Research Progress and Potential Functions of AMF and GRSP in the Ecological Remediation of Metal Tailings
Abstract
1. Introduction
2. Overview of AMF
3. Analysis of the Physiological Functions of AMF Symbiosis for Ecological Remediation of Tailings from Metals Mining
3.1. Promoting Plant Growth
3.2. Improving Drought Resistance in Plants
3.3. Improving Metal Tolerance in Plants
3.4. Altering the Microbial Composition of the Soil Environment
4. Application of AMF Symbionts in the Ecological Remediation of Tailings from Metals Mining
5. Environmental Functions of GRSP, a Release Product of Arbuscular Mycorrhizal Fungi
5.1. Characterization of GRSP
5.2. Structural Composition of GRSP
5.3. Analysis of the Potential Functions of Glomalin-Related Soil Proteins in the Ecological Remediation of Tailings from Metal Mining
5.3.1. Carbon Fixation Function
5.3.2. Immobilizing Heavy Metals
5.3.3. Increasing the Stability of Soil Aggregates
5.3.4. Improving Drought Tolerance in Plants
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.Y. Study on Process and Properties of Porous Ceramics Prepared by Fine Grained Iron Tailing from Beijing Area. Master’s Thesis, Beijing Jiaotong University, Beijing, China, 2017. (In Chinese). [Google Scholar]
- Sun, W.; Ji, B.; Khoso, S.A.; Tang, H.H.; Liu, R.Q.; Wang, L.; Hu, Y.H. An extensive review on restoration technologies for mining tailings. Environ. Sci. Pollut. R 2018, 25, 33911–33925. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, S.; Wang, B. Biochar as a potential strategy for remediation of contaminated mining soils: Mechanisms, applications, and future perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Won, S.; Ha, M.G.; Nguyen, D.D.; Kang, H.Y. Bioleaching for environmental remediation of toxic metals and metalloids: A review on soils, sediments, and mine tailings. Chemosphere 2021, 282, 131108. [Google Scholar] [CrossRef] [PubMed]
- Oades, J.M. Soil organic matter and structural stability: Mechanisms and implications for management. Plant Soil 1984, 76, 319–337. [Google Scholar] [CrossRef]
- Schnitzer, M. Soil organic matter: The next 75 years. Soil Sci. 1991, 151, 41–58. [Google Scholar] [CrossRef]
- Gil-Loaiza, J.; Field, J.P.; White, S.A.; Csavina, J.; Felix, O.; Betterton, E.A.; Saez, A.E.; Maier, R.M. Phytoremediation reduces dust emissions from metal(ioid)-contaminated mine tailings. Environ. Sci. Technol. 2018, 52, 5851–5858. [Google Scholar] [CrossRef] [PubMed]
- Madejón, P.; Domínguez, M.T.; Girón, I.; Burgos, P.; López-Fernández, M.T.; Porras, Ó.G.; Madejón, E. Assessment of the phytoremediation effectiveness in the restoration of uranium mine tailings. Ecol. Eng. 2022, 180, 106669. [Google Scholar] [CrossRef]
- Babu, A.G.; Shim, J.; Shea, P.J.; Oh, B.T. Penicillium aculeatum pdr-4 and trichoderma sp. Pdr-16 promote phytoremediation of mine tailing soil and bioenergy production with sorghum-sudangrass. Ecol. Eng. 2014, 69, 186–191. [Google Scholar] [CrossRef]
- Afonso, T.F.; Demarco, C.F.; Pieniz, S.; Camargo, F.A.O.; Quadro, M.S.; Andreazza, R. Potential of solanum viarum dunal in use for phytoremediation of heavy metals to mining areas, southern brazil. Environ. Sci. Pollut. R 2019, 26, 24132–24142. [Google Scholar] [CrossRef]
- Ali, A.; Guo, D.; Mahar, A.; Wang, Z.; Muhammad, D.; Li, R.; Wang, P.; Shen, F.; Xue, Q.; Zhang, Z. Role of streptomyces pactum in phytoremediation of trace elements by brassica juncea in mine polluted soils. Ecotox. Environ. Safe 2017, 144, 387–395. [Google Scholar] [CrossRef]
- Ziedan, E.S.; Elewa, I.; Mostafa, M.; Sahab, A. Application of mycorrhizae for controlling root diseases of sesame. J. Plant Prot. Res. 2011, 51, 355–361. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L.X. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling arbuscular mycorrhiza-induced changes in plant primary and secondary metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, M.; Tripathi, B.N. Glomalin: An arbuscular mycorrhizal fungal soil protein. Protoplasma 2013, 250, 663–669. [Google Scholar] [CrossRef]
- Malekzadeh, E.; Aliasgharzad, N.; Majidi, J.; Abdolalizadeh, J.; Aghebati-Maleki, L. Contribution of glomalin to pb sequestration by arbuscular mycorrhizal fungus in a sand culture system with clover plant. Eur. J. Soil Biol. 2016, 74, 45–51. [Google Scholar] [CrossRef]
