Active Teaching Methodologies Improve Cognitive Performance and Attention-Concentration in University Students
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Anthropometry
2.2.2. Physical Fitness
2.2.3. D2 Attention Test
2.2.4. Active Methodologies to Improve Physical Fitness
2.2.5. Procedure
2.2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, O.; Bennie, J.; Vergeer, I.; Bosselut, G.; Biddle, S.J.H. How sedentary are university students? A systematic review and meta-analysis. Prev. Sci. 2020, 21, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.H.; Kolle, E.; Dyrstad, S.M.; Holme, I.; Anderssen, S.A. Accelerometer-determined physical activity in adults and older people. Med. Sci. Sports Exerc. 2012, 44, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Chen, K.Y.; Freedson, P.S.; Buchowski, M.S.; Beech, B.M.; Pate, R.R.; Troiano, R.P. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am. J. Epidemiol. 2008, 167, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Liu, B.; Sun, Y.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Trends in adherence to the physical activity guidelines for Americans for aerobic activity and time spent on sedentary behavior among US adults, 2007 to 2016. JAMA Netw. Open 2019, 2, e197597. [Google Scholar] [CrossRef] [Green Version]
- Peterson, N.E.; Sirard, J.R.; Kulbok, P.A.; DeBoer, M.D.; Erickson, J.M. Sedentary behavior and physical activity of young adult university students. Res. Nurs. Health 2018, 41, 30–38. [Google Scholar] [CrossRef]
- Jamnick, N.A.; Pettitt, R.W.; Granata, C.; Pyne, D.B.; Bishop, D.J. An examination and critique of current methods to determine exercise intensity. Sports Med. 2020, 50, 1729–1756. [Google Scholar] [CrossRef]
- Patterson, R.; McNamara, E.; Tainio, M.; de Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J.H. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia 2012, 55, 2895–2905. [Google Scholar] [CrossRef]
- Teychenne, M.; Costigan, S.A.; Parker, K. The association between sedentary behaviour and risk of anxiety: A systematic review. BMC Public Health 2015, 15, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, L.; Zhang, Y.; Zhang, D. Sedentary behaviour and the risk of depression: A meta-analysis. Br. J. Sports Med. 2015, 49, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Thorp, A.A.; Owen, N.; Neuhaus, M.; Dunstan, D.W. Sedentary behaviors and subsequent health outcomes in adults: A systematic review of longitudinal studies, 1996–2011. Am. J. Prev. Med. 2011, 41, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Stathokostas, L. Sedentary behavior and physical activity are independent predictors of successful aging in middleaged and older adults. J. Aging Res. 2012, 2012, 190654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A. Lancet Sedentary Behaviour Working Group. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Lakerveld, J.; Loyen, A.; Schotman, N.; Peeters, C.F.; Cardon, G.; van der Ploeg, H.P.; Lien, N.; Chastin, S.; Brug, J. Sitting too much: A hierarchy of sociodemographic correlates. Prev. Med. 2017, 101, 77–83. [Google Scholar] [CrossRef]
