Abstract
Land subsidence caused by underground coal mining critically influences the structure and function of ecosystems in mining areas. However, knowledge on the aquatic bacterial community structure and interspecies interactions in subsidence lakes are still limited. To address this issue, we collected water samples from a coal mining subsidence lake and its connected river and investigated the spatial distribution and co-occurrence patterns of the bacterial community using 16S rRNA amplicon sequencing. The results revealed that the bacterial α-diversity in the subsidence lake was higher than that in the river. The bacterial community composition was also significantly different between the subsidence lake and its connected river. Total nitrogen explained 21.4% of the bacterial community composition variation, while sulfate explained 38.4% of the bacterial functional composition variation. Co-occurrence network analysis indicated that the modularity indices and stability of the microbial network in the subsidence lake were significantly higher than those in rivers, which presented more resistance to environmental disturbance. Keystone bacterial taxa in the subsidence lake and river included the Clostridiaceae 1 family, and the Shewanella, Flavobacterium, and Limnohabitans genera, which play vital roles in the carbon, sulfur, and nitrogen cycles. Moreover, functional analysis showed that assimilatory sulfate reduction processes had a major role in the sulfur cycle of the subsidence lake and its connected river ecosystem. Overall, our findings provide new insights into the microbial community structure and assembly in subsidence lakes and its connected river ecosystems, with significant implications for the responsible utilization of water resources and the promotion of sustainable development in mining areas.
1. Introduction
Coal is an important source of the energy worldwide [1]. With the rapid development of society and the economy, the energy demand is increasing and coal exploitation activities have increased accordingly [2]. Although coal resource exploitation has made a significant contribution to the national economy, it has increased pollution in various forms, such as mining subsidence and coal gangue accumulation, as well as a series of ecological and environmental problems [3,4,5]. Underground mining currently accounts for a larger ratio of global coal production than open cut mining [6]. Underground coal mining leads to ground displacement and deformation, forming large-scale subsidence areas and ultimately forming subsidence lakes under the action of high diving levels and surface runoff [7,8]. Due to the low topography of subsidence lakes, leachate water from coal gangue piles, agricultural drainage, and domestic sewage are more likely to flow into those water bodies, releasing a large number of nutrients, such as nitrogen and phosphorus into the lakes [8,9]. Moreover, some subsidence areas that are affected by long-term anthropogenic activities and rainfall continue to expand the range of subsidence and gradually connect with adjacent rivers, resulting in the enrichment of river pollution sources and biodiversity reduction, which in turn has a serious impact on the overall structure and function of aquatic ecosystems in mining areas [10]. Microorganisms play a vital role in water quality purification and in the biogeochemical cycle of nutrients (e.g., C, N, and P) in aquatic ecosystems [11,12]. Therefore, a better understanding of the structure and function of the microbial community in coal mining subsidence lakes is of great significance for the responsible utilization of water resources and the promotion of sustainable development practices in mining areas. In recent years, studies on coal mining subsidence lakes have mainly focused on the source and transformation of heavy metals [13], nutrients [10], and polycyclic aromatic hydrocarbons [9], while the structure and function of the microbial communities have not been fully explored.
As essential components of microorganisms, bacterial communities play a crucial role in organic matter processing, energy flow, and nutrient cycling in aquatic ecosystems [14,15,16]. Furthermore, bacterial communities are extremely sensitive to changes in physicochemical states in water bodies and usually form specific community structures to cope with various environmental pressures [17,18]. Therefore, the bacterial community can be used as a potential comprehensive index to monitor the influence of natural factors and anthropogenic activities on aquatic environments [19]. Most studies on aquatic bacterial communities are focused on glaciers [20,21], oceans [22,23], rivers [19,24], and eutrophic lakes [25,26]. With respect to coal mining subsidence areas, a few studies have explored the effects of land reclamation and soil remediation on soil bacterial communities in low diving level subsidence areas [27,28,29]. However, few studies have explored the spatial and temporal distribution characteristics of bacterial community structure and diversity in coal mining subsidence lakes with high diving levels. As reported previously, the bacterial community structure in eutrophic lakes varies with season and location under the influence of various natural and anthropogenic pressures, with spatial and temporal heterogeneity [30,31]. However, it is unknown whether the bacterial communities in subsidence lakes also show spatial heterogeneity under the influence of various anions and cations, heavy metals, and nutrients. Currently, there is still a knowledge gap on the spatial pattern and interspecific interaction of the bacterial community structure in coal mining subsidence lakes.
Aquatic microorganisms are not isolated in the aquatic ecosystem but exist in the complex interaction system that shapes the structure of the microbial community [32]. A variety of microorganisms interact directly or indirectly to form a complicated ecological network through various processes, such as predation, competition, inhibition, and promotion [33], which is of great significance for controlling the community assembly and maintaining community stability [34]. Network analysis is a vital method to uncover the interactions among bacterial communities in ecosystems [35]. Network analysis was applied to explore the assembly patterns of complex bacterial communities and their relationship with the habitat environment in eutrophic lakes [36,37], rivers [38,39], soils [40,41], and oceans [42,43]. These studies have shown that the co-occurrence pattern of bacterial communities has a nonrandom and modular structure. Further research showed that keystone bacterial taxa in the co-occurrence network exert significant impacts on the structure and function of entire bacterial communities, and their disappearance may induce substantial pressures on community composition and succession [33]. Therefore, revealing the co-occurrence patterns and keystone taxa of aquatic bacterial communities is of great significance to better understand community assembly. However, relatively little is known about the co-occurrence patterns of bacterial communities and which keystone bacterial taxa maintain community stability in coal mining subsidence lakes.
In this study, 16S rRNA gene sequencing combined with function prediction and network analysis was applied to analyze the spatial distribution and co-occurrence patterns of the aquatic bacterial community in subsidence lakes and its connected rivers in the Linhuan mining area of Huaibei. We hypothesized that the spatial distribution and co-occurrence pattern of the aquatic bacterial community would be significantly different between the subsidence lake and its connected river. Moreover, we hypothesized that nutrients are the main factors affecting the spatial distribution and predicted function of aquatic bacterial communities in a subsidence lake and its connected river. The results of this study revealed the spatial distribution and co-occurrence pattern of the bacterial community in a subsidence lake and made a fundamental contribution to predicting the ecological function of subsidence lakes.
2. Materials and Methods
2.1. Site Description and Sample Collection
The Linhuan mining area is in the city of Huaibei in Anhui Province, China, and its geographical location is between 116°34′25″–116°44′27″ E and 33°36′50″–34°40′47″ N [44] (Figure 1). The Huihe River is the largest river flowing through the area from west to the east and is a small to medium-sized seasonal river. The Linhuan subsidence lake is shaped by a coal mining collapse, and its shape is irregular. The accumulation of water in the subsidence area mainly comes from atmospheric precipitation and surface runoff. The average water depth is approximately 3.45 m, the maximum water depth is 9.0 m, and the subsidence time of the subsidence lake is more than 17 years [45]. One part of the subsidence lake serves as a water source for the Linhuan industrial park, and the water surface is covered with photovoltaic panels for solar power generation, while the other part has been developed as a fish pond. A fly ash field and a gangue mountain are located to the east of the subsidence lake. To the southwest of the lake are the Linhuan industrial park and a small gangue mountain, and a large number of water bodies are collected at the gangue mountain due to rainfall and surface runoff. To the north, the Xiangshun channel connects the subsidence lake with the Huihe River, which is the water source of the subsidence lake [46]. With the advance of coal mining, the subsidence area continues to increase. While the ecological environment in this area has changed from a terrestrial ecosystem to a water–land composite ecosystem, it is also affected by mine acid wastewater and agricultural and domestic sewage, which have collectively seriously disturbed the structure and function of the ecosystem.
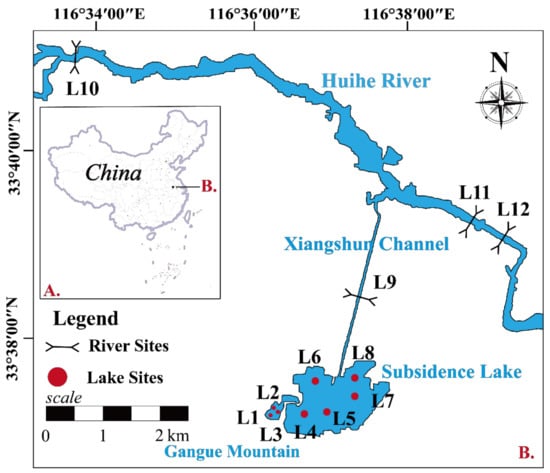
Figure 1.
Map of sampling sites in the coal mining subsidence lake and its connected river.
