Role of Tocochromanols in Tolerance of Cereals to Biotic Stresses: Specific Focus on Pathogenic and Toxigenic Fungal Species
Abstract
:1. Introduction
2. Tocochromanols in Cereals
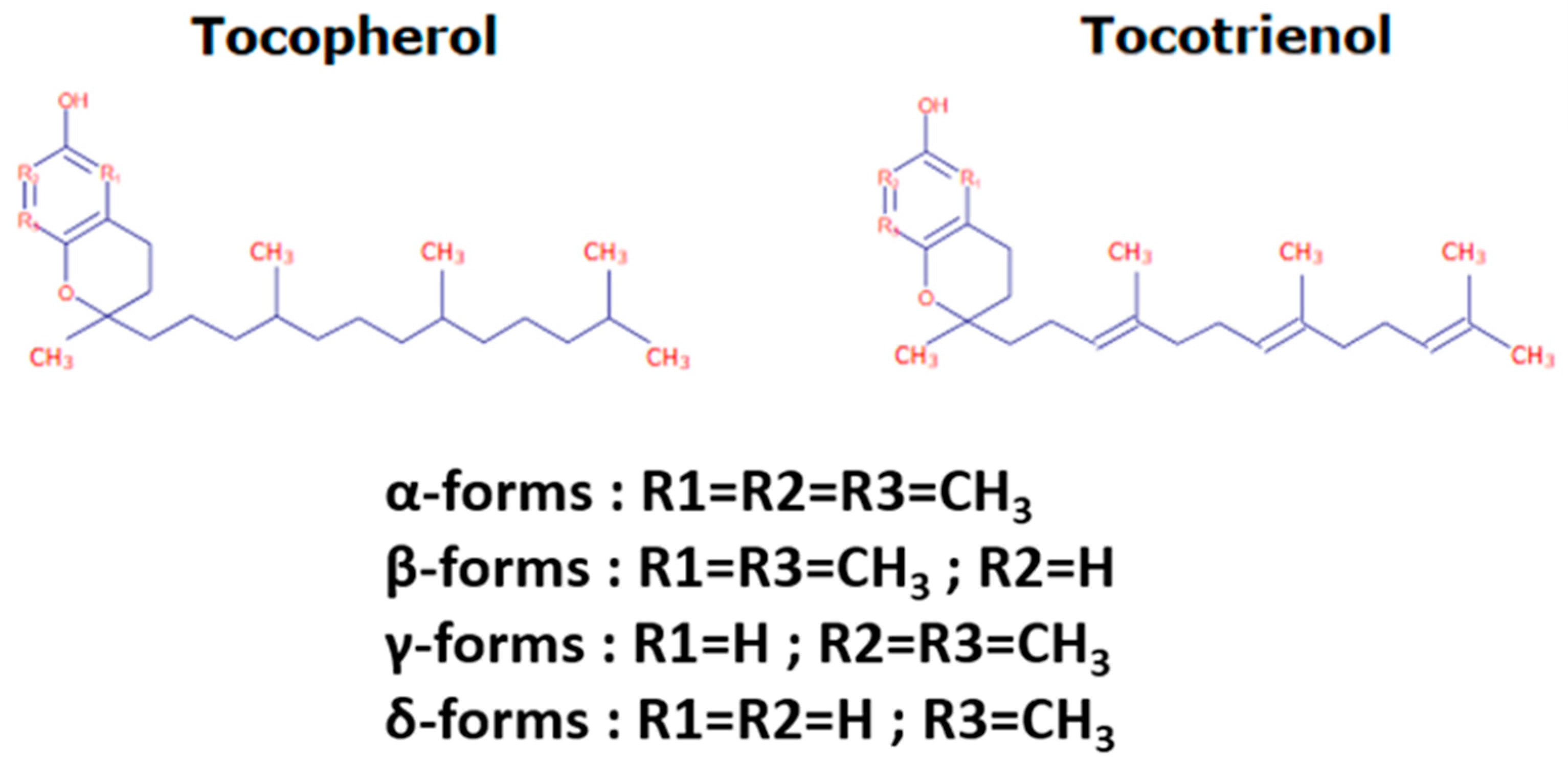
2.1. Tocochromanol Structure
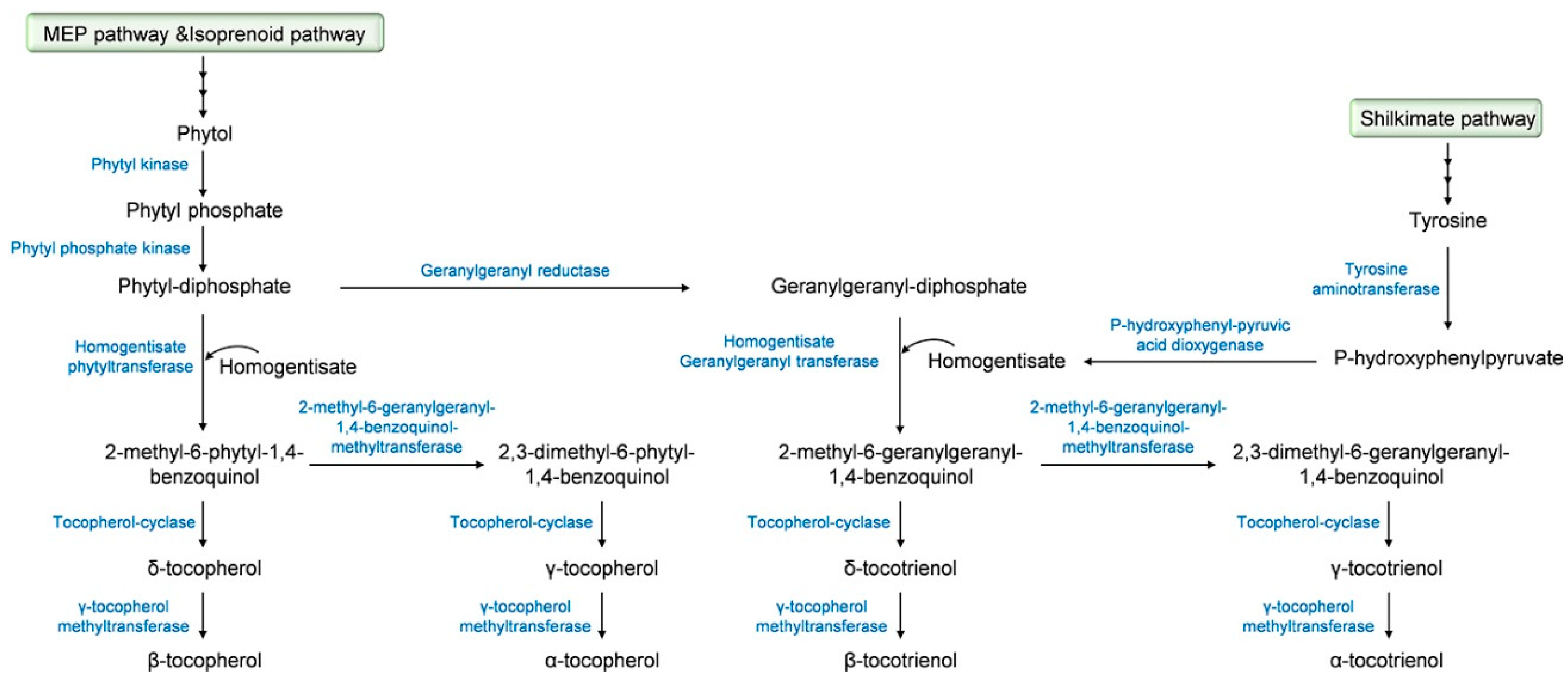
2.2. Tocochromanol Biosynthesis
2.3. Tocochromanol Composition of Major Cereal Crops
2.4. Distribution of Tocochromanols within Cereal Kernels
2.5. Kinetics of Tocochromanol Accumulation during Maturation of Cereal Kernels
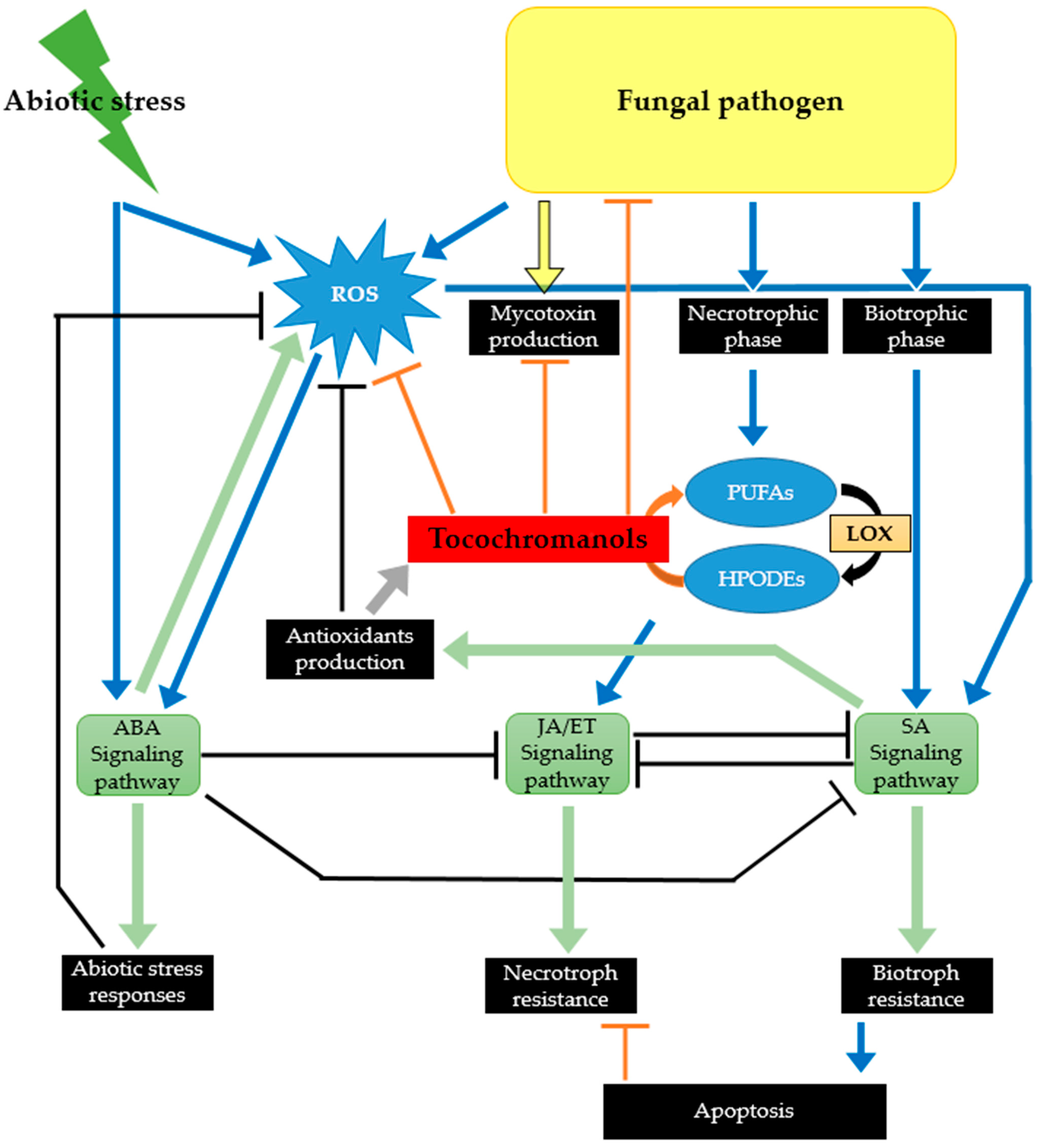
3. Tocochromanols as Part of the Plant Chemical Defense Arsenal against Phytopathogenic and Toxigenic Fungi
3.1. By Protecting Crops against Environmental Stresses, Tocochromanols Can Indirectly Affect Their Susceptibility to Fungal Pathogens
3.2. Tocochromanols Can Mitigate the Damage Caused by ROS Produced in Response to Infection by Toxigenic Fungal Pathogens
3.3. Tocochromanols Can Interfere with Fatty Acid Metabolism and Consequently with Plant Signaling Networks
3.4. Tocochromanols Display Antifungal and Antimycotoxin Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD Agriculture Statistics. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/data/oecd-agriculture-statistics_agr-data-en (accessed on 27 June 2022).