- Loyen, A.; van der Ploeg, H.P.; Bauman, A.; Brug, J.; Lakerveld, J. European sitting championship: Prevalence and correlates of self-reported sitting time in the 28 European Union member states. PLoS ONE 2016, 11, e0149320. [Google Scholar] [CrossRef] [Green Version]
- Owen, N.; Sugiyama, T.; Eakin, E.E.; Gardiner, P.A.; Tremblay, M.S.; Sallis, J.F. Adults’ sedentary behavior: Determinants and interventions. Am. J. Prev. Med. 2011, 41, 189–196. [Google Scholar] [CrossRef]
- Gardner, B.; Smith, L.; Lorencatto, F.; Hamer, M.; Biddle, S.J.H. How to reduce sitting time? A review of behaviour change strategies used in sedentary behaviour reduction interventions among adults. Health Psychol. Rev. 2016, 10, 89–112. [Google Scholar] [CrossRef] [Green Version]
- Oria, H.M.; Fernández, F.T.G.; Fernández, A.S.S. Metodologías Activas en la Práctica de la Educación Física, 1st ed.; Ediciones Morata: Las Rozas, Spain, 2020; pp. 11–15. [Google Scholar]
- Dragoescu, R.M. An overview of higher education at the European level. Comput. Methods Soc. Sci. 2013, 1, 21–29. [Google Scholar]
- Universities UK. Patterns and Trends in UK Higher Education 2016; Higher Education Statistics Agency: London, UK, 2017. [Google Scholar]
- Cotten, E.; Prapavessis, H. Increasing nonsedentary behaviors in university students using text messages: Randomized controlled trial. JMIR mHealth uHealth 2016, 4, e99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussi, F.C.; Pitanga, F.J.G.; Pires, C.G.D.S. Cumulative sitting time as discriminator of overweight, obesity, abdominal obesity and lipid disorders in nursing university. Rev. Bras. Cineantropometria Desempenho Hum. 2017, 19, 40–49. [Google Scholar] [CrossRef]
- Clark, B.K.; Pavey, T.G.; Lim, R.F.; Gomersall, S.R.; Brown, W.J. Past-day recall of sedentary time: Validity of a self-reported measure of sedentary time in a university population. J. Sci. Med. Sport 2016, 19, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Conroy, D.E.; Maher, J.P.; Elavsky, S.; Hyde, A.L.; Doerksen, S.E. Sedentary behavior as a daily process regulated by habits and intentions. Health Psychol. 2013, 32, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouse, P.C.; Biddle, S.J. An ecological momentary assessment of the physical activity and sedentary behaviour patterns of university students. Health Educ. J. 2010, 69, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Farinola, M.G.; Bazán, N.E. Sedentary behavior and physical activity in university students: A pilot study. Argent. J. Cardiol. 2011, 79, 351–354. [Google Scholar]
- Moulin, M.S.; Irwin, J.D. An assessment of sedentary time among undergraduate students at a Canadian university. Int. J. Exerc. Sci. 2017, 10, 1116–1129. [Google Scholar]
