopia. Weed Biol. Manag. 2010, 10, 143–152. [Google Scholar] [CrossRef]
- Riaz, T.; Javaid, A. Prevalence of invasive parthenium weed in district Hafizabad, Pakistan. J. Anim. Plant Sci. 2010, 20, 90–93. [Google Scholar]
- Murphy, S.T.; Cheesman, O.D. The Aid Trade—International Assistance Programmes as Pathways for the Introduction of IAS (a Preliminary Report); World Bank Paper 109. A paper prepared by CABI Bioscience; UK Centre: London, UK, 2006; 38p. [Google Scholar]
- Bajwa, A.A.; Chauhan, B.S.; Adkins, S.W. Morpho-physiological responses of two Australian biotypes of parthenium weed (Parthenium hysterophorus L.) to soil moisture stress. In Proceedings of the 20th Australasian Weeds Conference, Perth, WA, Australia, 11–15 September 2016; pp. 11–15. [Google Scholar]
- Shabbir, A.; McConnachie, A.; Adkins, S.W. Spread. In Parthenium Weed: Biology, Ecology and Management; Adkins, S.W., Shabbir, A., Dhileepan, K., Eds.; CABI: Boston, MA, USA, 2019; Volume 7, pp. 40–56. [Google Scholar]
- Khan, N.; Fahad, S. Economic Review of Parthenium Hysterophorus L. Plant in the World. Plant World 2020. [Google Scholar] [CrossRef]
- Kaur, M.; Aggarwal, N.K.; Kumar, V.; Dhiman, R. Effects and management of Parthenium hysterophorus: A weed of global significance. Int. Sch. Res. Not. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Tamado, T.; Ohlander, L.; Milberg, P. Interference by the weed Parthenium hysterophorus L. with grain sorghum: Influence of weed density and duration of competition. Int. J. Pest Manag. 2002, 48, 183–188. [Google Scholar] [CrossRef]
- Mitiku, W. Competitive study between parthenium (Parthenium hysterophorus L.) and common bean (Phaseolus vulgaris L.). Master’s Thesis, Haramaya University, Addis Ababa, Ethiopia, 2011. [Google Scholar]
- Seifu, A.; Seboka, N.; Misganaw, M.; Bekele, T.; Merawi, E.; Ayenew, A.; Faris, G. Impact of invasive alien plant, Xanthium strumarium, on species diversity and composition of invaded plant communities in Borena Zone, Ethiopia. Biodivers. Int. J. 2017, 1, 00004. [Google Scholar] [CrossRef]
- Etana, A.; Kelbessa, E.; Soromessa, T. Impact of Parthenium hysterophorus L. (Asteraceae) on herbaceous plant biodiversity of Awash National Park (ANP), Ethiopia. Manag. Biol. Invasions 2011, 2, 69–80. [Google Scholar] [CrossRef]
- Seta, T.; Assefa, A.; Mesfin, F.; Balcha, A. Distribution status and the impact of Parthenium weed (Parthenium hysterophorus L.) at Gedeo Zone (southern Ethiopia). Afr. J. Agric. Res. 2013, 8, 386–397. [Google Scholar]
- Cunningham, A.B. Applied Ethnobotany: People, Wild Plant Use and Conservation; Earthscan: London, UK, 2001; 300p. [Google Scholar]
- Wittenberg, R. Instruments and tools for assessing the impact of invasive plant species in Africa. In Report Submitted under the PDF-B Phase of the UNEP/GEF Project: Removing Barriers to Invasive Plant Management in Africa; CABI Africa Regional Centre: Nairobi, Kenya, 2004. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Karki, D. Ecological and Socio-Economic Impacts of Parthenium hysterophorus L. Invasion in Two Urban Areas in Nepal. Ph.D. Thesis, Tribhuvan University, Kathmandu, Nepal, 2009. [Google Scholar]
- Dorca, A.M.; Emily, W.; Paul, K.M.; John, O.A. Distribution of Parthenium hysterophorus Linn and its Impacts on Biodiversity in Nyando Sub-County, Kisumu County, Kenya. Eur. Acad. Res. 2018, 6, 1599–1621. [Google Scholar]
- Gebrehiwot, N.; Berhanu, L. Impact of parthenium on species diversity in Gamo Gofa, Ethiopia. Sch. J. Agric. Sci. 2015, 5, 226–231. [Google Scholar]
