Response of Wheat Cultivars to Organic and Inorganic Nutrition: Effect on the Yield and Soil Biological Properties
Abstract
:1. Introduction
2. Materials and Methods
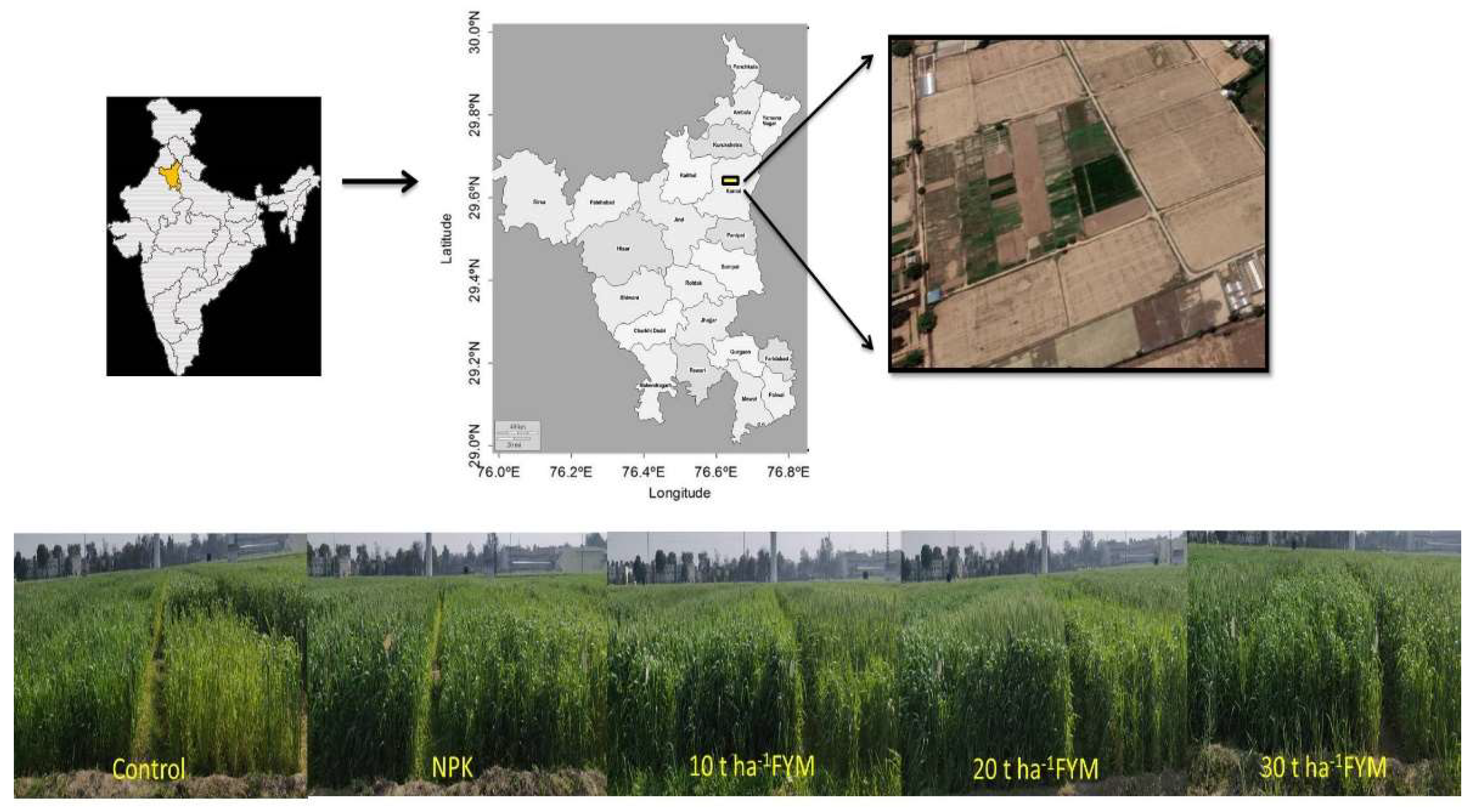
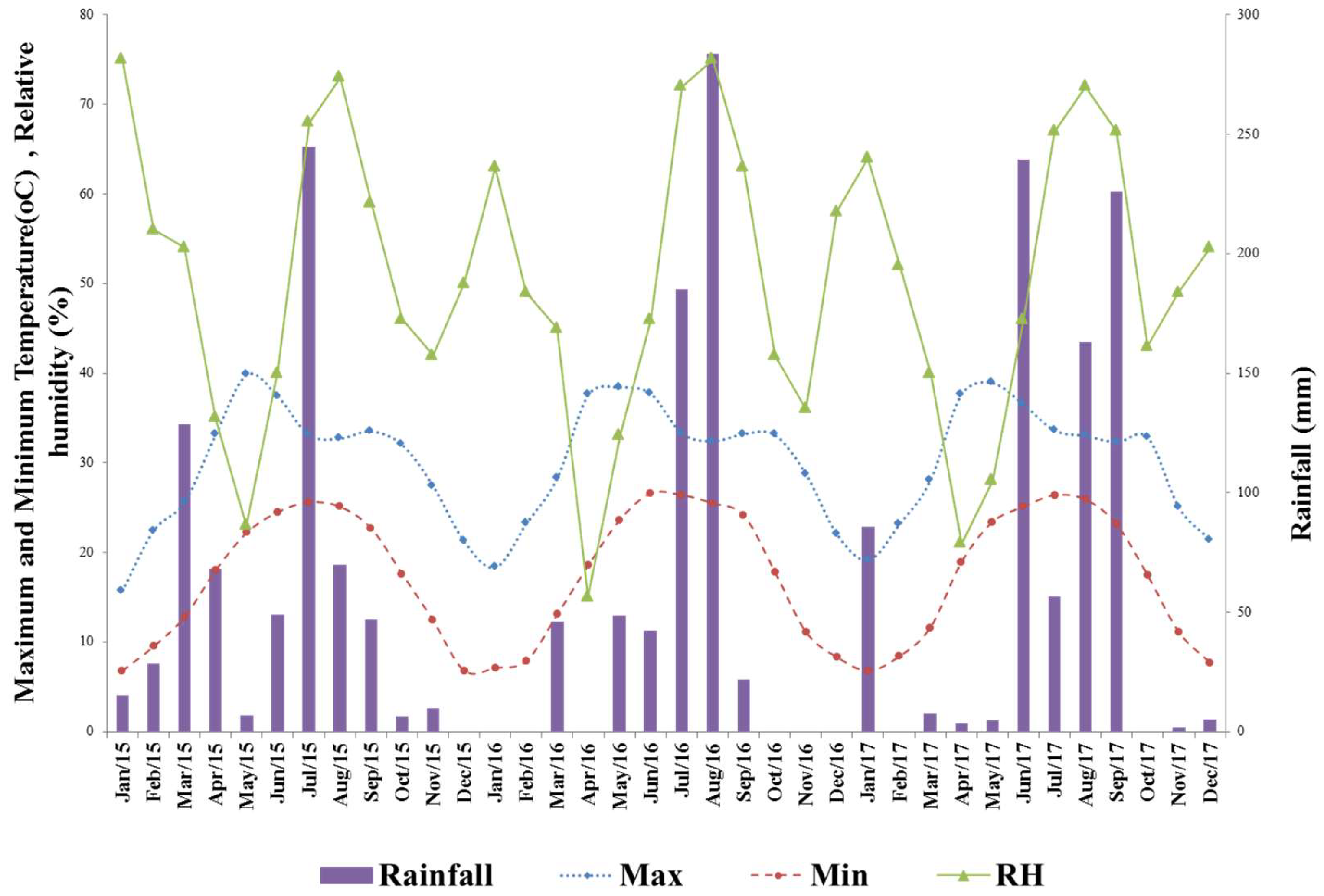
2.1. Site Description and Weather Condition
2.2. Experimental Design and Treatments
2.3. Soil Biological Properties
2.4. Computation of Soil Biological Index (SBI)
2.5. Yield and Validation of SBI
2.6. Statistical Analysis
3. Results
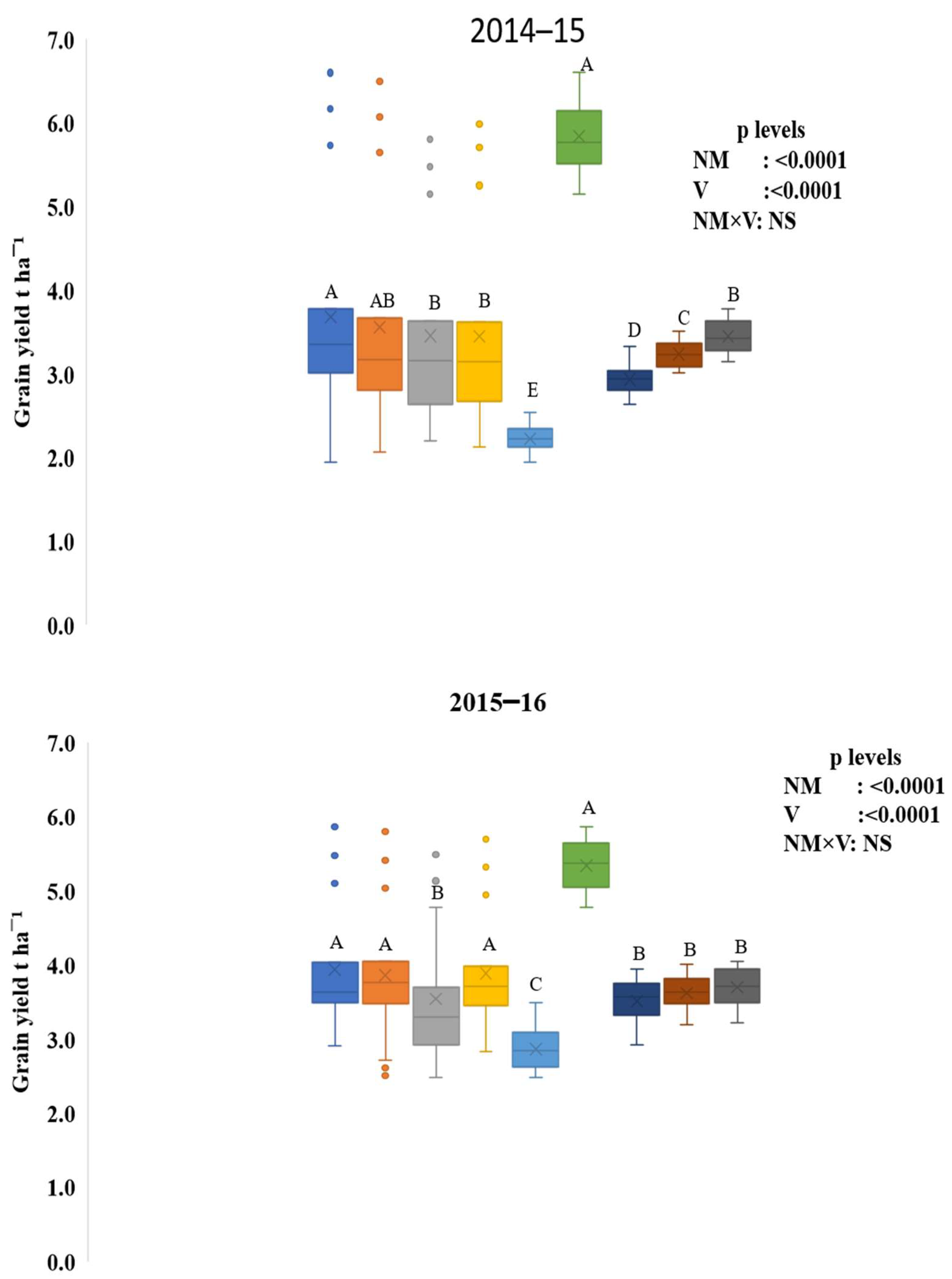
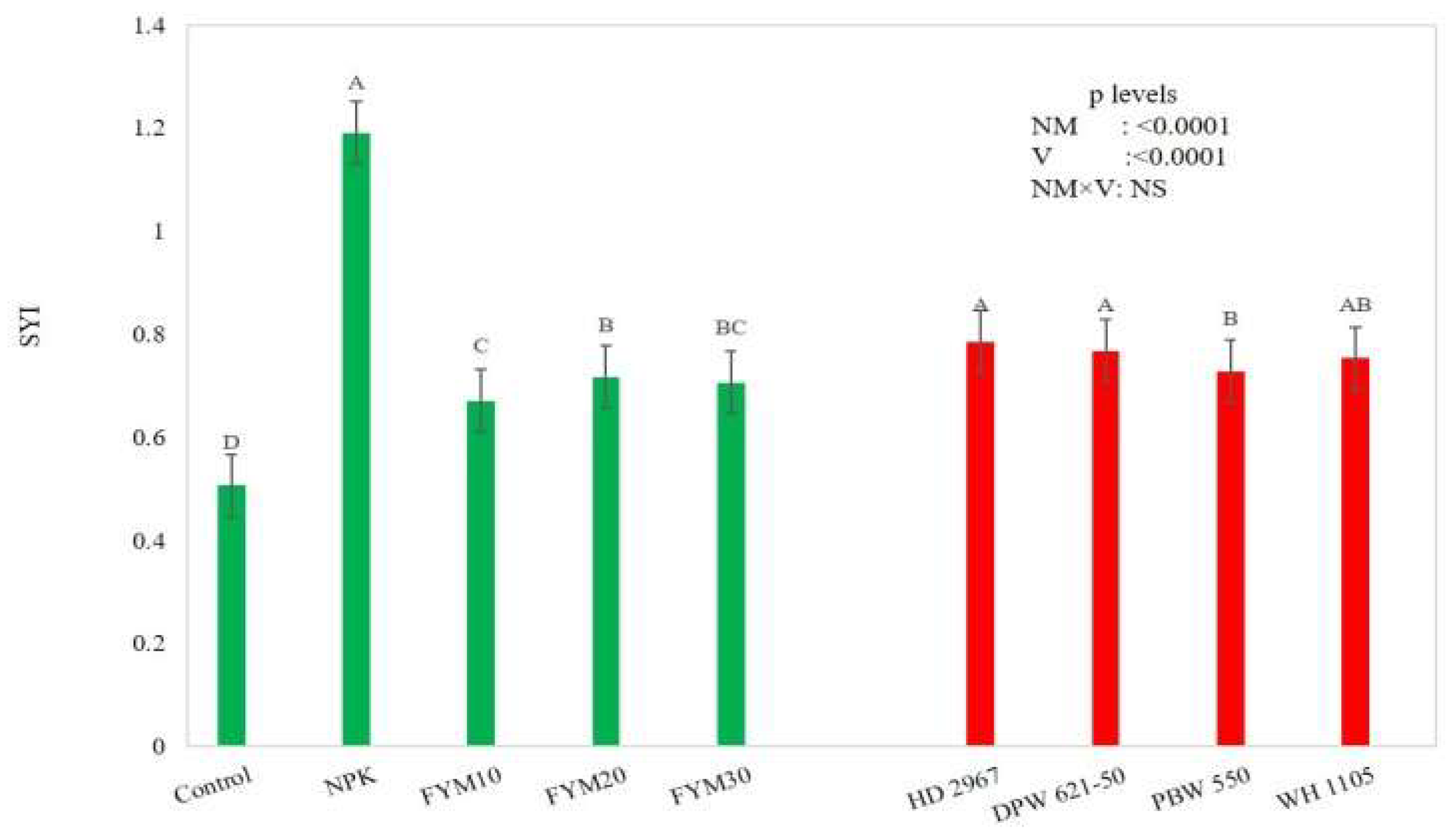
3.1. Crop yield and Sustainability Yield Index
3.2. Microbial Biomass Carbon (MBC) and Microbial Population
3.3. Soil Microbial Enzymes and Soil Respiration
3.4. Microbial Indicators
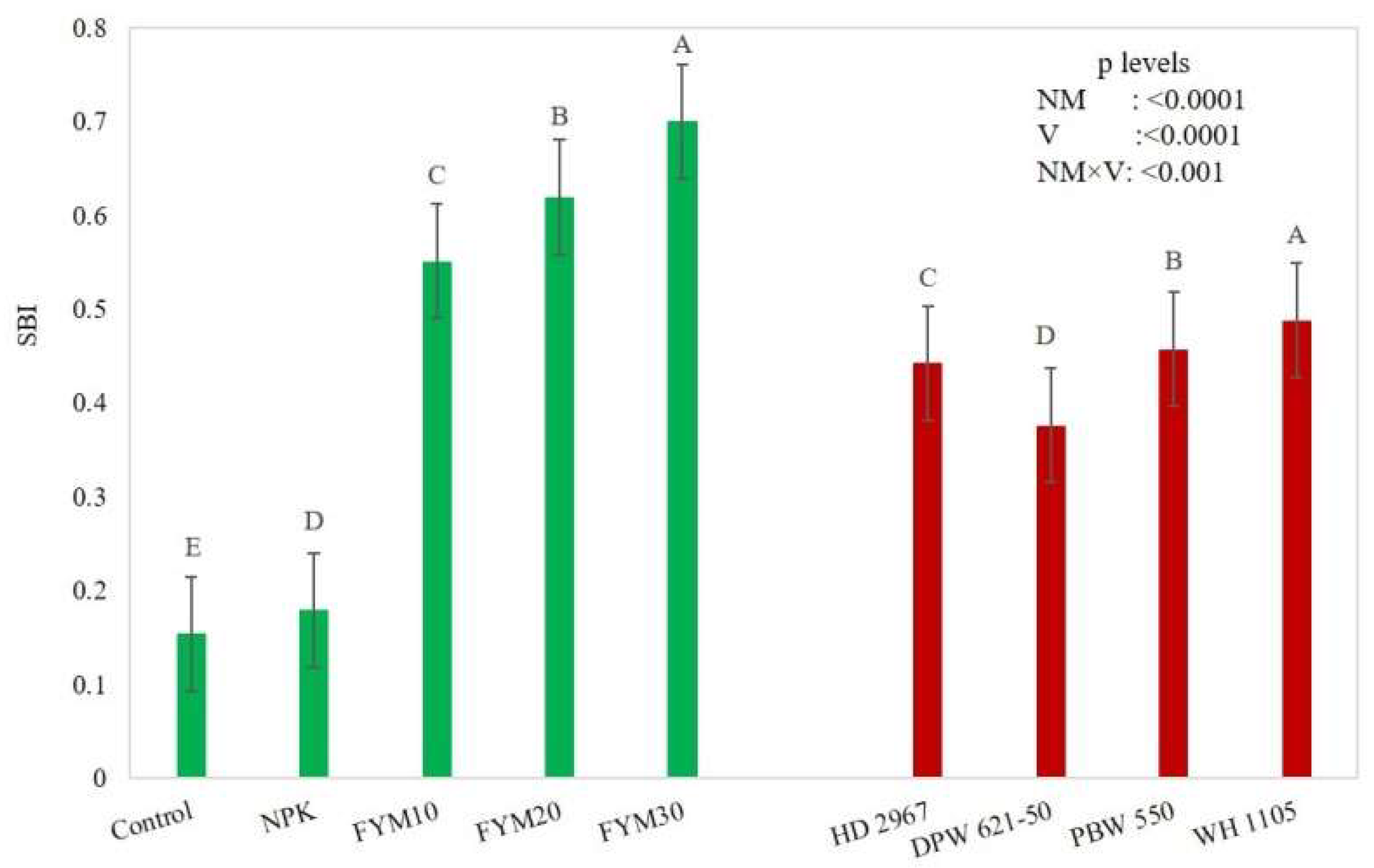
3.5. Soil Biological Index (SBI)
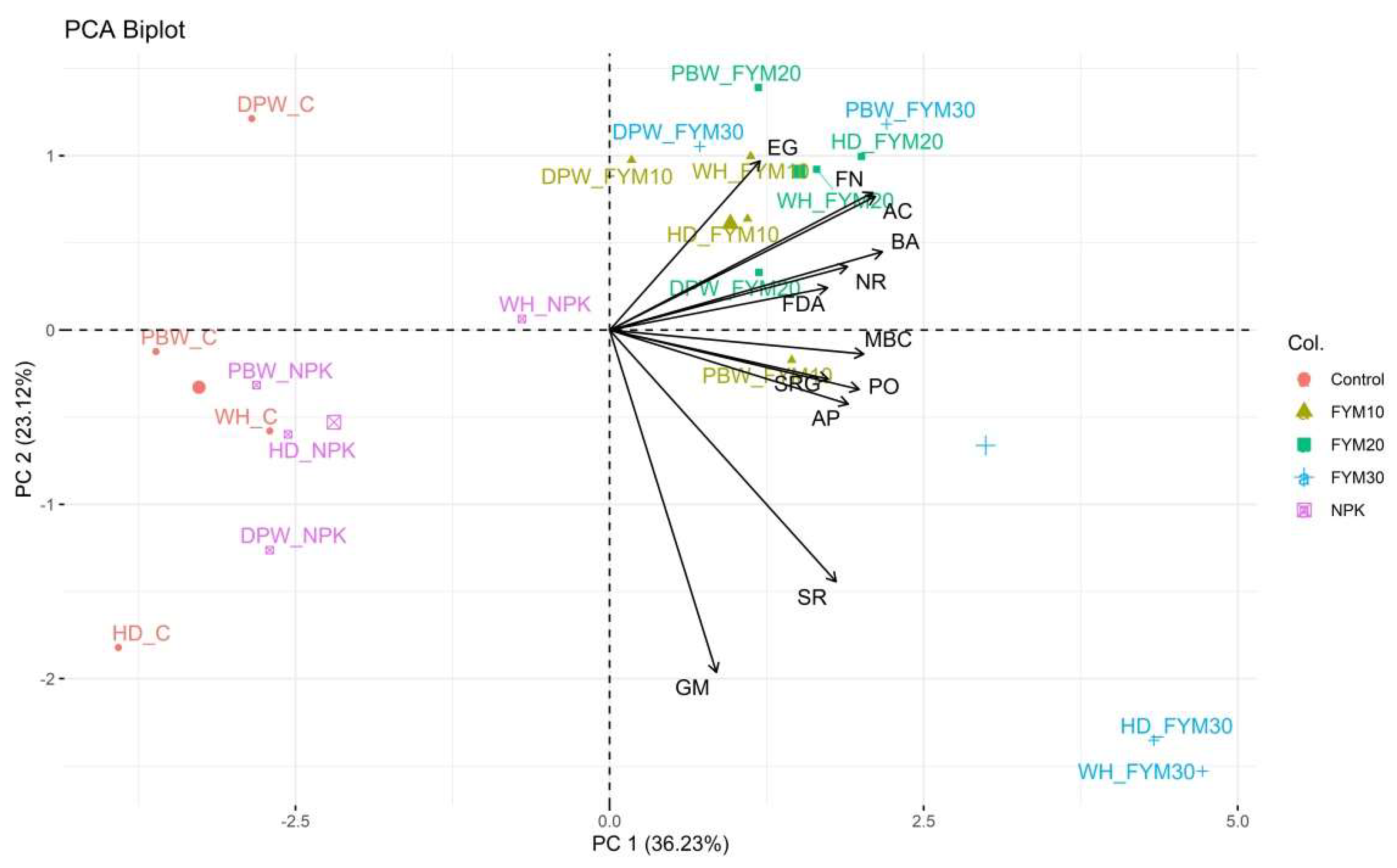
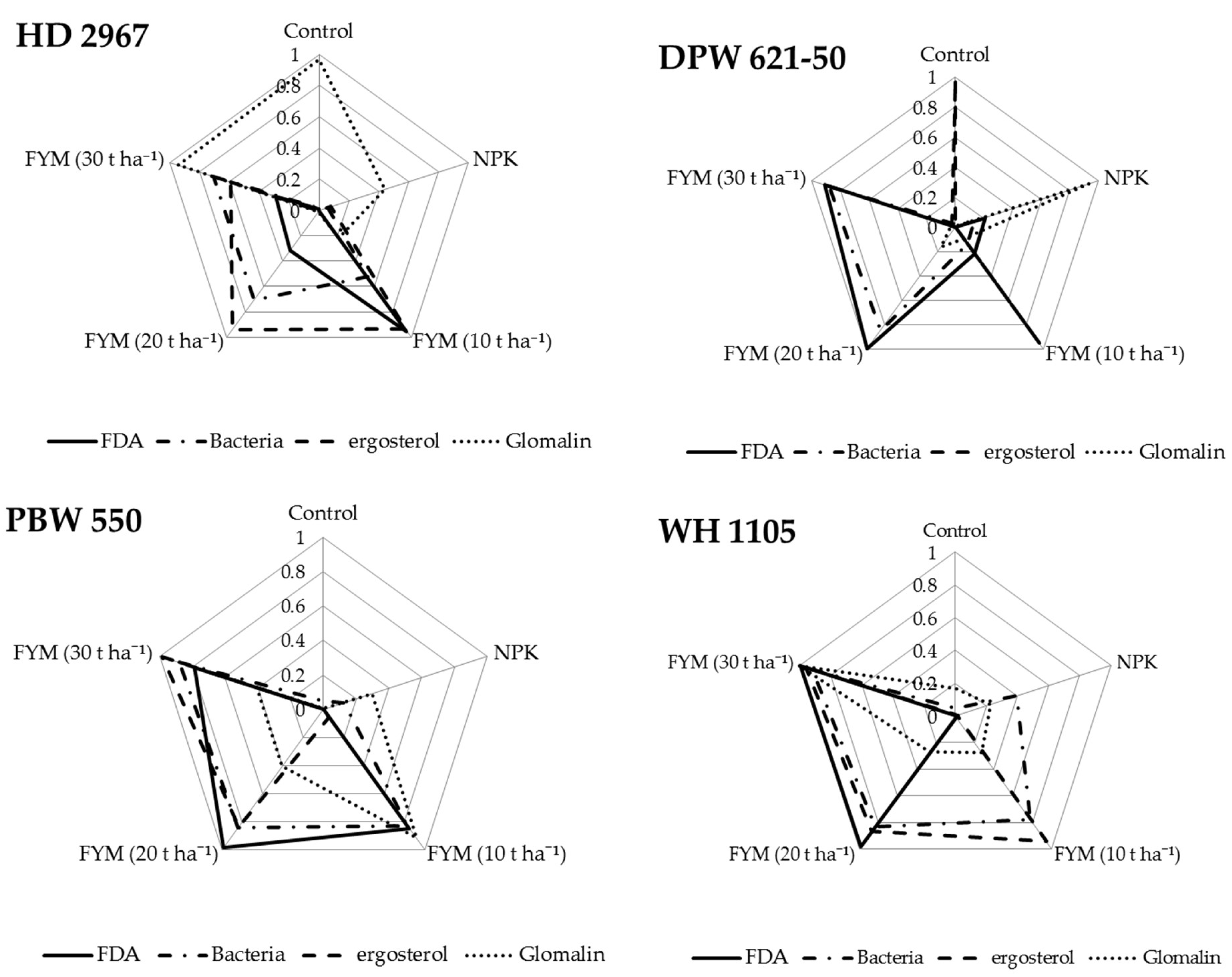
3.6. Interrelationship between SBI and Soil Biological Properties
4. Discussion
4.1. Crop Yield and Sustainability Yield Index
4.2. Microbial Biomass Carbon (MBC) and Microbial Population
4.3. Soil Biological Indicators
4.4. Soil Biological Index
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vance, E.; Brookes, P.; Jenkinson, D. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Chandra, P.; Dhuli, P.; Verma, P.; Singh, A.; Choudhary, M.; Prajapat, K.; Rai, A.K.; Yadav, R.K. Culturable microbial diversity in the rhizosphere of different biotypes under variable salinity. Trop. Ecol. 2020, 61, 291–300. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties, 5.2; SSSA Book Series; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Ali Tabatabai, A.W., Eds.; Wiley: Hoboken, NJ, USA, 1994; pp. 775–833. ISBN 9780891188650. [Google Scholar]
- Sinsabaugh, R. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Han, J.-G.; Dou, L.; Zhu, Y.; Li, P. Estimates of potential nitrate reductase activity in sediments: Comparisons of two incubation methods and four inhibitors. Environ. Earth Sci. 2013, 71, 419–425. [Google Scholar] [CrossRef]