- US Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health; US Government Printing: Washington, DC, USA, 2000; Volume 2011.
- Loprinzi, P.D.; Kane, C.J. Exercise and cognitive function: A randomized controlled trial examining acute exercise and free-living physical activity and sedentary effects. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2015; Volume 90, pp. 450–460. [Google Scholar]
- Loprinzi, P.D.; Herod, S.M.; Cardinal, B.J.; Noakes, T.D. Physical activity and the brain: A review of this dynamic, bi-directional relationship. Brain Res. 2013, 1539, 95–104. [Google Scholar] [CrossRef]
- Ratey, J.J.; Loehr, J.E. The positive impact of physical activity on cognition during adulthood: A review of underlying mechanisms, evidence and recommendations. Rev. Neurosci. 2011, 22, 171–185. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Hillman, C.H.; Fernhall, B.O.; Thompson, K.M.; Valentini, T.A. The effect of acute aerobic and resistance exercise on working memory. Med. Sci. Sports Exerc. 2009, 41, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.-J.; Liu, J.-Y.; Chang, H.; Chen, P.-J. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res. 2008, 1210, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-P.; Kim, H.-B.; Jang, M.-H.; Lim, B.-V.; Kim, Y.-J.; Kim, H.; Kim, S.-S.; Kim, E.-H.; Kim, C.-J. Magnitude-and time-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Int. J. Sports Med. 2003, 24, 114–117. [Google Scholar] [CrossRef] [PubMed]
- McQuail, J.A.; Frazier, C.J.; Bizon, J.L. Molecular aspects of age-related cognitive decline: The role of GABA signaling. Trends Mol. Med. 2015, 21, 450–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Páez-Maldonado, J.A.; Reigal, R.E.; Morillo-Baro, J.P.; Carrasco-Beltrán, H.; Hernández-Mendo, A.; Morales-Sánchez, V. Physical fitness, selective attention and academic performance in a pre-adolescent sample. Int. J. Environ. Res. Public Health 2020, 17, 6216. [Google Scholar] [CrossRef] [PubMed]
- Alberts, J.L.; Linder, S.M.; Penko, A.L.; Lowe, M.J.; Phillips, M. It is not about the bike, it is about the pedaling: Forced exercise and Parkinson’s disease. Exerc. Sport Sci. Rev. 2011, 39, 177–186. [Google Scholar] [CrossRef]
- Tillerson, J.L.; Caudle, W.M.; Reveron, M.E.; Miller, G.W. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 2003, 119, 899–911. [Google Scholar] [CrossRef]
- Ke, Z.; Yip, S.P.; Li, L.; Zheng, X.-X.; Tong, K.-Y. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: A rat brain ischemia model. PLoS ONE 2011, 6, e16643. [Google Scholar] [CrossRef] [Green Version]
- Kinni, H.; Guo, M.; Ding, J.Y.; Konakondla, S.; Dornbos, D., III; Tran, R.; Guthikonda, M.; Ding, Y. Cerebral metabolism after forced or voluntary physical exercise. Brain Res. 2011, 1388, 48–55. [Google Scholar] [CrossRef]