- Yang, H.J. The Study on the Role and Effects of AMF in Iron Tailings Reclamation of Inner Mongolia Grasslands. Master’s Thesis, Inner Mongolia University, Huhehaote, China, 2012. (In Chinese). [Google Scholar]
- Koide, R.T.; Mosse, B. A history of research on arbuscular mycorrhiza. Mycorrhiza 2004, 14, 145–163. [Google Scholar] [CrossRef]
- Estaún, V.; Calvet, C.; Camprubí, A. Effect of differences among crop species and cultivars on the arbuscular mycorrhizal symbiosis. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Dordrecht, The Netherlands, 2010; pp. 279–295. [Google Scholar]
- Mathur, N.; Bohra, J.S.S.; Quaizi, A.; Vyas, A. Arbuscular mycorrhizal fungi: A potential tool for phytoremediation. J. Plant Sci. 2007, 2, 127–140. [Google Scholar] [CrossRef]
- Wężowicz, K.; Rozpądek, P.; Turnau, K. Interactions of arbuscular mycorrhizal and endophytic fungi improve seedling survival and growth in post-mining waste. Mycorrhiza 2017, 27, 499–511. [Google Scholar] [CrossRef]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Gu, H.H.; Zhou, Z.; Gao, Y.Q.; Yuan, X.T.; Ai, Y.J.; Zhang, J.Y.; Zuo, W.Z.; Taylor, A.A.; Nan, S.Q.; Li, F.P. The influences of arbuscular mycorrhizal fungus on phytostabilization of lead/zinc tailings using four plant species. Int. J. Phytoremediat. 2017, 19, 739–745. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.J.; Qin, M.S.; Zhang, Q.; Pan, J.B.; Liu, Y.J.; Feng, H.Y. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Ur-Rahman, S.; Hassani, D.; Hayat, K.; Zhou, P.; Hui, N. Advances in fungal-assisted phytoremediation of heavy metals: A review. Pedosphere 2021, 31, 475–495. [Google Scholar] [CrossRef]
- Miransari, M. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl. Microbiol. Biot. 2011, 89, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Giovannetti, M.; Sbrana, C. Appressoria and phosphorus fluxes in mycorrhizal plants: Connections between soil- and plant-based hyphae. Mycorrhiza 2020, 30, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; He, C. Arbuscular mycorrhizal influence on growth, photosynthetic pigments, osmotic adjustment and oxidative stress in tomato plants subjected to low temperature stress. Acta Physiol. Plant. 2011, 33, 1217–1225. [Google Scholar] [CrossRef]
- Sun, M.F.; Yuan, D.; Hu, X.C.; Zhang, D.J.; Li, Y.Y. Effects of mycorrhizal fungi on plant growth, nutrient absorption and phytohormones levels in tea under shading condition. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2006–2020. [Google Scholar] [CrossRef]
- Lee, B.R.; Muneer, S.; Avice, J.C.; Jung, W.J.; Kim, T.H. Mycorrhizal colonisation and p-supplement effects on n uptake and n assimilation in perennial ryegrass under well-watered and drought-stressed conditions. Mycorrhiza 2012, 22, 525–534. [Google Scholar] [CrossRef]
- Mitra, D.; Saritha, B.; Janeeshma, E.; Gusain, P.; Khoshru, B.; Abo Nouh, F.A.; Rani, A.; Olatunbosun, A.N.; Ruparelia, J.; Rabari, A.; et al. Arbuscular mycorrhizal fungal association boosted the arsenic resistance in crops with special responsiveness to rice plant. Environ. Exp. Bot. 2022, 193, 104681. [Google Scholar] [CrossRef]
- Kumar, B.; Rathore, M.; Ranganatha, A.R.G. Weeds as a source of genetic material for crop improvement under adverse conditions. In Plant Acclimation to Environmental Stress; Springer: New York, NY, USA, 2013; pp. 323–342. [Google Scholar]
- Pagano, M.C. Drought stress and mycorrhizal plant. In Use of Microbes for the Alleviation of Soil Stresses, Volume 1; Springer: New York, NY, USA, 2014; pp. 97–110. [Google Scholar]
- Rapparini, F.; Peñuelas, J. Mycorrhizal fungi to alleviate drought stress on plant growth. In Use of Microbes for the Alleviation of Soil Stresses, Volume 1; Springer: New York, NY, USA, 2014; pp. 21–42. [Google Scholar]
- Begum, N.; Ahanger, M.A.; Su, Y.Y.; Lei, Y.F.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L.X. Improved drought tolerance by amf inoculation in maize (zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Abdalla, M.; Ahmed, M.A. Arbuscular mycorrhiza symbiosis enhances water status and soil-plant hydraulic conductance under drought. Front. Plant Sci. 2021, 12, 722954. [Google Scholar] [CrossRef]