- Stotzky, G. Microbial Respiration. In Methods of Soil Analysis; Agronomy Monographs; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 2016; pp. 1550–1572. ISBN 9780891182047. [Google Scholar]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Young, J.C. Microwave-assisted extraction of the fungal metabolite ergosterol and total fatty acids. J. Agric. Food Chem. 1995, 43, 2904–2910. [Google Scholar] [CrossRef]
- Green, V.; Stott, D.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.; Mitchell, J. A comparison of soil quality indexing methods for vegetable production systems in Northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Bastida, F.; Moreno, J.; Hernández, T.; Garcia, C. Microbiological degradation index of soils in a semiarid climate. Soil Biol. Biochem. 2006, 38, 3463–3473. [Google Scholar] [CrossRef]
- Barman, A.; Pandey, R.N.; Singh, B.; Das, B. Manganese deficiency in wheat genotypes: Physiological responses and manganese deficiency tolerance index. J. Plant Nutr. 2017, 40, 2691–2708. [Google Scholar] [CrossRef]
- Dutta, J.; Sharma, S.P.; Sharma, S.K.; Sharma, G.D.; Sankhyan, N.K. Indexing soil quality under long-term maize-wheat cropping system in an acidic alfisol. Commun. Soil Sci. Plant Anal. 2015, 46, 1841–1862. [Google Scholar] [CrossRef]
- Ljubičić, N.; Popović, V.; Ćirić, V.; Kostić, M.; Ivošević, B.; Popović, D.; Pandžić, M.; El Musafah, S.; Janković, S. Multivariate Interaction Analysis of Winter Wheat Grown in Environment of Limited Soil Conditions. Plants 2021, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Ikanović, J.; Popović, D.; Popović, V.; Jaćimović, G.; Đurović, I.; Kolarić, L.; Ćosić, M.; Rakaščan, N. Analysis of Genotype-by-Year interaction for Secale cereale L. productive traits and circular economy. Agric. For. 2022, 68, 297–317. [Google Scholar] [CrossRef]
- Popović, V.; Ljubičić, N.; Kostić, M.; Radulović, M.; Blagojević, D.; Ugrenović, V.; Popović, D.; Ivošević, B. Genotype × Environment Interaction for Wheat Yield Traits Suitable for Selection in Different Seed Priming Conditions. Plants 2020, 9, 1804. [Google Scholar] [CrossRef]