- Nanda, B.; Balde, J.; Manjunatha, S. The acute effects of a single bout of moderate-intensity aerobic exercise on cognitive functions in healthy aduk males. J. Clin. Diagn. Res. JCDR 2013, 7, 1883–1885. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Chen, F.-C.; Pan, C.-Y.; Wang, C.-H.; Huang, T.-H.; Chen, T.-C. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology 2014, 41, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hinojo-Lucena, F.J.; Mingorance-Estrada, Á.C.; Trujillo-Torres, J.M.; Aznar-Díaz, I.; Cáceres Reche, M.P. Incidence of the Flipped Classroom in the Physical Education Students’ Academic Performance in University Contexts. Sustainability 2018, 10, 1334. [Google Scholar] [CrossRef] [Green Version]
- Crisol-Moya, E.; Caurcel-Cara, M.J. Active Methodologies in Physical Education: Perception and Opinion of Students on the Pedagogical Model Used by Their Teachers. Int. J. Environ. Res. Public Health 2021, 18, 1438. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams & Wilkins: London, UK, 2010. [Google Scholar]
- Astrand, P.O. Work Tests with the Bicycle Ergometer; AB Cykelfabriken Monark: Varberg, Sweden, 1965. [Google Scholar]
- Myers, J.; Ashley, E. Dangerous curves. A perspective on exercise, lactate, and the anaerobic threshold. Chest 1997, 111, 787–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, M.P.; Gardner, R.M.; Adams, T.D.; Yanowitz, F.G.; Crapo, R.O. “Anaerobic threshold”: Problems of determination and validation. J. Appl. Physiol. 1983, 55, 1178–1186. [Google Scholar] [CrossRef]
- Brickenkamp, R. The D2 Test. Test of Attention under Pressure (Trad. Al Castellano Por N. Seisdedos); TEA Ediciones: Madrid, Spain, 2002. [Google Scholar]
- Janssen, M.; Toussaint, H.M.; van Mechelen, W.; Verhagen, E.A.L.M. Effects of acute bouts of physical activity on children’s attention: A systematic review of the literature. In SpringerPlus; Springer: Berlin/Heidelberg, Germany, 2014; Volume 3. [Google Scholar] [CrossRef] [Green Version]
- Kulinna, P.H.; Stylianou, M.; Dyson, B.; Banville, D.; Dryden, C.; Colby, R. The Effect of an Authentic Acute Physical Education Session of Dance on Elementary Students’ Selective Attention. BioMed. Res. Int. 2018, 2018, 8790283. [Google Scholar] [CrossRef] [Green Version]
- Reigal, R.E.; Moral-Campillo, L.; Morillo-Baro, J.P.; de Mier, R.J.-R.; Hernández-Mendo, A.; Morales-Sánchez, V. Physical exercise, fitness, cognitive functioning, and psychosocial variables in an adolescent sample. Int. J. Environ. Res. Public Health 2020, 17, 1100. [Google Scholar] [CrossRef] [Green Version]
- Arboix-Alió, J.; Sagristà, F.; Marcaida, S.; Aguilera-Castells, J.; Peralta-Geis, M.; Solà, J.; Buscà, B. Relationship between physical fitness and the physical activity habit with the selective attention capacity in secondary school students. Cuad. Psicol. Deporte 2022, 22, 1–13. [Google Scholar] [CrossRef]