- Shi, S.M.; Chen, K.; Gao, Y.; Liu, B.; Yang, X.H.; Huang, X.Z.; Liu, G.X.; Zhu, L.Q.; He, X.H. Arbuscular mycorrhizal fungus species dependency governs better plant physiological characteristics and leaf quality of mulberry (Morus alba L.) seedlings. Front. Microbiol. 2016, 7, 1030. [Google Scholar] [CrossRef]
- Yang, Y.R.; Tang, M.; Sulpice, R.; Chen, H.; Tian, S.; Ban, Y.H. Arbuscular mycorrhizal fungi alter fractal dimension characteristics of robinia pseudoacacia l. Seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J. Plant Growth Regul. 2014, 33, 612–625. [Google Scholar]
- Fan, Q.J.; Liu, J.H. Colonization with arbuscular mycorrhizal fungus affects growth, drought tolerance and expression of stress-responsive genes in poncirus trifoliata. Acta Physiol. Plant. 2011, 33, 1533–1542. [Google Scholar] [CrossRef]
- Aganchich, B.; Wahbi, S.; Yaakoubi, A.; El-Aououad, H.; Bota, J. Effect of arbuscular mycorrhizal fungi inoculation on growth and physiology performance of olive tree under regulated deficit irrigation and partial rootzone drying. S. Afr. J. Bot. 2022, 148, 1–10. [Google Scholar] [CrossRef]
- Azcón, R.; Medina, A.; Aroca, R.; Ruiz-Lozano, J.M. Abiotic Stress Remediation by the Arbuscular Mycorrhizal Symbiosis and Rhizosphere Bacteria/Yeast Interactions, Molecular Microbial Ecology of the Rhizosphere, 1 & 2; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Bioremediation of adverse impact of cadmium toxicity on cassia italica mill by arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2016, 23, 39–47. [Google Scholar] [CrossRef]
- Wang, H.R.; Zhao, X.Y.; Zhang, J.M.; Lu, C.; Feng, F.J. Arbuscular mycorrhizal fungus regulates cadmium accumulation, migration, transport, and tolerance in Medicago sativa. J. Hazard. Mater. 2022, 435, 129077. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K.; Bhattacharjee, M.K.; Dutta, S. Role of arbuscular mycorrhiza in heavy metal tolerance in plants: Prospects for phytoremidiation. J. Phytol. 2010, 2, 16–27. [Google Scholar]
- Riaz, M.; Kamran, M.; Fang, Y.Z.; Wang, Q.Q.; Cao, H.Y.; Yang, G.L.; Deng, L.L.; Wang, Y.J.; Zhou, Y.Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Qiu, L.; Lin, H.Z.; Song, B.R.; Kong, T.L.; Sun, W.M.; Sun, X.X.; Zhang, Y.X.; Li, B.Q. Glomalin-related soil protein (grsp) in metal sequestration at pb/zn-contaminated sites. J. Soil Sediment 2022, 22, 577–593. [Google Scholar] [CrossRef]
- Toler, H.D.; Morton, J.B.; Cumming, J.R. Growth and metal accumulation of mycorrhizal sorghum exposed to elevated copper and zinc. Water Air Soil Pollut. 2005, 164, 155–172. [Google Scholar] [CrossRef]
- Galli, U.; Schuepp, H.; Brunold, C. Heavy-metal binding by mycorrhizal fungi. Physiol. Plant. 1994, 92, 364–368. [Google Scholar] [CrossRef]
- Nafady, N.A.; Elgharably, A. Mycorrhizal symbiosis and phosphorus fertilization effects on zea mays growth and heavy metals uptake. Int. J. Phytoremediat. 2018, 20, 869–875. [Google Scholar] [CrossRef]
- Lance, A.C.; Burke, D.J.; Hausman, C.E.; Burns, J.H. Microbial inoculation influences arbuscular mycorrhizal fungi community structure and nutrient dynamics in temperate tree restoration. Restor. Ecol. 2019, 27, 1084–1093. [Google Scholar] [CrossRef]
- Zhan, F.D.; Li, B.; Jiang, M.; Li, T.G.; He, Y.M.; Li, Y.; Wang, Y.S. Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass cynodon dactylon (L.) pers. Grown in a lead-zinc mine wasteland. Int. J. Phytoremediat. 2019, 21, 849–856. [Google Scholar] [CrossRef]
- Malekzadeh, E.; Alikhani, H.A.; Savaghebifiroozabadi, G.R.; Zarei, M. Influence of arbuscular mycorrhizal fungi and an improving growth bacterium on cd uptake and maize growth in cd-polluted soils. Span. J. Agric. Res. 2011, 9, 1213–1223. [Google Scholar] [CrossRef]
- De Oliveira, V.H.; Ullah, I.; Dunwell, J.M.; Tibbett, M. Mycorrhizal symbiosis induces divergent patterns of transport and partitioning of cd and zn in populus trichocarpa. Environ. Exp. Bot. 2020, 171, 103925. [Google Scholar] [CrossRef]
- Motaharpoor, Z.; Taheri, H.; Nadian, H. Rhizophagus irregularis modulates cadmium uptake, metal transporter, and chelator gene expression in Medicago sativa. Mycorrhiza 2019, 29, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Zhang, Y.L.; Li, P.J.; Gong, Z.Q. Effects of arbuscular mycorrhizal fungi (Glomus mosseae) on Cd Accumulation in Maize Plants. Chin. J. Soil Sci. 2011, 42, 568–572. (In Chinese) [Google Scholar]