- Prajapat, K.; Vyas, A.K.; Dhar, S. Productivity, profitability and land-use efficiency of soybean (Glycine max)-based cropping systems under different nutrient-management practices. Indian J. Agron. 2014, 59, 229–234. [Google Scholar]
- Uwah, D.; Eneji, A.; Uj, E. Organic and mineral fertilizers effects on the performance of sweet Maize (Zea Mays L. Saccharata Strut) in South Eastern rainforest zone of Nigeria. Int. J. Agric. Sci. 2011, 3, 54–61. [Google Scholar] [CrossRef]
- Behera, U.K.; Sharma, A.R.; Pandey, H.N. Sustaining productivity of wheat–soybean cropping system through integrated nutrient management practices on the Vertisols of central India. Plant Soil 2007, 297, 185–199. [Google Scholar] [CrossRef]
- Srinivasrao, C.; Kundu, S.; Grover, M.; Manjunath, M.; Kasbe, S.; Patel, J.; Singh, S.; Singh, R.; Patel, M.; Arunachalam, A.; et al. Effect of long term application of organic and inorganic fertilizers on soil microbial activities in semi-arid and sub-humid rainfed agricultural systems. Trop. Ecol. 2018, 59, 99–108. [Google Scholar]
- Bandyopadhyay, K.; Misra, A.K.; Ghosh, P.K.; Hati, K. Effect of integrated use of farmyard manure and chemical fertilizers on soil physical properties and productivity of soybean. Soil Tillage Res. 2010, 110, 115–125. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Gebremikael, M.T.; Wu, H.; Cai, D.; Wang, B.; Li, B.; Zhang, J.; Li, Y.; Xi, J. Response of soil organic carbon fractions, microbial community composition and carbon mineralization to high-input fertilizer practices under an intensive agricultural system. PLoS ONE 2018, 13, e0195144. [Google Scholar] [CrossRef] [Green Version]
- Moharana, P.; Sharma, B.M.; Biswas, D.; Dwivedi, B.; Singh, R. V Long-term effect of nutrient management on soil fertility and soil organic carbon pools under a 6-year-old pearl millet-wheat cropping system in an Inceptisol of subtropical India. F. Crop. Res. 2012, 136, 32–41. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-term effect of manure and fertilizer on soil organic carbon pools in dryland farming in Northwest China. PLoS ONE 2013, 8, e56536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, A.; de Melo, W.J. Soil microbial biomass in organic farming system. Ciência Rural 2010, 40, 2419–2426. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Zheljazkov, V.D.; Gollany, H.T.; Kleber, M.; Xing, B.; Astatkie, T. Macronutrient in soils and wheat from long-term agroexperiments reflects variations in residue and fertilizer inputs. Sci. Rep. 2020, 10, 3263. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, G.; Tripathi, S.; Singh, A.K. Litterfall, litter decomposition and nutrient dynamics in a subtropical natural oak forest and managed plantation in Northeastern India. For. Ecol. Manage. 2007, 240, 96–104. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.P.; Prabha, R.; Renu, S.; Sahu, P.K.; Singh, V. Agrowaste bioconversion and microbial fortification have prospects for soil health, crop productivity, and eco-enterprising. Int. J. Recycl. Org. Waste Agric. 2019, 8, 457–472. [Google Scholar] [CrossRef] [Green Version]
- Tazisong, I.; Senwo, Z.; He, Z. Phosphatase Hydrolysis of Organic Phosphorus Compounds. Adv. Enzym. Res. 2015, 3, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.K.; Dinkar, A.; Basak, N.; Dixit, A.K.; Das, S.K.; Dev, I.; Sundha, P.; Chandra, P.; Kumar, S. Phosphorus nutrition of oats genotypes in acidic soils: Exploiting responsive plant-microbe partnership. Appl. Soil Ecol. 2021, 167, 104094. [Google Scholar] [CrossRef]
- Machado, D.; Pereira, M.; Santos, L.; Diniz, A.; Guareschi, R. Organic matter and soil fertility in different successional stages of seasonal semidecidual forest. Rev. Caatinga 2019, 32, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Clark, I.M.; Buchkina, N.; Jhurreea, D.; Goulding, K.W.T.; Hirsch, P.R. Impacts of nitrogen application rates on the activity and diversity of denitrifying bacteria in the Broadbalk Wheat Experiment. Philos. Trans. Biol. Sci. 2012, 367, 1235–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, M.; Morley, N.; Baggs, E.; Daniell, T. Soil nitrate reducing processes–drivers, mechanisms for spatial variation, and significance for nitrous oxide production. Front. Microbiol. 