Abstract
Aroma compounds are important in the food and beverage industry, as they contribute to the quality of fermented products. Yeasts produce several aroma compounds during fermentation. In recent decades, production of many aroma compounds by yeasts obtained through adaptive laboratory evolution has become prevalent, due to consumer demand for yeast strains in the industry. This review presents general aspects of yeast, aroma production and adaptive laboratory evolution and focuses on the recent advances of yeast strains obtained by adaptive laboratory evolution to enhance the production of aroma compounds.
1. Introduction
Aromas are one of the most important compounds that stimulate the olfactory and gustatory senses [1,2,3] and contribute to the quality of beverages, food and fragrance [3]. The structure of aroma compounds are heterogenous and can have all kinds of functional groups [1]. They are chemicals of value in the chemical, food, chemical, cosmetic and pharmaceutical industries. Microorganisms produce several aroma compounds by fermentation. These compounds affect the flavour of the products. Aroma compounds are important in the fermentation of wine, beer and ciders [3,4]. They contribute to the quality of fermented beverages and there is an increasing interest in beverages with various aromas and distinct flavours [3,5]. Higher alcohols, esters, aldehydes and terpenes are examples of metabolites that have considerable impact on the aroma and flavour of fermented products [6,7]. During fermentation, yeasts produce acetic acid, acetaldehyde, organic acids, ethyl acetate, volatile fatty acids, acetoin, glycerol, esters, and carbonyl compounds [3], all of which are important for the secondary aroma of beverages [3,4,8]. Saccharomyces cerevisiae produces most of the aroma compounds and affects the quality of fermented beverages [7,9,10]. This review presents recent advances of yeast strains obtained by adaptive laboratory evolution (ALE) and, in turn, their production of aroma compounds. This review also includes general aspects of aroma compounds, strain improvement by ALE and the effect of conventional and non-conventional yeasts on fermented beverages. It further focuses on the cases of adaptive laboratory evolution of yeast strains to achieve aroma enhancement. It also highlights a few Turkish fermented beverages.
2. Aroma Compounds
Aroma compounds consist of higher alcohols, terpenes, volatile fatty acids, esters, aldehydes and flavonoids. One of the aroma compounds that yeasts produce through fermentation is higher alcohols (fusel alcohols). Higher alcohols are one of the largest and most important groups of aroma compounds [3,7,11]. They are biosynthesised via carbohydrate metabolism [12] and the Ehrlich pathway, which is an amino acid catabolism pathway [7,13,14]. Examples of higher alcohols that give different aromas and flavours are 1-butanol associated with fusel odour, 2-phenylethanol (2-PE) associated with rose notes, isobutanol associated with alcoholic flavour, 2-methyl-1-butanol associated with brandy aroma and isoamyl alcohol associated with marzipan flavours [3,7,15]. Aroma compounds should be kept within certain limits to balance the flavour of fermented products [16]. For example, a study found that, in wine, higher alcohols effuse a pleasant aroma when the concentration is about 300 mg/L [3]. However, when concentrations of higher alcohols exceed 400 mg/L, the aroma of wine is adversely affected [3,7]. Meilgaard [17] showed that there were threshold values for higher alcohols and the other aroma compounds in beer. In cases where high concentrations of higher alcohols give off flavours, the industry may prefer strains that produce low amounts of higher alcohols [7,18]. The levels of concentrations of aroma compounds are also related to the strains that are used for fermentation [7,9]. 2-PE is one of the more important higher alcohols that is widely used in the cosmetic, food and pharmaceutical industries [1,19]. It effuses a floral and rose-like scent [1,3,20]. 2-PE is the fusel alcohol of L-phenyalanine and of the four phenylpropanoid aroma compounds [2]. S. cerevisiae can produce 2-PE from catabolism of L-phenyalanine by the Ehrlich pathway [2,3,21]. 2-PE production was increased using 2-phenyalanine as a precursor. Extraction of 2-PE from flowers is inefficient and costly. Biological processes are advantageous as they are environmentally friendly, have mild reaction conditions and have short ferment cycles. The market demand for 2-PE continues to grow, but the production of 2-PE is not enough to meet this demand [19].
Volatile fatty acids can be responsible for defects in wine. Saturated, straight-chain fatty acids are prevalent in alcoholic drinks. The production of volatile fatty acids, such as acetic acid, may indicate that that there is bacterial growth in the product [3,22]. For example, in one study, it was observed that unpleasant aroma of fermented beverages can be triggered when the concentration of volatile fatty acids exceeds 700 mg/L [3,23]. Formic acid, acetic acid, propionic acid, isobutyric acid, hexanoic, octanoic, and decanoic acids are some of the volatile fatty acids produced by yeast, and they are responsible for both pleasant and unpleasant odours in wine and beer [23].
The primary aroma includes terpenes, which contribute to a mix of appealing descriptors, such as honey, floral and citrus notes. Linalool and α-terpineol contribute floral notes and their odours are perceptible at low concentrations [24]. Most primary aroma compounds are found in grapes in odourless and non-volatile bound forms, which can be hydrolysed during fermentation through the action of wine yeasts, thus releasing odour-active compounds [25]. All terpenoids come from the intermediary metabolite isopentyl pyrophosphate. Terpenoids in beer come primarily from hops, and they pass on organoleptic properties to beer. Monoterpenoids and sesquiterpenoids are terpenoids that exhibit robust sensory qualities and are particularly valued for their flavours and fragrances [26]. For example, geraniol is ascribed a rose-like “citrus” odour, whereas the cis-isomer nerol is regarded to have a fresh, “green” odour [26,27]. Terpenoids (terpenes or isoprenoids) have had an important role in medical treatments since ancient times [28]. They are the most diverse group of natural compounds and have exceptional flavour qualities [2]. They are in the primary aroma group in the food industry and they effuse highly desirable floral, citrus and honey notes [3]. Linalool and α-terpineol also provide floral aroma and their odour thresholds are low. Linalool is used in the cosmetic industry and is also used as a flavouring ingredient [22,24].
Esters that are synthesised by S. cerevisiae during fermentation contribute highly desirable aromas, such as fruitness to fermented beverages, which makes them favourable and desirable to the industry [7,29,30]. They can also be used in artificial fragrances and perfumes [4]. There are two important ester groups, namely the ethyl esters of medium-chain fatty acids (MCFA) and the acetate esters in fermented beverages. Compounds within the acetate esters group are associated with various flavours; for example, ethyl acetate is associated with fruity flavour, isoamyl acetate is associated with banana and pear flavours, isobutyl acetate is associated with banana flavour, phenylethyl acetate is associated with rose, honey, fruity and flowery flavours, ethyl hexanoate is associated with apple and banana flavours, ethyl octanoate is associated with pineapple and pear flavours, ethyl propionate is associated with banana and apple flavours and ethyl decanoate is associated with floral flavour [29,31,32]. Even a small amount of ester is enough to have a significant impact on fermented beverages [3,19,29]. For example, in wine, the aroma of the product may be spoiled if the concentration of ethyl acetate exceeds 200 mg/L [3,23] and, conversely, ethyl acetate emits a desirable aroma when its concentration is below 150 mg/L [7,23]. The concentration of most esters is low in fermented beverages, and they are very close to the scent perceptibility limit of the human nose. For this reason, minimal changes in the concentration of esters have a significant impact on fermented products [7,29,33].
Flavonoids are polyphenolic compounds produced by yeast and are important to human health due to their anti-oxidant, anti-inflammatory, anti-oncogenic and anti-allergic properties. These properties make flavonoid production important. L-phenylalanine and L-tyrosine have a role in the production of flavonoids [1]. β-glucosidases of yeast have an important role in aroma production and they are used to release the odour-active compounds that are found in non-volatile precursors during the wine-making process [3,25]. Release of terpene glycoside is one of the indicators for the quality of wine [34]. The β-glucosidases enzyme hydrolyses monoterpenes and noriseprenoids, which are non-volatile and flavourless glycosides, and this enhances the aroma of wines [35].