- Wu, S.L.; Vosatka, M.; Vogel-Mikus, K.; Kavcic, A.; Kelemen, M.; Sepec, L.; Pelicon, P.; Skala, R.; Powter, A.R.V.; Teodoro, M.; et al. Nano zero-valent iron mediated metal(loid) uptake and translocation by arbuscular mycorrhizal symbioses. Environ. Sci. Technol. 2018, 52, 7640–7651. [Google Scholar] [CrossRef]
- Luo, L.; Guo, M.; Wang, E.; Yin, C.; Wang, Y.; He, H.; Zhao, C. Effects of mycorrhiza and hyphae on the response of soil microbial community to warming in eastern Tibetan Plateau. Sci. Total Environ. 2022, 837, 155498. [Google Scholar] [CrossRef]
- Fabianska, I.; Sosa-Lopez, E.; Bucher, M. The role of nutrient balance in shaping plant root-fungal interactions: Facts and speculation. Curr. Opin. Microbiol. 2019, 49, 90–96. [Google Scholar] [CrossRef]
- Hao, L.J.; Zhang, Z.C.; Hao, B.H.; Diao, F.W.; Zhang, J.X.; Bao, Z.H.; Guo, W. Arbuscular mycorrhizal fungi alter microbiome structure of rhizosphere soil to enhance maize tolerance to La. Ecotox. Environ. Safe 2021, 212, 111996. [Google Scholar] [CrossRef]
- Fu, L. The Remediation of Copper Contaminated Soils Combined with Tagetes patula, L., Earthworm and Arbuscular Mycorrhizal Fungus. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2016. (In Chinese). [Google Scholar]
- Gao, Y.Z.; Cheng, Z.X.; Ling, W.T.; Huang, J. Arbuscular mycorrhizal fungal hyphae contribute to the uptake of polycyclic aromatic hydrocarbons by plant roots. Bioresour. Technol. 2010, 101, 6895–6901. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- Xia, J.B.; Zhao, Z.G.; Fang, Y. Soil hydro-physical characteristics and water retention function of typical shrubbery stands in the yellow river delta of China. Catena 2017, 156, 315–324. [Google Scholar] [CrossRef]
- Yang, Y.R.; He, C.J.; Huang, L.; Ban, Y.H.; Tang, M. The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS ONE 2017, 12, 19. [Google Scholar] [CrossRef]
- Meier, S.; Borie, F.; Bolan, N.; Cornejo, P. Phytoremediation of metal-polluted soils by arbuscular mycorrhizal fungi. Crit. Rev. Environ. Sci. Technol. 2012, 42, 741–775. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, Z.; Bai, Z.; Hou, H.; Yuan, Y.; Guo, A.; Li, Y. Effects of mycorrhizae and water conditions on perennial ryegrass growth in rare earth tailings. RSC Adv. 2019, 9, 10881–10888. [Google Scholar] [CrossRef]
- Husna Tuheteru, F.D.; Arif, A. The potential of arbuscular mycorrhizal fungi to conserve kalappia celebica, an endangered endemic legume on gold mine tailings in sulawesi, indonesia. J. For. Res. 2021, 32, 675–682. [Google Scholar] [CrossRef]
- Verdugo, C.; Sánchez, P.; Santibáñez, C.; Urrestarazu, P.; Bustamante, E.; Silva, Y.; Gourdon, D.; Ginocchio, R. Efficacy of lime, biosolids, and mycorrhiza for the phytostabilization of sulfidic copper tailings in chile: A greenhouse experiment. Int. J. Phytoremediat. 2011, 13, 107–125. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Bi, Y.L.; Yu, M.; Chen, B.D. The modified effect of arbuscular mycorrhizal fungi on the Fe tailings substrate. Met. Mine 2010, 39, 171–174. (In Chinese) [Google Scholar]
- Guo, W.; Zhao, R.X.; Yang, H.J.; Zhao, J.; Zhang, J. Using native plants to evaluate the effect of arbuscular mycorrhizal fungi on revegetation of iron tailings in grasslands. Biol. Fert. Soils 2013, 49, 617–626. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.W.; Wu, S.L.; Li, Z.R.; Feng, H.M.; Jiang, Z.P. Effect of arbuscular mycorrhizae on growth, heavy metal uptake and accumulation of Zenia insignis chun seedlings. Environ. Sci. 2014, 1, 3142–3148. (In Chinese) [Google Scholar]
- Nayuki, K.; Chen, B.; Ohtomo, R.; Kuga, Y. Cellular imaging of cadmium in resin sections of arbuscular mycorrhizas using synchrotron micro x-ray fluorescence. Microbes Environ. 2014, 29, 60–66. [Google Scholar] [CrossRef]
- Perez, R.; Tapia, Y.; Antilen, M.; Casanova, M.; Vidal, C.; Santander, C.; Aponte, H.; Cornejo, P. Interactive effect of compost application and inoculation with the fungus claroideoglomus claroideum in oenothera picensis plants growing in mine tailings. Ecotox Environ. Safe 2021, 208, 111495. [Google Scholar] [CrossRef]
- El, F.A.; Duponnois, R.; Winterton, P.; Ouhammou, A.; Meddich, A.; Boularbah, A.; Hafidi, M. Effect of different amendments on growing of canna indica l. Inoculated with amf on mining substrate. Int. J. Phytoremediat. 2015, 17, 503–513. [Google Scholar]