- Musese, L.J.; Andrew, J.M.; Shirima, D.D.; Witt, A.; Kilewa, R. Effects of the abundance of Parthenium hysterophorus on the composition and diversity of other herbaceous plant species in Simanjiro Rangeland, Tanzania. Int. J. Eng. Technol. Manag. Res. 2020, 7, 11–20. [Google Scholar] [CrossRef]
- ECZ (Environmental Council of Zambia). Implementation of Invasive Plant Prevention and Control Programmes in Zambia, Report Submitted to the CAB International Africa Regional Centre under the PDF-B Phase of the UNEP/GEF Project; Environmental Council of Zambia: Lusaka, Zambia, 2004. [Google Scholar]
- Hedberg, I.; Edwards, S.; Nemomissa, S. (Eds.) Flora of Ethiopia and Eritrea; The National Herbarium, Addis Ababa University: Uppsala, Sweden, 2003; Volume 4, pp. lxiii, 1–532. [Google Scholar]
- Mesfin, T. Flora of Ethiopia and Eritrea Volume 4 Part 2, Asteraceae (Compositae); The National Herbarium: Addis Ababa, Ethiopio; Uppsala, Sweden, 2004; p. 408. [Google Scholar]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University of Illinois: Chicago, IL, USA, 1949. [Google Scholar]
- Nagendra, H. Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl. Geogr. 2002, 22, 175–186. [Google Scholar] [CrossRef]
- Kent, M.; Coker, P. Vegetation Description and Analysis: A Practical Approach; Belhaven Press: London, UK, 1992; 438p. [Google Scholar]
- Whittaker, H.G. Communities and Ecosystem, 2nd ed.; Macmillan Publishing Co., Willey and Sons: New York, NY, USA, 1975; 547p. [Google Scholar]
- Girma, M.; Maryo, M. The diversity and composition of woody plants in chebera churcura national park (ccnp), southern Ethiopia. Open J. For. 2018, 8, 439. [Google Scholar] [CrossRef]
- Jaccard, P. Distribution comparée de la flore alpine dans quelques régions des Alpes occidentales et orientales. Bull. Murithienne 1902, 31, 81–92. [Google Scholar]
- Sorensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skar. 1948, 5, 1–34. [Google Scholar]
- Anteneh, N.; Esayas, M. Allelopathic effects of Parthenium hysterophorus L. aqueous extracts on soybean (Glycine max L.) and haricot bean (Phaseolus vulgaris L.) seed germination, shoot and root growth and dry matter production. J. Appl. Bot. Food Qual. 2011, 84, 219–222. [Google Scholar]
- Dalip, K.; Ahmed, J.; Singh, S. Distribution and effect of Parthenium hysterophorus L. Mehari sub-watershed of Rajouri forest range. Int. J. Sci. Res. 2013, 2, 304–306. [Google Scholar]
- Anil, K. Parthenium hysterophorus L. and its Impact on living World. Indian J. Sci. Res. 2014, 4, 08–14. [Google Scholar]
- Belz, R.G.; Reinhardt, C.F.; Foxcroft, L.C.; Hurle, K. Residue allelopathy in Parthenium hysterophorus L.—Does parthenin play a leading role? Crop Prot. 2007, 26, 237–245. [Google Scholar] [CrossRef]
- Joshi, B.; Pandey, N.; Rao, P.B. Allelopathic effect of weed species extracts on germination, growth and biochemical aspects in different varieties of wheat (Triticum aestivum L.). Indian J. Agric. Res. 2009, 43, 79–87. [Google Scholar]
- Shabbir, A.; Bajwa, R. Distribution of parthenium weed (Parthenium hysterophorus L.), an alien invasive weed species threatening the biodiversity of Islamabad. Weed Biol. Manag. 2006, 6, 89–95. [Google Scholar] [CrossRef]
- Akter, A.; Zuberi, M.I. Invasive alien species in Northern Bangladesh: Identification, inventory and impacts. Int. J. Biodivers. Conserv. 2009, 1, 129–134. [Google Scholar]
- Dogra, K.S.; Kohli, R.K.; Sood, S.K. An assessment and impact of three invasive species in the Shivalik hills of Himachal Pradesh, India. Int. J. Biodivers. Conserv. 2009, 1, 4–10. [Google Scholar]
