Integrated Bioprocess for Cellulosic Ethanol Production from Wheat Straw: New Ternary Deep-Eutectic-Solvent Pretreatment, Enzymatic Saccharification, and Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DES Pretreatment of WS
2.3. Enzymatic Saccharification and Fermentation
2.4. Analytical Methods
3. Results and Discussion
3.1. The Properties of DES
3.2. DES Pretreatment of WS at Different Temperatures
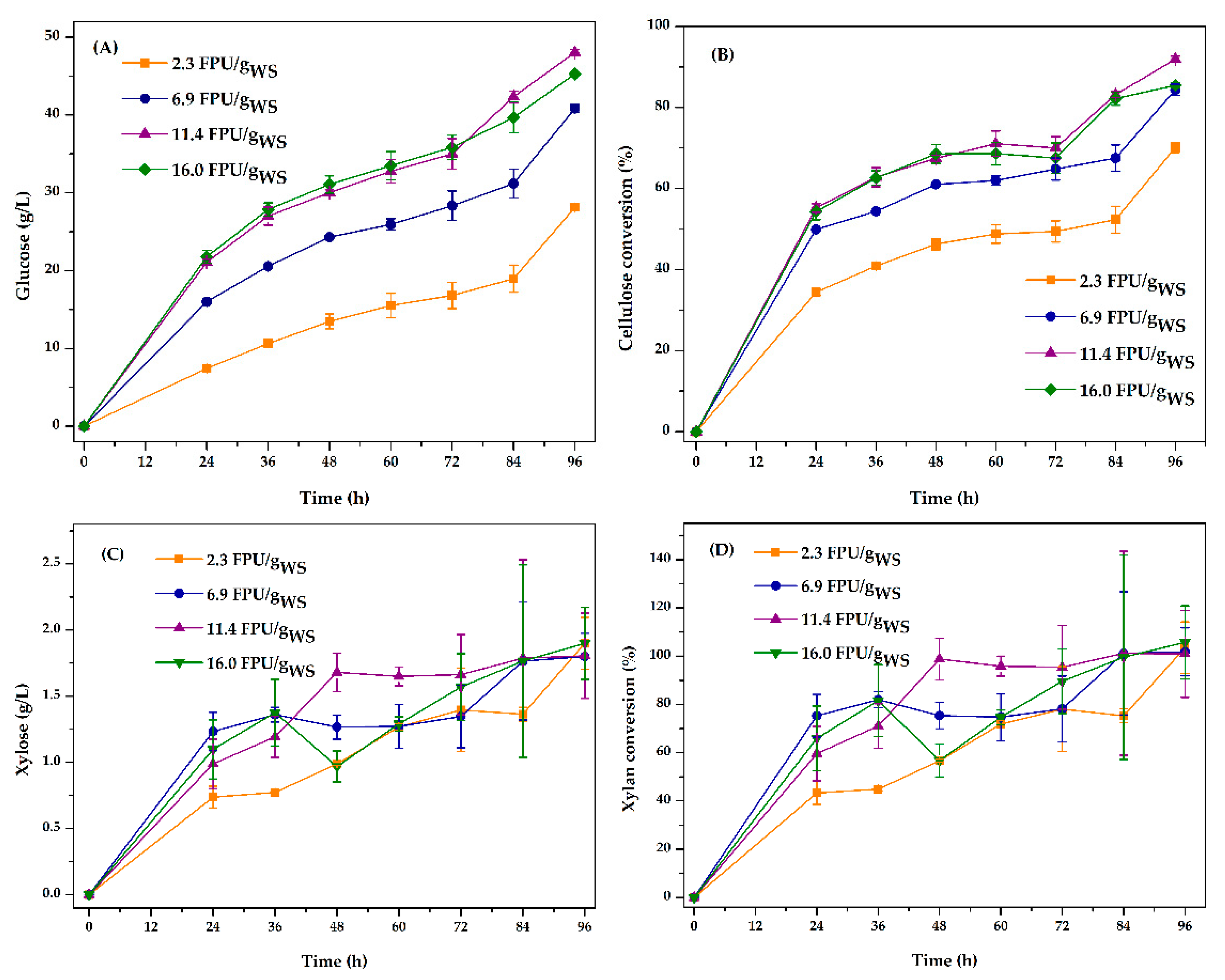
3.3. Optimization of Enzyme Loading
3.4. Optimization Analysis of Ethanol Fermentation Process
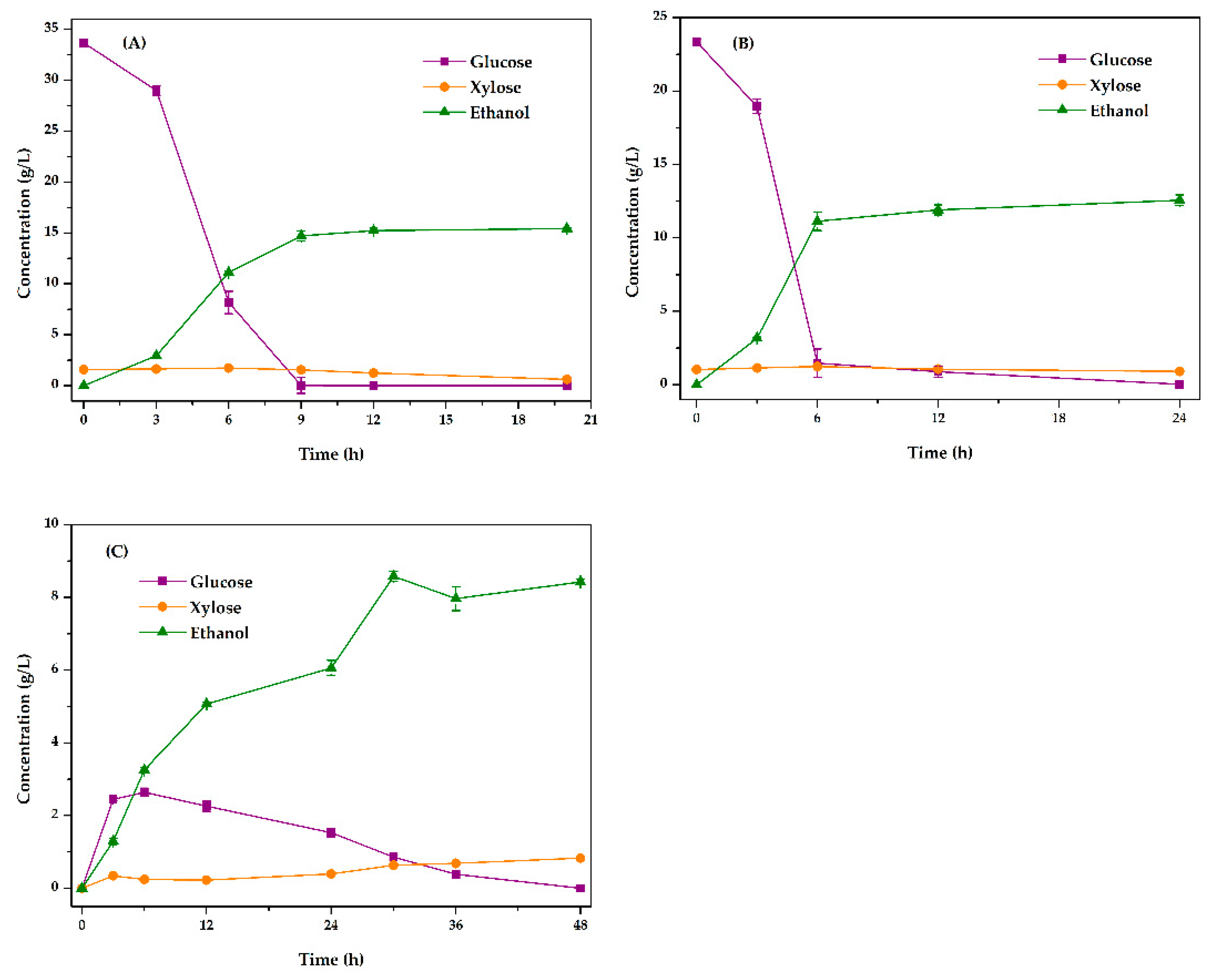
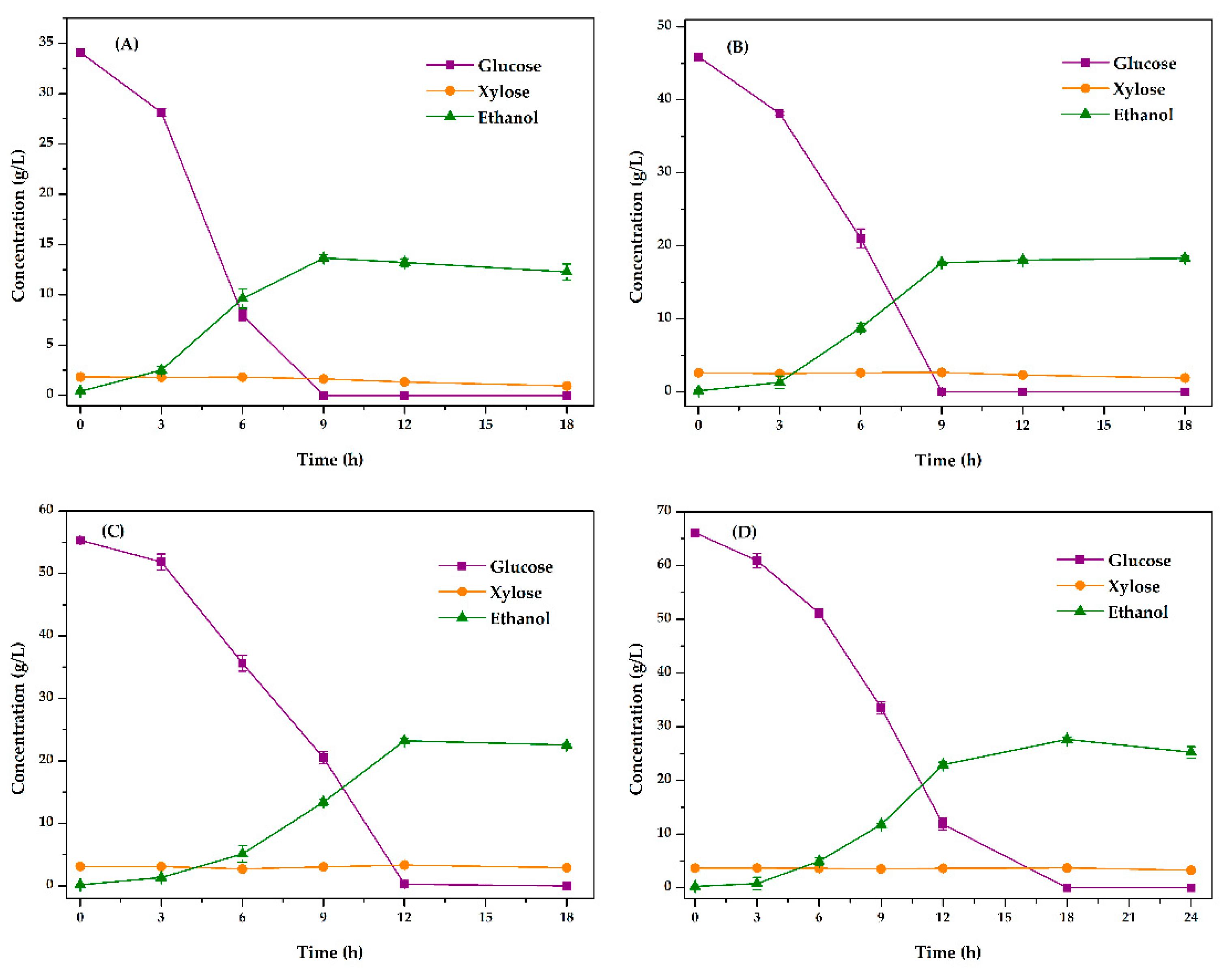
3.4.1. Optimization of Fermentation Method
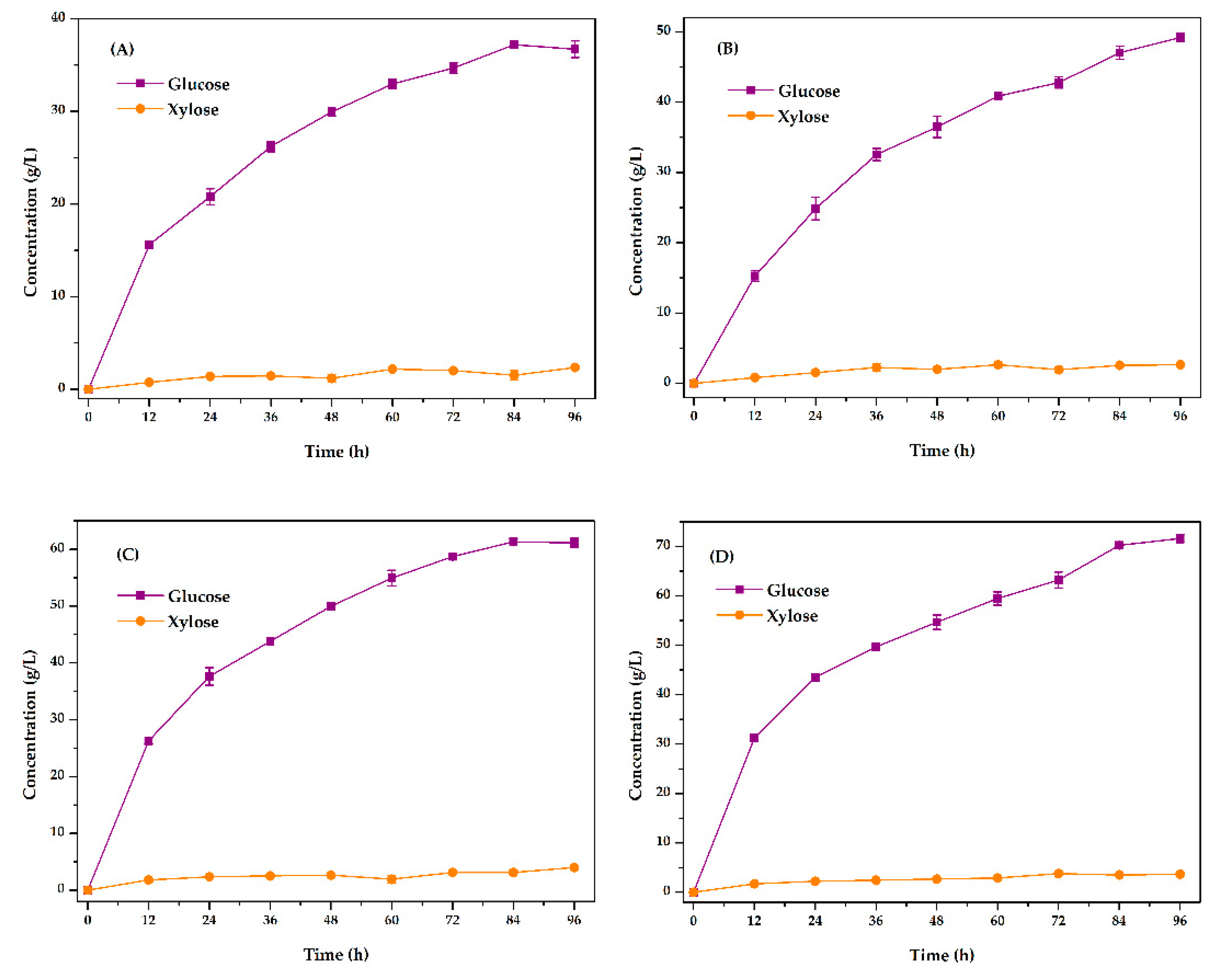
3.4.2. Optimization of Fermentation Substrate Content Conditions
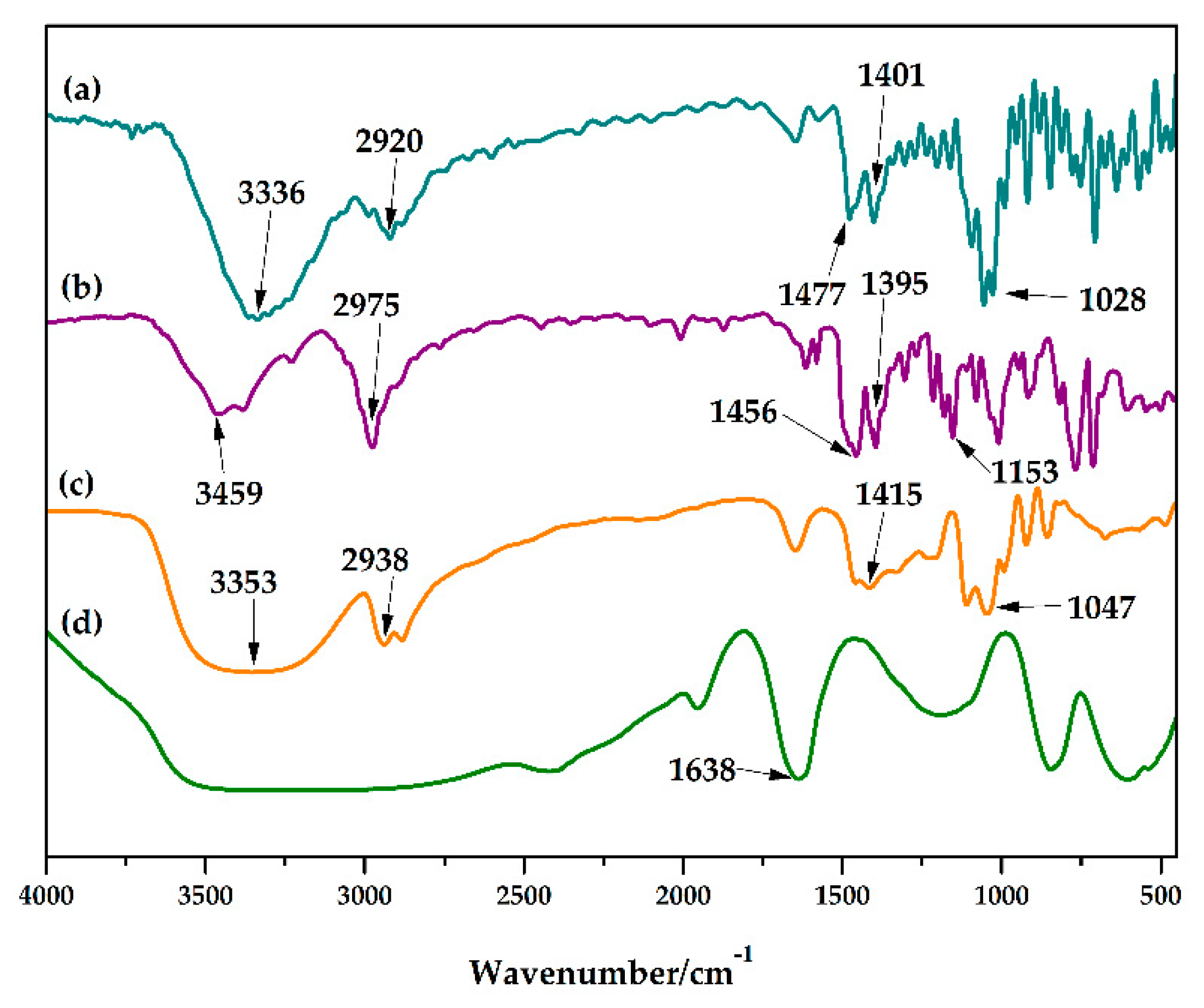
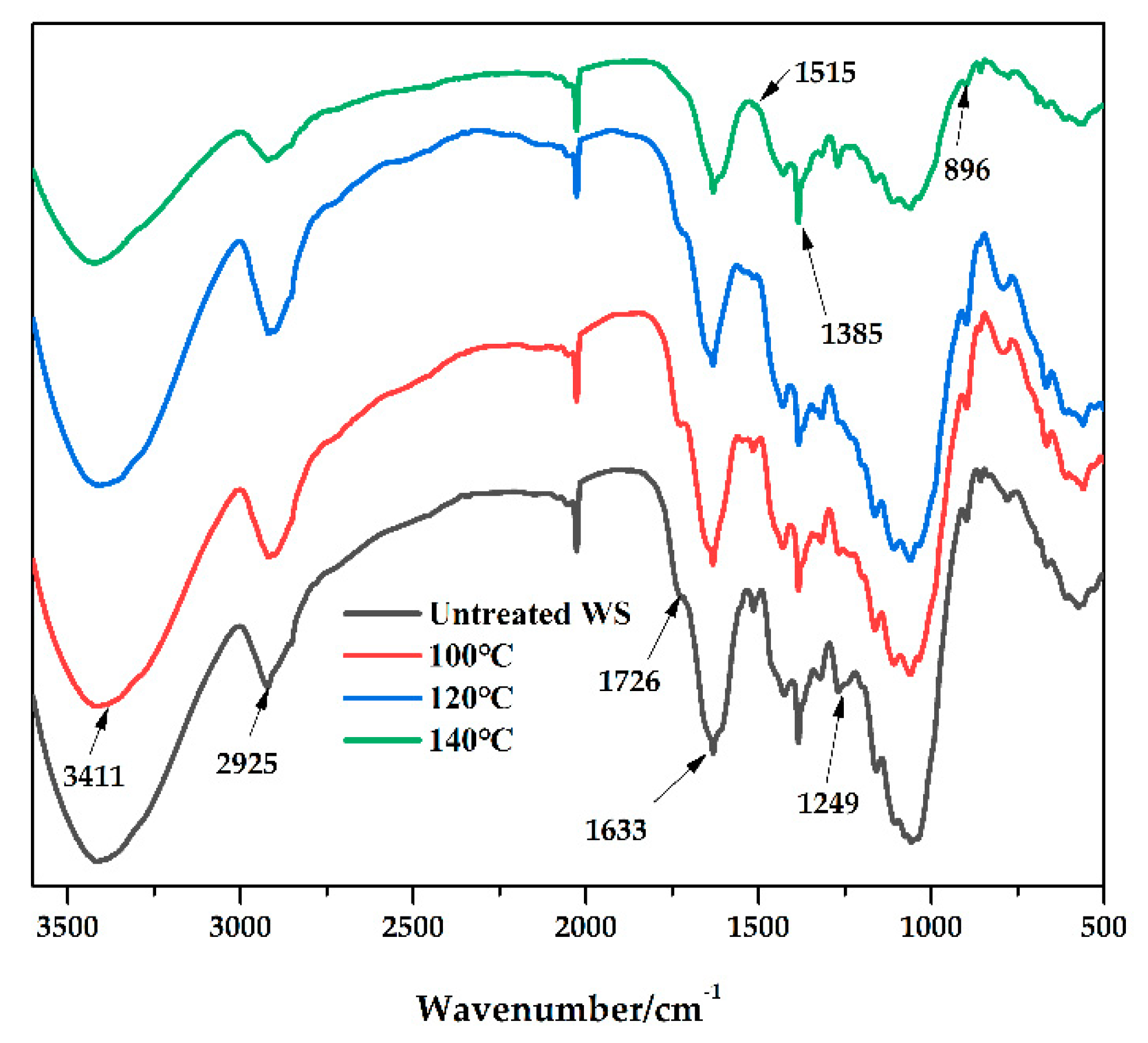
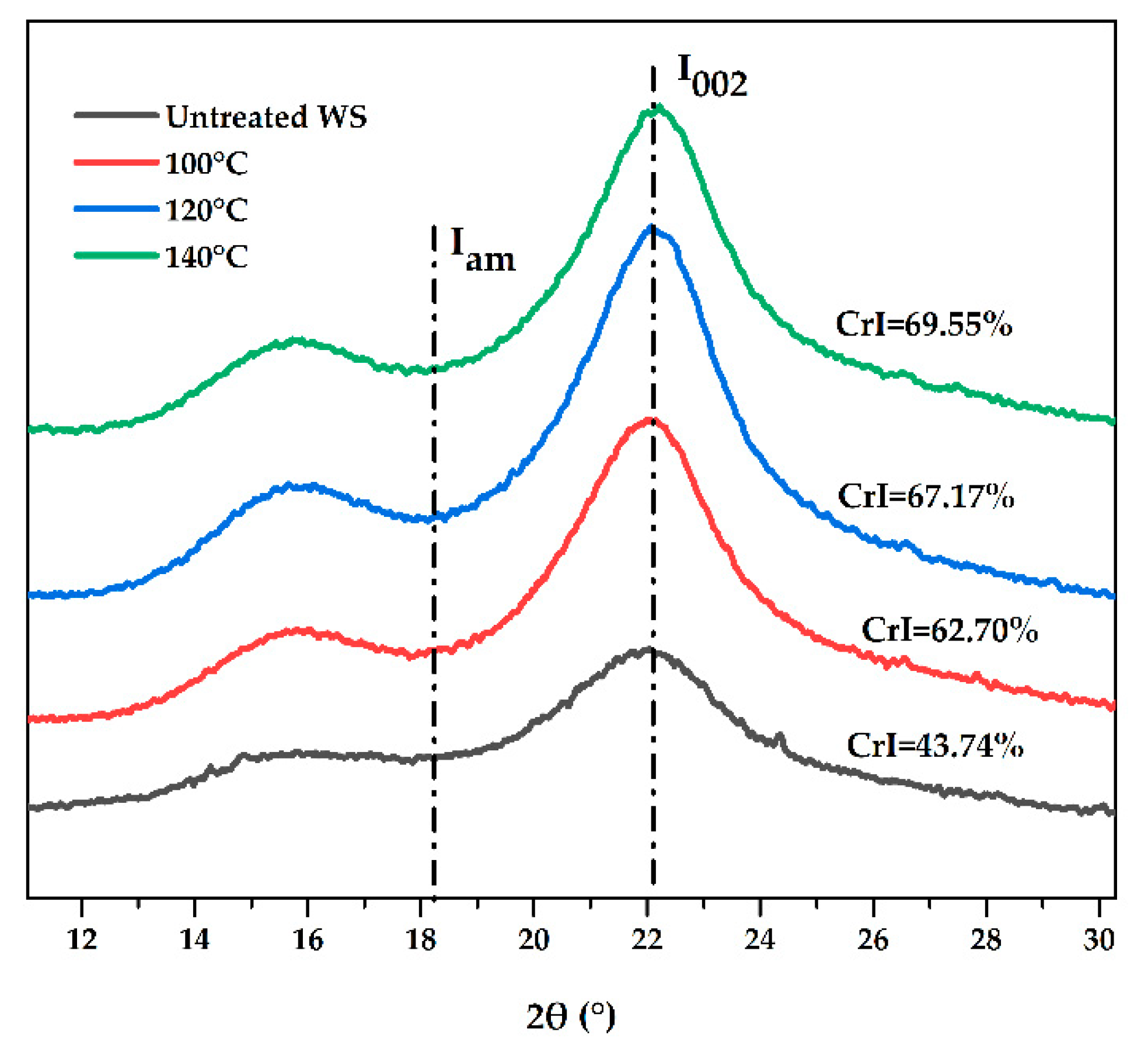
3.5. Characterization of WS before and after Pretreatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Carbon catabolite repression occurrence in photo fermentation of ethanol-rich substrates. J. Environ. Manag. 2021, 297, 113371. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.; Karimi, K. Bioethanol: Market and Production Processes. In Biofuels Refining and Perfomance; McGraw-Hill: New York, NY, USA, 2008; pp. 69–106. [Google Scholar]
- Mączyńska, J.; Krzywonos, M.; Kupczyk, A.; Tucki, K.; Sikora, M.; Pińkowska, H.; Bączyk, A.; Wielewska, I. Production and use of biofuels for transport in Poland and Brazil—The case of bioethanol. Fuel 2019, 241, 989–996. [Google Scholar] [CrossRef]