S. cerevisiae synthesise acetaldehydes during fermentation and this is the major aldehyde that constitutes over 90% of the total aldehyde content of wine [8,36]. The low concentrations of acetaldehyde give a pleasant fruity aroma. However, when its concentration is high, it produces green and grassy off-flavours. Acetaldehyde has a direct effect on the aromatic profile of fermented beverages [7]. Acetaldehyde can have high reactivity with other compounds, such as SO2 in wine [36]. When acetaldehyde binds with SO2, which is an antimicrobial and antioxidant agent, it provides limited protection to wine [37]. While different types of wine have different concentrations of acetaldehyde [36], different S.cerevisiae strains synthesise different amounts of acetaldehyde. Strains should be chosen based on the type of wine produced [7].
Aromatics used in industry are generally produced by chemical synthesis or are obtained by extracting natural compounds from plants or animals [1,2]. When these aroma compounds are isolated from plants or animals, they are named bioflavours. When aroma compounds are chemically synthesised, these compounds may cause environmental pollution and unwanted racemic mixtures may also occur. Low yield in the isolation of product from plants or animals and the high cost of isolation processes are examples of the disadvantages of extraction from natural sources. Isolation aroma compounds from plants and animals need multiple purification steps that cause product loss and degradation. The market prefers chemically synthesised aroma products for their cheap prices [2]. There is an increasing demand for new production sources for aroma compounds that offer more renewable and sustainable processes. An alternative way is to use microbial-cell mediated bio-production. Saccharomyces cerevisiae and other non-conventional yeast species are used to synthesise aromatics. This is still very much a developing field [1].
3. Strain Improvement by Adaptive Laboratory Evolution
Yeasts have been central to the production of fermented foods and beverages throughout the course of human history, and possibly for this reason, the choice of a species or strain of yeast for a particular industrial application is still often grounded more in tradition rather than science. It is possible to generate yeast strains that have enhanced performance with biotechnological applications [38]. A great number of genes and biosynthetic pathways, and various parameters, such as growth medium strains and conditions involved in fermentation processes, make aroma production complex [6]. Genetic techniques to develop S. cerevisiae strains with better traits have been proposed since the beginning of the century [39]; however, it is not preferred by the industry, as consumers prefer non-GM strains in the food industry [7].
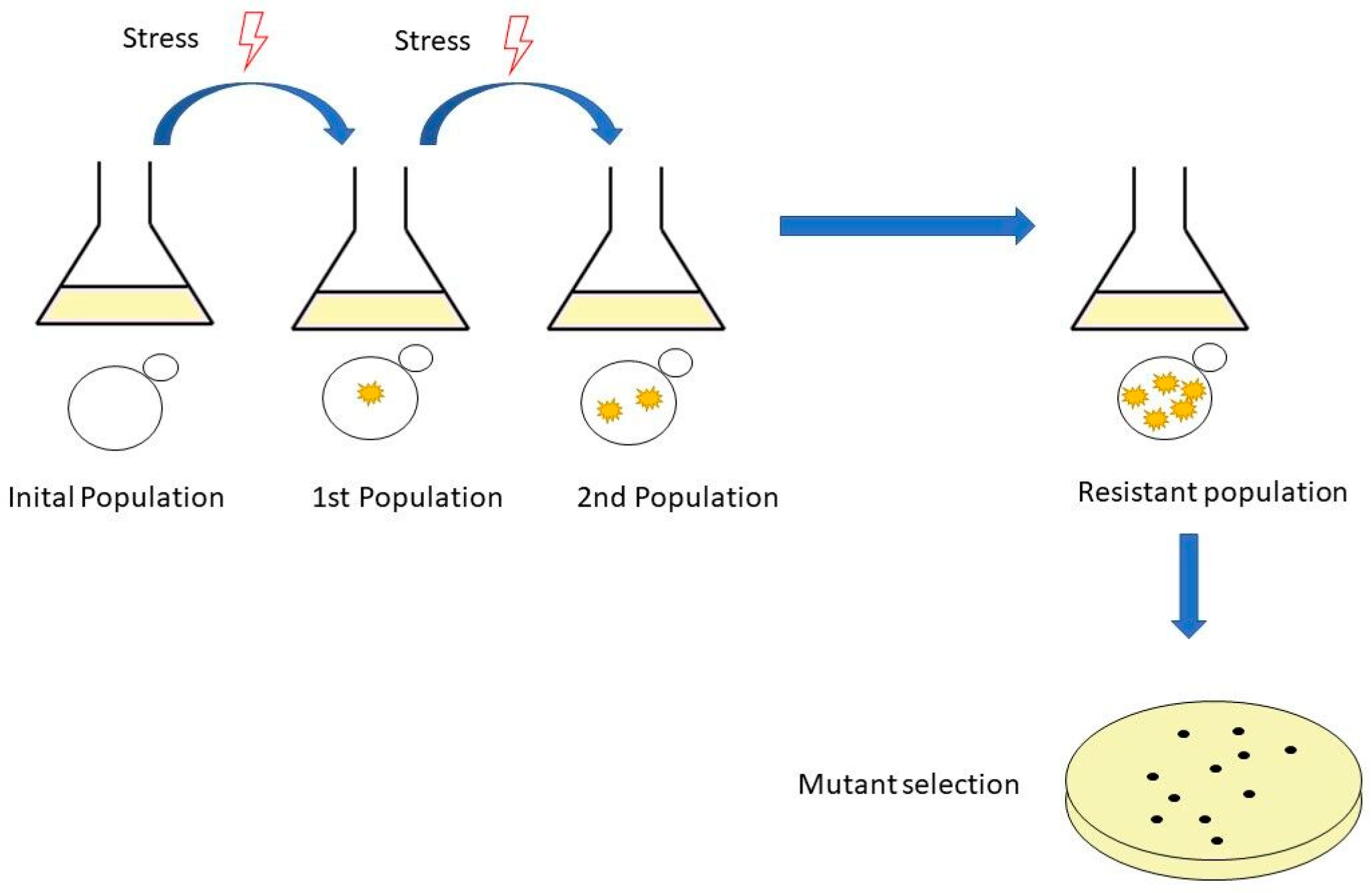
Adaptive laboratory evolution is a method used widely to study the basic mechanisms of molecular evolution and adaptive changes in populations of microorganisms subjected to specific growth conditions [40]. This method can improve strains by subjecting them to selective stresses, and after serial inoculations with these stress conditions, the strains develop spontaneous mutants that provide resistance to stress conditions [41] (Figure 1).
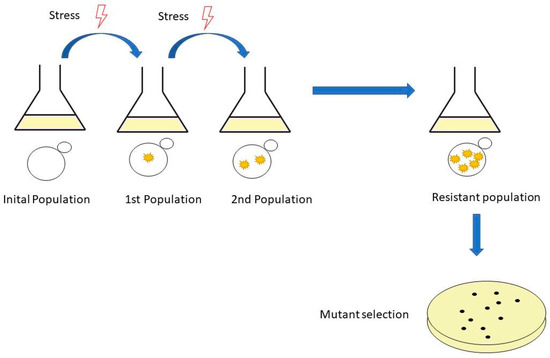
Figure 1.
The workflow of adaptive laboratory evolution (ALE). It is performed by serial passages in shake flasks. The experiment starts with an initial population, followed by a population with mutations and, finally, a resistant population and mutant selection.
ALE creates an advantage under selective environmental conditions. In ALE experiments, spontaneous mutations help the resistance or advantage of the strain, and the spontaneous mutation rate can be increased by physical and chemical mutagens. Ploidy of the strain affects the mutation rate in ALE experiments. ALE requires genetic diversity and availability of a selectable trait. A shortened lag phase, increased growth rate, prototrophy for particular nutrients, improved substrate consumption, and flocculation are examples of selectable traits that are important in ALE. Stress tolerance is needed for yeast strain during fermentation, as it affects the quality of the product [42]. The common stresses that yeast strains are exposed to in industry are ethanol stress, osmotic stress, oxidative stress, thermal stress, freezing, salinity stress and thawing stress, among others [42,43]. A fitness advantage is needed for the strain to grow under these stresses. However, fitness advantage is not the only target of ALE to produce flavour and aroma compounds. ALE can be challenging if the phenotype targeted for improvement is not directly selectable, e.g., higher yield of a particular product. In such cases, making use of genetic modifications or a smart experimental design can combine the non-selectable feature with a selectable one, e.g., product formation to growth rate. Production of volatile flavour compounds is one such trait, and yeast cells do not necessarily exhibit a fitness advantage due to the phenotype. Indirect methods are used to adapt and select yeast cells, and carbonyl compound production has been modified in this way. Significant opportunity remains for further customisation of flavour profiles through the use of chemicals that modify biochemical pathways [42].