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Mahpara, F.; Safdar, A.; Yasir, S.; Muhammad, U.; Muhammad, H.Z.; Khalida, B. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Gauthier, L.; Atanasova-Penichon, V.; Chéreau, S.; Richard-Forget, F. Metabolomics to Decipher the Chemical Defense of Cereals against Fusarium Graminearum and Deoxynivalenol Accumulation. Int. J. Mol. Sci. 2015, 16, 24839–24872. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Vincenzo, L.; Veronica, M.T.L.; Angela, C. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Trivandrum, Kerala, 2006; ISBN 978-81-308-0034-9. [Google Scholar]
- Sattler, S.E.; Funnell-Harris, D.L. Modifying Lignin to Improve Bioenergy Feedstocks: Strengthening the Barrier against Pathogens? Front. Plant Sci. 2013, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant Secondary Metabolites in Cereals: Potential Involvement in Resistance to Fusarium and Mycotoxin Accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef]
- Bollina, V.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S. Identification of Metabolites Related to Mechanisms of Resistance in Barley against Fusarium Graminearum, Based on Mass Spectrometry. Plant Mol. Biol. 2011, 77, 355. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile Terpenoids: Multiple Functions, Biosynthesis, Modulation and Manipulation by Genetic Engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, Elicitation and Roles of Monocot Terpenoid Phytoalexins. Plant J. Cell Mol. Biol. 2014, 79, 659–678. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Mauch, F. The Role of Abscisic Acid in Plant–Pathogen Interactions. Curr. Opin. Plant Biol. 2005, 8, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Navarro, L.; Bari, R.; Jones, J.D. Pathological Hormone Imbalances. Curr. Opin. Plant Biol. 2007, 10, 372–379. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Kasper, R.; Gil-Serna, J.; Marín, S.; Sanchis, V.; Ramos, A.J. Effect of Capsicum Carotenoids on Growth and Ochratoxin A Production by Chilli and Paprika Aspergillus Spp. Isolates. Int. J. Food Microbiol. 2010, 142, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.-Å. The Chemistry and Antioxidant Properties of Tocopherols and Tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Alegre, L.; Munné-Bosch, S. Drought-Induced Changes in Flavonoids and Other Low Molecular Weight Antioxidants in Cistus Clusii Grown under Mediterranean Field Conditions. Tree Physiol. 2004, 24, 1303–1311. [Google Scholar] [CrossRef]
- Chennupati, P.; Seguin, P.; Liu, W. Effects of High Temperature Stress at Different Development Stages on Soybean Isoflavone and Tocopherol Concentrations. J. Agric. Food Chem. 2011, 59, 13081–13088. [Google Scholar] [CrossRef]
- Kodad, O.; Socias i Company, R.; Alonso, J.M. Genotypic and Environmental Effects on Tocopherol Content in Almond. Antioxidants 2018, 9, 6. [Google Scholar] [CrossRef]
- Xiang, N.; Li, C.; Li, G.; Yu, Y.; Hu, J.; Guo, X. Comparative Evaluation on Vitamin E and Carotenoid Accumulation in Sweet Corn (Zea mays L.) Seedlings under Temperature Stress. J. Agric. Food Chem. 2019, 67, 9772–9781. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.A.; Ammon, A.; Sonnewald, U.; Munné-Bosch, S.; Voll, L.M. Tocopherol Deficiency Reduces Sucrose Export from Salt- Stressed Potato Leaves Independently of Oxidative Stress and Symplastic Obstruction by Callose. J. Exp. Bot. 2015, 66, 957–971. [Google Scholar] [CrossRef]
- Kruk, J.; Holländer-Czytko, H.; Oettmeier, W.; Trebst, A. Tocopherol as Singlet Oxygen Scavenger in Photosystem II. J. Plant Physiol. 2005, 162, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in Plants: Biosynthesis, Transport, and Function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (ATEAC), DPPH Assay and Peroxyl Radical Scavenging Assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Mène-Saffrané, L.; DellaPenna, D. Biosynthesis, Regulation and Functions of Tocochromanols in Plants. Plant Physiol. Biochem. 2010, 48, 301–309. [Google Scholar] [CrossRef]
- Hussain, N.; Irshad, F.; Jabeen, Z.; Shamsi, I.H.; Li, Z.; Jiang, L. Biosynthesis, Structural, and Functional Attributes of Tocopherols in Planta; Past, Present, and Future Perspectives. J. Agric. Food Chem. 2013, 61, 6137–6149. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.E.; Gore, M.A.; Magallanes-Lundback, M.; Mesberg, A.; Lin, H.; Tiede, T.; Chen, C.; Buell, C.R.; Buckler, E.S.; Rocheford, T.; et al. Genome-Wide Association Study and Pathway-Level Analysis of Tocochromanol Levels in Maize Grain. G3 GenesGenomesGenetics 2013, 3, 1287–1299. [Google Scholar] [CrossRef]
- Fritsche, S.; Wang, X.; Jung, C. Recent Advances in Our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops. Antioxidants 2017, 6, 99. [Google Scholar] [CrossRef]
- Ma, J.; Qiu, D.; Pang, Y.; Gao, H.; Wang, X.; Qin, Y. Diverse Roles of Tocopherols in Response to Abiotic and Biotic Stresses and Strategies for Genetic Biofortification in Plants. Mol. Breed. 2020, 40, 18. [Google Scholar] [CrossRef]
- Mène-Saffrané, L. Vitamin E Biosynthesis and Its Regulation in Plants. Antioxidants 2017, 7, 2. [Google Scholar] [CrossRef]
- Valentin, H.E.; Lincoln, K.; Moshiri, F.; Jensen, P.K.; Qi, Q.; Venkatesh, T.V.; Karunanandaa, B.; Baszis, S.R.; Norris, S.R.; Savidge, B.; et al. The Arabidopsis Vitamin E Pathway Gene5-1 Mutant Reveals a Critical Role for Phytol Kinase in Seed Tocopherol Biosynthesis. Plant Cell 2006, 18, 212–224. [Google Scholar] [CrossRef]