- Daft, M.J.; Hacskaylo, E. Growth of endomycorrhizal and nonmycorrhizal red maple seedlings in sand and anthracite spoil. For. Sci. 1977, 23, 207–216. [Google Scholar]
- Victoria, K.S.; Aggangan, N.S. Effect of mycorrhizal inoculation on growth, nutrient status, and rhizosphere microbes of acacia mangium and eucalyptus urophylla. Philipp. J. Crop Sci. 2019, 44, 9–17. [Google Scholar]
- Di, L.; Zheng, K.Y.; Wang, Y.; Zhang, Y.; Lao, R.M.; Qin, Z.Y.; Li, T.; Zhao, Z.W. Harnessing an arbuscular mycorrhizal fungus to improve the adaptability of a facultative metallophytic poplar (Populus yunnanensis) to cadmium stress: Physiological and molecular responses. J. Hazard. Mater. 2022, 424, 127430. [Google Scholar]
- Straker, C.J.; Weiersbye, I.M.; Witkowski, E.T.F. Arbuscular mycorrhiza status of gold and uranium tailings and surrounding soils of South Africa’s deep level gold mines: I. Root colonization and spore levels. S. Afr. J. Bot. 2007, 73, 218–225. [Google Scholar] [CrossRef]
- Straker, C.J.; Freeman, A.J.; Witkowski, E.T.F.; Weiersbye, I.M. Arbuscular mycorrhiza status of gold and uranium tailings and surrounding soils of South Africa’s deep level gold mines. II. Infectivity. S. Afr. J. Bot. 2008, 74, 197–207. [Google Scholar] [CrossRef][Green Version]
- Zanchi, C.S.; Batista, E.R.; Silva, A.O.; Barbosa, M.V.; Pinto, F.A.; dos Santos, J.V.; Carneiro, M.A.C. Recovering soils affected by iron mining tailing using herbaceous species with mycorrhizal inoculation. Water Air Soil Poll. 2021, 232, 110. [Google Scholar] [CrossRef]
- Jin, Z.X.; Li, J.M.; Li, Y.L. Interactive effects of arbuscular mycorrhizal fungi and copper stress on flowering phenology and reproduction of elsholtzia splendens. PLoS ONE 2015, 10, e0145793. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Chen, C.M.; Song, M. Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS ONE 2019, 14, e0223994. [Google Scholar] [CrossRef]
- Wu, Q.S.; Cao, M.Q.; Zou, Y.N.; He, X.H. Direct and indirect effects of glomalin, mycorrhizal hyphae, and roots on aggregate stabililety in rhizosphere of trifoliate orange. Sci. Rep. UK 2014, 4, 5823. [Google Scholar] [CrossRef]
- Wright, S.F.; Nichols, K.A.; Schmidt, W.F. Comparison of efficacy of three extractants to solubilize glomalin on hyphae and in soil. Chemosphere 2006, 64, 1219–1224. [Google Scholar] [CrossRef]
- Emran, M.; Gispert, M.; Pardini, G. Patterns of soil organic carbon, glomalin and structural stability in abandoned mediterranean terraced lands. Eur. J. Soil Sci. 2012, 63, 637–649. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A.; Buyer, J.S. Comparison of n-linked oligosaccharides of glomalin from arbuscular mycorrhizal fungi and soils by capillary electrophoresis. Soil Biol. Biochem. 1998, 30, 1853–1857. [Google Scholar] [CrossRef]
- Tian, H.; Liu, X.L.; Gai, J.P.; Zhang, J.L.; Li, X.L. Review of glomalin-related soil protein and its function. Chin. J. Soil Sci. 2009, 40, 1215–1220. (In Chinese) [Google Scholar]
- Gillespie, A.W.; Farrell, R.E.; Walley, F.L.; Ross, A.R.S.; Leinweber, P.; Eckhardt, K.U.; Regier, T.Z.; Blyth, R.I.R. Glomalin-related soil protein contains non-mycorrhizal-related heat-stable proteins, lipids and humic materials. Soil Biol. Biochem. 2011, 43, 766–777. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Zhong, S.; Yin, G.; Gao, Y.; He, X. Recalcitrant carbon components in glomalin-related soil protein facilitate soil organic carbon preservation in tropical forests. Sci. Rep. UK 2017, 7, 2391. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Wang, H.; Nie, S.; Liang, Z. Effects of soil salinity on the content, composition, and ion binding capacity of glomalin-related soil protein (grsp). Sci. Total Environ. 2017, 581, 657–665. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.Y.; Chen, S.; Qian, L.; Yuan, B.; Tian, Y.; Wang, Y.Z.; Liu, J.C.; Yan, C.L.; Lu, H.L. Terrestrial-derived soil protein in coastal water: Metal sequestration mechanism and ecological function. J. Hazard. Mater. 2020, 386, 121655. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, H.L.; Chen, J.Y.; Jiang, Y.C.; Williams, M.A.; Wu, S.J.; Li, J.W.; Liu, J.C.; Yang, G.S.; Yan, C.L. Interactions of soil metals with glomalin-related soil protein as soil pollution bioindicators in mangrove wetland ecosystems. Sci. Total Environ. 2020, 709, 136051–136059. [Google Scholar] [CrossRef]