2012, 3, 407. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Marschner, P. Soil respiration, microbial biomass and nutrient availability in soil after repeated addition of low and high C/N plant residues. Biol. Fertil. Soils 2016, 52, 165–176. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, D.; Sharma, P. Effect of organics, biofertilizers and crop residue application on soil microbial activity in rice–wheat and rice-wheat mungbean cropping systems in the Indo-Gangetic plains. Cogent Geosci. 2015, 1, 1085296. [Google Scholar] [CrossRef]
- Treseder, K.; Turner, K. Glomalin in Ecosystems. Soil Sci. Soc. Am. J.-SSSAJ 2007, 71, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Bhanwaria, R.; Singh, B.; Musarella, C.M. Effect of organic manure and moisture regimes on soil physiochemical properties, microbial biomass Cmic:Nmic:Pmic turnover and yield of mustard grains in arid climate. Plants 2022, 11, 722. [Google Scholar] [CrossRef]
- Quilliam, R.; Hodge, A.; Jones, D. Sporulation of arbuscular mycorrhizal fungi in organic-rich patches following host excision. Appl. Soil Ecol. 2010, 46, 247–250. [Google Scholar] [CrossRef]
- Angela, H.; Alastair, H.F. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Bhattacharyya, R.; Das, T.K.; Sharma, A.; Ghosh, A.; Das, S.; Jha, P. Crop rotation and residue management effects on soil enzyme activities, glomalin and aggregate stability under zero tillage in the Indo-Gangetic Plains. Soil Tillage Res. 2018, 184, 291–300. [Google Scholar] [CrossRef]
- Thirkell, T.; Cameron, D.; Hodge, A. Contrasting nitrogen fertilisation rates alter mycorrhizal contribution to barley nutrition in a field trial. Front. Plant Sci. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Mille-Lindblom, C.; Wachenfeldt, E.; Tranvik, L. Ergosterol as a measure of living fungal biomass: Persistence in environmental samples after fungal death. J. Microbiol. Methods 2004, 59, 253–262. [Google Scholar] [CrossRef]