- Vom Dorp, K.; Hölzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.; Hanson, A.D.; Dörmann, P. Remobilization of Phytol from Chlorophyll Degradation Is Essential for Tocopherol Synthesis and Growth of Arabidopsis. Plant Cell 2015, 27, 2846–2859. [Google Scholar] [CrossRef]
- Bergmüller, E.; Porfirova, S.; Dörmann, P. Characterization of an Arabidopsis Mutant Deficient in γ-Tocopherol Methyltransferase. Plant Mol. Biol. 2003, 52, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Porfirova, S.; Bergmüller, E.; Tropf, S.; Lemke, R.; Dörmann, P. Isolation of an Arabidopsis Mutant Lacking Vitamin E and Identification of a Cyclase Essential for All Tocopherol Biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 12495–12500. [Google Scholar] [CrossRef] [PubMed]
- Diepenbrock, C.H.; Kandianis, C.B.; Lipka, A.E.; Magallanes-Lundback, M.; Vaillancourt, B.; Góngora-Castillo, E.; Wallace, J.G.; Cepela, J.; Mesberg, A.; Bradbury, P.J.; et al. Novel Loci Underlie Natural Variation in Vitamin E Levels in Maize Grain. Plant Cell 2017, 29, 2374–2392. [Google Scholar] [CrossRef] [PubMed]
- Schuy, C.; Groth, J.; Ammon, A.; Eydam, J.; Baier, S.; Schweizer, G.; Hanemann, A.; Herz, M.; Voll, L.M.; Sonnewald, U. Deciphering the Genetic Basis for Vitamin E Accumulation in Leaves and Grains of Different Barley Accessions. Sci. Rep. 2019, 9, 9470. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Garvin, D.F. Subgenome-Specific Assembly of Vitamin E Biosynthesis Genes and Expression Patterns during Seed Development Provide Insight into the Evolution of Oat Genome. Plant Biotechnol. J. 2016, 14, 2147–2157. [Google Scholar] [CrossRef]
- Chaudhary, N.; Khurana, P. Vitamin E Biosynthesis Genes in Rice: Molecular Characterization, Expression Profiling and Comparative Phylogenetic Analysis. Plant Sci. 2009, 177, 479–491. [Google Scholar] [CrossRef]
- Kimura, E.; Abe, T.; Murata, K.; Kimura, T.; Otoki, Y.; Yoshida, T.; Miyazawa, T.; Nakagawa, K. Identification of OsGGR2, a Second Geranylgeranyl Reductase Involved in α-Tocopherol Synthesis in Rice. Sci. Rep. 2018, 8, 1870. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Yoon, M.-Y.; He, Q.; Kim, T.-S.; Tong, W.; Choi, B.-W.; Lee, Y.-S.; Park, Y.-J. Natural Variations in OsγTMT Contribute to Diversity of the α-Tocopherol Content in Rice. Mol. Genet. Genom. 2015, 290, 2121–2135. [Google Scholar] [CrossRef]
- Tanaka, R.; Rothbart, M.; Oka, S.; Takabayashi, A.; Takahashi, K.; Shibata, M.; Myouga, F.; Motohashi, R.; Shinozaki, K.; Grimm, B.; et al. LIL3, a Light-Harvesting-like Protein, Plays an Essential Role in Chlorophyll and Tocopherol Biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 16721–16725. [Google Scholar] [CrossRef]
- Zhan, W.; Liu, J.; Pan, Q.; Wang, H.; Yan, S.; Li, K.; Deng, M.; Li, W.; Liu, N.; Kong, Q.; et al. An Allele of ZmPORB2 Encoding a Protochlorophyllide Oxidoreductase Promotes Tocopherol Accumulation in Both Leaves and Kernels of Maize. Plant J. 2019, 100, 114–127. [Google Scholar] [CrossRef]
- Wang, H.; Xu, S.; Fan, Y.; Liu, N.; Zhan, W.; Liu, H.; Xiao, Y.; Li, K.; Pan, Q.; Li, W.; et al. Beyond Pathways: Genetic Dissection of Tocopherol Content in Maize Kernels by Combining Linkage and Association Analyses. Plant Biotechnol. J. 2018, 16, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.R.; Shen, X.; Della Penna, D. Complementation of the Arabidopsis Pds1 Mutation with the Gene Encoding P-Hydroxyphenylpyruvate Dioxygenase. Plant Physiol. 1998, 117, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Keller, Y.; Bouvier, F.; d’Harlingue, A.; Camara, B. Metabolic Compartmentation of Plastid Prenyllipid Biosynthesis. Eur. J. Biochem. 1998, 251, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Sattler, S.; Maeda, H.; Sakuragi, Y.; Bryant, D.A.; DellaPenna, D. Highly Divergent Methyltransferases Catalyze a Conserved Reaction in Tocopherol and Plastoquinone Synthesis in Cyanobacteria and Photosynthetic Eukaryotes. Plant Cell 2003, 15, 2343–2356. [Google Scholar] [CrossRef]
- Zhang, C.; Cahoon, R.E.; Hunter, S.C.; Chen, M.; Han, J.; Cahoon, E.B. Genetic and Biochemical Basis for Alternative Routes of Tocotrienol Biosynthesis for Enhanced Vitamin E Antioxidant Production. Plant J. 2013, 73, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Graebner, R.C.; Wise, M.; Cuesta-Marcos, A.; Geniza, M.; Blake, T.; Blake, V.C.; Butler, J.; Chao, S.; Hole, D.J.; Horsley, R.; et al. Quantitative Trait Loci Associated with the Tocochromanol (Vitamin E) Pathway in Barley. PLoS ONE 2015, 10, e0133767. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Long, W.; Niu, M.; Zhao, Z.; Teng, X.; Zhu, X.; Zhu, J.; Hao, Y.; Wang, Y.; et al. SGD1, a Key Enzyme in Tocopherol Biosynthesis, Is Essential for Plant Development and Cold Tolerance in Rice. Plant Sci. Int. J. Exp. Plant Biol. 2017, 260, 90–100. [Google Scholar] [CrossRef]
- Yunhui, Z.; Kai, L.; Xiaomei, Z.; Yan, W.; Suobing, Z.; Haiyuan, C.; Jing, L.; Yingjie, W.; Xianwen, F. Rice Tocopherol Deficiency 1 Encodes a Homogentisate Phytyltransferase Essential for Tocopherol Biosynthesis and Plant Development in Rice. Plant Cell Rep. 2018, 37, 775–787. [Google Scholar]
- Lachman, J.; Hejtmánková, A.; Orsák, M.; Popov, M.; Martinek, P. Tocotrienols and Tocopherols in Colored-Grain Wheat, Tritordeum and Barley. Food Chem. 2018, 240, 725–735. [Google Scholar] [CrossRef]