In this study, combined with hydrogeological conditions and on-site investigations, a total of 12 sampling points were established in the coal mining subsidence waters of the Linhuan mining area and the Huihe River (Figure 1). To explore the impact of underground coal mining activities and the leaching process of coal gangue on the microorganisms of subsidence lakes, five sampling points (L4, L5, L6, L7, and L8) in the subsidence lake (SL) and three sampling points (L1, L2, and L3) in the coal gangue mountain waters (GM) were selected. To characterize the effect of natural river recharge on the microorganisms of subsidence lakes, three sampling points (L10, L11, and L12) were selected from upstream to downstream of the Huihe River, and one sampling point (L9) was selected in the Xiangshun channel. Water samples were collected at these sites in April 2021. At each site, three surface water samples (0–50 cm) were collected in parallel using a Perspex sampler and then mixed for homogenization. Then, 300–400 mL subsamples were filtered through polycarbonate filters (0.22 µm pore-size, Millipore, Burlington, MA, USA) for molecular analysis, and the filtered samples were stored in the field at −20 °C and subsequently stored in the laboratory at −80 °C for DNA extraction. The other samples were immediately transported to the laboratory for chemical analysis.
2.2. Analysis of Physicochemical Indices
At each site during sampling, pH, total dissolved solids (TDS), dissolved oxygen (DO), electrical conductivity (EC), and water temperature (T) were measured by a multiparameter water quality sonde (YSI 6600 V2, Yellow Springs Instruments Inc., Yellow Spring, OH, USA). Secchi transparency (SD) was determined using a Secchi disk. Total nitrogen (TN), total phosphorus (TP), chemical oxygen demand (CODcr), ammonia nitrogen (NH4+-N), nitrate (NO3−), orthophosphate (PO43−), and sulfate (SO42−) were measured in the laboratory according to standard methods [47]. F−, Cl−, K+, Ca2+, Na+, and Mg2+ were determined using ion chromatography (930 Compact IC Flex).
2.3. DNA Extraction and PCR Amplification
According to the manufacturer’s instructions, total DNA was extracted from the homogenized water samples using a FastDNA® Spin Kit for Soil (MP Biomedicals, Solon, OH, USA). The primer sets 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used to amplify the V3-V4 hypervariable regions of the bacterial 16S rRNA genes [48]. PCR amplification was conducted in triplicate for each sample. The PCR program was as follows: 98 °C for 3 min, followed by 30 cycles at 98 °C for 45 s, 55 °C for 45 s, 72 °C for 45 s, and a final extension at 72 °C for 7 min. Then, the PCR amplification product was purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and pooled in equimolar amounts and finally sequenced (2 × 300 bp) on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) by Personal Biotechnology Co., Ltd., Shanghai, China.
2.4. Sequence Analyses
The 16S rRNA gene amplicon sequence data were processed using the Qiime2 pipeline [49] and the DADA2 algorithm [50]. After quality trimming, merging of paired sequences and removal of chimeric sequences, amplicon sequence variant (ASV) tables were generated based on 100% sequence similarity for 16S rRNA. The representative ASVs were subsequently annotated using the Silva database [51] (version 132) to identify the taxonomy of each ASV. The ASVs annotated as chloroplasts or mitochondria were excluded from further analyses.
2.5. Statistical Analyses
The statistical analyses and visualization were conducted by “ggplot2” and “heatmap” in R 3.5.3 and RStudio 1.1.463. To determine the alpha and beta diversity of water bacteria, the Chao 1 and Shannon indices were calculated and principal coordinates analysis (PCoA) was performed using the “vegan” package in R. The Kruskal–Wallis test was conducted to test the variation in the alpha diversity index and the microbial community at the phylum and genus levels between the subsidence lake and its connected river. The variance inflation factor (VIF) for each selected significant variable was calculated, and variables with VIF values greater than 20 (indicating strong collinearity) were removed from the model. The significant effects of physicochemical indices (p < 0.05) on the variation in the microbial community structure and function were determined by using redundancy analysis (RDA), Pearson’s correlation analysis, and variation partition analysis (VPA) [52]. The bacterial functional composition (BFC) was predicted through PICRUSt2 annotation based on the ASV table [53].
Co-occurrence networks were constructed by using the “psych” package in R based on Spearman’s correlation [54]. To simplify the dataset, only ASVs with a relative abundance >0.05% in the water samples were used in the analyses. To be statistically significant, microbial ASVs that occurred simultaneously in each sample of the group were included in the analysis. Robust correlations with Spearman’s correlation coefficients |r| > 0.9 and false discovery rate-corrected p-values < 0.01 were used to construct networks, and the p values were corrected at the same time [41]. In networks, ASVs represent nodes, while correlations among ASVs are represented as edges. The topological properties of the co-occurrence networks and random networks were also determined using the package “igraph” in R [55]. Nodes with a high degree value and a low betweenness centrality value were defined as keystone bacterial taxa [56]. The co-occurrence networks were visualized by the interactive platform Gephi (v0.9.2). In addition, this study uses the UPGMA method to carry out hierarchical cluster analysis on the correlation matrix of ASVs (with relative abundance > 0.05%), supporting network analysis from the side. The detailed analysis of hierarchical cluster analysis is described in the supplemental information.
3. Results
3.1. Alpha and Beta Diversity of the Bacterial Community
The spatial distribution of the microbial communities in the coal mining subsidence lake and its connected river was profiled by high-throughput sequencing technology. After quality filtering, 784,413 high-quality reads were acquired from the 12 sites, averaging 65,367 sequences per sample, and the average length was 425 bp. Those reads were clustered into 28,496 amplicon sequence variants (ASVs) through the 12 samples that were obtained from a coal mining subsidence lake and its connected river. The rarefaction curves approached saturation, and the coverage values of all the samples were >0.97, which suggested that the sequencing depth was sufficient to detect most bacterial taxa, even some scarce species (Figure S1; Table S1). Based on the statistical analysis of all the samples, the results of bacterial diversity (Chao 1 and Shannon indices) ranked as follows: gangue mountain (GM) (mean = 3496.18 and 7.342, respectively) > subsidence lake (SL) (mean = 3452.61 and 7.39, respectively) > Huihe River (HR) (mean = 1612.81 and 6.98, respectively). The Chao 1 index in the SL and GM was significantly higher than that in the HR (p < 0.001, Kruskal–Wallis test) (Figure 2a). There were no significant differences (p > 0.05, Kruskal–Wallis test) in the Shannon index among the three distinct types of sampling points (Figure 2a).
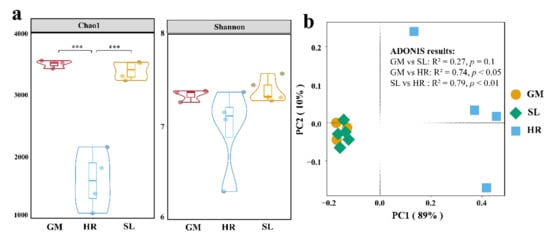
Figure 2.
Alpha and beta diversity of water samples in a coal mining subsidence lake and its connected river. (a) Chao 1 and Shannon diversity indices; (b) principal coordinates analysis (PCoA); SL: subsidence lakes; GM: coal gangue mountain waters; HR: Huihe River. *** p < 0.001.
Bray–Curtis similarity among bacterial communities at different sites was explored by principal coordinates analysis (PCoA) (Figure 2b). Adonis showed that the bacterial communities in the Huihe River were significantly separated from those in the subsidence lake and gangue mountain (p < 0.05). However, there were no significant differences between the subsidence lake and gangue mountain communities (p > 0.05, Figure 2b).
3.2. Taxonomic Composition of the Bacterial Community
The bacterial community composition at the phylum and genus levels was analyzed in the coal mining subsidence lake and its connected river (Figure 3). In the subsidence lake and gangue mountain waters, the dominant phyla were Proteobacteria (35.2% and 34.4%, respectively), followed by Firmicutes (35.4% and 35.2%, respectively) and Bacteroidetes (23.7% and 23.4%, respectively) (Figure 3a). In the HR, however, the most dominant bacterial phyla were Proteobacteria (36.1%), Bacteroidetes (20.8%), Actinobacteria (19.1%), and Cyanobacteria (14.4%) (Figure 3a). Cyanobacteria gradually decreased in the HR from upstream to downstream (Figure 3a). The most dominated bacterial community at phylum levels in different sampling types show significant differences (Figure 4a). The relative abundance of Firmicutes in the SL was significantly higher than that in the HR, while the relative abundance of Cyanobacteria and Actinobacteria in the HR was higher than that in the SL (Figure 4a), which had the highest relative abundance at L10 (23.1%) and L12 (24.1%), respectively (Figure 4a) (p < 0.05; Kruskal–Wallis test).