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Enhancing photo fermentative hydrogen production using ethanol rich dark fermentation effluents. Int. J. Hydrogen Energy 2022, 47, 117–126. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Yang, M.; Singh, S.; Cheng, G. Transforming lignocellulosic biomass into biofuels enabled by ionic liquid pretreatment. Bioresour. Technol. 2021, 322, 124522. [Google Scholar] [CrossRef]
- Nghiem, N.; O’Connor, J.; Hums, M. Integrated Process for Extraction of Wax as a Value-Added Co-Product and Improved Ethanol Production by Converting Both Starch and Cellulosic Components in Sorghum Grains. Fermentation 2018, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Mabee, W.E.; McFarlane, P.N.; Saddler, J.N. Biomass availability for lignocellulosic ethanol production. Biomass. Bioenerg. 2011, 35, 4519–4529. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Bior. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Norvell, K.; Nghiem, N. Soaking in Aqueous Ammonia (SAA) Pretreatment of Whole Corn Kernels for Cellulosic Ethanol Production from the Fiber Fractions. Fermentation 2018, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Huang, Y.; Yuan, H.; Yin, X.; Wu, C. Life cycle assessment of biofuels in China: Status and challenges. Renew. Sustain. Energ. Rev. 2018, 97, 301–322. [Google Scholar] [CrossRef]
- Ruiz-Duenas, F.J.; Martinez, A.T. Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Liu, Y.; Zheng, X.; Qureshi, N. High-efficient cellulosic butanol production from deep eutectic solvent pretreated corn stover without detoxification. Ind. Crops Prod. 2021, 162, 113258. [Google Scholar] [CrossRef]
- Lynd, L.R. The grand challenge of cellulosic biofuels. Nat. Biotechnol. 2017, 35, 912–915. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Wen, J.; Mei, Q.-Q.; Chen, X.; Sun, D.; Yuan, T.; Sun, R. Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Thuy Lan Chi, N. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef]