- Chen, W.; Gu, X.; Chen, J.; Wang, X. Association of Cardiorespiratory Fitness and Cognitive Function with Psychological Well-Being in School-Aged Children. Int. J. Environ. Res. Public Health 2022, 19, 1434. [Google Scholar] [CrossRef] [PubMed]
- Pirrie, A.M.; Lodewyk, K.R. Investigating links between moderate-to-vigorous physical activity and cognitive performance in elementary school students. Ment. Health Phys. Act. 2012, 5, 93–98. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.; Lambourne, K.; Szabo-Reed, A.N. Physical activity, fitness, cognitive function, and academic achievement in children: A systematic review. Med. Sci. Sports Exerc. 2016, 48, 1197–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozdowska, A.; Falkenstein, M.; Jendrusch, G.; Platen, P.; Lücke, T.; Kersting, M.; Sinningen, K. Interrelations of physical fitness and cognitive functions in German schoolchildren. Children 2021, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; Raine, L.B.; Johnson, C.R.; Chaddock, L.; Voss, M.W.; Cohen, N.J.; Kramer, A.F.; Hillman, C.H. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J. Cogn. Neurosci. 2011, 23, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Takehara, K.; Ganchimeg, T.; Kikuchi, A.; Gundegmaa, L.; Altantsetseg, L.; Aoki, A.; Fukuie, T.; Suwabe, K.; Bat-Erdene, S.; Mikami, M.; et al. The effectiveness of exercise intervention for academic achievement, cognitive function, and physical health among children in Mongolia: A cluster RCT study protocol. BMC Public Health 2019, 19, 697. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.; de Sousa, A.; Medeiros, A.R.; del Rosso, S.; Stults-Kolehmainen, M.; Boullosa, D.A. The influence of exercise and physical fitness status on attention: A systematic review. Int. Rev. Sport Exerc. Psychol. 2019, 12, 202–234. [Google Scholar] [CrossRef]
- Sabarit, A.; Reigal, R.E.; Morillo-Baro, J.P.; de Mier, R.J.R.; Franquelo, A.; Hernández-Mendo, A.; Falcó, C.; Morales-Sánchez, V. Cognitive functioning, physical fitness, and game performance in a sample of adolescent soccer players. Sustainability 2020, 12, 5245. [Google Scholar] [CrossRef]
- Papanikolaou, Z. Attention in Young Soccer Players: The Development of an Attentional Focus Training Program. J. Life Sci. 2011, 3, 1–12. [Google Scholar] [CrossRef]
- Gunnell, K.E.; Poitras, V.J.; LeBlanc, A.; Schibli, K.; Barbeau, K.; Hedayati, N.; Ponitfex, M.B.; Goldfield, G.S.; Dunlap, C.; Lehan, E.; et al. Physical activity and brain structure, brain function, and cognition in children and youth: A systematic review of randomized controlled trials. Ment. Health Phys. Act. 2019, 16, 105–127. [Google Scholar] [CrossRef]
- Valkenborghs, S.R.; Noetel, M.; Hillman, C.H.; Nilsson, M.; Smith, J.J.; Ortega, F.B.; Lubans, D.R. The impact of physical activity on brain structure and function in youth: A systematic review. Pediatrics 2019, 144, e20184032. [Google Scholar] [CrossRef]