- Sookwong, P.; Murata, K.; Nakagawa, K.; Shibata, A.; Kimura, T.; Yamaguchi, M.; Kojima, Y.; Miyazawa, T. Cross-Fertilization for Enhancing Tocotrienol Biosynthesis in Rice Plants and QTL Analysis of Their F2 Progenies. J. Agric. Food Chem. 2009, 57, 4620–4625. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Brandolini, A. Protein, Ash, Lutein and Tocols Distribution in Einkorn (Triticum monococcum L. Subsp. Monococcum) Seed Fractions. Food Chem. 2008, 107, 444–448. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice Antioxidants: Phenolic Acids, Flavonoids, Anthocyanins, Proanthocyanidins, Tocopherols, Tocotrienols, γ-Oryzanol, and Phytic Acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yu, Y.; Mao, J.; Liu, H.; Hu, J.; Li, T.; Guo, X.; Liu, R. Evaluation of Biosynthesis, Accumulation and Antioxidant Activity of Vitamin E in Sweet Corn (Zea mays L.) during Kernel Development. Int. J. Mol. Sci. 2017, 18, 2780. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Juvik, J.A. Quantification of Carotenoid and Tocopherol Antioxidants in Zea mays. J. Agric. Food Chem. 1999, 47, 1948–1955. [Google Scholar] [CrossRef]
- Sun, X.; Ma, L.; Lux, P.E.; Wang, X.; Stuetz, W.; Frank, J.; Liang, J. The Distribution of Phosphorus, Carotenoids and Tocochromanols in Grains of Four Chinese Maize (Zea mays L.) Varieties. Food Chem. 2022, 367, 130725. [Google Scholar] [CrossRef] [PubMed]
- Franzen, J.; Haaß, M.M. Vitamin E Content during Development of Some Seedlings. Phytochemistry 1991, 30, 2911–2913. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Wise, M.L.; Garvin, D.F. A Developmental Profile of Tocol Accumulation in Oat Seeds. J. Cereal Sci. 2013, 57, 79–83. [Google Scholar] [CrossRef]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Differential Distribution of Tocopherols and Tocotrienols in Photosynthetic and Non-Photosynthetic Tissues. Phytochemistry 2006, 67, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, H.; Ciska, E.; Kozlowska, H. The Cereal Grains: Focus on Vitamin E. Czech J. Food Sci. 2001, 19, 182–188. [Google Scholar] [CrossRef]
- Yu, L.; Li, G.; Li, M.; Xu, F.; Beta, T.; Bao, J. Genotypic Variation in Phenolic Acids, Vitamin E and Fatty Acids in Whole Grain Rice. Food Chem. 2016, 197, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Bergman, C.J.; Xu, Z. Genotype and Environment Effects on Tocopherol, Tocotrienol, and γ-Oryzanol Contents of Southern U.S. Rice. Cereal Chem. 2003, 80, 446–449. [Google Scholar] [CrossRef]
- Heinemann, R.J.B.; Xu, Z.; Godber, J.S.; Lanfer-Marquez, U.M. Tocopherols, Tocotrienols, and γ-Oryzanol Contents in Japonica and Indica Subspecies of Rice (Oryza sativa L.) Cultivated in Brazil. Cereal Chem. 2008, 85, 243–247. [Google Scholar] [CrossRef]
- Kim, N.H.; Kwak, J.; Baik, J.Y.; Yoon, M.-R.; Lee, J.-S.; Yoon, S.W.; Kim, I.-H. Changes in Lipid Substances in Rice during Grain Development. Phytochemistry 2015, 116, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Lampi, A.-M.; Nurmi, T.; Ollilainen, V.; Piironen, V. Tocopherols and Tocotrienols in Wheat Genotypes in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9716–9721. [Google Scholar] [CrossRef]
- Labuschagne, M.; Mkhatywa, N.; Johansson, E.; Wentzel, B.; van Biljon, A. The Content of Tocols in South African Wheat; Impact on Nutritional Benefits. Foods 2017, 6, 95. [Google Scholar] [CrossRef]
- Munne-Bosch, S. The Role of alpha-Tocopherol in Plant Stress Tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- Fisk, I.D.; White, D.A.; Carvalho, A.; Gray, D.A. Tocopherol—An Intrinsic Component of Sunflower Seed Oil Bodies. J. Am. Oil Chem. Soc. 2006, 83, 341–344. [Google Scholar] [CrossRef]
- Siles, L.; Cela, J.; Munné-Bosch, S. Vitamin E Analyses in Seeds Reveal a Dominant Presence of Tocotrienols over Tocopherols in the Arecaceae Family. Phytochemistry 2013, 95, 207–214. [Google Scholar] [CrossRef]
- Grams, G.W.; Blessin, C.W.; Inglett, G.E. Distribution of Tocopherols within the Corn Kernel. J. Am. Oil Chem. Soc. 1970, 47, 337–339. [Google Scholar] [CrossRef]
- Ko, S.-N.; Kim, C.-J.; Kim, H.; Kim, C.-T.; Chung, S.-H.; Tae, B.-S.; Kim, I.-H. Tocol Levels in Milling Fractions of Some Cereal Grains and Soybean. J. Am. Oil Chem. Soc. 2003, 80, 585–589. [Google Scholar] [CrossRef]
- Morrison, W.R.; Coventry, A.M.; Barnes, P.J. The Distribution of Acyl Lipids and Tocopherols in Flours Millstreams. J. Sci. Food Agric. 1982, 33, 925–933. [Google Scholar] [CrossRef]
- Picot, A.; Atanasova-Pénichon, V.; Pons, S.; Marchegay, G.; Barreau, C.; Pinson-Gadais, L.; Roucolle, J.; Daveau, F.; Caron, D.; Richard-Forget, F. Maize Kernel Antioxidants and Their Potential Involvement in Fusarium Ear Rot Resistance. J. Agric. Food Chem. 2013, 61, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.; Krahnstöver, A.; van der Kooij, T.A.W.; Schlensog, M.; Krupinska, K. Tocopherol and Tocotrienol Accumulation during Development of Caryopses from Barley (Hordeum vulgare L.). Phytochemistry 2004, 65, 2977–2985. [Google Scholar] [CrossRef]
- Matringe, M.; Ksas, B.; Rey, P.; Havaux, M. Tocotrienols, the Unsaturated Forms of Vitamin E, Can Function as Antioxidants and Lipid Protectors in Tobacco Leaves. Plant Physiol. 2008, 147, 764–778. [Google Scholar] [CrossRef]
- Kim, S.-E.; Lee, C.-J.; Ji, C.Y.; Kim, H.S.; Park, S.-U.; Lim, Y.-H.; Park, W.S.; Ahn, M.-J.; Bian, X.; Xie, Y.; et al. Transgenic Sweetpotato Plants Overexpressing Tocopherol Cyclase Display Enhanced α-Tocopherol Content and Abiotic Stress Tolerance. Plant Physiol. Biochem. 2019, 144, 436–444. [Google Scholar] [CrossRef]
- Spicher, L.; Almeida, J.; Gutbrod, K.; Pipitone, R.; Dörmann, P.; Glauser, G.; Rossi, M.; Kessler, F. Essential Role for Phytol Kinase and Tocopherol in Tolerance to Combined Light and Temperature Stress in Tomato. J. Exp. Bot. 2017, 68, 5845–5856. [Google Scholar] [CrossRef]