- Foglia, F.; Drapcho, C.; Nghiem, J. Effectiveness of Tannin Removal by Alkaline Pretreatment on Sorghum Ethanol Production. Fermentation 2022, 8, 274. [Google Scholar] [CrossRef]
- Fillat, Ú.; Ibarra, D.; Eugenio, M.; Moreno, A.; Tomás-Pejó, E.; Martín-Sampedro, R. Laccases as a Potential Tool for the Efficient Conversion of Lignocellulosic Biomass: A Review. Fermentation 2017, 3, 17. [Google Scholar] [CrossRef]
- Cuevas, M.; García Martín, J.F.; Bravo, V.; Sánchez, S. Ethanol Production from Olive Stones through Liquid Hot Water Pre-Treatment, Enzymatic Hydrolysis and Fermentation. Influence of Enzyme Loading, and Pre-Treatment Temperature and Time. Fermentation 2021, 7, 25. [Google Scholar] [CrossRef]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass. Bioenerg. 2017, 100, 10–16. [Google Scholar] [CrossRef]
- Qi, G.; Xiong, L.; Tian, L.; Luo, M.; Chen, X.; Huang, C.; Li, H.; Chen, X. Ammonium sulfite pretreatment of wheat straw for efficient enzymatic saccharification. Sustain. Energy. Technol. 2018, 29, 12–18. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Vo, D.N. Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour. Technol. 2022, 344, 126203. [Google Scholar] [CrossRef]
- Le, T.D.T.; Nguyen Truong, V.P.; Ngo, M.T.T.; Kim, T.H.; Oh, K.K. Pretreatment of Corn Stover Using an Extremely Low-Liquid Ammonia (ELLA) Method for the Effective Utilization of Sugars in Simultaneous Saccharification and Fermentation (SSF) of Ethanol. Fermentation 2021, 7, 191. [Google Scholar] [CrossRef]