Butler et al. [44] were the first to propose strain improvement by the concept of evolutionary engineering. This method is also known as adaptive, directed or experimental evolution and is the combination of natural variation and selection under artificial conditions [38]. Essentially, it involves obtaining mutant strains with desired properties [45].
Strain improvement became an interesting topic when penicillin-producing cultures were improved to enhance penicillin production by mutagenesis and screening. Genetic engineering of fermentation by yeast was applied to improve strains after 1985 [46]. According to Darwin theory, species undergo mutations to adapt to their environment so that they can survive and reproduce [47]. In adaptive laboratory evolution (ALE), the principles of natural evolution occur in the laboratory to achieve a population that has better fitness to resist a stress condition [40]. Variables such as selective stress conditions, the duration of the experiment to have serial transfer of the yeast, sexual or asexual reproduction of the population and clonal interference affect the results of ALE experiments [48]. When the population is grown under stress conditions for many generations to obtain a specific phenotype, random mutants will appear in the population [38]. Mutagens can be used to increase the genetic and phenotypic variabilities [38,45]. If a specific mutation (or mutations) becomes dominant in the population with a fitness advantage, this mutant can be selected and reproduced in the population [38]. The properties of microorganisms make them well-suited for evolutionary engineering studies, as generation time is short and they are easily manipulated and cultivated. Yeast strains with improved phenotypes can be generated quickly through evolutionary engineering [49]. There are several studies that show how yeast cells are improved by evolutionary engineering. Laboratory yeast strains and industrial yeast strains were used in evolutionary engineering to develop adaptation to specific stresses. The aim of these studies was to understand the genetic background and the reproducibility of the adaptation [50,51]. Industrial yeast strains are polyploid and are, therefore, more difficult to improve by evolutionary engineering, as compared to laboratory strains. Nevertheless, there are studies that were successful in achieving improvement by means of evolutionary engineering [52].
S. cerevisiae is the predominant yeast species in baking. It has been used as a starter culture in fermented products since the 19th century. The first compressed yeasts became available in England in the 18th century and in northern Europe in the 19th century. Compressed yeasts were used for large-scale bread production in 1868 in the USA [7]. Consumers value aroma and flavour as very important properties in bread. Alcohols, aldehydes, ketones (volatile aromatic compounds), acids, esters, sugars (non-volatile compounds), fatty acids and lipids are the aromatic compounds that are produced during baking. Volatile and secondary metabolites of yeast affect the taste of bread; thus, these metabolites affect the preference of consumers [20,53,54]. Reduction of dough pH also affects flavour. The ability of strains to produce CO2 enables strain selection in baking. Ethanol production, cell growth rate, volume of the final product, structure, colour, fermentation performance, shelf-life, stress tolerance and adaptation to utilise other sugars are selectable traits for yeast in baking. The amount of aroma produced by S. cerevisiae can be limited compared to other yeast strains in fermented foods and beverages [7]. ALE can be used to improve flavour and aroma in baked products [20,54]. Although commercial strains do not fully meet the demands of the industry, producers nevertheless use these commercial baking strains [38,54]. Improved fermentation in sweet doughs, tolerance to salt toxicity, better freeze-dry stress tolerance, microbial stability, enhanced qualities, such as taste, aroma and texture, and synthesis of metabolites, including antioxidants, phenols and anthocyanins, are examples of traits of commercial baking strains that need to be optimised for use in industry [55]. Consumers and producers desire more flavourful, nutritious products and those that exhibit resistance to stress without the loss of fermentation capacity [54].
Non-Saccharomyces yeasts are another option that can be used for fermented products, as baker’s yeast (S. cerevisiae) can have disadvantages, such as poor aromatic profiles and less stress resistance. However, the scientific community and consumers prefer S. cerevisiae, since it qualifies as GRAS (generally regarded as safe) and QPS (qualified presumption of safety). Non-conventional yeast can be used to produce more flavours [6,7,10,21,28]. The global yeast market is expected to grow to about USD 6 billion by 2025, which corresponds to an annual growth rate of about 10% over four years. The growth of the market is an indication that the need for other yeasts has also increased. An alternative to baker’s yeast may be sought due to the increasing demand for novel bakery products [43]. Non-Saccharomyces yeast are undomesticated strains, and they have different fermentation characteristics compared to Saccharomyces yeasts. Furthermore, our understanding of non-Saccharomyces yeast is limited. They also have the undesirable effect of producing off-flavours in fermented beverages. Non-Saccharomyces yeast have been regarded as contaminants in the fermented beverage industry. They have been used with S. cerevisiae during fermentation, as non-conventional yeast produces less ethanol [56]. Brettanomyces (=Dekkera) bruxellensis, Kazachstania exigua, Kazachstania gamospora, Kazachstania humilis, Kazachstania zonat, Kluyveromyces marxianus, Lachancea thermotolerans, Pichia kluyveri, Pichia kudriavzevii, Saccharomyces cerevisiae var. diastaticus, Saccharomycopsis fibuligera, Saccharomycodes ludwigii, Torulospora delbrueckii, Zygosaccharomyces rouxii, Wickerhamomyces anomalus, Wickerhamomyces subpellicullosus Candida yeasts, Kloeckera yeasts and Hanseniaspora yeasts are the non-conventional yeasts used to achieve aroma complexity [4,43,56]. Saccharomyces pastorianus has cold resistance, utilises maltose and generates pleasant aroma profiles. Lachancea thermotolerans is a strain used in the production of sour beers. It produces lactic acid, which acidifies the beer, while simultaneously producing ethanol. This strain can be used in industry when the lactic acid bacteria that is used for beer souring is undesirable in the fermentation facility. Lachancea fermentation, Hanseniaspora vineae and Schizossacchoramyces japonicus can be used for beer souring and to produce low alcohol beers. Strains isolated from fermentation environments have different traits, such as fitness advantage, ethanol and osmotic stress tolerance compared to the wild-type [42].
S. cerevisiae is the predominant yeast used in evolutionary engineering studies; however, other yeast strains are also used [38]. UV- or EMS-mutagenized populations are used as a starting population to increase genetic variability in the ALE method [38]. These mutagenesis approaches are applied not only to S. cerevisiae but also to non-conventional yeasts. S. cerevisiae was developed by EMS mutagenesis and ALE with high ethanol production [57]. For example, EMS mutagenesis was applied to Kluyveromyces marxianus to enhance ethanol production [58]. ALE can also be used on yeast strains that are obtained by other genetic methods, such as genome shuffling. In ALE, it is important that the evolved strain remains stable when the selective stress is not applied. The whole genome of the evolved strain can be sequenced, and this can show how the phenotype is established. This information can be used to improve strains by reverse metabolic engineering [38]. ALE is a valuable methodology to obtain yeast strains with improved properties [45]. Yeast strains can be improved by ALE to develop stress tolerance, to produce new products, increase product yield and to improve fermentation rates [43,59]. Adaptive laboratory evolution is advantageous compared to rational metabolic engineering, as it does not require much genetic information about the strain [60]. As it is considered to be a non-genetic modification strategy for yeast strains, this method is also more acceptable to consumers. Furthermore, it is an inexpensive and simple strategy, as less laboratory equipment are required and growth is achieved through serial transfer in shake flasks [43].
Examples of phenotypes improved through adaptive laboratory evolution are listed in Table 1.

Table 1.
Phenotypes improved by adaptive laboratory evolution.
4. Applications of Yeast in the Industry
Wine, beer and other fermented products are traditionally and economically valuable products. Yeast activity and fermentation activity affect the quality of the product and their influence on aroma, as yeast produces aroma compounds [76].
S. cerevisiae is involved in the fermentation of alcoholic beverages, as well as less known non-alcoholic beverages around the world, such as shalgam, boza, koumiss and kefir [7,77]. The traditional fermentation yeast is S. cerevisiae; however, non-S. cerevisiae yeast species make a valuable contribution to fermentation by producing volatile compounds. Evolutionary engineering can be used to maintain the characteristics of fermented products and enhance flavour profiles. An example is yeast that was engineered to produce hop flavours in the brewing industry. A company named Berkeley Yeast has engineered wine and beer yeasts with enhanced flavour and performance [76].