- Cela, J.; Chang, C.; Munne-Bosch, S. Accumulation of G- Rather than a-Tocopherol Alters Ethylene Signaling Gene Expression in the Vte4 Mutant of Arabidopsis Thaliana. Plant Cell Physiol. 2011, 52, 1389–1400. [Google Scholar] [CrossRef]
- Maeda, H.; Song, W.; Sage, T.L.; DellaPenna, D. Tocopherols Play a Crucial Role in Low-Temperature Adaptation and Phloem Loading in Arabidopsis. Plant Cell 2006, 18, 2710–2732. [Google Scholar] [CrossRef]
- Chapman, J.M.; Muhlemann, J.K.; Gayomba, S.R.; Muday, G.K. RBOH-Dependent ROS Synthesis and ROS Scavenging by Plant Specialized Metabolites To Modulate Plant Development and Stress Responses. Chem. Res. Toxicol. 2019, 32, 370–396. [Google Scholar] [CrossRef]
- Mène-Saffrané, L.; Jones, A.D.; DellaPenna, D. Plastochromanol-8 and Tocopherols Are Essential Lipid-Soluble Antioxidants during Seed Desiccation and Quiescence in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 17815–17820. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Weiler, E.W.; Alegre, L.; Müller, M.; Düchting, P.; Falk, J. A-Tocopherol May Influence Cellular Signaling by Modulating Jasmonic Acid Levels in Plants. Planta 2007, 225, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhao, G.; Zhang, S.; Li, Y.; Gu, H.; Li, Y.; Zhao, Q.; Qi, Y. Chloroplast-to-Nucleus Signaling Regulates MicroRNA Biogenesis in Arabidopsis. Dev. Cell 2019, 48, 371–382.e4. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The Interaction of Plant Biotic and Abiotic Stresses: From Genes to the Field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Balmer, D.; Flors, V.; Glauser, G.; Mauch-Mani, B. Metabolomics of Cereals under Biotic Stress: Current Knowledge and Techniques. Front. Plant Sci. 2013, 4, 82. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-Morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic Fungi and Mycotoxins in a Climate Change Scenario: Ecology, Genomics, Distribution, Prediction and Prevention of the Risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef]
- Papendick, R.; Cook, R. Plant water stress and development of Fusarium foot rot in Wheat Subjected to Different Cultural Practices. Phytopathology 1974, 64, 358–363. [Google Scholar] [CrossRef]
- Wildermuth, G.B.; Morgan, J.M. Genotypic Differences in Partial Resistance to Crown Rot Caused by Fusarium Pseudograminearum in Relation to an Osmoregulation Gene in Wheat. Australas. Plant Pathol. 2004, 33, 121–123. [Google Scholar] [CrossRef]
- Guo, B.; Chen, Z.-Y.; Lee, R.D.; Scully, B.T. Drought Stress and Preharvest Aflatoxin Contamination in Agricultural Commodity: Genetics, Genomics and Proteomics. J. Integr. Plant Biol. 2008, 50, 1281–1291. [Google Scholar] [CrossRef]
- Vaughan, M.M.; Huffaker, A.; Schmelz, E.A.; Dafoe, N.J.; Christensen, S.A.; McAuslane, H.J.; Alborn, H.T.; Allen, L.H.; Teal, P.E.A. Interactive Effects of Elevated [CO2] and Drought on the Maize Phytochemical Defense Response against Mycotoxigenic Fusarium Verticillioides. PLoS ONE 2016, 11, e0159270. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Mascagni, H.; Bruns, H.; Shier, W. Effect of Planting Density, Irrigation Regimes, and Maize Hybrids with Varying Ear Size on Yield, and Aflatoxin and Fumonisin Contamination Levels. Am. J. Plant Sci. 2012, 3, 1341–1354. [Google Scholar] [CrossRef]
- Desaint, H.; Aoun, N.; Deslandes, L.; Vailleau, F.; Roux, F.; Berthomé, R. Fight Hard or Die Trying: When Plants Face Pathogens under Heat Stress. New Phytol. 2021, 229, 712–734. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Kazan, K.; Praud, S.; Torney, F.J.; Rusu, A.; Manners, J.M. Early Activation of Wheat Polyamine Biosynthesis during Fusarium Head Blight Implicates Putrescine as an Inducer of Trichothecene Mycotoxin Production. BMC Plant Biol. 2010, 10, 289. [Google Scholar] [CrossRef]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A Major Player in the Multifaceted Response of Fusarium to Its Environment. Toxins 2013, 6, 1–19. [Google Scholar] [CrossRef]
- Gupta, A.; Hisano, H.; Hojo, Y.; Matsuura, T.; Ikeda, Y.; Mori, I.C.; Senthil-Kumar, M. Global Profiling of Phytohormone Dynamics during Combined Drought and Pathogen Stress in Arabidopsis Thaliana Reveals ABA and JA as Major Regulators. Sci. Rep. 2017, 7, 4017. [Google Scholar] [CrossRef]
- Vellosillo, T.; Vicente, J.; Kulasekaran, S.; Hamberg, M.; Castresana, C. Emerging Complexity in Reactive Oxygen Species Production and Signaling during the Response of Plants to Pathogens. Plant Physiol. 2010, 154, 444–448. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as Key Players in Plant Stress Signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Morkunas, I.; Bednarski, W.; Mai, V.C.; Formela, M.; Beszterda, M.; Wiśniewska, H.; Goliński, P. Deoxynivalenol and Oxidative Stress Indicators in Winter Wheat Inoculated with Fusarium Graminearum. Toxins 2014, 6, 575–591. [Google Scholar] [CrossRef]
- Desmond, O.J.; Manners, J.M.; Stephens, A.E.; Maclean, D.J.; Schenk, P.M.; Gardiner, D.M.; Munn, A.L.; Kazan, K. The Fusarium Mycotoxin Deoxynivalenol Elicits Hydrogen Peroxide Production, Programmed Cell Death and Defence Responses in Wheat. Mol. Plant Pathol. 2008, 9, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, J.; Szczerba, A.; Dziurka, M.; Hornyák, M.; Kopeć, P.; Szklarczyk, M.; Płażek, A. Physiological and Biochemical Response to Fusarium Culmorum Infection in Three Durum Wheat Genotypes at Seedling and Full Anthesis Stage. Int. J. Mol. Sci. 2021, 22, 7433. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, H.; Wang, Y.; Wang, H.; Chen, S.; Yin, Z. Comparative Transcriptome Profiling and Co-Expression Network Analysis Uncover the Key Genes Associated Withearly-Stage Resistance to Aspergillus Flavus in Maize. BMC Plant Biol. 2021, 21, 216. [Google Scholar] [CrossRef] [PubMed]