- Salazar Alekseyeva, K.; Mähnert, B.; Berthiller, F.; Breyer, E.; Herndl, G.J.; Baltar, F. Adapting an Ergosterol Extraction Method with Marine Yeasts for the Quantification of Oceanic Fungal Biomass. J. Fungi 2021, 7, 690. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Martinez, M.; Gutiérrez-Romero, V.; Jannsens, M.; Ortega-Blu, R. Biological soil quality indicators: A review. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Microbiology Series Nº 2; FORMATEX: Badajoz, Spain, 2010; Volume 2, pp. 319–328. ISBN -13 978-84-614-6195-0. [Google Scholar]
- Stockdale, E.A.; Watson, C.A. Biological indicators of soil quality in organic farming systems. Renew. Agric. Food Syst. 2009, 24, 308–318. [Google Scholar] [CrossRef]
- Lal, R. Soil organic matter and water retention. Agron. J. 2020, 112, 3265–3277. [Google Scholar] [CrossRef]
- Veum, K.; Goyne, K.; Kremer, R.; Miles, R.; Sudduth, K. Biological indicators of soil quality and soil organic matter characteristics in an agricultural management continuum. Biogeochemistry 2014, 117, 81–99. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amezaga, I.; Garbisu, C. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef]



| HD 2967 | DPW 650-21 | PBW 550 | WH 1105 | ||
|---|---|---|---|---|---|
| 1. | Morphological features | ||||
| i. | Plant height (cm) | 98 | 97 | 86 | 99 |
| ii. | Days to maturity (days) | 143 | 144 | 135 | 142 |
| iii. | 1000-grain weight (g) | 42.1 | 38 | 39.1 | 41.1 |
| iv. | Average yield (t ha−1) | 5.04 | 5.13 | 4.77 | 5.25 |
| v. | Potential yield (t ha−1) | 6.61 | 6.98 | 6.24 | 7.16 |
| 2. | Special features |
|
|
|
|
| Treatments | MBC (μg MBC g−1 Soil) | BA (CFUs × 105 g−1 Soil) | FN (CFUs × 102 g−1 Soil) | AC (CFUs × 103 g−1 Soil) |
|---|---|---|---|---|
| Nutrient management (NM) | ||||
| Control | 139.18 E | 124.33 E | 49.67 C | 42.83 C |
| NPK | 205.81 D | 159.25 D | 62.25 B | 64.33 B |