ALE may be needed to maintain familiar flavour profiles, as traditional winemaking regions are being affected by climate change, resulting in grapes with higher sugar content due to global warming. The alcohol concentration of wines is expected to rise by about 2% due to global warming [78]. When the harvest time of grapes is extended to increase grape maturity, fruit flavour and colour intensity, this enhanced maturity also increases the alcohol concentration in wine. Grapes that are more ripe have higher sugar concentrations (>240 g/LFf) and result in higher alcohol concentration (>13.5% v/v) in the final wine [78]. Alcohol concentration in wine is controlled in the interest of economic, health and quality factors. Some countries charge a high tax rate on wine with high alcohol concentrations. Too much alcohol can negatively affect aroma and, more importantly, excessive alcohol consumption adversely affects health. The balance of aroma compounds, alcohol and sweetness is important. ALE can be used to decrease the alcohol content of wine.
Chocolate is a fermentated product and is produced from the beans of the tropical plant Theobroma cacao. However, these beans are bitter and inedible. The aroma and flavour of raw cocoa beans are not similar to chocolate [79,80,81]. The levels of polyphenols and alcaloids in raw cocoa beans are reduced through fermentation to develop the flavours that determine the quality of cocoa and chocolate. Fermentation processes are carried out spontaneously in cocoa pulp [82]. Fermentation by yeast is important for the quality of chocolate. In a study, cocoa fermentation is compared in the presence and absence of yeast. It is shown that the production of ethanol, higher alcohols and esters was increased in the presence of yeast during fermentation [83]. Chocolate produced by yeast fermentation has a higher quality. It is observed that lactic acid bacteria and acetic acid bacteria are not important for the fermentation of cocoa compared to yeast [84,85]. S. cerevisiae is the most prevalent among the yeasts used in the fermentation of cocoa beans in several studies. S. cerevisiae has pectinolytic activity, rapidly grows even when the pH slightly increases and can adapt to grow at high temperatures and high ethanol concentrations. These properties make S. cerevisiae favourable for the fermentation of cocoa [7]. As cocoa fermentation is carried out spontaneously, it is also poorly controlled. ALE can be used to obtain resistant S. cerevisiae strains in cocoa fermentation [75].
Shalgam, boza, koumiss and kefir are examples of traditional, fermented beverages that are well-known in Turkey and are gaining wider recognition around the world [77,86,87,88]. Shalgam is a fermented beverage that is produced from turnips, broken wheat flour, salt and water [77,86]. It contains amino acids, polyphenol contents, acetic acid, ethanol and volatile aroma compounds [77]. It consists of lactic acid bacteria Saccharomyces and non-Saccharomyces yeasts [77,86]. Boza is a cereal food and a non-alcoholic fermented beverage produced from wheat or maize. It is popular in Turkey, Bulgaria and other Eastern European countries, as well as in Egypt, Nigeria and Kenya [77,87]. Lactic acid and yeast fermentation are observed in boza [77]. In a study, S. uvarum and S. cerevisiae were found to be the predominant yeasts in Turkish boza [89]. In another study, Uyghur boza samples were collected and yeast strains belonging to Saccharomyces cerevisiae, Pichia membranifaciens, and Pichia fermentans were found [87]. Kefir is a fermented beverage produced from the milk of cows, sheep, goats or other animals. The geographic origin of kefir is the Caucasus Mountains and it is produced by yeast and lactic acid fermentation [77]. Kefir product consists of lactic acid, ethanol, carbon dioxide and other flavour compounds, such as diacetyl, acetoin and acetaldehyde resulting from fermentation. The microbial content of kefir grains is homofermentative and heterofermentative Leuconostoc spp., Lactococcus spp., Lactobacillus spp., Leuconostoc spp. and acetic bacteria and yeasts such as Candida spp. and S. cerevisae [77,90,91]. Koumiss is naturally fermented mare’s milk. It is a product of yeast-lactic fermentation. Koumiss contains essential fatty acids, such as linolenic acid and linoleic [92]. In a study, Lactococcus raffinolactis, Lactobacillus helveticus and Citrobacter freundii and Lactobacillus kefiranofaciens bacteria and Dekkera anomala, Meyerozyma caribbic, Kazachstania unispora, Kluyveromyces marxianus, uncultured Guehomyces, and Pichia sp. BZ159 yeast were observed in koumiss [88]. Since these fermented beverages contain yeast, they can be used in evolutionary engineering studies.
5. Studies of Adaptive Laboratory Evolution of Saccharomyces cerevisiae and Non-Conventional Yeasts
Glycerol has a role when osmotic stress is applied to S. cerevisiae. Glycerol is the compatible solute in yeast, and by providing sweetness, it has a positive effect on fermented beverages. Tilloy et al. 2014 [68] demonstrated that glycerol formation in yeast can be improved by ALE. When osmotolerant S. cerevisiae were generated by ALE using increasing KCl concentrations, the evolved strain became resistant to high osmotic stress and had higher glycerol production compared to the parental strain [68]. In another study, EMS mutagenesis and ALE, by gradually increasing NaCl concentrations, were used together to obtain salt-resistant S. cerevisiae strains with a higher growth rate and higher glycerol levels [71]. In another study for the improvement of glycerol formation, adaptive laboratory evolution (ALE) experiments showed that yeasts have the capacity to grow efficiently on glycerol. An ALE experiment demonstrated that S. cerevisiae strains can grow in medium containing 10% glycerol. In evolved lineages, PBS2, HOG1 and GUT1 are observed to be mutated. PBS2 and HOG1 are part of the high-osmolarity glycerol (HOG) pathway. GUT1, the glycerol kinase encoding gene, is effective in glycerol utilisation. GUT1 is a target for adaptive mutations and catalyses the first step in glycerol metabolism. KGD1, UBC13 and GUT1 are important for glycerol utilisation in industrial yeasts [58]. The growth of S. cerevisiae in a medium containing sulfite can direct the metabolism into glycerol by binding with acetaldehydes. In an ALE experiment with sulfite, an evolved strain produced more glycerol (46%); however, its ethanol production was the same as the parental strain [73].
S. cerevisiae faces high osmotic stress, high temperature and high ethanol levels during industrial processes. In a study that focuses on thermotolerance, a strain was subjected to fermentation processes at 40 °C to increase ethanol production. S. cerevisiae strains achieved thermotolerance by long-term adaptive evolution (1200 generations) at 39.5 °C. Their ethanol yield was increased by 10% and 70% in 2% glucose fermentations. The evolved strain used high glucose concentration (200 g/L glucose) and produced 93.1 g/L ethanol at 40 °C [74,83].
Osmotic stress is not only studied on S. cerevisiae but also on non-conventional yeasts. Another study for osmotic stress resistance was performed to obtain a salt-resistant yeast, Wickerhamomyces anomalus, which is an aroma-producing yeast for soy sauce fermentation. A combination of mutagenesis and ALE experimentation was successful in obtaining an evolved strain. Yeast cells were subjected to atmospheric and room-temperature plasma (ARTP), and an ALE experiment with a sodium chloride concentration of 18 (w/w) was conducted. The evolved strain produced more ethanol, acid and acetaldehyde in the soy sauce, and also increased the variety of esters [69]. In a study, S. pastorianus (lager yeast strain) was obtained by ALE with improved growth under hyperosmotic conditions, and the evolved strain exhibited shorter fermentation time and higher amounts of diacetyl, pentanedione, 3-methylbutyl acetate and 2-phenylethyl acetate. It led to a small change in flavour [70].
As breweries use high-gravity brewing, it is important to obtain yeast strains with better fermentation rates. Multi-stress resistant lager yeast strains were obtained with improved fermentation capacity and higher ethanol content under high-gravity conditions and UV treatment [38,93,94].
Zygosaccharomyces rouxii is among the salt-tolerant yeasts and is used to enhance the aroma compounds’ formation during soy sauce fermentation. Atmospheric and room temperature plasma (ARTP) mutagenesis was used to improve salt-tolerant Z. rouxii. The evolved strain has better growth and glucose metabolism than the wild-type strain. Ethanol produces a precursor to many aromatic esters in soy sauce. The evolved strain leads to an enhanced amount of ethanol that triggers ester production. Acids are an indicator of quality in soy sauce, regulate soy sauce flavour and contribute to its aroma components [72].