- Xu, J.; Tang, M. Relationship between arbuscular mycorrhizal fungi and soil factors in the rhizosphere of different tree species in Pb-Zn polluted mine. J. Northwest A F Univ. 2013, 5, 75–80. (In Chinese) [Google Scholar]
- Rillig, M.C.; Wright, S.F.; Nichols, K.A.; Schmidt, W.F.; Torn, M.S. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil 2001, 233, 167–177. [Google Scholar] [CrossRef]
- Wu, Q.S.; Li, Y.; Zou, Y.N.; He, X.H. Effects of arbuscular mycorrhizal fungi on aggregate stability, GRSP, and carbohydrates of white clover. Mycorrhiza 2015, 25, 121–130. [Google Scholar] [CrossRef]
- Staunton, S.; Saby, N.P.A.; Arrouays, D.; Quiquampoix, H. Can soil properties and land use explain glomalin-related soil protein (grsp) accumulation? A nationwide survey in france. Catena 2020, 193, 104620. [Google Scholar] [CrossRef]
- Matos, P.S.; Figueira Da Silva, C.; Pereira, M.G.; da Silva, E.M.R.; Tarré, R.M.; Custódio Franco, A.L.; Zonta, E. Short-term modifications of mycorrhizal fungi, glomalin and soil attributes in a tropical agroforestry. Acta Oecol. 2022, 114, 103815. [Google Scholar] [CrossRef]
- Guo, L.D.; Tian, C.J. Progress of the function of mycorrhizal fungi in the cycle of carbon and nitrogen. Microbiol. China 2013, 1, 158–171. (In Chinese) [Google Scholar]
- Bai, C.M.; He, X.L.; Tang, H.T.; Shan, B.Q.; Zhao, L.L. Spatial distribution of arbuscular mycorrhizal fungi, glomalin and soil enzymes under the canopy of astragalus adsurgens pall. In the mu us sandland, China. Soil Biol. Biochem. 2009, 41, 941–947. [Google Scholar] [CrossRef]
- Buyer, J.S.; Zuberer, D.A.; Nichols, K.A.; Franzluebbers, A.J. Soil microbial community function, structure, and glomalin in response to tall fescue endophyte infection. Plant Soil 2011, 339, 401–412. [Google Scholar] [CrossRef]
- Singh, A.K.; Jiang, X.J.; Yang, B.; Li, H.M.; Liu, W.J.; Singh, N. Effect of root-glomalin on soil carbon storage in trees’ rhizosphere and interspace of a tropical dry forest. Land Degrad. Devt. 2021, 32, 5281–5291. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.K.; Ghosh, P. Distribution of soil organic carbon and glomalin related soil protein in reclaimed coal mine-land chronosequence under tropical condition. Sci. Total Environ. 2018, 625C, 1341–1350. [Google Scholar] [CrossRef]
- Li, B.W. Effect of Fertilization on GRSP and Environmental Factors in Alpine Meadows on the Qinghai-Tibetan Plateau. Master’s Thesis, Lanzhou University, Lanzhou, China, 2016. (In Chinese). [Google Scholar]
- Wang, J.; Zhou, Z.Y.; Ling, W.T. Disrtibution and enviromental function of glomalin-related soil protein: A review. Chin. J. Appl. Ecol. 2016, 27, 634–642. (In Chinese) [Google Scholar]
- Wang, M.Y.; Xia, R.X.; Wang, P. Effects of arbuscular mycorrhizal fungi on available iron and metals sequestered by glomalin in different rhizospheric soil of Poncirus trifoliata. J. Fujian Agr. For. Univ. (Nat. Sci. Edit.) 2010, 39, 42–46. (In Chinese) [Google Scholar]
- Khan, A.G. Mycorrhizoremediation-an enhanced form of phytoremediation. J. Zhejiang Univ. Sci. B 2006, 7, 503–514. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.H.; Liu, T.; Huang, S.P.; Chang, Y.F. Elevated CO2 increases glomalin-related soil protein (grsp) in the rhizosphere of robinia pseudoacacia l. Seedlings in Pb- and Cd-contaminated soils. Environ. Pollut. 2016, 218, 349–357. [Google Scholar] [CrossRef]
- Bano, S.A.; Ashfaq, D. Role of mycorrhiza to reduce heavy metal stress. Nat. Sci. 2013, 5, 16–20. [Google Scholar] [CrossRef]
- Yuan, W.; Su, Q.; Sun, Z.J.; Shen, Y.Q.; Li, J.N.; Zhu, X.L.; Hong, H.; Chen, Z.P.; Feng, C.W. The role of arbuscular mycorrhizal fungi in plant uptake, fractions, and speciation of antimony. Appl. Soil. Ecol. 2016, 107, 244–250. [Google Scholar]
- Tang, H.L.; Liu, L.; Wang, L.; Ba, C.J. Effect of land use type on profile distribution of glomalin. Chin. J. Eco-Agric. 2009, 17, 1137–1142. (In Chinese) [Google Scholar] [CrossRef]
- Gujre, N.; Agnihotri, R.; Rangan, L.; Sharma, M.P.; Mitra, S. Deciphering the dynamics of glomalin and heavy metals in soils contaminated with hazardous municipal solid wastes. J. Hazard. Mater. 2021, 416, 125869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.G.; Wang, H.; Gao, B.; Yuan, Z.L. Effects of inoculation with Glomus mosseae on toxicity in tobacco under Pb stress. J. Henan Agric. Univ. 2015, 2, 153–157. (In Chinese) [Google Scholar]