- Brandt, A.; Ray, M.J.; To, T.Q.; Leak, D.J.; Murphy, R.J.; Welton, T. Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid–water mixtures. Green Chem. 2011, 13, 2489. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J. Pretreatment of biomass using ionic liquids: Research updates. Renew. Energy 2017, 111, 77–84. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Application of deep eutectic solvents in biomass pretreatment and conversion. Green Energy Environ. 2019, 4, 95–115. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Kim, K.H.; Dutta, T.; Sun, J.; Simmons, B.; Singh, S. Biomass pretreatment using deep eutectic solvents from lignin derived phenols. Green Chem. 2018, 20, 809–815. [Google Scholar] [CrossRef]
- Shen, X.; Chen, T.; Wang, H.; Mei, Q.; Yue, F.; Sun, S.; Wen, J.; Yuan, T.; Sun, R. Structural and Morphological Transformations of Lignin Macromolecules during Bio-Based Deep Eutectic Solvent (DES) Pretreatment. ACS Sustain. Chem. Eng. 2019, 8, 2130–2137. [Google Scholar] [CrossRef]
- Chen, Z.; Ragauskas, A.; Wan, C. Lignin extraction and upgrading using deep eutectic solvents. Ind. Crops Prod. 2020, 147, 112241. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Liu, Y.; Su, Z.; Li, B.; Gao, C.; Tian, W. Comprehensive analysis of important parameters of choline chloride-based deep eutectic solvent pretreatment of lignocellulosic biomass. Bioresour. Technol. 2021, 319, 124209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Ali, M.F.; Abdeltawab, A.A.; Yakout, S.M.; Yu, G. Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis. Bioresour. Technol. 2018, 263, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, Q.; You, T.; Zhang, X.; Xu, F.; Wu, Y. Short-time deep eutectic solvent pretreatment for enhanced enzymatic saccharification and lignin valorization. Green Chem. 2019, 21, 3099–3108. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Xiao, J.; He, X.; Zhang, K.; Yuan, S.; Peng, Z.; Chen, Z.; Lin, X. Enhanced Enzymatic Hydrolysis and Lignin Extraction of Wheat Straw by Triethylbenzyl Ammonium Chloride/Lactic Acid-Based Deep Eutectic Solvent Pretreatment. ACS Omega 2019, 4, 19829–19839. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Jérôme, F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010, 12, 1127. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Fang, C.; Thomsen, M.H.; Frankær, C.G.; Brudecki, G.P.; Schmidt, J.E.; AlNashef, I.M. Reviving Pretreatment Effectiveness of Deep Eutectic Solvents on Lignocellulosic Date Palm Residues by Prior Recalcitrance Reduction. Ind. Eng. Chem. Res. 2017, 56, 3167–3174. [Google Scholar] [CrossRef]
- Wang, Z.K.; Li, H.; Lin, X.C.; Tang, L.; Chen, J.J.; Mo, J.W.; Yu, R.S.; Shen, X.J. Novel recyclable deep eutectic solvent boost biomass pretreatment for enzymatic hydrolysis. Bioresour. Technol. 2020, 307, 123237. [Google Scholar] [CrossRef]
- Ci, Y.; Yu, F.; Zhou, C.; Mo, H.; Li, Z.; Ma, Y.; Zang, L. New ternary deep eutectic solvents for effective wheat straw deconstruction into its high-value utilization under near-neutral conditions. Green Chem. 2020, 22, 8713–8720. [Google Scholar] [CrossRef]
- Wang, Z.K.; Shen, X.J.; Chen, J.J.; Jiang, Y.Q.; Hu, Z.Y.; Wang, X.; Liu, L. Lignocellulose fractionation into furfural and glucose by AlCl3-catalyzed DES/MIBK biphasic pretreatment. Int. J. Biol. Macromol. 2018, 117, 721–726. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of structural carbohydrates and lignin in biomass national renewable. Energy Lab. 2011, 10, 1–15. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Saputra, R.; Walvekar, R.; Khalid, M.; Mubarak, N.M. Synthesis and thermophysical properties of ethylammonium chloride-glycerol-ZnCl2 ternary deep eutectic solvent. J. Mol. Liq. 2020, 310, 113232. [Google Scholar] [CrossRef]