There are studies that focus on enhancing yeast tolerance to acidity. Industrialisation of fruit wines are problematic due to high acidity. Low pH adaptation is crucial for yeasts in the wine production industry. S. cerevisiae can grow in pH ranging from 3.8 to 6.0; however, during wine fermentation, the pH values fall below 3.0. Low pH affects the growth and duration of fermentation, and it causes poor taste and quality. A combination of ALE strategy and mutagenesis enhances yeast tolerance to acidic environments in wine production of greengage fruits. The levels of aroma compounds in the evolved strain are higher than the wild-type strain. The evolved strain can grow at pH 2.5, while the survival rate of the parental strain is very low (9.73%) at this pH. Fermentation time of the evolved strain was shortened [61].
Mutagenesis and ALE were used together to obtain acetic acid resistant yeast culture (S. cerevisiae). It is observed that ASG1, ADH3 and SKS1 contributed to the development of acetic acid tolerance. The presence of acetic acid during bioethanol production can inhibit yeast growth and alcoholic fermentation. If the strain exhibits high acetic acid tolerance, ethanol production will be more productive [62].
In a study, thermo-resistant S. cerevisiae was obtained by ALE to be used in cocoa fermentation. Thermotolerance is a useful property in cocoa fermentation; application of high temperatures to yeast cells has a positive effect on growth and fermentation. The mechanism of thermotolerance has not yet been solved, although there are genes known to be related to thermotolerance in S. cerevisiae. Cocoa beans are fermented to obtain chocolate flavour precursors in the production of chocolate. During this process, yeasts are subjected to high temperature (45–48 °C), elevated ethanol and low pH. ALE was used to obtain thermo-tolerant diploid S. cerevisiae. The evolved strain can grow and ferment at 40 °C [75].
Chinese rice wine is an alcoholic beverage and is produced by saccharification and fermentation of glutinous rice using “wheat Qu” as the saccharifying agent and S. cerevisiae as the fermentation starter. During the process, yeast cells are exposed to high ethanol and high sugar stress. GMO is restricted by legal codes and is not looked upon favourably by consumers. Application of adaptive laboratory evolution is a good strategy to be able to select strains with certain desirable properties. An ethanol-tolerant S. cerevisiae strain was evolved by EMS and ALE using serial batch transfer fermentation over 200 generations. Although this evolved strain had improved fermentation characteristics and better ethanol tolerance, the aroma compound was not changed in this strain. The ethanol content upon completion of the fermentation process was 20% (v/v). Ethanol tolerance is needed for this wine fermentation [64].
The concentration of alcohol in the wine increased due to the usage of mature grapes, which naturally have higher sugar content. However, yeast growth and fermentation are restrained by high ethanol levels; therefore, the aroma of the wine decreased. The aim is to develop yeast strains that produce less ethanol with the same amount of sugar. The carbon metabolism of yeast is directed from ethanol to glycerol to obtain a yeast strain with low alcohol [95]. NaCl-resistant S. cerevisiae is developed using ALE methodology. The metabolism of this evolved strain produced less ethanol by directing the metabolism towards glycerol [96].
Esters are important aroma compounds for fermented alcoholic beverages. They add fruity and flowery notes to beer. Ethyl acetate provides flavour and fragrance. It is observed that free amino nitrogen in the medium increased the final amount of ethyl acetate in the fermentation process. Acetyl-coA is important for the production of ethyl acetate. ALE is used to obtain acetic acid resistant S. pastarinous with high expression of acetyl-coA genes and to increase the production of ethyl acetate. They demonstrated that higher acetic acid triggers the production of ethyl acetate [63]. Wine yeast strains developed by ALE can grow on gluconate as the sole carbon source and these evolved strains demonstrated better fermentation rates and produced less volatile acidity and more desirable volatile esters. This evolved strain enhances the taste of wine [38,97].
Limonene is an important natural plant product that has a pleasant aroma and is used in food, cosmetics and the pharmaceutical and chemical industries. The value of the market for limonene is expected to rise to USD 9 billion by 2024. Limonene is highly toxic to cells. The microorganism must be engineered to become resistant to the toxic effect of limonene. ALE is an efficient and powerful methodology for studying tolerance mechanisms. The tolerance to and titer of limonene in Yarrowia lipolytica was improved by short-term ALE. In this study, omics studies, tolerance engineering and evolutionary engineering were implemented to enhance the limonene tolerance and titer in Y. lipolytica. Transcriptomics data for gene candidates and efficacy of the gene candidates were tested. YALIOF19492g is the key beneficial gene. The strain is resistant to the other terpenoids, linalool which is a monoterpenoid and (+)-nootkatone, and β-ionone which are sesquiterperoids. Eight different strategies of short-term ALE were used and evaluated to achieve improved tolerance. Heterologous transporter expression also affects limonene production. Increase in tolerance does not enhance the production yield of interest. Limonene is a terpenoid. YALIOF19492p is a protein of unknown function. The results showed that the evolved strains had higher cell membrane permeability in the presence of limonene [57].
Torulaspora delbrueckii, which is a yeast species that can positively affect the aromatic properties of wines, is involved in the early stages of fermentation processes. It cannot tolerate a high ethanol concentration and cannot complete fermentation. T. delbrueckii has a positive effect on the aromatic properties of wines. T. delbrueckii is resistant to alcohol concentration up to 9%. It cannot remain in the late stages of fermentation when ethanol levels are higher than 12%. A T. delbrueckii strain, obtained by ALE with increasing concentrations of ethanol through serial batch cultivation, demonstrated resistance to SO2 and ethanol at concentration levels of 11.5% (v/v). The evolved strain improved the aromatic profile by producing more aldehydes, 2-ethylhexanol, ethanol, sulphur compounds and phenolic derivates and it was able to remain until the end of the fermentation process [70]. In another study for non-conventional yeast, K. marxianus was used for ALE studies. K. marxianus obtained ethanol tolerance by ALE [67] and also improved osmotic, oxidative and temperature tolerance. This method also increased the ethanol production of K. marxianus [67].
Yeast strains are stored frozen to be activated later for fermentation in the beer and baking industries. Yeast cells are discarded after they are used for 5–20 fermentations, after which fresh cultures are used from storage. Brewing yeast cells cannot adapt to stresses and evolve due to the practice of frozen storage. During brewing, yeast face fermentation stresses, such as osmotic stress, CO2 accumulation, ethanol toxicity and oxygen availability. Saccharomyces pastorianus brewing yeast variants were obtained by ALE with improved fermentation capacity. The yeast strains were selected at high osmotic stress. The evolved strain had a shorter fermentation time and produced 6.45% alcohol by volume of beer. It produced higher diacetyl and pentanedione, 3-methylbutyl acetate and 2-phenylethyl acetate contents compared to the original strain and it led to a small change in flavour. The fermentation rate of ethanol tolerance and osmotic tolerance enhanced strains and product quality of the strain under these stresses were both acceptable [70]. In another study, EMS was applied to S. cerevisiae and the dough fermentation capacity of S. cerevisiae was increased [98].
Jabuticaba is a fruit grown in South America. Syrups, wine, liquor, vinegar and jelly are produced from this fruit. Adaptive laboratory evolution was applied to obtain the ethanol-resistant S. cerevisiae T73 strain, and its behaviour evolved when it was co-cultured with Saccharomyces kudriavzevii CR85 during the fermentation of Myrciaria jaboticaba. The ethanol tolerance of S. cerevisiae T73 was improved and the ethanol production of the evolved strain in co-culture with S. kudriavzevii CR85 was increased [66].
There are studies for non-Saccharomyces yeast strains that produce aroma compounds. Lai et al. [3] investigated the characteristics of yeast strains other than Saccharomyces and their effect on aroma compounds in the fermentation processes of wine-making. Forty-two yeast strains were isolated from fruits, and among these, S. cerevisiae G112 exhibited better ethanol tolerance, thermal tolerance, and β-glucosidase activity. Non-Saccharamoyces yeast, such as H. uvarum, P. kluvyeri and H. guilliermondii, produced good amounts of esters, including ethyl acetate and 2-phenethyl acetate. P. kluvyeri exhibited the best sulphur dioxide tolerance [3].