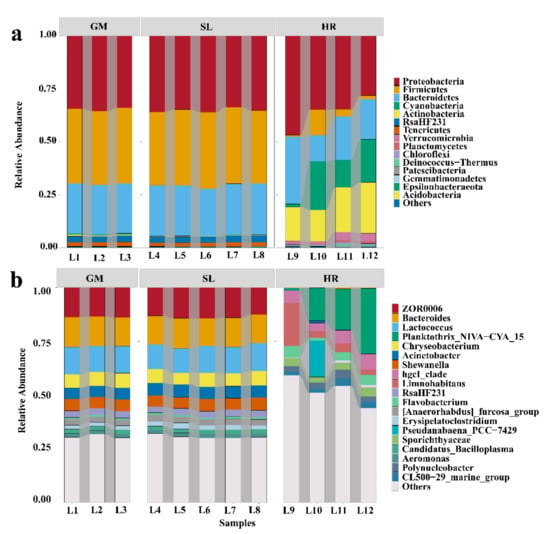
Figure 3.
Relative abundance of reads of bacteria at the phylum (a) and genus (b) levels in the water samples; SL: subsidence lakes; GM: coal gangue mountain waters; HR: Huihe River.
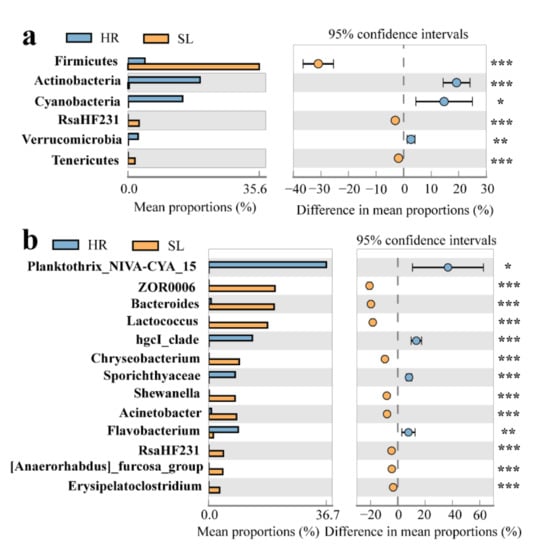
Figure 4.
Significantly different bacterial community compositions among the subsidence lake and its connected river. (a) phylum level; (b) genus level; SL: subsidence lake; GM: coal gangue mountain waters; HR: Huihe River; * p < 0.05, ** p < 0.01, *** p < 0.001.
At the genus level, in the SL and HR, the bacterial community composition differed greatly among different samples. However, the composition of bacterial communities was similar among the samples in the GM and SL (Figure 3b). In the SL and GM, the dominant genera were ZOR0006 (13.6% and 13.7%, respectively), Bacteroides (13.4% and 13.6%, respectively), Lactococcus (12.1% and 12.4%, respectively), Chryseobacterium (6.2% and 6.5%, respectively), Acinetobacter (5.7% and 5.2%, respectively), and Shewanella (5.7% and 5.2%, respectively) (Figure 3b). In the HR, the dominant genera were Planktothrix_NIVA-CYA_15 (16.4%), hgcI_clade (5.7%), and Flavobacterium (3.8%) (Figure 3b). The abundances of the Limnohabitans (7.4%) and Pseudanabaena_PCC-7429 (4.8%) genera at L9 and L10, respectively, were significantly higher than those at other sites. The most dominant bacterial community at the genus level also showed significant differences among the different sampling types (Figure 4b). ZOR0006, Bacteroides, Lactococcus, Acinetobacter, and Chryseobacterium were the dominant genera in the SL, while the relative abundances of Planktothrix_NIVA-CYA_15 and hgcI_clade were significantly higher in the HR (Figure 4b) (p < 0.05; Kruskal–Wallis test).
3.3. Physiochemical Properties and Redundancy Analysis
The physicochemical parameters of the coal mining subsidence lake and its connected river are summarized in Table S2. The concentrations of EC, CODcr, TP, TN, NH4+, and PO43− in the GM were higher than those in the HR and SL. For the TN concentrations, all the samples in the coal mining subsidence lake and its connected river were above the medium eutrophication level (0.8–1.2 mg/L), with values ranging from 0.87 to 2.71 mg/L. According to the evaluation criteria for TP, the TP concentrations in all the samples ranged from 0.045 to 0.268 mg/L, which indicated medium eutrophication. The highest TN and TP concentrations were 2.71 (L2) and 0.268 (L1) mg/L, respectively. There were few fluctuations in the other physiochemical parameters among the different sampling types (Table S2).
Redundancy analysis (RDA) indicated that the bacterial community composition was significantly affected by three environmental variables: TN, TDS, and T (Figure 5a). Among these three environmental variables, TN was the most important factor, which explained 21.4% of the total bacterial community composition variation (Figure 5c). The bacterial community function was also significantly affected by three environmental variables: SO42−, T, and PO43− (Figure 5b). Among those variables, SO42− was the most crucial factor, which alone accounted for 38.4% of the total bacterial community function variation (Figure 5d).
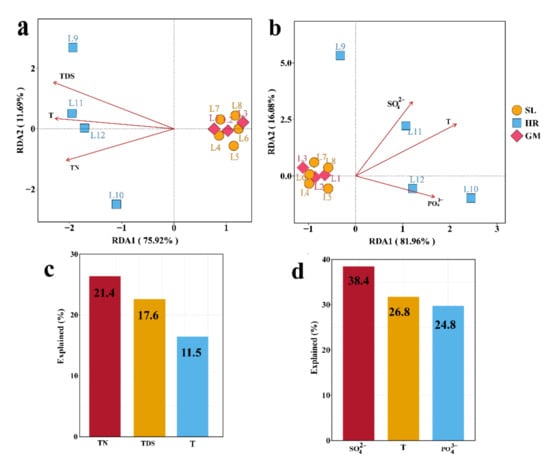
Figure 5.
Redundancy analyses (RDA) show the influence of significant environmental factors on (a) bacterial community composition and (b) bacterial functional composition. Bar plots (c,d) presenting the variation explained by each factor are shown below the RDA; SL: subsidence lakes; GM: coal gangue mountain waters; HR: Huihe River.
Spearman’s correlations among nineteen environmental parameters, bacterial community composition, and diversity indices for the water samples are shown in Figure S2. The results indicated that the Shannon index was positively correlated with Ca2+, K+, TP, and CODcr but was negatively correlated with pH, DO, TDS, and EC. The Chao 1 index was positively correlated with NH4+, K+, TP, Ca2+, and F− and negatively correlated with T, DO, TDS, and EC. Among the genera with a high relative abundance, ZOR0006, Bacteroides, Lactococcus, Chryseobacterium, Acinetobacter, and Shewanella were positively correlated with NH4+, F−, TP, NO3−, and SD but were negatively correlated with T, DO, and pH (Figure S2). However, the Limnohabitans hgcI_clade, Planktothrix_NIVA-CYA_15, and Flavobacterium genera were positively correlated with T, pH, DO, TDS, and EC and negatively correlated with NH4+ and F− (Figure S2).
3.4. Distribution Characteristics of Sulfur Metabolism Genes in Different Samples
In this study, PICRUSt2 was used to predict the functions of microbial communities, and the sulfur metabolism-related functional gene KO (KEGG Orthology) was obtained by comparison with the KEGG database (Table S3). The genes related to sulfur metabolism in the coal mining subsidence lake and its connected river were mainly involved in sulfate transport, assimilatory sulfate reduction, dissimilatory sulfate reduction and oxidation, and sulfur oxidation (Figure 6). The abundance of dissimilatory sulfate reduction and oxidation genes in the water was significantly lower than that of other sulfur metabolizing genes (Table S3). The abundances of the assimilatory sulfate reduction genes CysJ, cysI, CysH, Sir, cysN, cysD, and cysC were higher in all the samples (except Sir in the GM and SL samples) (Figure 6). The abundances of CysH and Sir were significantly higher in the HR than in the GM and SL samples (p < 0.01; Kruskal–Wallis test). The sulfate transport genes cysA, cysU, cysW, and cysP encode proteins that can transfer bacterial extracellular sulfate to the intracellular space. The abundance of these genes was high in all the samples, especially the abundance of cysP, which was higher than that of the other genes in the three types of samples (Figure 6). The dissimilatory sulfate reduction and oxidation genes aprA, aprB, and Sat were lower in all the samples. The genes sseA, sorA, and glpE participate in sulfur oxidation, and the abundances of sseA and glpE were significantly higher in the HR and the SL than in the other samples (p < 0.01; Kruskal–Wallis test) (Table S3).