- Paveglio, G.; Milani, F.; Sauer, A.; Roman, D.; Meyer, A.; Pizzuti, L. Structure-Physical Properties Relationship of Eutectic Solvents Prepared from Benzyltriethylammonium Chloride and Carboxylic Acids. J. Brazil. Chem. Soc. 2021, 32, 542–551. [Google Scholar] [CrossRef]
- Jablonsky, M.; Haz, A.; Majova, V. Assessing the opportunities for applying deep eutectic solvents for fractionation of beech wood and wheat straw. Cellulose 2019, 26, 7675–7684. [Google Scholar] [CrossRef]
- Wu, M.; Gong, L.; Ma, C.; He, Y.C. Enhanced enzymatic saccharification of sorghum straw by effective delignification via combined pretreatment with alkali extraction and deep eutectic solvent soaking. Bioresour. Technol. 2021, 340, 125695. [Google Scholar] [CrossRef]
- Zheng, X.; Xian, X.; Hu, L.; Tao, S.; Zhang, X.; Liu, Y.; Lin, X. Efficient short-time hydrothermal depolymerization of sugarcane bagasse in one-pot for cellulosic ethanol production without solid-liquid separation, water washing, and detoxification. Bioresour. Technol. 2021, 339, 125575. [Google Scholar] [CrossRef]
- Rodrigues, T.H.; de Barros, E.M.; de Sa Brigido, J.; da Silva, W.M.; Rocha, M.V.; Goncalves, L.R. The Bioconversion of Pretreated Cashew Apple Bagasse into Ethanol by SHF and SSF Processes. Appl. Biochem. Biotech. 2016, 178, 1167–1183. [Google Scholar] [CrossRef]
- Lu, J.; Song, F.; Liu, H.; Chang, C.; Cheng, Y.; Wang, H. Production of high concentration bioethanol from reed by combined liquid hot water and sodium carbonate-oxygen pretreatment. Energy 2021, 217, 119332. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. The use of high-solids loadings in biomass pretreatment—A review. Biotechnol. Bioeng. 