6. Conclusions
Adaptive laboratory evolution offers a method to generate novel yeast strains to improve both yeast strain performance and production of aroma compounds. Saccharomyces cerevisiae strains are popular host organisms in industrial biotechnology; however, non-Saccharomyces yeast is also an option for ALE studies to produce compounds that affect the aroma profile of fermented beverages. Non-Saccharomyces yeast strains present the industry with an alternative to develop yeast with tolerance-enhanced properties. Consumer expectations can be fulfilled with the production of fermented products that have achieved aroma complexity through the use of these strains.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Liu, Q.; Liu, Y.; Chen, Y.; Nielsen, J. Current State of Aromatics Production Using Yeast: Achievements and Challenges. Curr. Opin. Biotechnol. 2020, 65, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, N.; Kroukamp, H.; Pretorius, I.S. The Smell of Synthetic Biology: Engineering Strategies for Aroma Compound Production in Yeast. Fermentation 2018, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.-T.; Hsieh, C.-W.; Lo, Y.-C.; Liou, B.-K.; Lin, H.-W.; Hou, C.-Y.; Cheng, K.-C. Isolation and Identification of Aroma-Producing Non-Saccharomyces Yeast Strains and the Enological Characteristic Comparison in Wine Making. LWT 2022, 154, 112653. [Google Scholar] [CrossRef]
- Escalante, W.D.E. Perspectives and Uses of Non-Saccharomyces Yeasts in Fermented Beverages. In Frontiers and New Trends in the Science Fermented Food Beverages; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-1-4757-6255-6. [Google Scholar]
- Mina, M.; Tsaltas, D. Contribution of Yeast in Wine Aroma and Flavour. In Yeast-Industrial Applications; IntechOpen: London, UK, 2017; ISBN 978-953-51-3599-9. [Google Scholar] [CrossRef] [Green Version]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine Flavor and Aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Farina, L.; Boido, E.; Henschke, P.A.; Dellacassa, E. Production of Fermentation Aroma Compounds by Saccharomyces cerevisiae Wine Yeasts: Effects of Yeast Assimilable Nitrogen on Two Model Strains. FEMS Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef] [Green Version]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-Active Wine Yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [Green Version]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.-C. Olfactory Impact of Higher Alcohols on Red Wine Fruity Ester Aroma Expression in Model Solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef]
- Stewart, G.G. The Production of Secondary Metabolites with Flavour Potential during Brewing and Distilling Wort Fermentations. Fermentation 2017, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, F. Über Die Bedingungen Der Fuselölbildung Und Über Ihren Zusammenhang Mit Dem Eiweißaufbau Der Hefe. Ber. Der Dtsch. Chem. Ges. 1907, 40, 1027–1047. [Google Scholar] [CrossRef] [Green Version]
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitrov, D.; Haygarov, V.; Yoncheva, T. Aromatic Profile of Red Wines from Grapevine Varieties Rubin, Storgozia, Bouquet, Trapezitsa, Kaylashky Rubin and Pinot Noir, Cultivated in the Region of Central Northern Bulgaria. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 885–889. [Google Scholar] [CrossRef]
- Pires, E.; Teixeira, J.; Brányik, T.; Vicente, A. Yeast: The Soul of Beer’s Aroma—A Review of Flavour-Active Esters and Higher Alcohols Produced by the Brewing Yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meilgaard, M. Flavor Chemistry of Beer: Part II: Flavor and Threshold of 239 Aroma Volatiles. Tech. Q. Master Brew. Assoc. Am. 1975, 12, 151–168. [Google Scholar]
- Eldarov, M.A.; Kishkovskaia, S.A.; Tanaschuk, T.N.; Mardanov, A.V. Genomics and Biochemistry of Saccharomyces cerevisiae Wine Yeast Strains. Biochem. Mosc. 2016, 81, 1650–1668. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, S.; Li, Y.; Shi, G. Improvement of 2-Phenylethanol Production in Saccharomyces cerevisiae by Evolutionary and Rational Metabolic Engineering. PLoS ONE 2021, 16, e0258180. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, Ecology and Industrial Applications of Aroma Formation in Yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef] [Green Version]
- Hassing, E.-J.; de Groot, P.A.; Marquenie, V.R.; Pronk, J.; Daran, J. Connecting Central Carbon and Aromatic Amino Acid Metabolisms to Improve de Novo 2-Phenylethanol Production in Saccharomyces cerevisiae. Metab. Eng. 2019, 56, 165–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, Z.; Qin, Y.; Ye, D.-Q.; Song, Y.-Y.; Liu, Y.-L. Efficient Display of Aspergillus niger β-Glucosidase on Saccharomyces Cerevisiae Cell Wall for Aroma Enhancement in Wine. J. Agric. Food Chem. 2019, 67, 5169–5176. [Google Scholar] [CrossRef]
- Lambrechts, M.; Pretorius, I. Yeast and Its Importance to Wine Aroma. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.; Buica, A. Comparison of an Offline SPE-GC-MS and Online HS-SPME-GC-MS Method for the Analysis of Volatile Terpenoids in Wine. Molecules 2020, 25, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mele, M.A.; Kang, H.-M.; Lee, Y.-T.; Islam, M.Z. Grape Terpenoids: Flavor Importance, Genetic Regulation, and Future Potential. Crit. Rev. Food Sci. Nutr. 2021, 61, 1429–1447. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.R. 31-Terpenoids in Beer. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 327–332. ISBN 978-0-12-373891-2. [Google Scholar]
- Bauer, K. Common Fragrance and Flavor Materials: Preparation, Properties, and Uses; Vch Pub: New York, NY, USA, 1990; ISBN 978-0-89573-919-3. [Google Scholar]
- Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast. Pharmaceuticals 2021, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and Biological Function of Volatile Esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstrepen, K.J.; Van Laere, S.D.M.; Vanderhaegen, B.M.P.; Derdelinckx, G.; Dufour, J.-P.; Pretorius, I.S.; Winderickx, J.; Thevelein, J.M.; Delvaux, F.R. Expression Levels of the Yeast Alcohol Acetyltransferase Genes ATF1, Lg-ATF1, and ATF2 Control the Formation of a Broad Range of Volatile Esters. Appl. Environ. Microbiol. 2003, 69, 5228–5237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.; Jin, G.-J.; Xu, Y.-H.; Xue, S.-J.; Qiao, S.-J.; Teng, Y.-X.; Tao, Y.-S. Enhancing Wine Ester Biosynthesis in Mixed Hanseniaspora uvarum/Saccharomyces cerevisiae Fermentation by Nitrogen Nutrient Addition. Food Res. Int. 2019, 123, 559–566. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical Study of Aromatic Series in Sherry Wines Subjected to Biological Aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Saerens, S.M.G.; Pretorius, I.S. Novel Yeast Strains as Tools for Adjusting the Flavor of Fermented Beverages to Market Specifications. In Biotechnology in Flavor Production; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 62–132. ISBN 978-1-118-35405-6. [Google Scholar]
- Villena, M.A.; Iranzo, J.F.Ú.; Pérez, A.I.B. β-Glucosidase Activity in Wine Yeasts: Application in Enology. Enzyme Microb. Technol. 2007, 40, 420–425. [Google Scholar] [CrossRef]
- Fernandes, T.; Silva-Sousa, F.; Pereira, F.; Rito, T.; Soares, P.; Franco-Duarte, R.; Sousa, M.J. Biotechnological Importance of Torulaspora delbrueckii: From the Obscurity to the Spotlight. J. Fungi 2021, 7, 712. [Google Scholar] [CrossRef]