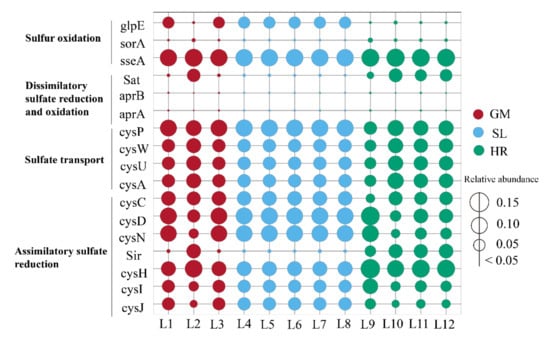
Figure 6.
Relative abundances of sulfur metabolizing genes in the subsidence lake and its connected river; SL: subsidence lakes; GM: coal gangue mountain waters; HR: Huihe River.
3.5. Bacterial Community Co-Occurrence Network Analysis
The correlation-based network was composed of 153 nodes with 825 edges for subsidence lakes and 201 nodes with 731 edges for the Huihe River (Figure 7; Table S4). Random networks were constructed with the same nodes and edges in each group to guarantee that the real networks were nonrandom. Moreover, the value of the structural properties (modularity and clustering coefficient (avgCC)) in subsidence lake and Huihe River networks were higher than those in Erdös-Réyni random networks, and the value of small-world coefficients (σ) in subsidence lake and Huihe River networks with σ > 1 (Table S4), all suggesting that both networks have “small world” properties. The co-occurrence network in the subsidence lake and the Huihe River were mostly positively correlated (60.36% and 71.27%, respectively), which indicated mutualistic and cooperative relationships. For the subsidence lake network, Proteobacteria (56.86%), Firmicutes (22.88%), Bacteroidetes (12.42%), Tenericutes (3.92%), and Rsahf231 (1.96%) mainly occupied the nodes (Figure 7a). Nodes in the Huihe River network mainly pertained to Proteobacteria (29. 85%), Cyanobacteria (24.38%), Bacteroidetes (20.9%), Actinobacteria (13.3%), and Verrucomicrobia (4.48%) (Figure 7b). Keystone bacterial taxa play a crucial role in maintaining microbial community stability. Based on the low betweenness centrality and high degree value, the top species identified as keystone taxa were the Clostridiaceae_1 family and Citrobacter, Shewanella genera for the subsidence lake network, and the Flavobacterium, Limnohabitans, and Sphingorhabdus genera for the Huihe River network.
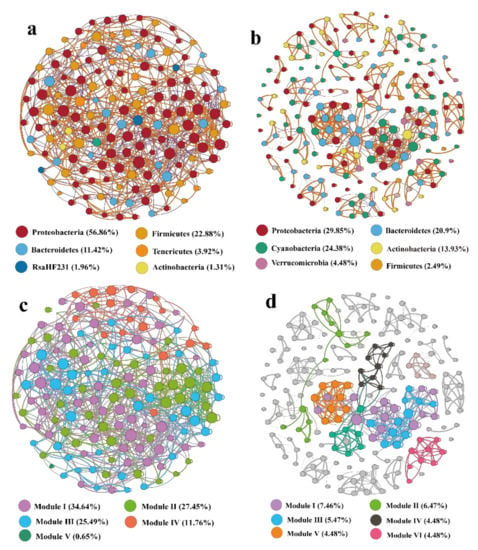
Figure 7.
Spearman’s correlation-based network analysis of frequent ASVs (relative abundance > 0.05%) for (a,c) subsidence lakes and (b,d) river water samples. The size of each node is proportional to the number of connections (i.e., degree), and the nodes are colored according to different phyla. Orange and blue edges indicate positive and negative correlations, respectively.
Furthermore, a module is regarded as a group of ASVs that are linked more tightly together. In this study, both the subsidence lake and Huihe River network were mainly divided into six modules. In the subsidence lake network, modules I, II, and III occupied 34.64%, 27.45%, and 25.49%, respectively (Figure 7c). In the Huihe River network, modules I, II, and III occupied 7.46%, 6.47%, and 5.47%, respectively (Figure 7d). In the subsidence lake network, the ASVs from modules I and II mainly belonged to Proteobacteria, Firmicutes, and Bacteroidetes. In the Huihe River network, the ASVs from module I and Module II belonged to Actinobacteria, Cyanobacteria, Proteobacteria, and Bacteroidetes.
4. Discussion
4.1. Variation of Bacterial Diversity in Subsidence Lake and Its Connected River
In this study, we found that the bacterial α-diversity in the gangue mountain and subsidence lake was much higher than that in the Huihe River (Figure 2). The high richness observed in the gangue mountain and subsidence lake is likely attributed to the increase in nutrients and resources and/or because of the increased niche availability for aquatic bacteria at an intermediate level of nutrients. This concept is supported by the fact that NH4+ and TP were positively correlated with the Chao 1 and Shannon indices, and the concentrations of nutrients in the gangue mountains and subsidence lake were much higher than those in the Huihe River (Figure S2, Table S2). Nutrient availability plays a crucial role in the variation in bacterial diversity [57]. Our result on the relationship between bacterial diversity and nutrients is consistent with previous research, which found that the bacterial diversity indices increased with increasing nutrients [58,59]. However, the impact of nutrients on bacterial diversity may depend on the scale, as a previous study showed that nutrients significantly reduce bacterial diversity across a large eutrophication scale [60]. This phenomenon is consistent with the intermediate-disturbance hypothesis, which holds that a mildly disturbed ecosystem would have maximal community diversity [61]. On the other hand, a previous study suggested that network modules can be explained as overlapping niches and that groups of microbes are more closely connected with each other [62,63]. Here, network analysis suggested that the modularity index in the subsidence lake was higher than that in the Huihe River (Table S4), indicating that there are more ecological niches in the subsidence lake for a variety of bacteria to occupy.
4.2. Variations of Bacterial Community Composition at Different Sites
The major bacterial communities that we found in the waters of the subsidence lake and its connected river were consistent with previous studies of lakes and rivers performed around the world [64,65], but the relative abundances differed among the different sites. The results of the bacterial community composition analysis showed that the dominant bacteria at the phylum and genus levels between the subsidence lake and gangue mountain samples were similar. There are several possible reasons for this observation. For example, sampling sites that are close to each other tend to have similar environmental conditions for bacterial communities [66]. Moreover, anthropogenic activities such as the frequent photovoltaic power generation in this area can accelerate the homogenization process. Proteobacteria, specifically Gammaproteobacteria and Alphaproteobacteria, were most abundant in the subsidence lake and its connected river. This result was consistent with other studies performed in eutrophic lakes and urban rivers, where Proteobacteria also played a dominant role in aquatic environments [56,67]. Gammaproteobacteria are widely distributed in oligotrophic and eutrophic environments, and their abundance is influenced by pH and nutrient availability [11,68]. Alphaproteobacteria typically prevail in marine environments and participate in biogeochemical cycling [69]. Numerous species of Proteobacteria are implicated in the biogeochemical cycles of C, N, and S in subsidence lakes and its connected rivers.
The second most abundant phylum in the subsidence lake and gangue mountains were Firmicutes, which are associated with human activities [11]. For example, the genus ZOR0006, which is affiliated with Firmicutes, was the most enriched microbial taxon related to fish gills and guts [70]. Lactococcus (Firmicutes) has a beneficial influence on the growth performance, disease resistance, nutrient metabolism, and intestinal microbial diversity of fish [71,72]. Moreover, the genus has also been observed in human patients with urinary tract infections and endocarditis [36]. The significantly higher abundance of Firmicutes in the subsidence lake may be associated with the comprehensive utilization mode of photovoltaic power generation on the surface and the underwater fishery in the coal mining subsidence lake. Bacteroidetes, as the third dominant community, was significantly enriched in the coal mining subsidence lake and gangue mountains. This result was consistent with the study of Hou et al., who found that Bacteroidetes was enriched in mining area sediment samples and positively correlated with metal content [73]. Coal gangue gradually disintegrates under the influence of external factors such as weathering and rainfall, and internal heavy metals migrate and transform into the surrounding environment [74]. This may be the reason for the high abundance of Bacteroidetes in the coal mining subsidence lake and gangue mountain waters. Furthermore, it has been reported that Bacteroidetes are able to disintegrate hydrolytic cellulose and starch polysaccharides and respond to the enrichment of various dissolved organic matter in aquatic environments [75,76]. During the formation of the coal mining subsidence lake, a large amount of plant litter and soil organic matter would have entered the lake along with surface runoff, which could have stimulated the enrichment of Bacteroidetes.