2012, 109, 1430–1442. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Jacoby, W.A.; Wan, C. Ternary deep eutectic solvents for effective biomass deconstruction at high solids and low enzyme loadings. Bioresour. Technol. 2019, 279, 281–286. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, H.; Li, L.; Wang, H.; He, Z.; Jiang, E.; Sun, Y.; Li, Z.; Yi, W.; Xu, X. Pretreatment Influence of an Imitative Deep Eutectic Solvent Composed of Biomass Light Oil and Choline Chloride on Boosting Selective Saccharification during Corn Stalk Pyrolysis. ACS Sustain. Chem. Eng. 2021, 9, 12813–12824. [Google Scholar] [CrossRef]


| Pretreatment | Solid Recovery (%) | Content (%) | ||
|---|---|---|---|---|
| Cellulose | Xylan | Lignin | ||
| Untreated WS | 100 | 33.02 ± 0.27 | 17.26 ± 0.23 | 18.58 ± 0.45 |
| 100 °C | 55.09 ± 0.15 a, * | 57.29 ± 0.44 * | 10.35 ± 0.70 * | 9.94 ± 0.21 * |
| 120 °C | 45.80 ± 0.38 * | 68.29 ± 0.40 * | 3.94 ± 0.07 * | 8.09 ± 0.46 * |
| 140 °C | 41.11 ± 0.20 * | 60.94 ± 0.34 * | 1.37 ± 0.32 * | 7.67 ± 0.46 * |
| Pretreatment | Cellulose Recovery (%) | Xylan Removal (%) | Lignin Removal (%) | Cellulose Conversion b (%) | Glucose Recovery c (%) |
|---|---|---|---|---|---|
| Untreated WS | 100 | 0 | 0 | 22.48 ± 0.38 | 22.48 ± 0.38 |
| 100 °C | 95.61 ± 0.42 a, * | 66.97 ± 0.20 * | 70.53 ± 0.32 * | 77.65 ± 1.53 * | 58.61 ± 0.27 * |
| 120 °C | 94.73 ± 0.22 * | 89.53 ± 0.36 * | 80.05 ± 0.62 * | 91.15 ± 1.07 * | 71.58 ± 1.34 * |
| 140 °C | 75.86 ± 0.59 * | 96.74 ± 0.37 * | 83.02 ± 0.68 * | 97.49 ± 0.80 * | 57.98 ± 1.70 * |
| DES | Molar Ratio | DES Pretreatment Condition | Recovery of Glucan (%) | Biopolymer Removal (%) | Reference | ||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Lignin | Hemicellulose | ||||