- Liu, S.; Pilone, G. An Overview of Formation and Role of Acetaldehyde in Wine Making with Emphasis on Microbiological Implications. Int. J. Food Sci. Technol. 2001, 35, 49–61. [Google Scholar] [CrossRef]
- Li, E.; de Orduña Heidinger, R.M. Acetaldehyde Metabolism in Industrial Strains of Saccharomyces cerevisiae Inhibited by SO2 and Cooling during Alcoholic Fermentation. OENO One 2020, 54, 351–358. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Picca Nicolino, M.; Voordeckers, K.; Verstrepen, K.J. Improving Industrial Yeast Strains: Exploiting Natural and Artificial Diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuller, D.; Casal, M. The Use of Genetically Modified Saccharomyces cerevisiae Strains in the Wine Industry. Appl. Microbiol. Biotechnol. 2005, 68, 292–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragosits, M.; Mattanovich, D. Adaptive Laboratory Evolution–Principles and Applications for Biotechnology. Microb. Cell Factories 2013, 12, 64. [Google Scholar] [CrossRef] [Green Version]
- Deparis, Q.; Claes, A.; Foulquie-Moreno, M.; Thevelein, J. Engineering Tolerance to Industrially Relevant Stress Factors in Yeast Cell Factories. FEMS Yeast Res. 2017, 17, fox036. [Google Scholar] [CrossRef]
- Gibson, B.; Dahabieh, M.; Krogerus, K.; Jouhten, P.; Magalhães, F.; Pereira, R.; Siewers, V.; Vidgren, V. Adaptive Laboratory Evolution of Ale and Lager Yeasts for Improved Brewing Efficiency and Beer Quality. Annu. Rev. Food Sci. Technol. 2020, 11, 23–44. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Semumu, T.; Gamero, A. Non-Conventional Yeasts as Alternatives in Modern Baking for Improved Performance and Aroma Enhancement. Fermentation 2021, 7, 102. [Google Scholar] [CrossRef]
- Butler, D.K.; Yasuda, L.E.; Yao, M.-C. Induction of Large DNA Palindrome Formation in Yeast: Implications for Gene Amplification and Genome Stability in Eukaryotes. Cell 1996, 87, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Sauer, U. Evolutionary Engineering of Industrially Important Microbial Phenotypes. Adv. Biochem. Eng. Biotechnol. 2001, 73, 129–169. [Google Scholar] [CrossRef]
- Demain, A.L.; Vandamme, E.J.; Collins, J.; Buchholz, K. History of Industrial Biotechnology. In Industrial Biotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–84. ISBN 978-3-527-80779-6. [Google Scholar]
- Darwin, C.; Murray, J.; William Clowes and Sons; Evans, B. On the Origin of Species by Means of Natural Selection, or, The Preservation of Favoured Races in the Struggle for Life; John Murray, Albemarle Street: London, UK, 1859; pp. 1–564. [Google Scholar]
- Mavrommati, M.; Daskalaki, A.; Papanikolaou, S.; Aggelis, G. Adaptive Laboratory Evolution Principles and Applications in Industrial Biotechnology. Biotechnol. Adv. 2022, 54, 107795. [Google Scholar] [CrossRef]
- Elena, S.F.; Lenski, R.E. Evolution Experiments with Microorganisms: The Dynamics and Genetic Bases of Adaptation. Nat. Rev. Genet. 2003, 4, 457–469. [Google Scholar] [CrossRef] [PubMed]
- de Melo, A.H.F.; Lopes, A.M.M.; Dezotti, N.; Santos, I.L.; Teixeira, G.S.; Goldbeck, R. Evolutionary Engineering of Two Robust Brazilian Industrial Yeast Strains for Thermotolerance and Second-Generation Biofuels. Ind. Biotechnol. 2020, 16, 91–98. [Google Scholar] [CrossRef]
- López-Malo, M.; García-Rios, E.; Melgar, B.; Sanchez, M.R.; Dunham, M.J.; Guillamón, J.M. Evolutionary Engineering of a Wine Yeast Strain Revealed a Key Role of Inositol and Mannoprotein Metabolism during Low-Temperature Fermentation. BMC Genomics 2015, 16, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teunissen, A.; Dumortier, F.; Gorwa, M.-F.; Bauer, J.; Tanghe, A.; Loïez, A.; Smet, P.; Van Dijck, P.; Thevelein, J.M. Isolation and Characterization of a Freeze-Tolerant Diploid Derivative of an Industrial Baker’s Yeast Strain and Its Use in Frozen Doughs. Appl. Environ. Microbiol. 2002, 68, 4780–4787. [Google Scholar] [CrossRef] [Green Version]
- Aslankoohi, E.; Herrera-Malaver, B.; Rezaei, M.N.; Steensels, J.; Courtin, C.M.; Verstrepen, K.J. Non-Conventional Yeast Strains Increase the Aroma Complexity of Bread. PLoS ONE 2016, 11, e0165126. [Google Scholar] [CrossRef] [Green Version]
- Lahue, C.; Madden, A.A.; Dunn, R.R.; Smukowski Heil, C. History and Domestication of Saccharomyces cerevisiae in Bread Baking. Front. Genet. 2020, 11, 584718. [Google Scholar] [CrossRef]
- Palla, M.; Blandino, M.; Grassi, A.; Giordano, D.; Sgherri, C.; Quartacci, M.F.; Reyneri, A.; Agnolucci, M.; Giovannetti, M. Characterization and Selection of Functional Yeast Strains during Sourdough Fermentation of Different Cereal Wholegrain Flours. Sci. Rep. 2020, 10, 12856. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhu, K.; Miao, L.; Rong, L.; Zhao, Y.; Li, S.; Ma, L.; Li, J.; Zhang, C.; Xiao, D.; et al. Simultaneous Improvement of Limonene Production and Tolerance in Yarrowia Lipolytica through Tolerance Engineering and Evolutionary Engineering. ACS Synth. Biol. 2021, 10, 884–896. [Google Scholar] [CrossRef]
- Strucko, T.; Zirngibl, K.; Pereira, F.; Kafkia, E.; Mohamed, E.T.; Rettel, M.; Stein, F.; Feist, A.M.; Jouhten, P.; Patil, K.R.; et al. Laboratory Evolution Reveals Regulatory and Metabolic Trade-Offs of Glycerol Utilization in Saccharomyces cerevisiae. Metab. Eng. 2018, 47, 73–82. [Google Scholar] [CrossRef]
- Swamy, K.B.S.; Zhou, N. Experimental Evolution: Its Principles and Applications in Developing Stress-Tolerant Yeasts. Appl. Microbiol. Biotechnol. 2019, 103, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, T.W.; Jin, Y.-S. Metabolic Engineering for Improved Fermentation of Pentoses by Yeasts. Appl. Microbiol. Biotechnol. 2004, 63, 495–509. [Google Scholar] [CrossRef]
- Tian, T.; Wu, D.; Ng, C.-T.; Yang, H.; Sun, J.; Liu, J.; Lu, J. A Multiple-Step Strategy for Screening Saccharomyces cerevisiae Strains with Improved Acid Tolerance and Aroma Profiles. Appl. Microbiol. Biotechnol. 2020, 104, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- González-Ramos, D.; Gorter de Vries, A.R.; Grijseels, S.S.; van Berkum, M.C.; Swinnen, S.; van den Broek, M.; Nevoigt, E.; Daran, J.-M.G.; Pronk, J.T.; van Maris, A.J.A. A New Laboratory Evolution Approach to Select for Constitutive Acetic Acid Tolerance in Saccharomyces cerevisiae and Identification of Causal Mutations. Biotechnol. Biofuels 2016, 9, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Niu, C.; Liu, C.; Wang, J.; Zheng, F.; Li, Q. Screening Lager Yeast with Higher Ethyl-Acetate Production by Adaptive Laboratory Evolution in High Concentration of Acetic Acid. World J. Microbiol. Biotechnol. 2021, 37, 125. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Y. Adaptive Evolution of Saccharomyces cerevisiae with Enhanced Ethanol Tolerance for Chinese Rice Wine Fermentation. Appl. Biochem. Biotechnol. 2014, 173, 1940–1954. [Google Scholar] [CrossRef] [PubMed]
- Catrileo, D.; Acuña-Fontecilla, A.; Godoy, L. Adaptive Laboratory Evolution of Native Torulaspora delbrueckii YCPUC10 With Enhanced Ethanol Resistance and Evaluation in Co-Inoculated Fermentation. Front. Microbiol. 2020, 11, 595023. [Google Scholar] [CrossRef]
- dos Santos, J.E.; Oliveira, T.F.; de Freitas, F.F.; Silva, M.C.S.; Castiglioni, G.L. Adaptive Evolution of Saccharomyces cerevisiae and Its Application in Co-Culture with Saccharomyces kudriavzevii in the Production of Fermented Myrciaria jaboticaba. Res. Soc. Dev. 2021, 10, e52010212525. [Google Scholar] [CrossRef]