In this study, bloom-forming Cyanobacteria were observed only in the Huihe River (L10, L11, and L12) area, and their relative abundance gradually decreased from upstream to downstream (Figure 3a). Cyanobacteria are generally found in eutrophic environments and consistently occur with increased N and P in the water [77,78]. Luo et al. also reported that Cyanobacteria were significantly enriched in rivers, with higher nitrogen and phosphorus concentrations in summer [79]. Therefore, the enrichment of Cyanobacteria reflects the high nitrogen and phosphorus content in the Huihe River, indicating that the river is at risk of eutrophication. There is a large amount of farmland in the Huihe River area. Agricultural production and related human activities may be important sources of nitrogen and phosphorus in the Huihe River [45]. Furthermore, we observed that Cyanobacteria gradually decreased in the Huihe River from upstream to downstream (Figure 3a). The co-occurrence network analysis showed that some numbers of Cyanobacteria were significantly negatively correlated with the numbers of Proteobacteria, Bacteroidetes, and Actinobacteria (Figure 7). Our observation was consistent with that of Osman et al., who found that heterotrophic bacteria, such as Proteobacteria, Bacteroides, Actinobacteria, and Firmicutes, exhibit antagonistic activity against various Cyanobacteria [80]. However, our results contrast with the findings of Lu et al., who studied bacterial community composition in eutrophic rivers and found that Cyanobacteria could promote the growth of Proteobacteria, Actinobacteria, and Bacteroidetes [12]. This discrepancy may be attributed to the different relative abundances of Cyanobacteria: the dominant species in our study, and a lower relative abundance in the study by Lu et al. [12].
4.3. Predicted Functional Potential of Bacterial Communities Associated with Sulfur Metabolism
Our data illustrated that sulfate had strong impact on the predicted bacterial functional composition (Figure 5b,d). Thus, we analyzed the sulfur cycle in the subsidence lake and its connected river. The spectrum of major sulfur-related functional genes found in the subsidence lake and the river are shown in Table S3. The abundances of assimilatory sulfate reduction genes and sulfate transport genes were significantly higher than those of dissimilatory sulfate reduction and oxidation genes and sulfur oxidation genes in all the samples (Table S3). Our results agree with those of Cai et al. [81], who reported that sulfate-reducing genes were the most abundant and were far more numerous than sulfur oxidation genes and sulfur assimilation genes in urban rivers. Our data indicated that SO42− concentrations in subsidence lakes and rivers are higher (Table S3). When sulfates are significantly enriched, the bacterial community may absorb the enriched sulfates into the cell via sulfate transport genes and then reduce sulfates to sulfides by assimilatory sulfate reduction genes. This may be an adaptive mechanism of microorganisms during sulfate enrichment in water bodies.
4.4. Differences in Microbiome Complexity and Keystone Taxa in the Subsidence Lake and Its Connected River
Co-occurrence network-based analysis is an effective tool for characterizing complex interaction networks and ecological processes that transcend microbial community diversity and composition [82]. In this study, the resulting networks had modular and small-world properties, reflecting the essential network attributes of a complex ecosystem [83]. The lower graph density, average degree, and average clustering coefficient values combined with the higher average path length in the subsidence lake networks indicated that the coupling between the community nodes and the network complexity was lower. Previous studies illustrated that increased environmental pressure reduces the connectivity and complexity of microbial community networks [84]. The higher concentration of nutrients in subsidence lakes can specifically select microbial populations with fewer interactions through niche differentiation. However, the modularity indices in the subsidence lake were significantly higher than those in the Huihe River (Table S4). Higher modularity has been considered crucial for promoting the stability of microbial networks [85], and the higher modularity indices in the subsidence lake illustrated a stable community structure. In addition, we found that the subsidence lake network had a higher ratio of negative links than did the Huihe River (Table S4). This was consistent with previous results, which found that negative links within the network can enhance the stability of the microbial community structure [86]. Compared with that of the subsidence lake network, the Huihe River community diversity was lower, but there were slightly higher values of the average clustering coefficient, graph density, and average degree, and the network was a tighter cluster (Table S4). This indicated that an appropriate amount of diversity loss may improve the metabolic efficiency of water microorganisms, although the microbial diversity is low [87]. Furthermore, the low values of average path length in the Huihe River network suggested that the information and substances were rapidly disseminated among species in the microbial communities [88]. This signified that the microbes had a higher transmission capacity in the river than in the subsidence lake.
ASVs, which are thought by some to be keystone bacterial taxa, are nodes with high degree and low betweenness centrality values, which may have a considerable impact on the entire microbial community [89]. The influence is potentially due to powerful ecological associations rather than high abundance [90]. In the subsidence lake co-occurrence network, keystone bacterial taxa included the Clostridiaceae_1 family and the Shewanella genus. The Huihe River network included the Flavobacterium and Limnohabitans genera. Clostridiaceae_1 is a strictly anaerobic fermenting bacterium and can utilize a variety of organic substrates (e.g., sucrose, glucose, and cellulose) to produce volatile fatty acids [91]. The genus Shewanella is known for its wide range of electron accepting capacities, which play an essential role in organic matter reduction [92]. The genus Flavobacterium, found in many nutrient-rich environments, can mineralize high molecular weight organic (e.g., polysaccharides and proteins) compounds into low molecular weight organic compounds [93]. It has been reported that Limnohabitans are sensitive to salinity, which plays a key role in the scavenging of sulfur and nitrogen pollutants [94,95]. Consequently, the niche spaces occupied by these keystone bacterial taxa may be relatively complementary, playing indispensable roles in the C, S, and N cycles, and are of ecological significance in preserving community structure and functional stability in the subsidence lake and its connected river ecosystem. The co-occurrence network consisted of six major modules in the subsidence lake and Huihe River, (Figure 7c,d). Closely related phylogenetic species tend to occur in the same module and may have similar or complementary functions [34]. In the subsidence lake co-occurrence network, the bacteria in module I were mainly associated with the pathogenicity of fish and humans. The Erysipelotrichaceae family and the genera Lactococcus, Flavobacterium, and Aeromonas inhabit various aquatic environments and are increasingly recognized as fish pathogens, which may have serious impacts on freshwater and marine fisheries [96,97,98,99]. The pneumonia of the Klebsiella genus causes a wide range of infections in humans, including urinary tract infections, pneumonia, liver abscesses, and bacteremia [100]. In the Huihe River co-occurrence network, nutrient-degrading bacteria in the module I had a high degree. The CL50029_marine_group genus plays crucial roles during the processes of salt ionic (including Ca2+, Mg2+, Cl−, and SO42−) conversion in river ecology [101]. The genus hgcI_clade is mainly related to denitrification and nitrogen fixation [102].
Niche theory illustrates that bacterial community structure is controlled by deterministic factors such as environmental filtering and interspecies interactions, which are often referred to as deterministic processes [103]. In this study, network analysis showed nonrandom co-occurrence patterns of bacterial communities in the subsidence lake and its connected river, indicating the role of deterministic processes in community assembly. This pattern was driven by environmental filtering and ecological functions. However, in this study, only bacterial communities were considered when constructing microbial co-occurrence networks. Considering a wider range of microbial communities (such as fungi and archaea) is crucial to research the response of aquatic microbial community structures, and their ecological functions to anthropogenic activities and environmental changes in coal mining subsidence areas. In addition, the co-occurrence patterns of aquatic microorganisms change with the seasons [56,103]. Future studies are needed to explore changes in microbial communities at multiple seasonal scales or time series.
5. Conclusions
In this study, microbial community diversity and function were investigated in a coal mining subsidence lake and its connected river ecosystem by using high-throughput sequencing. Our findings illustrated that the aquatic bacterial community α-diversity in the subsidence lake was higher than that in its connected river. Among the physicochemical factors, nutrients could better explain the differences in bacterial communities. Co-occurrence network analysis revealed that the environmental factors (especially nutrients) and ecological functions were the primary factors driving the assembly of bacterial communities in the subsidence lake and its connected river. Functional analysis showed that assimilatory sulfate reduction processes were major players in the sulfur cycle of the subsidence lake and its connected river ecosystem. Overall, our results provide new insight into the spatial distribution characteristics and co-occurrence patterns of bacterial communities in a coal mining subsidence lake and its connected river. Future research will be needed to comprehensively analyze the variation in microbial diversity and co-occurrence patterns in coal mining subsidence lakes on a spatiotemporal scale. In addition, the microbial ecological processes in the sediments of the subsidence lake should also be revealed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14080674/s1, Figure S1: Rarefaction curves of richness across water samples in coal mining subsidence lakes and its connected rivers; Figure S2: Spearman’s correlations between concentration of each environmental factors and bacterial relative abundance at genus level. * p < 0.05, ** p < 0.01, *** p < 0.001; Figure S3: Cluster analysis of frequent ASVs (relative abundance > 0.05%) correlation in subsidence lake samples; Figure S4: Cluster analysis of frequent ASVs (relative abundance > 0.05%) correlation in Huihe river samples; Table S1: Estimates of richness and diversity for amplicon sequence variants (ASVs) for 12 water samples obtained from coal mining subsidence lakes and its connected rivers; Table S2: Physical and chemical indices of water samples in different sites in coal mining subsidence lakes and its connected rivers; Table S3: Distribution of sulfur metabolism genes in each group of samples; Table S4: Topological properties of bacterial community co-occurrence networks and their Erdős-Rényi random networks in different habitats.