| ChCl/MEA | 1:6 | 70 | 9 | 93.7 | 71.4 | 42.1 | [34] |
| ChCl/MEA a | 1:6 | 90 | 12 | 90.8 | 81.0 | 47.3 | [34] |
| TEBAC/LA | 1:9 | 100 | 10 | - | 79.7 | - | [36] |
| Alanine/LA | 1:9 | 60 | 24 | - | 23.7 | - | [50] |
| ChCl/BA/PEG-200 b | 1:1:1.5 | 120 | 4 | - | 88.4 | 84.4 | [43] |
| TEBAC/GL/ACH | 1: 2: 0.05 | 120 | 1 | 94.7 | 80.1 | 89.5 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, X.; Fang, L.; Zhou, Y.; Li, B.; Zheng, X.; Liu, Y.; Lin, X. Integrated Bioprocess for Cellulosic Ethanol Production from Wheat Straw: New Ternary Deep-Eutectic-Solvent Pretreatment, Enzymatic Saccharification, and Fermentation. Fermentation 2022, 8, 371. https://doi.org/10.3390/fermentation8080371
Xian X, Fang L, Zhou Y, Li B, Zheng X, Liu Y, Lin X. Integrated Bioprocess for Cellulosic Ethanol Production from Wheat Straw: New Ternary Deep-Eutectic-Solvent Pretreatment, Enzymatic Saccharification, and Fermentation. Fermentation. 2022; 8(8):371. https://doi.org/10.3390/fermentation8080371
Chicago/Turabian StyleXian, Xiaoling, Lv Fang, Yongxing Zhou, Biying Li, Xiaojie Zheng, Yao Liu, and Xiaoqing Lin. 2022. "Integrated Bioprocess for Cellulosic Ethanol Production from Wheat Straw: New Ternary Deep-Eutectic-Solvent Pretreatment, Enzymatic Saccharification, and Fermentation" Fermentation 8, no. 8: 371. https://doi.org/10.3390/fermentation8080371
APA StyleXian, X., Fang, L., Zhou, Y., Li, B., Zheng, X., Liu, Y., & Lin, X. (2022). Integrated Bioprocess for Cellulosic Ethanol Production from Wheat Straw: New Ternary Deep-Eutectic-Solvent Pretreatment, Enzymatic Saccharification, and Fermentation. Fermentation, 8(8), 371. https://doi.org/10.3390/fermentation8080371