- da Silveira, F.A.; de Oliveira Soares, D.L.; Bang, K.W.; Balbino, T.R.; de Moura Ferreira, M.A.; Diniz, R.H.S.; de Lima, L.A.; Brandão, M.M.; Villas-Bôas, S.G.; da Silveira, W.B. Assessment of Ethanol Tolerance of Kluyveromyces marxianus CCT 7735 Selected by Adaptive Laboratory Evolution. Appl. Microbiol. Biotechnol. 2020, 104, 7483–7494. [Google Scholar] [CrossRef]
- Tilloy, V.; Ortiz-Julien, A.; Dequin, S. Reduction of Ethanol Yield and Improvement of Glycerol Formation by Adaptive Evolution of the Wine Yeast Saccharomyces cerevisiae under Hyperosmotic Conditions. Appl. Environ. Microbiol. 2014, 80, 2623–2632. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-C.; Rao, J.-W.; Meng, F.-B.; Wang, Z.-W.; Liu, D.-Y.; Yu, H. Combination of Mutagenesis and Adaptive Evolution to Engineer Salt-Tolerant and Aroma-Producing Yeast for Soy Sauce Fermentation. J. Sci. Food Agric. 2021, 101, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, J.; Rautio, J.; Mattinen, L.; Vidgren, V.; Londesborough, J.; Gibson, B.R. Adaptive Evolution of the Lager Brewing Yeast Saccharomyces pastorianus for Improved Growth under Hyperosmotic Conditions and Its Influence on Fermentation Performance. FEMS Yeast Res. 2013, 13, 335–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tekarslan-Sahin, S.H.; Alkim, C.; Sezgin, T. Physiological and Transcriptomic Analysis of a Salt-Resistant Saccharomyces cerevisiae Mutant Obtained by Evolutionary Engineering. Bosn. J. Basic Med. Sci. 2018, 18, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Luo, W.; Wu, X.-M.; Fan, J.; Zhang, W.-X.; Suyama, T. Improving RNA Content of Salt-Tolerant Zygosaccharomyces rouxii by Atmospheric and Room Temperature Plasma (ARTP) Mutagenesis and Its Application in Soy Sauce Brewing. World J. Microbiol. Biotechnol. 2019, 35, 180. [Google Scholar] [CrossRef] [PubMed]
- Kutyna, D.R.; Varela, C.; Stanley, G.A.; Borneman, A.R.; Henschke, P.A.; Chambers, P.J. Adaptive Evolution of Saccharomyces cerevisiae to Generate Strains with Enhanced Glycerol Production. Appl. Microbiol. Biotechnol. 2012, 93, 1175–1184. [Google Scholar] [CrossRef]
- Caspeta, L.; Coronel, J.; Montes de Oca, A.; Abarca, E.; González, L.; Martínez, A. Engineering High-Gravity Fermentations for Ethanol Production at Elevated Temperature with Saccharomyces cerevisiae. Biotechnol. Bioeng. 2019, 116, 2587–2597. [Google Scholar] [CrossRef]
- García-Ríos, E.; Lairón-Peris, M.; Muñiz-Calvo, S.; Heras, J.M.; Ortiz-Julien, A.; Poirot, P.; Rozès, N.; Querol, A.; Guillamón, J.M. Thermo-Adaptive Evolution to Generate Improved Saccharomyces cerevisiae Strains for Cocoa Pulp Fermentations. Int. J. Food Microbiol. 2021, 342, 109077. [Google Scholar] [CrossRef]
- Dixon, T.A.; Williams, T.C.; Pretorius, I.S. Bioinformational Trends in Grape and Wine Biotechnology. Trends Biotechnol. 2022, 40, 124–135. [Google Scholar] [CrossRef]
- Altay, F.; Karbancıoglu-Güler, F.; Daskaya-Dikmen, C.; Heperkan, D. A Review on Traditional Turkish Fermented Non-Alcoholic Beverages: Microbiota, Fermentation Process and Quality Characteristics. Int. J. Food Microbiol. 2013, 167, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Varela, C.; Dry, P.R.; Kutyna, D.R.; Francis, I.L.; Henschke, P.A.; Curtin, C.D.; Chambers, P.J. Strategies for Reducing Alcohol Concentration in Wine. Aust. J. Grape Wine Res. 2015, 21, 670–679. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and Its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Weckx, S. The Cocoa Bean Fermentation Process: From Ecosystem Analysis to Starter Culture Development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products—An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J. State-of-the-Art Chocolate Manufacture: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1313–1344. [Google Scholar] [CrossRef] [Green Version]
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts Are Essential for Cocoa Bean Fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. The Effect of Lactic Acid Bacteria on Cocoa Bean Fermentation. Int. J. Food Microbiol. 2015, 205, 54–67. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Fleet, G.H.; Zhao, J. Unravelling the Contribution of Lactic Acid Bacteria and Acetic Acid Bacteria to Cocoa Fermentation Using Inoculated Organisms. Int. J. Food Microbiol. 2018, 279, 43–56. [Google Scholar] [CrossRef]
- Coskun, F. A Traditional Turkish Fermented Non-Alcoholic Beverage, “Shalgam”. Beverages 2017, 3, 49. [Google Scholar] [CrossRef] [Green Version]
- Aila, R.; Alim, A.; Mahemuti, A.; Kelimu, A. Separation, Purification and Identification of Excellent Yeasts from the Natural Fermented Beverage of Boza. J. Food Nutr. Res. 2020, 8, 450–458. [Google Scholar] [CrossRef]
- Tang, H.; Ma, H.; Hou, Q.; Li, W.; Xu, H.; Liu, W.; Sun, Z.; Haobisi, H.; Menghe, B. Profiling of Koumiss Microbiota and Organic Acids and Their Effects on Koumiss Taste. BMC Microbiol. 2020, 20, 85. [Google Scholar] [CrossRef]
- Hancioğlu, O.; Karapinar, M. Microflora of Boza, a Traditional Fermented Turkish Beverage. Int. J. Food Microbiol. 1997, 35, 271–274. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.; Wyffels, J.T.; Seydim, A.C.; Greene, A.K. Turkish Kefir and Kefir Grains: Microbial Enumeration and Electron Microscobic Observation†. Int. J. Dairy Technol. 2005, 58, 25–29. [Google Scholar] [CrossRef]
- Magalhães, K.T.; de Melo Pereira, G.V.; Campos, C.R.; Dragone, G.; Schwan, R.F. Brazilian Kefir: Structure, Microbial Communities and Chemical Composition. Braz. J. Microbiol. 2011, 42, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Afzaal, M.; Saeed, F.; Anjum, F.; Waris, N.; Husaain, M.; Ikram, A.; Ateeq, H.; Muhammad Anjum, F.; Suleria, H. Nutritional and Ethnomedicinal Scenario of Koumiss: A Concurrent Review. Food Sci. Nutr. 2021, 9, 6421–6428. [Google Scholar] [CrossRef] [PubMed]
- Blieck, L.; Toye, G.; Dumortier, F.; Verstrepen, K.J.; Delvaux, F.R.; Thevelein, J.M.; Van Dijck, P. Isolation and Characterization of Brewer’s Yeast Variants with Improved Fermentation Performance under High-Gravity Conditions. Appl. Environ. Microbiol. 2007, 73, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Huuskonen, A.; Markkula, T.; Vidgren, V.; Lima, L.; Mulder, L.; Geurts, W.; Walsh, M.; Londesborough, J. Selection from Industrial Lager Yeast Strains of Variants with Improved Fermentation Performance in Very-High-Gravity Worts. Appl. Environ. Microbiol. 2010, 76, 1563–1573. [Google Scholar] [CrossRef] [Green Version]
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s Balancing Act between Ethanol and Glycerol Production in Low-Alcohol Wines. Microb. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Tilloy, V.; Cadière, A.; Ehsani, M.; Dequin, S. Reducing Alcohol Levels in Wines through Rational and Evolutionary Engineering of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2015, 213, 49–58. [Google Scholar] [CrossRef]
- Cadière, A.; Aguera, E.; Caillé, S.; Ortiz-Julien, A.; Dequin, S. Pilot-Scale Evaluation the Enological Traits of a Novel, Aromatic Wine Yeast Strain Obtained by Adaptive Evolution. Food Microbiol. 2012, 32, 332–337. [Google Scholar] [CrossRef]
- Angelov, A.I.; Karadjov, G.I.; Roshkova, Z.G. Strains Selection of Baker’s Yeast with Improved Technological Properties. Food Res. Int. 1996, 29, 235–239. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).