Author Contributions
Conceptualization, Resources, Funding acquisition, T.F.; Data Curation, Writing-Original Draft, Supervision, W.F.; Formal analysis, Supervision, Y.Z.; Investigation, Resources, Project administration, A.L.; Validation, Visualization, S.W.; Software, Investigation, X.W. (Xingming Wang); Supervision, Validation, L.X.; Investigation, Resources, X.W. (Xiangping Wei); Software, Investigation, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (51878004), Research Foundation of Huaibei Mining Group in 2021, Research Foundation of Huainan Mining Group in 2021, China Energy Investment Corporation 2030 Pilot Project (grant numbers GJNY2030XDXM-19-03.2), Research Foundation of Huaibei Mining Group in 2022, and Research Foundation of the Institute of Environment-friendly Materials and Occupational Health (Wuhu), Anhui University of Science and Technology (ALW2020YF08).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All raw sequencing reads in this study have been deposited at the NCBI Sequence Read Archive under accession number PRJNA864567.
Acknowledgments
Anonymous reviewers are acknowledged for their constructive comments and helpful suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Zhu, J.; Ye, C.; Zhu, P.; Ba, Q.; Pang, J.; Shu, L. Effects of biochar application on the abundance and community composition of denitrifying bacteria in a reclaimed soil from coal mining subsidence area. Sci. Total Environ. 2018, 625, 1218–1224. [Google Scholar] [CrossRef]
- Sun, S.; Sun, H.; Zhang, D.; Zhang, J.; Cai, Z.; Qin, G.; Song, Y. Response of Soil Microbes to Vegetation Restoration in Coal Mining Subsidence Areas at Huaibei Coal Mine, China. Int. J. Environ. Res. Public Health 2019, 16, 1757. [Google Scholar] [CrossRef]
- HU, Z.-Q.; WANG, X.-J.; HE, A.-M. Distribution Characteristic and Development Rules of Ground Fissures Due to Coal Mining in Windy and Sandy Region. J. China Coal Soc. 2014, 39, 11–18. [Google Scholar]
- Zhou, D.-W.; Wu, K.; Cheng, G.-L.; Li, L. Mechanism of mining subsidence in coal mining area with thick alluvium soil in China. Arab. J. Geosci. 2014, 8, 1855–1867. [Google Scholar] [CrossRef]
- Pei, W.; Yao, S.; Knight, J.F.; Dong, S.; Pelletier, K.; Rampi, L.P.; Wang, Y.; Klassen, J. Mapping and detection of land use change in a coal mining area using object-based image analysis. Environ. Earth Sci. 2017, 76, 125. [Google Scholar] [CrossRef]
- Mason, T.J.; Krogh, M.; Popovic, G.C.; Glamore, W.; Keith, D.A. Persistent effects of underground longwall coal mining on freshwater wetland hydrology. Sci. Total Environ. 2021, 772, 144772. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, G.; Zheng, L.; Chou, C.-L. Characteristics of coal quality and their relationship with coal-forming environment: A case study from the Zhuji exploration area, Huainan coalfield, Anhui, China. Energy 2010, 35, 423–435. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, X.; Tang, Q.; Ou, J. Lead Pollution and Isotope Tracing of Surface Sediments in the Huainan Panji Coal Mining Subsidence Area, Anhui, China. Bull. Environ. Contam. Toxicol. 2019, 103, 10–15. [Google Scholar] [CrossRef]
- Ouyang, Z.; Gao, L.; Yang, C. Distribution, sources and influence factors of polycyclic aromatic hydrocarbon at different depths of the soil and sediments of two typical coal mining subsidence areas in Huainan, China. Ecotoxicol. Environ. Saf. 2018, 163, 255–265. [Google Scholar] [CrossRef]
- Hu, J.; Chen, X.; Chen, Y.; Li, C.; Ren, M.; Jiang, C.; Chen, Y.; An, S.; Xu, Y.; Zheng, L. Nitrate sources and transformations in surface water of a mining area due to intensive mining activities: Emphasis on effects on distinct subsidence waters. J. Environ. Manag. 2021, 298, 113451. [Google Scholar] [CrossRef]
- Ge, Y.; Lou, Y.; Xu, M.; Wu, C.; Meng, J.; Shi, L.; Xia, F.; Xu, Y. Spatial distribution and influencing factors on the variation of bacterial communities in an urban river sediment. Environ. Pollut. 2020, 272, 115984. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Song, Y.; Mao, G.; Lin, B.; Wang, Y.; Gao, G. Spatial variation in bacterial biomass, community composition and driving factors across a eutrophic river. Ecotoxicol. Environ. Saf. 2020, 205, 111113. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Wang, R.; Liu, G. Health risk assessment of potentially harmful elements in subsidence water bodies using a Monte Carlo approach: An example from the Huainan coal mining area, China. Ecotoxicol. Environ. Saf. 2019, 171, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I. Ecosystem processes and interactions in a morass of diversity. FEMS Microbiol. Ecol. 2012, 81, 507–519. [Google Scholar] [CrossRef]
- Madsen, E.L. Microorganisms and their roles in fundamental biogeochemical cycles. Curr. Opin. Biotechnol. 2011, 22, 456–464. [Google Scholar] [CrossRef]
- Besemer, K.; Luef, B.; Preiner, S.; Eichberger, B.; Agis, M.; Peduzzi, P. Sources and composition of organic matter for bacterial growth in a large European river floodplain system (Danube, Austria). Org. Geochem. 2009, 40, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-P.; Yang, Y.; Niu, Z.-S.; Lu, D.-P.; Zhu, C.-H.; Feng, J.-N.; Wu, J.-Y.; Chen, Y.-R.; Tou, F.-Y.; Liu, M.; et al. Characteristics of microbial community indicate anthropogenic impact on the sediments along the Yangtze Estuary and its coastal area, China. Sci. Total Environ. 2018, 648, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tu, D.; Li, X.; Lu, W.; Li, J. Impact of long-term industrial contamination on the bacterial communities in urban river sediments. BMC Microbiol. 2020, 20, 254. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, M.; Li, Y.; Wang, P.; Wang, C.; Gao, Y.; Wu, H.; Xu, C.; Niu, L.; Wang, L.; et al. Determination of vertical and horizontal assemblage drivers of bacterial community in a heavily polluted urban river. Water Res. 2019, 161, 98–107. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y.; Hu, Y.; Cai, J.; Liu, X.; Bai, C.; Tang, X.; Zhang, Y.; Jang, K.-S.; Spencer, R.G.; et al. Microbial production and consumption of dissolved organic matter in glacial ecosystems on the Tibetan Plateau. Water Res. 2019, 160, 18–28. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Jiao, N.; Xu, B.; Gu, Z.; Xing, T.; Xiong, J. Bacterial community composition and diversity in Kalakuli, an alpine glacial-fed lake in Muztagh Ata of the westernmost Tibetan Plateau. FEMS Microbiol. Ecol. 2017, 93, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Tang, Y.; Wang, S.; Lu, X.; Cheng, Z.; Zhang, X.; Wu, P.; Chang, X.; Xia, Y. Typhoon-induced turbulence redistributed microplastics in coastal areas and reformed plastisphere community. Water Res. 2021, 204, 117580. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Liu, P.; Sun, Y.; Song, Z.; Hu, X. Characteristics of bacterial community structure and function associated with nutrients and heavy metals in coastal aquaculture area. Environ. Pollut. 2021, 275, 116639. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Zhang, W.; Lin, L.; Wang, L.; Niu, L.; Zhang, H.; Wang, P.; Wang, C. Response of bacterial community in composition and function to the various DOM at river confluences in the urban area. Water Res. 2020, 169, 115293. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Li, B.-B.; Yang, Z.-C.; Cheng, Y.-Y.; Liu, D.-F.; Yu, H.-Q. Mediation of functional gene and bacterial community profiles in the sediments of eutrophic Chaohu Lake by total nitrogen and season. Environ. Pollut. 2019, 250, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, W.; Zuo, Y.; Lin, L.; Song, L. Heavy metal migration and risk transference associated with cyanobacterial blooms in eutrophic freshwater. Sci. Total Environ. 2018, 613–614, 1324–1330. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Gao, W.; Guo, Z.; Xue, C.; Pang, J.; Shu, L. Effects of biochar amendment on bacterial and fungal communities in the reclaimed soil from a mining subsidence area. Environ. Sci. Pollut. Res. 2019, 26, 34368–34376. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Meng, H.; Xie, Y.; Zhang, J.; Hong, J. Effects of Seven-Year Fertilization Reclamation on Bacterial Community in a Coal Mining Subsidence Area in Shanxi, China. Int. J. Environ. Res. Public Health 2021, 18, 12504. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Wei, Y.; Meng, H.; Cao, Y.; Lead, J.R.; Hong, J. Effects of fertilization and reclamation time on soil bacterial communities in coal mining subsidence areas. Sci. Total Environ. 2020, 739, 139882. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Nawaz, M.Z.; Mahboob, S.; Al-Ghanim, K.A.; Khan, I.A.; Lu, Z.; Chen, T. Seasonal succession and spatial distribution of bacterial community structure in a eutrophic freshwater Lake, Lake Taihu. Sci. Total Environ. 2019, 669, 29–40. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Y.; Gao, G.; Jiang, J. Spatial-Temporal Variation of Bacterial Communities in Sediments in Lake Chaohu, a Large, Shallow Eutrophic Lake in China. Int. J. Environ. Res. Public Health 2019, 16, 3966. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Dini-Andreote, F.; Zhang, N.; Liang, C.; Yao, Z.; Zhang, H.; Zhang, D. Divergent Co-occurrence Patterns and Assembly Processes Structure the Abundant and Rare Bacterial Communities in a Salt Marsh Ecosystem. Appl. Environ. Microbiol. 2020, 86, e00322-20. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Zhao, D.; Zeng, J.; Guo, L.; Yu, Z. Disentangling the seasonal co-occurrence patterns and ecological stochasticity of planktonic and benthic bacterial communities within multiple lakes. Sci. Total Environ. 2020, 740, 140010. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Zhang, H.; Xie, W.; Zhou, X.; Zhu, X.; Zhang, D. Co-occurrence patterns and assembly processes of microeukaryotic communities in an early-spring diatom bloom. Sci. Total Environ. 2020, 711, 134624. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, J.; Chen, F.; Li, X.; Zhang, Q.; Yang, Y. Adaptive Development of Soil Bacterial Communities to Ecological Processes Caused by Mining Activities in the Loess Plateau, China. Microorganisms 2020, 8, 477. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, W.; Li, X.; Gao, G.; Jiang, J. Linking bacterial community shifts with changes in the dissolved organic matter pool in a eutrophic lake. Sci. Total Environ. 2020, 719, 137387. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Shen, Y.; Wang, C.; Wang, P.; Zhang, W.; Gao, Y.; Niu, L. Statistical determination of crucial taxa indicative of pollution gradients in sediments of Lake Taihu, China. Environ. Pollut. 2019, 246, 753–762. [Google Scholar] [CrossRef]
- Hu, A.; Ju, F.; Hou, L.; Li, J.; Yang, X.; Wang, H.; Mulla, S.I.; Sun, Q.; Bürgmann, H.; Yu, C.-P. Strong impact of anthropogenic contamination on the co-occurrence patterns of a riverine microbial community. Environ. Microbiol. 2017, 19, 4993–5009. [Google Scholar] [CrossRef]
- Yan, Z.; Hao, Z.; Wu, H.; Jiang, H.; Yang, M.; Wang, C. Co-occurrence patterns of the microbial community in polycyclic aromatic hydrocarbon-contaminated riverine sediments. J. Hazard. Mater. 2019, 367, 99–108. [Google Scholar] [CrossRef]
- Jiao, S.; Liu, Z.; Lin, Y.; Yang, J.; Chen, W.; Wei, G. Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biol. Biochem. 2016, 98, 64–73. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Milici, M.; Deng, Z.-L.; Tomasch, J.; Decelle, J.; Wos-Oxley, M.L.; Wang, H.; Jáuregui, R.; Plumeier, I.; Giebel, H.-A.; Badewien, T.H. Co-Occurrence Analysis of Microbial Taxa in the Atlantic Ocean Reveals High Connectivity in the Free-Living Bacterioplankton. Front. Microbiol. 2016, 7, 649. [Google Scholar] [CrossRef] [PubMed]
- Cram, J.A.; Xia, L.C.; Needham, D.M.; Sachdeva, R.; Sun, F.; Fuhrman, J.A. Cross-depth analysis of marine bacterial networks suggests downward propagation of temporal changes. ISME J. 2015, 9, 2573–2586. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, L.; Wei, X.; Dong, X.; Chen, X. Eutrophication Characteristics and Sources of Nitrogen in Surface Water in Huaibei Mining Area, Anhui. Adm. Tech. Environ. Monit. 2021, 02, 1006–2009. [Google Scholar]
- Chen, X.; Zheng, L.; Jiang, C.; Huang, W.; Dong, X. Characteristics of Surface Water Chemistry and Sulfur Hydrogen Oxygen Isotope Composition in Linhuan Mining Area of Huaibei City, Anhui Province. Earth Environ. 2019, 47, 9. [Google Scholar]
- Kong, L.; Jiang, C.; Zheng, L.; Cheng, H.; Ren, M.; Min, F.; Fang, L. Characters of Hydrochemistry and Their Influenced Factors of Different Waters in the Linhuan Coal Mining Subsidence Area of Huaibei City. J. Lake Sci. 2017, 29, 10. [Google Scholar]
- Jin, X.C.; Tu, Q.Y. The Standard Methods for Observation and Analysis in Lake Eutrophication; Chinese Environment Science Press: Beijing, China, 1990; p. 240. [Google Scholar]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Liu, J.; He, X.-X.; Lin, X.-R.; Chen, W.-C.; Zhou, Q.-X.; Shu, W.-S.; Huang, L.-N. Ecological Effects of Combined Pollution Associated with E-Waste Recycling on the Composition and Diversity of Soil Microbial Communities. Environ. Sci. Technol. 2015, 49, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2: An Improved and Extensible Approach for Metagenome Inference. BioRxiv 2019, 672295. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Chen, X.; Jiang, Q.; Liu, Y.; Xie, S. Cyanobacterial bloom induces structural and functional succession of microbial communities in eutrophic lake sediments. Environ. Pollut. 2021, 284, 117157. [Google Scholar] [CrossRef] [PubMed]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. InterJournal Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Zhang, L.; Fang, W.; Li, X.; Lu, W.; Li, J. Strong linkages between dissolved organic matter and the aquatic bacterial community in an urban river. Water Res. 2020, 184, 116089. [Google Scholar] [CrossRef]
- Tang, X.; Xie, G.; Shao, K.; Tian, W.; Gao, G.; Qin, B. Aquatic Bacterial Diversity, Community Composition and Assembly in the Semi-Arid Inner Mongolia Plateau: Combined Effects of Salinity and Nutrient Levels. Microorganisms 2021, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Zwirglmaier, K.; Keiz, K.; Engel, M.; Geist, J.; Raeder, U. Seasonal and spatial patterns of microbial diversity along a trophic gradient in the interconnected lakes of the Osterseen Lake District, Bavaria. Front. Microbiol. 2015, 6, 1168. [Google Scholar] [CrossRef]
- Hewson, I.; Vargo, G.A.; Fuhrman, J.A. Bacterial Diversity in Shallow Oligotrophic Marine Benthos and Overlying Waters: Effects of Virus Infection, Containment, and Nutrient Enrichment. Microb. Ecol. 2003, 46, 322–336. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, G.; Jiang, X.; Shao, K.; Tang, X.; Gao, G. The Relationships Between the Free-Living and Particle-Attached Bacterial Communities in Response to Elevated Eutrophication. Front. Microbiol. 2020, 11, 423. [Google Scholar] [CrossRef]
- Fox, J.W. The intermediate disturbance hypothesis should be abandoned. Trends Ecol. Evol. 2013, 28, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Shiri, F.; Anat, K.; Isacc, M.; Uri, G.; Roded, S.; Eytan, R. The Large-Scale Organization of the Bacterial Network of Ecological Co-Occurrence Interactions. Nucleic Acids Res. 2010, 38, 3857–3868. [Google Scholar]
- Roberto, A.A.; Van Gray, J.B.; Leff, L.G. Sediment bacteria in an urban stream: Spatiotemporal patterns in community composition. Water Res. 2018, 134, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Londono, N.; Donovan, A.R.; Shi, H.; Geisler, M.; Liang, Y. Impact of TiO2 and ZnO nanoparticles on an aquatic microbial community: Effect at environmentally relevant concentrations. Nanotoxicology 2017, 11, 1140–1156. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, Z.; Zhang, C.; Wei, Q.; Zhang, S.; Li, M. Spatial and seasonal variations of sediment bacterial communities in a river-bay system in South China. Appl. Microbiol. Biotechnol. 2021, 105, 1979–1989. [Google Scholar] [CrossRef]
- Chalifour, A.; Walser, J.-C.; Pomati, F.; Fenner, K. Temperature, Phytoplankton Density and Bacteria Diversity Drive the Biotransformation of Micropollutants in a Lake Ecosystem. Water Res. 2021, 202, 117412. [Google Scholar] [CrossRef]