| FYM10 | 316.04 C | 214.92 C | 87.83 A | 87.58 A |
| FYM20 | 465.24 B | 250.67 B | 87.42A | 88.92 A |
| FYM30 | 707.49 A | 264.58 A | 85.75 A | 88.00 A |
| Cultivar (V) | ||||
| HD 2967 | 448.88 A | 183.27 B | 68.33 C | 66.80 B |
| DPW 621-50 | 276.69 B | 191.47 B | 73.47 B | 75.27 A |
| PBW 550 | 273.52 B | 214.20 A | 76.20 AB | 75.47 A |
| WH 1105 | 467.92 A | 220.07 A | 80.33 A | 79.80 A |
| NM | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| V | <0.0001 | <0.0001 | NS | NS |
| NM×V | <0.0001 | <0.0001 | NS | NS |
| AP (μg pNPP g−1 Soil h−1) | PO (Units µg−1) | NR (µgml−1 h−1) | SR (mg CO2-C g−1 Soil Week−1) | SRG (mg CO2-C g−1 Soil Week−1) | |
|---|---|---|---|---|---|
| Nutrient management (NM) | |||||
| Control | 21.89 E | 1669.14 D | 7.80 D | 45.31 C | 59.94 E |
| NPK | 38.58 D | 1437.61 E | 8.85 CD | 41.25 D | 68.35 D |
| FYM10 | 64.09 B | 2283.88 C | 11.64 BC | 69.60 A | 73.19 C |
| FYM20 | 52.48 C | 2598.25 B | 13.04 AB | 60.22 B | 79.48 B |
| FYM30 | 79.18 A | 2812.12 A | 15.42 A | 68.84 A | 107.30 A |
| Cultivar (V) | |||||
| HD 2967 | 65.46 A | 2424.99 A | 11.11 B | 66.55 A | 86.47 A |
| DPW 621-50 | 52.50 B | 1890.91 C | 9.55 B | 47.04 D | 67.86 C |
| PBW 550 | 42.99 C | 2261.14 A | 10.58 B | 51.06 C | 70.23 B |
| WH 1105 | 44.02 C | 2063.76 B | 14.15 A | 63.52 B | 86.05 A |
| NM | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| V | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| NM×V | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatments | FDA (µg Fluoresceing−1 Soil h−1) | Glomalin (µg kg−1) | Ergosterol (µg g−1 Soil) |
|---|---|---|---|
| Nutrient management (NM) | |||
| Control | 1.1 E | 28.73 C | 12.55 C |
| NPK | 1.4 D | 35.18 B | 7.57 D |
| FYM10 | 2.74 C | 32.36 B | 16.37 B |
| FYM20 | 4.32 A | 26.36 C | 18.74 A |
| FYM30 | 3.63 BA | 46.78 A | 19.39 A |
| Cultivar (V) | |||
| HD 2967 | 2.18 B | 36.75 B | 16.30 A |
| DPW 621-50 | 2.81 A | 27.68 D | 15.12 B |
| PBW 550 | 2.74 A | 31.59 C | 14.47 BC |
| WH 1105 | 2.83A | 39.51 A | 13.80 C |
| NM | <0.0001 | <0.0001 | <0.0001 |