- Chaddock-Heyman, L.; Erickson, K.I.; Chappell, M.A.; Johnson, C.L.; Kienzler, C.; Knecht, A.; Drollette, E.S.; Raine, L.B.; Scudder, M.R.; Kao, S.-C.; et al. Aerobic fitness is associated with greater hippocampal cerebral blood flow in children. Dev. Cogn. Neurosci. 2016, 20, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Esteban-Cornejo, I.; Rodriguez-Ayllon, M.; Verdejo-Roman, J.; Cadenas-Sanchez, C.; Mora-Gonzalez, J.; Chaddock-Heyman, L.; Raine, L.B.; Stillman, C.M.; Kramer, A.F.; Erickson, K.I.; et al. Physical fitness, white matter volume and academic performance in children: Findings from the activebrains and FITKids2 projects. Front. Psychol. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| D2 Attention Test | ||
|---|---|---|
| Variables | Acronyms | |
| 1 | Processed elements | TR |
| 2 | Successes | TA |
| 3 | Omissions | O |
| 4 | Commissions or errors | C |
| 5 | Last stimulus analysed in the row with the most attempted elements | TR+ |
| 6 | Last stimulus analysed in the row with the least attempted elements | TR− |
| All Group (n = 44) | Low-Level Group (n = 22) | High-Level Group (n = 22) | F | p | d | CI 95% Lower | CI | CI 95% Upper | |
|---|---|---|---|---|---|---|---|
| Anthropometric measures | |||||||
| Height (cm) | 170.77 ± 6.50 | 168.00 ± 7.64 | 173.55 ± 9.77 | F = 4.40 | p = 0.04 * | η2 = 0.09 | 170.70 | 2.71 | 173.49 |
| Weight (kg) | 68.84 ± 8.29 | 68.63 ± 12.19 | 69.06 ± 8.92 | F < 1 | 65.38 | 3.46 | 72.31 |
| BMI (%) | 23.51 ± 1.54 | 24.22 ± 3.40 | 22.80 ± 1.44 | F = 3.25 | p = 0.08 | η2 = 0.07 | 22.86 | 0.64 | 24.15 |
| Physical fitness | |||||||
| HRmax (bpm) | 173.41 ± 16.10 | 175.68 ± 17.29 | 171.14 ± 14.87 | F < 1 | 168.24 | 5.17 | 178.58 |
| VT (mL·kg−1 min−1) | 31.07 ± 8.08 | 25.25 ± 5.81 | 36.89 ± 5.37 | F = 47.70 | p = 0.001 ** | η2 = 0.53 | 29.23 | 1.84 | 32.90 |
| Load (w) | 191.28 ± 49.67 | 172.21 ± 43.69 | 211.36 ± 47.39 | F = 8.12 | p = 0.01 * | η2 = 0.16 | 191.45 | 13.49 | 205.28 |
| W/kg | 2.80 ± 0.64 | 2.53 ± 0.57 | 3.06 ± 0.59 | F = 9.19 | p = 0.001 * | η2 = 0.18 | 2.62 | 0.18 | 2.98 |
| Handgrip ND (kg) | 40.80 ± 8.18 | 39.35 ± 8.45 | 42.25 ± 7.77 | F = 1.40 | p = 0.24 | η2 = 0.03 | 38.27 | 2.54 | 43.34 |
| Handgrip D (kg) | 47.28 ± 7.95 | 45.79 ± 8.13 | 48.78 ± 7.51 | F = 1.60 | p = 0.21 *| η2 = 0.04 | 44.71 | 2.57 | 48.86 |
| D2 test | |||||||
| TR | 382.16 ± 75.74 | 369.55 ± 76.70 | 394.77 ± 74.37 | F = 1.23 | p = 0.27 | η2 = 0.03 | 359.79 | 22.37 | 404.52 |
| TA | 138.23 ± 35.46 | 129.36 ± 35.40 | 147.09 ± 34.02 | F = 2.87 | p = 0.10 | η2 = 0.06 | 128.49 | 9.73 | 147.96 |
| O | 22.70 ± 17.24 | 26.77 ± 19.68 | 18.64 ± 13.65 | F = 2.54 | p = 0.12 | η2 = 0.06 | 36.79 | 4.54 | 40.62 |
| C | 5.75 ± 6.72 | 8.14 ± 8.25 | 3.36 ± 3.51 | F = 6.23 | p = 0.02 * | η2 = 0.13 | 4.01 | 1.89 | 7.49 |
| TR+ | 38.70 ± 6.60 | 39.09 ± 7.12 | 38.32 ± 6.18 | F < 1 | 36.79 | 1.91 | 40.62 |
| TR− | 18.73 ± 6.92 | 17.59 ± 6.08 | 19.86 ± 7.64 | F = 1.19 | p = 0.28 | η2 = 0.03 | 16.83 | 1.89 | 20.62 |