- Chen, H.; Xiong, J.; Fang, L.; Han, F.; Zhao, X.; Fan, Q.; Tan, W. Sequestration of heavy metals in soil aggregates induced by glomalin-related soil protein: A five-year phytoremediation field study. J. Hazard. Mater. 2022, 437, 129445. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Gohre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef]
- Wang, Q.; Hong, H.L.; Yang, D.; Li, J.W.; Chen, S.; Pan, C.L.; Lu, H.L.; Liu, J.C.; Yan, C.L. Health risk assessment of heavy metal and its mitigation by glomalin-related soil protein in sediments along the south china coast. Environ. Pollut. 2020, 263, 114565. [Google Scholar] [CrossRef]
- Abbaslou, H.; Bakhtiari, S.; Hashemi, S.S. Rehabilitation of iron ore mine soil contaminated with heavy metals using rosemary phytoremediation-assisted mycorrhizal arbuscular fungi bioaugmentation and fibrous clay mineral immobilization. Iran J. Sci. Technol. 2018, A42, 431–441. [Google Scholar] [CrossRef]
- Siani, N.G.; Fallah, S.; Pokhrel, L.R.; Rostamnejadi, A. Natural amelioration of zinc oxide nanoparticle toxicity in fenugreek (trigonella foenum-gracum) by arbuscular mycorrhizal (Glomus intraradices) secretion of glomalin. Plant Physiol. Biochem. 2017, 112, 227–238. [Google Scholar] [CrossRef]
- Singh, G.; Bhattacharyya, R.; Das, T.K.; Sharma, A.R.; Ghosh, A.; Das, S.; Jha, P. Crop rotation and residue management effects on soil enzyme activities, glomalin and aggregate stability under zero tillage in the indo-gangetic plains. Soil Till. Res. 2018, 184, 291–300. [Google Scholar] [CrossRef]
- Huang, B.; Yan, G.; Liu, G.; Sun, X.; Wang, X.; Xing, Y.; Wang, Q. Effects of long-term nitrogen addition and precipitation reduction on glomalin-related soil protein and soil aggregate stability in a temperate forest. Catena 2022, 214, 106284. [Google Scholar] [CrossRef]
- Wright, S.F.; Anderson, R.L. Aggregate stability and glomalin in alternative crop rotations for the central great plains. Biol. Fert. Soils 2000, 31, 249–253. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Fokom, R.; Adamou, S.; Teugwa, M.C.; Boyogueno, A.D.B.; Nana, W.L.; Ngonkeu, M.E.L.; Tchameni, N.S.; Nwaga, D.; Ndzomo, G.T.; Zollo, P.H.A. Glomalin related soil protein, carbon, nitrogen and soil aggregate stability as affected by land use variation in the humid forest zone of south cameroon. Soil Till. Res. 2012, 120, 69–75. [Google Scholar] [CrossRef]
- Wu, Q.S.; Yuan, F.Y.; Fei, Y.J.; Li, L.; Huang, Y.M. Effects of mycorrhizal fungi on the stability of rhizosphere aggregates, soil proteins and saccharides related to the white trifoliate. Acta Prataculturae Sin. 2014, 4, 269–275. (In Chinese) [Google Scholar]
- Li, T.; Zhao, Z.W. Advances in researches on glomalin produced by arbuscular mycorrhizal fungi. Chin. J. Ecol. 2005, 24, 1080–1084. (In Chinese) [Google Scholar]
- Sekaran, U.; Sagar, K.L.; Kumar, S. Soil aggregates, aggregate-associated carbon and nitrogen, and water retention as influenced by short and long-term no-till systems. Soil Till. Res. 2021, 208, 104885. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Wright, S.F.; Starr, J.L.; Paltineanu, I.C. Changes in aggregate stability and concentration of glomalin during tillage management transition. Soil Sci. Soci. Am. J. 1999, 63, 1825–1829. [Google Scholar] [CrossRef]
- Galazka, A.; Gawryjolek, K. Glomalin-soil glicoprotein produced by arbuscular mycorhizal fungus. Postepy Mikrobiol. 2015, 54, 331–343. [Google Scholar]
- Nichols, K.A. Indirect contributions of am fungi and soil aggregation to plant growth and protection. In Mycorrhizae: Sustainable Agriculture and Forestry; Springer: Dordrecht, The Netherlands, 2008; pp. 177–194. [Google Scholar]
- Ji, L.L.; Tan, W.F.; Chen, X.H. Arbuscular mycorrhizal mycelial networks and glomalin-related soil protein increase soil aggregation in calcaric regosol under well-watered and drought stress conditions. Soil Till. Res. 2019, 185, 1–8. [Google Scholar] [CrossRef]
- Purin, S.; Rillig, M.C. The arbuscular mycorrhizal fungal protein glomalin: Limitations, progress, and a new hypothesis for its function. Pedobiologia 2008, 51, 123–130. [Google Scholar] [CrossRef]