- Shapovalova, A.A.; Khijniak, T.V.; Tourova, T.P.; Muyzer, G.; Sorokin, D.Y. Heterotrophic denitrification at extremely high salt and pH by haloalkaliphilic Gammaproteobacteria from hypersaline soda lakes. Extremophiles 2008, 12, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Allers, E.; Gómez-Consarnau, L.; Pinhassi, J.; Gasol, J.M.; Šimek, K.; Pernthaler, J. Response of Alteromonadaceae and Rhodobacteriaceae to Glucose and Phosphorus Manipulation in Marine Mesocosms. Environ. Microbiol. 2007, 9, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Kuang, T.; He, A.; Lin, Y.; Huang, X.; Liu, L.; Zhou, L. Comparative analysis of microbial communities associated with the gill, gut, and habitat of two filter-feeding fish. Aquac. Rep. 2020, 18, 100501. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Li, D.; Chen, W.-J.; Ban, S.-N.; Liu, T.; Wen, H.; Jiang, M. Effects of dietary host-associated Lactococcus lactis on growth performance, disease resistance, intestinal morphology and intestinal microbiota of mandarin fish. Aquaculture 2021, 540, 736702. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.; Liu, S.; Cai, Z.; Song, D.; Yang, L.; Nie, G. The probiotic properties of different preparations using Lactococcus lactis Z-2 on intestinal tract, blood and hepatopancreas in Cyprinus carpio. Aquaculture 2021, 543, 736911. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, P.; Zhang, J.; Zhou, Y.; Yang, Y.; Mao, Q.; Tsang, D.C.W.; Núñez-Delgado, A.; Luo, L. Spatial variation of sediment bacterial community in an acid mine drainage contaminated area and surrounding river basin. J. Environ. Manag. 2019, 251, 109542. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gao, L.; Zhou, X.; Zhao, H.; He, J.; Chen, J.; Wu, Q. Analysis of Physical and Chemical Characteristics of Coal Gangue and Ecological Risk Assessment in Grassland Coal Mine Area. Coal Sci. Technol. 2021, 1–9. [Google Scholar]
- Zhou, L.; Zhou, Y.; Tang, X.; Zhang, Y.; Jang, K.-S.; Székely, A.J.; Jeppesen, E. Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter. Water Res. 2020, 190, 116776. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Kube, M.; Teeling, H.; Richter, M.; Lombardot, T.; Allers, E.; Würdemann, C.A.; Quast, C.; Kuhl, H.; Knaust, F.; et al. Whole genome analysis of the marine Bacteroidetes ‘Gramella forsetii’ reveals adaptations to degradation of polymeric organic matter. Environ. Microbiol. 2006, 8, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Müllner, A.N.; Schagerl, M. Abundance and Vertical Distribution of the Phytobenthic Community within a Pool and Riffle Sequence of an Alpine Gravel Stream. Int. Rev. Hydrobiol. 2003, 88, 243–254. [Google Scholar] [CrossRef]
- Xie, L.; Xie, P. Long-term (1956–1999) dynamics of phosphorus in a shallow, subtropical Chinese lake with the possible effects of cyanobacterial blooms. Water Res. 2002, 36, 343–349. [Google Scholar] [CrossRef]
- Luo, X.; Xiang, X.; Yang, Y.; Huang, G.; Fu, K.; Che, R.; Chen, L. Seasonal effects of river flow on microbial community coalescence and diversity in a riverine network. FEMS Microbiol. Ecol. 2020, 96, fiaa132. [Google Scholar] [CrossRef]
- Osman, O.A.; Beier, S.; Grabherr, M.; Bertilsson, S. Interactions of Freshwater Cyanobacteria with Bacterial Antagonists. Appl. Environ. Microbiol. 2017, 83, e02634-16. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, Y.; Shen, Y.; Wang, C.; Wang, P.; Wang, L.; Niu, L.; Zhang, W. Vertical distribution and assemblages of microbial communities and their potential effects on sulfur metabolism in a black-odor urban river. J. Environ. Manag. 2019, 235, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Chen, H.; Yang, J.R.; Liu, M.; Huang, B.; Yang, J. Distinct Patterns and Processes of Abundant and Rare Eukaryotic Plankton Communities Following a Reservoir Cyanobacterial Bloom. ISME J. Emultidisciplinary J. Microb. Ecol. 2018, 12, 2263–2277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, J.; Yang, J.; Zhou, Z.; Pan, Y.; Li, M. Patterns and processes of free-living and particle-associated bacterioplankton and archaeaplankton communities in a subtropical river-bay system in South China. Limnol. Oceanogr. 2020, 65, S161–S179. [Google Scholar] [CrossRef]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.; Zhang, N.; Miao, Y.; Shen, Q.; Zhang, R. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 2021, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.E.; Frank, K.A.; Mason, D.M.; Ulanowicz, R.E.; Taylor, W.W. Compartments revealed in food-web structure. Nature 2003, 426, 282–285. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Xun, W.; Yan, R.; Ren, Y.; Jin, D.; Xiong, W.; Zhang, G.; Cui, Z.; Xin, X.; Zhang, R. Grazing-induced microbiome alterations drive soil organic carbon turnover and productivity in meadow steppe. Microbiome 2018, 6, 170. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, J.; Wu, Q.; Ai, Y.; Huang, Y.; Wei, W.; Sun, S.; Weng, Q. Co-occurrence pattern and function prediction of bacterial community in Karst cave. BMC Microbiol. 2020, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; Van Der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, B.; Zhang, Q.; Florentino, A.; Xu, R.; Zhang, Y.; Liu, Y. Co-digestion of blackwater with kitchen organic waste: Effects of mixing ratios and insights into microbial community. J. Clean. Prod. 2019, 236, 117703. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Romine, M.F.; Beliaev, A.S.; Auchtung, J.M.; Driscoll, M.E.; Gardner, T.S.; Nealson, K.H.; Osterman, A.L.; Pinchuk, G.; Reed, J.L.; et al. Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 2008, 6, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Tang, X.; Shao, K.; Zhu, G.; Gao, G. Bacterial diversity, community composition and metabolic function in Lake Tianmuhu and its dammed river: Effects of domestic wastewater and damming. Ecotoxicol. Environ. Saf. 2021, 213, 112069. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Bi, Y.; Deng, Y.; He, Z.; Wu, L.; van Nostrand, J.D.; Shi, Z.; Li, J.; Wang, X.; Hu, Z.; et al. Impacts of the Three Gorges Dam on microbial structure and potential function. Sci. Rep. 2015, 5, 8605. [Google Scholar] [CrossRef]
- Luo, J.; Tan, X.; Liu, K.; Lin, W. Survey of sulfur-oxidizing bacterial community in the Pearl River water using soxB, sqr, and dsrA as molecular biomarkers. 3 Biotech. 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Shaw, J.G. Aeromonas spp. clinical microbiology and disease. J. Infect. 2011, 62, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, D.; Balcázar, J.L.; Ruiz-Zarzuela, I.; de Blas, I.; Gironés, O.; Múzquiz, J.L. Lactococcus garvieae in fish: A review. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 177–198. [Google Scholar] [CrossRef]
- Wu, J.; Liu, M.; Zhou, M.; Wu, L.; Yang, H.; Huang, L.; Chen, C. Isolation and genomic characterization of five novel strains of Erysipelotrichaceae from commercial pigs. BMC Microbiol. 2021, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, A.; Decostere, A.; Pasmans, F.; Haesebrouck, F. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 2003, 26, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Xu, Z.; Te, S.H.; He, Y.; Gin, K.Y.-H. The Characteristics and Dynamics of Cyanobacteria–Heterotrophic Bacteria Between Two Estuarine Reservoirs—Tropical Versus Sub-Tropical Regions. Front. Microbiol. 2018, 9, 2531. [Google Scholar] [CrossRef]
- Ruprecht, J.E.; Birrer, S.C.; Dafforn, K.A.; Mitrovic, S.M.; Crane, S.L.; Johnston, E.L.; Wemheuer, F.; Navarro, A.; Harrison, A.J.; Turner, I.L.; et al. Wastewater effluents cause microbial community shifts and change trophic status. Water Res. 2021, 200, 117206. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Luo, G.; Guo, J.; Xiao, Y.; Zhang, F.; Guo, S.; Ling, N.; Shen, Q. Microbial generalist or specialist: Intraspecific variation and dormancy potential matter. Mol. Ecol. 2021, 31, 161–173. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).