| V | <0.0001 | <0.0001 | <0.0001 |
| NM×V | <0.0001 | <0.0001 | <0.0001 |
| PCs | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigen value | 4.35 | 2.77 | 2.36 |
| Percent variance | 36.23 | 23.12 | 19.68 |
| Cumulative percentage | 36.23 | 59.35 | 79.02 |
| Factor loading/eigen vector | |||
| FDA | 0.82 | 0.02 | 0.19 |
| MBC | 0.61 | 0.39 | 0.39 |
| AP | 0.59 | 0.26 | 0.48 |
| GM | 0.03 | −0.07 | 0.87 |
| EG | 0.09 | 0.92 | −0.14 |
| NR | 0.39 | 0.78 | 0.20 |
| PO | 0.44 | 0.56 | 0.47 |
| SRG | 0.25 | 0.68 | 0.41 |
| SR | 0.31 | 0.30 | 0.84 |
| BA | 0.92 | 0.23 | 0.19 |
| AC | 0.90 | 0.30 | 0.07 |
| FN | 0.89 | 0.32 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, P.; Gill, S.C.; Prajapat, K.; Barman, A.; Chhokar, R.S.; Tripathi, S.C.; Singh, G.; Kumar, R.; Rai, A.K.; Khobra, R.; et al. Response of Wheat Cultivars to Organic and Inorganic Nutrition: Effect on the Yield and Soil Biological Properties. Sustainability 2022, 14, 9578. https://doi.org/10.3390/su14159578
Chandra P, Gill SC, Prajapat K, Barman A, Chhokar RS, Tripathi SC, Singh G, Kumar R, Rai AK, Khobra R, et al. Response of Wheat Cultivars to Organic and Inorganic Nutrition: Effect on the Yield and Soil Biological Properties. Sustainability. 2022; 14(15):9578. https://doi.org/10.3390/su14159578
Chicago/Turabian StyleChandra, Priyanka, Subhash Chander Gill, Kailash Prajapat, Arijit Barman, Rajender Singh Chhokar, Subhash Chandra Tripathi, Geeta Singh, Raj Kumar, Arvind Kumar Rai, Rinki Khobra, and et al. 2022. "Response of Wheat Cultivars to Organic and Inorganic Nutrition: Effect on the Yield and Soil Biological Properties" Sustainability 14, no. 15: 9578. https://doi.org/10.3390/su14159578
APA StyleChandra, P., Gill, S. C., Prajapat, K., Barman, A., Chhokar, R. S., Tripathi, S. C., Singh, G., Kumar, R., Rai, A. K., Khobra, R., Jasrotia, P., & Singh, G. P. (2022). Response of Wheat Cultivars to Organic and Inorganic Nutrition: Effect on the Yield and Soil Biological Properties. Sustainability, 14(15), 9578. https://doi.org/10.3390/su14159578