| TR | TA | O | C | TR+ | TR− | |
|---|---|---|---|---|---|---|
| Age (yrs) | r = −0.08 | p = 0.56 | r = −0.01 | p = 0.97 | r = −0.12 | p = 0.40 | r = 0.09 | p = 0.54 | r = −0.10 | p = 0.47 | r = 0.09 | p = 0.53 |
| Height (cm) | r = 0.16 | p = 0.29 | r = 0.20 | p = 0.18 | r = −0.17 | p = 0.26 | r = −0.15| p = 0.31 | r = 0.11 | p = 0.45 | r = 0.22 | p = 0.13 |
| Weight (kg) | r= −0.05 | p = 0.72 | r= −0.01 | p = 0.94 | r = −0.10 | p = 0.51 | r = 0.05| p = 0.71 | r = −0.12 | p = 0.42 | r = 0.07 | p = 0.63 |
| BMI (%) | r = −0.21 | p = 0.17 | r= −0.17 | p = 0.24 | r = 0.01 | p = 0.99 | r = 0.22 | p = 0.13 | r = −0.28| p = 0.06 | r = −0.08 | p = 0.56 |
| TR | TA | O | C | TR+ | TR− | |
|---|---|---|---|---|---|---|
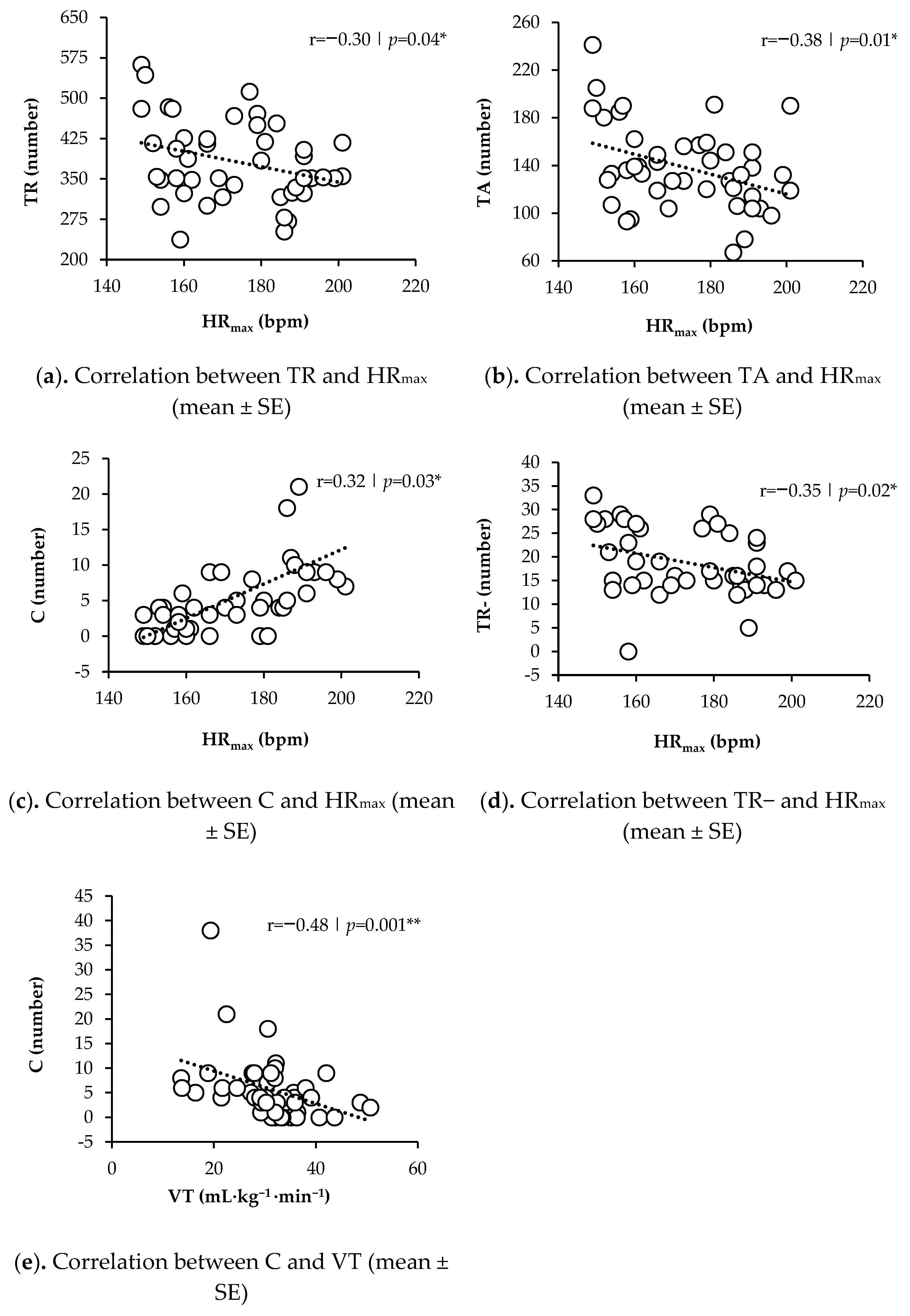
| HRmax | r = −0.30 | p = 0.04 * | r = −0.38 | p = 0.01 * | r = 0.27 | p = 0.074 | r = 0.32 | p = 0.03 * | r = 0.04 | p = 0.07 | r = −0.35 | p = 0.02 * |
| VT | r = 0.17 | p = 0.26 | r = 0.09| p = 0.52 | r= −0.01| p = 0.98 | r = −0.48| p = 0.001 ** | r = 0.10| p = 0.50 | r = 0.09 | p = 0.55 |
| Load | r = −0.01| p = 0.92 | r = −0.05| p = 0.71 | r = 0.02| p = 0.88 | r = −0.17| p = 0.26 | r = 0.05| p = 0.72 | r = 0.01| p = 0.93 |
| W/kg | r = 0.01| p = 0.96 | r = −0.07| p = 0.63 | r = 0.08| p = 0.56 | r = −0.20| p = 0.18 | r = 0.11| p = 0.044 | r = −0.03| p = 0.82 |
| R | R2 | Adjusted R2 | F | P | SE | ||
|---|---|---|---|---|---|---|---|
| HRmax | TR | 0.30 | 0.09 | 0.07 | 4.27 | 0.04 * | 73.01 |
| TA | 0.38 | 0.14 | 0.12 | 7.10 | 0.01 * | 33.18 | |
| C | 0.58 | 0.33 | 0.32 | 21.30 | 0.001 ** | 5.53 | |
| TR− | 0.35 | 0.12 | 0.10 | 5.94 | 0.01 * | 6.55 | |
| VT | C | 0.39 | 0.15 | 0.13 | 7.25 | 0.007 * | 6.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-García, L.; de la Cruz-Campos, J.-C.; Martín-Moya, R.; González-Fernández, F.-T. Active Teaching Methodologies Improve Cognitive Performance and Attention-Concentration in University Students. Educ. Sci. 2022, 12, 544. https://doi.org/10.3390/educsci12080544
Rodríguez-García L, de la Cruz-Campos J-C, Martín-Moya R, González-Fernández F-T. Active Teaching Methodologies Improve Cognitive Performance and Attention-Concentration in University Students. Education Sciences. 2022; 12(8):544. https://doi.org/10.3390/educsci12080544
Chicago/Turabian StyleRodríguez-García, Lorena, Juan-Carlos de la Cruz-Campos, Ricardo Martín-Moya, and Francisco-Tomás González-Fernández. 2022. "Active Teaching Methodologies Improve Cognitive Performance and Attention-Concentration in University Students" Education Sciences 12, no. 8: 544. https://doi.org/10.3390/educsci12080544
APA StyleRodríguez-García, L., de la Cruz-Campos, J.-C., Martín-Moya, R., & González-Fernández, F.-T. (2022). Active Teaching Methodologies Improve Cognitive Performance and Attention-Concentration in University Students. Education Sciences, 12(8), 544. https://doi.org/10.3390/educsci12080544