- Kohli, R.K.; Dogra, K.S.; Batish, D.R.; Singh, H.P. Impact of invasive plants on the structure and composition of natural vegetation of northwestern Indian Himalayas1. Weed Technol. 2004, 18 (Suppl. S1), 1296–1300. [Google Scholar] [CrossRef]
- Yaduraju, N.T.; Kumar, S.; Prasad Babu, M.B.B.; Gogoi, A.K. Parthenium hysterophorus—Distribution, problem and management strategies in India. In Proceedings of the 2nd International Conference on Parthenium Management, Bangalore, India, 5–7 December 2005; University of Agricultural Sciences: Bangalore, India, 2005; pp. 6–10. [Google Scholar]
- Bajwa, A.A.; Farooq, M.; Nawaz, A.; Yadav, L.; Chauhan, B.S.; Adkins, S. Impact of invasive plant species on the livelihoods of farming households: Evidence from Parthenium hysterophorus invasion in rural Punjab, Pakistan. Biol. Invasions 2019, 21, 3285–3304. [Google Scholar] [CrossRef]
- Kumari, P.; Sahu, P.K.; Soni, M.Y.; Awasthi, P. Impact of Parthenium hysterophorus L. invasion on species diversity of cultivated fields of Bilaspur (CG) India. Agric. Sci. 2014, 2014, 48207. [Google Scholar]
- Navie, S.C.; PANETTA, F.D.; McFADYEN, R.E.; Adkins, S.W. Germinable soil seedbanks of central Queensland rangelands invaded by the exotic weed Parthenium hysterophorus L. Weed Biol. Manag. 2004, 4, 154–167. [Google Scholar] [CrossRef]
- Dhileepan, K.; Strathie, L. Parthenium hysterophorus L. (Asteraceae). In Biological Control of Tropical Weeds Using Arthropods; Muniappan, R., Reddy, G., Raman, A., Eds.; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Timsina, B.; Shrestha, B.B.; Rokaya, M.B.; Münzbergová, Z. Impact of Parthenium hysterophorus L. invasion on plant species composition and soil properties of grassland communities in Nepal. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 233–240. [Google Scholar] [CrossRef]
- Nigatu, L.; Sharma, J. Parthenium weed invasion and biodiversity loss in Ethiopia: A literature. In African Crop Science Conference Proceedings; African Crop Science Society: Kampala, Uganda, 2013; Volume 11, pp. 377–381. [Google Scholar]
- Ayele, S.; Nigatu, L.; Tana, T.; Adkins, S.W. Impact of parthenium weed (Parthenium hysterophorus L.) on the above-ground and soil seed bank communities of rangelands in Southeast Ethiopia. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 262–274. [Google Scholar]
- Shabbir, A.; Bajwa, R. Parthenium invasion in Pakistan-a threat still unrecognized. Pak. J. Bot. 2007, 39, 2519–2526. [Google Scholar]
- Joshi, S. Reproductive Efficiency and Biomass Allocation of Invasive weed, Parthenium hysterophorus L. Ph.D. Thesis, Central Department of Botany, Tribhuvan University, Kathmandu, Nepal, 2005. [Google Scholar]
- Timsina, B. Impact of Parthenium hysterophorus L. Invasion on Soil and Plant Species Composition of Grasslands of Central Nepal. Master’s Thesis, Central Department of Botany, Tribhuvan University, Kathmandu, Nepal, 2007. [Google Scholar]
- Tiwari, S.; Adhikari, B.; Siwakoti, M.; Subedi, K. An Inventory and Assessment of Invasive Alien Plant Species of Nepal; IUCN World Conservation Union: Gland, Switzerland, 2005; 115p. [Google Scholar]
- Upendra, K.C.; Bharat, S. Invasion of Parthenium hysterophorus L. across land use types in Kirtipur Municipality. Int. J. Hum. Cap. Urban Manag. 2019, 4, 259–268. [Google Scholar]
- Kathiresan, R. Ecology and control of Parthenium hysterophorus invasion in veeranum command area. Indian J. Weed Sci 2008, 40, 78–80. [Google Scholar]
- Grice, A.C. The impacts of invasive plant species on the biodiversity of Australian rangelands. Rangel. J. 2006, 28, 27–35. [Google Scholar] [CrossRef]