- Lambarey, H.; Moola, N.; Veenstra, A.; Murray, S.; Suhail Rafudeen, M. Transcriptomic Analysis of a Susceptible African Maize Line to Fusarium Verticillioides Infection. Plants 2020, 9, 1112. [Google Scholar] [CrossRef]
- Kelley, R.Y.; Williams, W.P.; Mylroie, J.E.; Boykin, D.L.; Harper, J.W.; Windham, G.L.; Ankala, A.; Shan, X. Identification of Maize Genes Associated with Host Plant Resistance or Susceptibility to Aspergillus Flavus Infection and Aflatoxin Accumulation. PLoS ONE 2012, 7, e36892. [Google Scholar] [CrossRef]
- Rampersad, S.N. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef]
- Montibus, M.; Pinson-Gadais, L.; Richard-Forget, F.; Barreau, C.; Ponts, N. Coupling of Transcriptional Response to Oxidative Stress and Secondary Metabolism Regulation in Filamentous Fungi. Crit. Rev. Microbiol. 2015, 41, 295–308. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Genetic Regulation of Aflatoxin, Ochratoxin A, Trichothecene, and Fumonisin Biosynthesis: A Review. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2020, 23, 89–96. [Google Scholar] [CrossRef]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The Ascorbate-Glutathione-α-Tocopherol Triad in Abiotic Stress Response. Int. J. Mol. Sci. 2012, 13, 4458–4483. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Trebst, A. Tocopherol Is the Scavenger of Singlet Oxygen Produced by the Triplet States of Chlorophyll in the PSII Reaction Centre. J. Exp. Bot. 2006, 57, 1677–1684. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of Plant Hormones in Plant Defence Responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of Hormone Signaling Networks in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q.; Liu, Z.; Surendra, A.; Pan, Y.; Li, Y.; Zaharia, L.I.; Ouellet, T.; Fobert, P.R. Integrated Transcriptome and Hormone Profiling Highlight the Role of Multiple Phytohormone Pathways in Wheat Resistance against Fusarium Head Blight. PLoS ONE 2018, 13, e0207036. [Google Scholar] [CrossRef] [PubMed]
- Makandar, R.; Nalam, V.J.; Lee, H.; Trick, H.N.; Dong, Y.; Shah, J. Salicylic Acid Regulates Basal Resistance to Fusarium Head Blight in Wheat. Mol. Plant-Microbe Interact. 2012, 25, 431–439. [Google Scholar] [CrossRef]
- Li, G.; Yen, Y. Jasmonate and Ethylene Signaling Pathway May Mediate Fusarium Head Blight Resistance in Wheat. Crop Sci. 2008, 48, 1888–1896. [Google Scholar] [CrossRef]
- Gunnaiah, R.; Kushalappa, A.C.; Duggavathi, R.; Fox, S.; Somers, D.J. Integrated Metabolo-Proteomic Approach to Decipher the Mechanisms by Which Wheat QTL (Fhb1) Contributes to Resistance against Fusarium Graminearum. PLoS ONE 2012, 7, e40695. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Gao, J.; Wu, Y.; Xia, Z.; Zhang, H.; Wu, J. The Mechanisms of Maize Resistance to Fusarium Verticillioides by Comprehensive Analysis of RNA-Seq Data. Front. Plant Sci. 2016, 7, 1654. [Google Scholar] [CrossRef]
- Luo, M.; Brown, R.L.; Chen, Z.-Y.; Menkir, A.; Yu, J.; Bhatnagar, D. Transcriptional Profiles Uncover Aspergillus Flavus-Induced Resistance in Maize Kernels. Toxins 2011, 3, 766–786. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Yi, S.Y.; Shirasu, K.; Moon, J.S.; Lee, S.-G.; Kwon, S.-Y. The Activated SA and JA Signaling Pathways Have an Influence on Flg22-Triggered Oxidative Burst and Callose Deposition. PLoS ONE 2014, 9, e88951. [Google Scholar] [CrossRef]
- Kumaraswamy, K.G.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S. Mass Spectrometry Based Metabolomics to Identify Potential Biomarkers for Resistance in Barley against Fusarium Head Blight (Fusarium graminearum). J. Chem. Ecol. 2011, 37, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Tocochromanols: Rancid Lipids, Seed Longevity, and Beyond. Proc. Natl. Acad. Sci. USA 2010, 107, 17857–17858. [Google Scholar] [CrossRef] [PubMed]
- Reddanna, P.; Krishna Rao, M.; Channa Reddy, C. Inhibition of 5-Lipoxygenase by Vitamin E. FEBS Lett. 1985, 193, 39–43. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Ryu, H.; Bahadduri, P.; Swaan, P.W.; Ratan, R.R.; Sen, C.K. Molecular Basis of Vitamin E Action. Tocotrienol Modulates 12- Lipoxygenase, a Key Mediator of Glutamate-Induced Neurodegeneration. J. Biol. Chem. 2007, 23, 43508–43515. [Google Scholar]
- Gao, X.; Brodhagen, M.; Isakeit, T.; Brown, S.H.; Göbel, C.; Betran, J.; Feussner, I.; Keller, N.P.; Kolomiets, M.V. Inactivation of the Lipoxygenase ZmLOX3 Increases Susceptibility of Maize to Aspergillus Spp. Mol. Plant-Microbe Interact. 2009, 22, 222–231. [Google Scholar] [CrossRef]
- Nalam, V.J.; Alam, S.; Keereetaweep, J.; Venables, B.; Burdan, D.; Lee, H.; Trick, H.N.; Sarowar, S.; Makandar, R.; Shah, J. Facilitation of Fusarium Graminearum Infection by 9-Lipoxygenases in Arabidopsis and Wheat. Mol. Plant-Microbe Interact. 2015, 28, 1142–1152. [Google Scholar] [CrossRef]
- Brodhagen, M.; Tsitsigiannis, D.I.; Hornung, E.; Goebel, C.; Feussner, I.; Keller, N.P. Reciprocal Oxylipin-Mediated Cross-Talk in the Aspergillus-Seed Pathosystem. Mol. Microbiol. 2008, 67, 378–391. [Google Scholar] [CrossRef]