- Chen, S.T.; He, N.Y.; Chen, J.H.; Guo, F.Q. Identification of core subunits of photosystem II as action sites of hsp21 that is activated by the gun5-mediated retrograde pathway in arabidopsis. Plant J. 2017, 89, 1106–1118. [Google Scholar] [CrossRef]
- Chi, G.G.; Srivastava, A.K.; Wu, Q.S. Exogenous easily extractable glomalin-related soil protein improves drought tolerance of trifoliate orange. Arch. Agron Soil Sci. 2018, 64, 1341–1350. [Google Scholar] [CrossRef]
- Lobe, I.; Sandhage-Hofmann, A.; Brodowski, S.; Preez, C.C.D.; Amelung, W. Aggregate dynamics and associated soil organic matter contents as influenced by prolonged arable cropping in the south african highveld. Geoderma 2011, 162, 251–259. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Zhang, F. Soil aggregation and aggregate-associated carbon under four typical halophyte communities in an arid area. Environ. Sci. Pollut. Res. Int. 2016, 23, 23920–23929. [Google Scholar] [CrossRef]
- Lu, Y.N.; Xu, D.D.; Cheng, H.X.; Zhou, G.H.; Ma, L.L. Recent advances in studying characteristics of heavy metals enriched in soil aggregates. Chin. J. Soil Sci. 2014, 45, 1008–1013. (In Chinese) [Google Scholar]
- Deng, A.M.; Wang, L.; Chen, F.; Li, Z.G.; Liu, W.Z.; Liu, Y. Soil aggregate-associated heavy metals subjected to different types of land use in subtropical china. Glob. Ecol. Conserv. 2018, 16, e00465. [Google Scholar] [CrossRef]
- Sun, W.J.; Huang, Y.; Zhang, W.; Yu, Y.Q. Key issues on soil carbon sequestration potential in agricultural soils. Adv. Earth Sci. 2008, 23, 996–1004. (In Chinese) [Google Scholar]
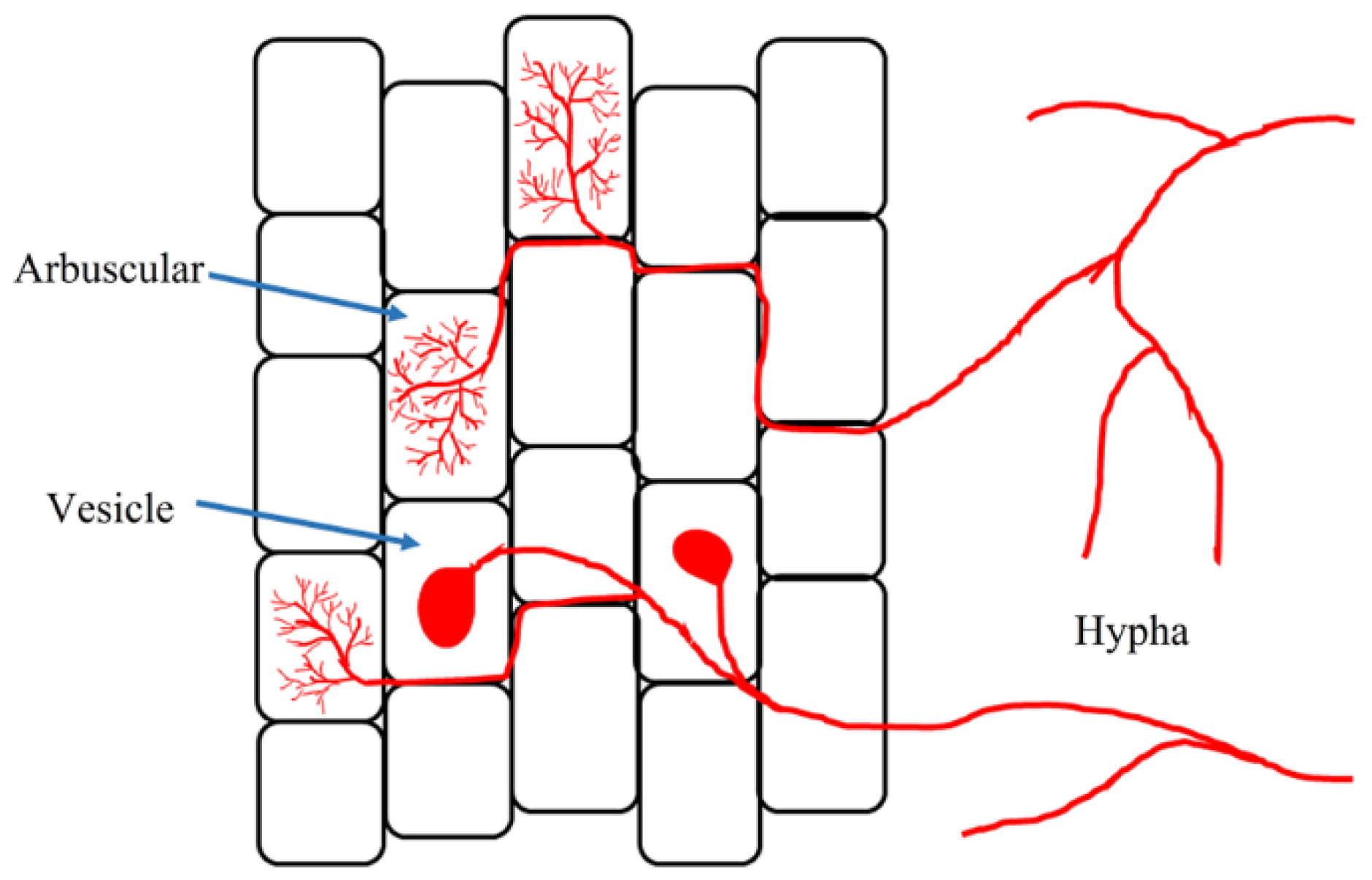

| Mycorrhizal Species | Host Plants | AMF Effects | References |
|---|---|---|---|
| Funneliformis mosseae | Lolium perenne L., Festuca arundinacea, Hylotelephium spectabile (Bor.) H. Ohba, Tradescantia pallida | Increased plant biomass, decreased heavy metals uptake | [23] |
| Funneliformis mosseae Diversispora spurcum | Cynodondactylon (L.) Pers. | Increased the pH, decreased Pb and Cd availability in tailings | [51] |
| Glomus mosses, Glomus etunicatum, Glomus versiforme | Lolium perenne L. | Increased plant growth, activities of CAT and SOD in plant | [66] |
| Glomus claroideum, Glomus coronatum | Kalappia celebica | Increased plant growth and N, P and K uptake | [67] |
| Glomus intraradices | Lolium perenne L. | Increased plant growth | [68] |
| Glomus mosseae, Glomus intraradices | Medicago sativa L. | Increased plant biomass and P uptake | [69] |
| Glomus versiforme, Glomus mosseae | Agropyron cristatum (L.) Gaertn., Elymus dahuricus Turcz. | Increased plant growth and N, P and K uptake, decreased heavy metals uptake | [70] |
| Glomus mosseae, Glomus intraradices | Zenia insignis Chun | Increased plant biomass and P uptake, decreased root to shoot Fe, Pb and Zn translocation | [71] |
| Gigaspora margarita, Rhizophagus irregularis | Allium cepa L., Lotus japonicus | Showed high signal of Cd in fungal cell | [72] |
| Claroideoglomus claroideum | Sorghum bicolor (L.) Moench, Trifolium repens L. | Increased plant biomass, promoted the production of photosynthetic pigments and decreased Cu availability in tailings | [73] |
| Glomus species | Canna indica L. | Increased plant biomass, decreased bioavailability of heavy metals | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, Y.-J.; Li, F.-P.; Yang, J.-Q.; Lu, S.; Gu, H.-H. Research Progress and Potential Functions of AMF and GRSP in the Ecological Remediation of Metal Tailings. Sustainability 2022, 14, 9611. https://doi.org/10.3390/su14159611
Ai Y-J, Li F-P, Yang J-Q, Lu S, Gu H-H. Research Progress and Potential Functions of AMF and GRSP in the Ecological Remediation of Metal Tailings. Sustainability. 2022; 14(15):9611. https://doi.org/10.3390/su14159611
Chicago/Turabian StyleAi, Yan-Jun, Fu-Ping Li, Jia-Qing Yang, Sai Lu, and Hai-Hong Gu. 2022. "Research Progress and Potential Functions of AMF and GRSP in the Ecological Remediation of Metal Tailings" Sustainability 14, no. 15: 9611. https://doi.org/10.3390/su14159611
APA StyleAi, Y.-J., Li, F.-P., Yang, J.-Q., Lu, S., & Gu, H.-H. (2022). Research Progress and Potential Functions of AMF and GRSP in the Ecological Remediation of Metal Tailings. Sustainability, 14(15), 9611. https://doi.org/10.3390/su14159611