- Navie, S.C.; McFadyen, R.E.; Panetta, F.D.; Adkins, S.W. The biology of Australian weeds. 27. Parthenium hysterophorus L. Plant Prot. Q. 1996, 11, 76–88. [Google Scholar]
- Stinson, K.; Kaufman, S.; Durbin, L.; Lowenstein, F. Impacts of garlic mustard invasion on a forest understory community. Northeast. Nat. 2007, 14, 73–88. [Google Scholar] [CrossRef]


| S.N | Kebeles | LUT | Abundance | Richness | Evenness | H′ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| IN | NI | IN | NI | IN | NI | IN | NI | |||
| 1 | Balekecha | Wood land | 111 | 265 | 27 | 42 | 0.865 | 0.869 | 2.85 | 3.247 |
| Ardatare | Wood land | 122 | 276 | 29 | 47 | 0.895 | 0.914 | 3.016 | 3.52 | |
| Mean | 116.5 ± 0.5 | 270.5 ± 0.5 | 28 ± 1.0 | 44.5 ± 2.5 | 0.88 ± 0.015 | 0.891 ± 0.0225 | 2.93 ± 0.083 | 3.38 ± 0.1365 | ||
| 2 | Balekecha | Roadside | 138 | 231 | 10 | 17 | 0.729 | 0.802 | 1.68 | 2.27 |
| Ardatare | Roadside | 72 | 133 | 8 | 11 | 0.85 | 0.77 | 1.77 | 1.849 | |
| Mean | 105 ± 0.3 | 182 ± 1.9 | 9 ± 1.0 | 14 ± 3.0 | 0.789 ± 0.6 | 0.786 ± 0.016 | 1.724 ± 0.045 | 2.059 ± 0.21 | ||
| 3 | Jamie | A. agri. land | 148 | 267 | 8 | 12 | 0.955 | 0.921 | 1.986 | 2.28 |
| Ardatare | A. agri. land | 128 | 250 | 10 | 11 | 0.961 | 0.967 | 2.21 | 2.27 | |
| Mean | 138 ± 1.0 | 258.5 ± 1.5 | 9 ± 1.0 | 11.5 ± 0.5 | 0.957 ± 0.003 | 0.944 ± 0.023 | 2.098 ± 0.112 | 2.275 ± 0.005 | ||
| 4 | Ardatare | G. land | 672 | 1296 | 19 | 27 | 0.74 | 0.688 | 2.18 | 2.27 |
| Jamie | G. land | 907 | 2108 | 17 | 29 | 0.787 | 0.734 | 2.229 | 2.47 | |
| Mean | 789.5 ± 1.17 | 1702 ± 2.6 | 18 ± 1.0 | 28 ± 1.0 | 0.763 ± 0.0235 | 0.711 ± 0.023 | 2.204 ± 0.0245 | 2.37 ± 0.1 | ||
| No. | Index of Similarity | Land Use Types | |||
|---|---|---|---|---|---|
| Woodland Vegetation | Abandoned Agricultural Land | Roadside Vegetation | Grassland Vegetation | ||
| 1 | Jaccard’s Similarity Index (ISj) | 56.52 | 76.47 | 50 | 52.17 |
| 2 | Sorenson’s Similarity Index (ISs) | 72.22 | 86.66 | 66.66 | 68.57 |
| S.N | LUT | Study Site | Density (Stem/m2) | Height (m) | % Cover | |||
|---|---|---|---|---|---|---|---|---|
| NI | IN | NI | IN | NI | IN | |||
| 1 | Acacia Wood land | Baliekecha | - | 15.825 | - | 0.53 | - | 60 |
| Ardatare | - | 12.335 | - | 0.535 | - | 53 | ||
| Mean | 14.08 ± 1.745 | 0.5325 ± 0.0025 | 56.5 ± 3.5 | |||||
| 2 | Roadside | Baliekecha | - | 34.9 | - | 0.695 | - | 69 |
| Ardatare | - | 33.5 | - | 0.565 | - | 75 | ||
| Mean | 34.2 ± 0.7 | 0.63 ± 0.065 | 72 ± 3.0 | |||||
| 3 | A. agri. land | Ardatare | - | 34 | - | 0.525 | - | 63 |
| Jamie | - | 30 | - | 0.685 | - | 69 | ||
| Mean | 32 ± 2.0 | 0.605 ± 0.08 | 66 ± 3.0 | |||||
| 4 | G. land | Ardatare | - | 27.3 | - | 0.65 | - | 72 |
| Jamie | - | 39.6 | - | 0.54 | - | 64 | ||
| Mean | 33.45 ± 6.15 | - | 0.595 ± 0.055 | - | 68 ± 4.0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boja, M.; Girma, Z.; Dalle, G. Impacts of Parthenium hysterophorus L. on Plant Species Diversity in Ginir District, Southeastern Ethiopia. Diversity 2022, 14, 675. https://doi.org/10.3390/d14080675
Boja M, Girma Z, Dalle G. Impacts of Parthenium hysterophorus L. on Plant Species Diversity in Ginir District, Southeastern Ethiopia. Diversity. 2022; 14(8):675. https://doi.org/10.3390/d14080675
Chicago/Turabian StyleBoja, Mesfin, Zerihun Girma, and Gemedo Dalle. 2022. "Impacts of Parthenium hysterophorus L. on Plant Species Diversity in Ginir District, Southeastern Ethiopia" Diversity 14, no. 8: 675. https://doi.org/10.3390/d14080675
APA StyleBoja, M., Girma, Z., & Dalle, G. (2022). Impacts of Parthenium hysterophorus L. on Plant Species Diversity in Ginir District, Southeastern Ethiopia. Diversity, 14(8), 675. https://doi.org/10.3390/d14080675