- Scarpari, M.; Punelli, M.; Scala, V.; Zaccaria, M.; Nobili, C.; Ludovici, M.; Camera, E.; Fabbri, A.A.; Reverberi, M.; Fanelli, C. Lipids in Aspergillus Flavus-Maize Interaction. Front. Microbiol. 2014, 5, 74. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, Y.; Sun, L.; Deng, Q.; Zhao, J. Fatty Acids and Oxylipins as Antifungal and Anti-Mycotoxin Agents in Food: A Review. Toxins 2021, 13, 852. [Google Scholar] [CrossRef]
- Burow, G.B.; Nesbitt, T.C.; Dunlap, J.; Keller, N.P. Seed Lipoxygenase Products Modulate Aspergillus Mycotoxin Biosynthesis. Mol. Plant-Microbe Interact. 1997, 10, 380–387. [Google Scholar] [CrossRef]
- Nobili, C.; D’Angeli, S.; Altamura, M.M.; Scala, V.; Fabbri, A.A.; Reverberi, M.; Fanelli, C. ROS and 9-Oxylipins Are Correlated with Deoxynivalenol Accumulation in the Germinating Caryopses of Triticum aestivum after Fusarium graminearum Infection. Eur. J. Plant Pathol. 2014, 139, 429–444. [Google Scholar] [CrossRef]
- Battilani, P.; Lanubile, A.; Scala, V.; Reverberi, M.; Gregori, R.; Falavigna, C.; Dall’asta, C.; Park, Y.-S.; Bennett, J.; Borrego, E.J.; et al. Oxylipins from Both Pathogen and Host Antagonize Jasmonic Acid-Mediated Defence via the 9-Lipoxygenase Pathway in Fusarium Verticillioides Infection of Maize. Mol. Plant Pathol. 2018, 19, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.C.; Morais-Braga, M.F.B.; Guedes, G.M.M.; Tintino, S.R.; Freitas, M.A.; Menezes, I.R.A.; Coutinho, H.D.M. Enhancement of the Antibiotic Activity of Aminoglycosides by Alpha-Tocopherol and Other Cholesterol Derivates. Biomed. Pharmacother. 2014, 68, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.K.; Seong, E.S.; Yu, C.Y.; Kim, S.-H.; Chung, I.-M. Evaluation of Phenolic Compounds and Antimicrobial Activities in Transgenic Codonopsis Lanceolata Plants via Overexpression of the γ-Tocopherol Methyltransferase (γ-Tmt) Gene. South Afr. J. Bot. 2017, 109, 25–33. [Google Scholar] [CrossRef]
- Hajji, A.; Bnejdi, F.; Saadoun, M.; Ben Salem, I.; Nehdi, I.; Sbihi, H.; Alharthi, F.A.; El Bok, S.; Boughalleb-M’Hamdi, N. High Reserve in δ-Tocopherol of Peganum Harmala Seeds Oil and Antifungal Activity of Oil against Ten Plant Pathogenic Fungi. Molecules 2020, 25, 4569. [Google Scholar] [CrossRef]
- Koval, D.; Plocková, M.; Kyselka, J.; Skřivan, P.; Sluková, M.; Horáčková, Š. Buckwheat Secondary Metabolites: Potential Antifungal Agents. J. Agric. Food Chem. 2020, 68, 11631–11643. [Google Scholar] [CrossRef]
- Norton, R.A. Inhibition of Aflatoxin B(1) Biosynthesis in Aspergillus Flavus by Anthocyanidins and Related Flavonoids. J. Agric. Food Chem. 1999, 47, 1230–1235. [Google Scholar] [CrossRef]
- Norton, R.A. Effect of Carotenoids on Aflatoxin B(1) Synthesis by Aspergillus Flavus. Phytopathology 1997, 87, 814–821. [Google Scholar] [CrossRef]

| Plant Species | Gene | Enzyme 1 | Ref. |
|---|---|---|---|
| Arabidopsis thaliana | |||
| PSD1 | HPPD | [45] | |
| GGR | GGR | [46] | |
| VTE1 | Tocopherol cyclase | [35] | |
| VTE2 | HPT | [35] | |
| VTE3 | MPBQ/MGGBQ MT | [47] | |
| VTE4 | γ-TMT | [34] | |
| VTE5 | Phytol kinase | [32] | |
| VTE6 | Phytyl-P kinase | [33] | |
| HGGT | HGGT | [48] | |
| Barley | |||
| VTE1 | Tocopherol cyclase | [49] | |
| VTE4 | γ-TMT | [49] | |
| HPT-7H | HPT | [37] | |
| HGGT | HGGT | [37] | |
| Maize | |||
| ZmVTE1 | Tocopherol cyclase | [28] | |
| ZmVTE2 | HPT | [28] | |
| ZmVTE3 | MPBQ/MGGBQ MT | [28] | |
| ZmVTE4 | γ-TMT | [28] | |
| ZmVTE5 | Phytol kinase | [28] | |
| ZmHPPD | HPPD | [28] | |
| Rice | |||
| OsGGR1 | GGR | [40] | |
| OsGGR2 | GGR | [40] | |
| OsγTMT | γ-TMT | [41] | |
| SGD1 | HPT | [50] | |
| RTD1 | HPT | [51] | |
| Oat | |||
| HPPD (3 homo) | HPPD | [38] | |
| VTE2_3/VTE2_4 | HPT | [38] | |
| VTE4_1 | γ-TMT | [38] | |
| α-T 1 (%) | β-T (%) | γ-T (%) | δ-T (%) | α-T3 1 (%) | β-T3 (%) | γ-T3 (%) | δ-T3 (%) | Total Tocopherol (%) | Total Tocotrienol (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Maize | ||||||||||
| Germ | 30 | 1 | 65 | 3 | 1 | 0 | 1 | 0 | 98 | 2 |
| Endosperm | 5 | 0 | 19 | 0 | 29 | 0 | 45 | 2 | 24 | 76 |
| Pericarp | 18 | 0 | 52 | 6 | 9 | 1 | 14 | 1 | 75 | 25 |
| Wheat | ||||||||||
| Germ | 69 | 26 | 0 | 0 | 2 | 3 | ND 2 | 0 | 95 | 5 |
| Endosperm | 5 | 3 | 13 | 0 | 11 | 68 | ND | 0 | 21 | 79 |
| Pericarp | 7 | 3 | 8 | 0 | 21 | 61 | ND | 0 | 18 | 82 |
| Barley | ||||||||||
| Germ | 68 | 3 | 16 | 1 | 6 | 2 | 3 | 0 | 89 | 11 |
| Endosperm | 14 | 1 | 2 | 1 | 41 | 25 | 15 | 3 | 17 | 83 |
| Pericarp | 15 | 1 | 5 | 1 | 47 | 12 | 17 | 3 | 21 | 79 |
| Rice | ||||||||||
| Germ | 81 | 3 | 5 | 0 | 6 | ND | 4 | 0 | 89 | 11 |
| Endosperm | 33 | 2 | 6 | 2 | 21 | ND | 33 | 4 | 42 | 58 |
| Pericarp | 37 | 1 | 4 | 0 | 27 | ND | 29 | 2 | 43 | 57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savignac, J.-M.; Atanasova, V.; Chéreau, S.; Ortéga, V.; Richard-Forget, F. Role of Tocochromanols in Tolerance of Cereals to Biotic Stresses: Specific Focus on Pathogenic and Toxigenic Fungal Species. Int. J. Mol. Sci. 2022, 23, 9303. https://doi.org/10.3390/ijms23169303
Savignac J-M, Atanasova V, Chéreau S, Ortéga V, Richard-Forget F. Role of Tocochromanols in Tolerance of Cereals to Biotic Stresses: Specific Focus on Pathogenic and Toxigenic Fungal Species. International Journal of Molecular Sciences. 2022; 23(16):9303. https://doi.org/10.3390/ijms23169303
Chicago/Turabian StyleSavignac, Jean-Marie, Vessela Atanasova, Sylvain Chéreau, Véronique Ortéga, and Florence Richard-Forget. 2022. "Role of Tocochromanols in Tolerance of Cereals to Biotic Stresses: Specific Focus on Pathogenic and Toxigenic Fungal Species" International Journal of Molecular Sciences 23, no. 16: 9303. https://doi.org/10.3390/ijms23169303






