Electroless Deposits of ZnO and Hybrid ZnO/Ag Nanoparticles on Mg-Ca0.3 Alloy Surface: Multiscale Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Solution Preparation
2.2. Electroless Deposits of ZnO-NPs and Hybrid ZnO/Ag-NPs on Mg-Ca0.3 Surface and Their Characterization
3. Results and Discussion
3.1. Surface Characterization of Mg-Ca0.3
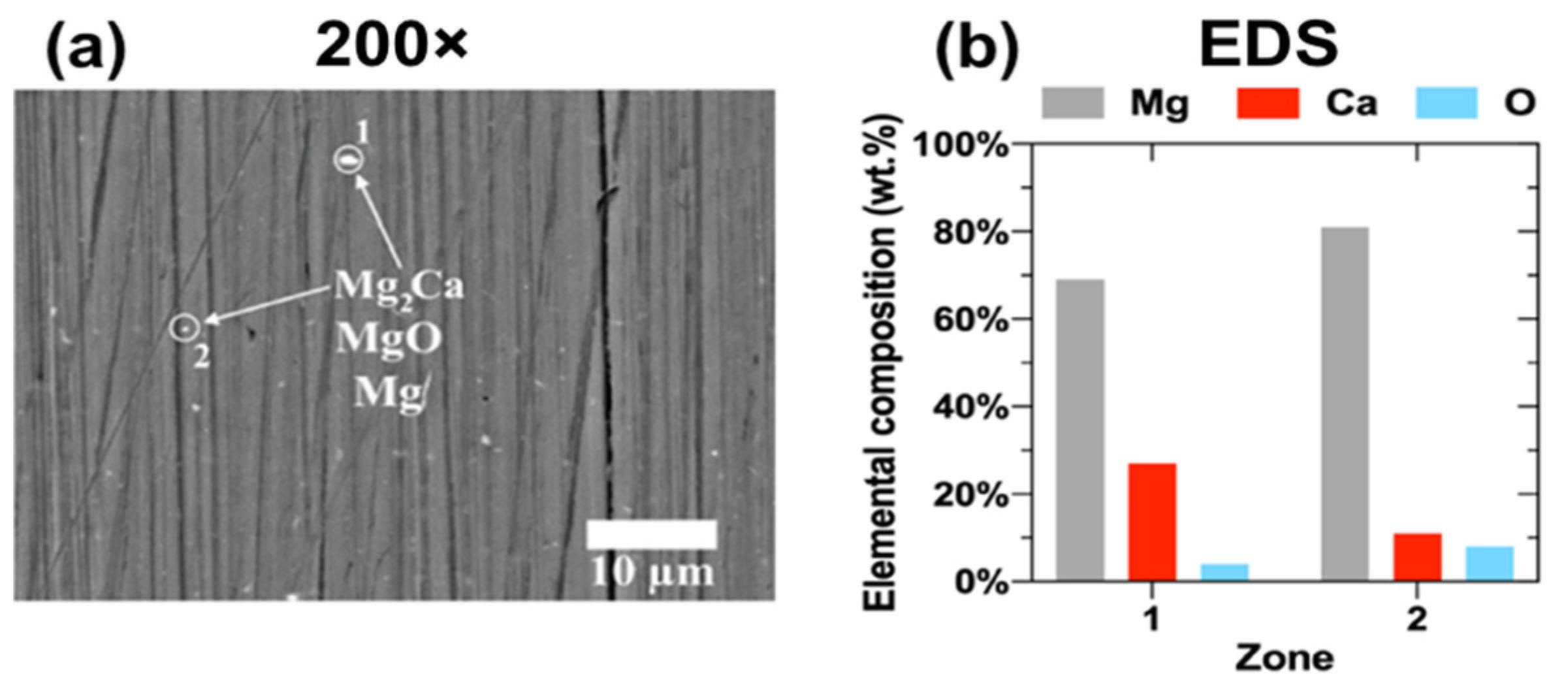
3.1.1. SEM-EDS Analysis
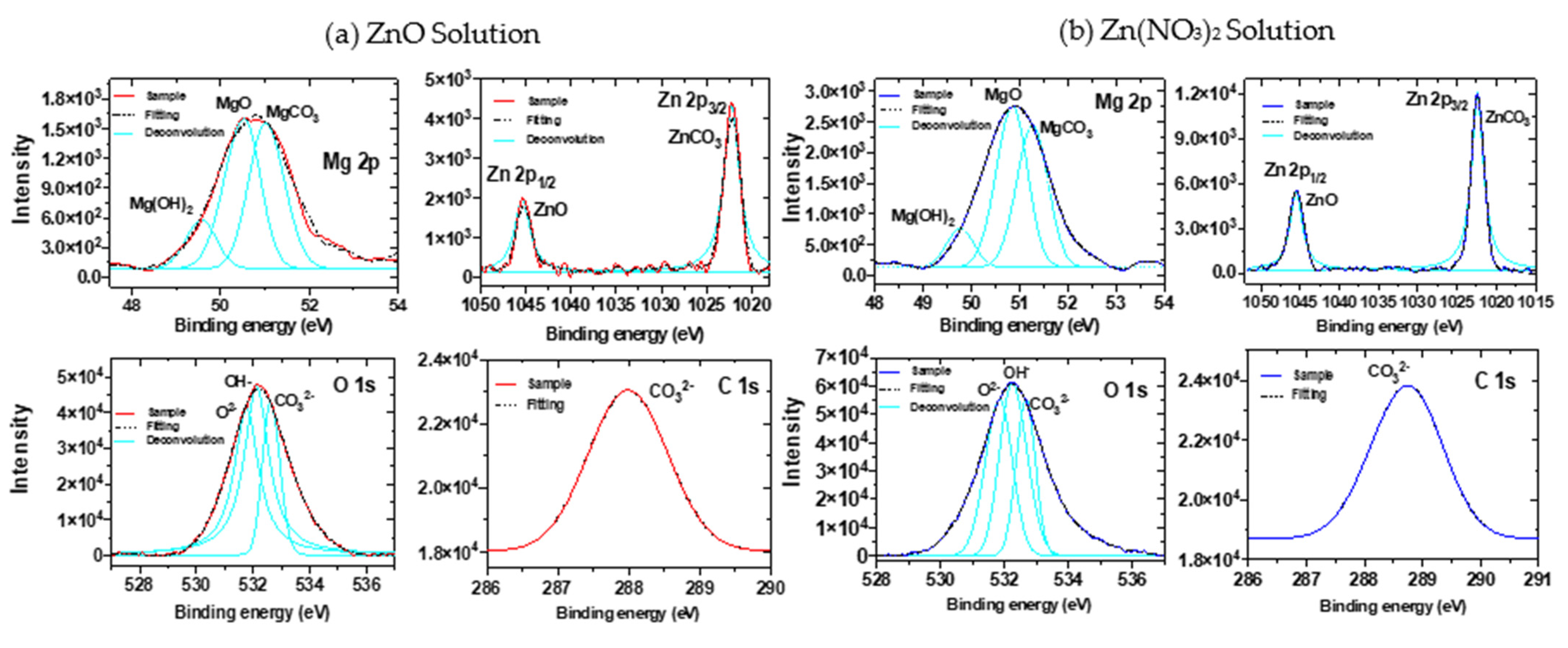
3.1.2. XPS Analysis
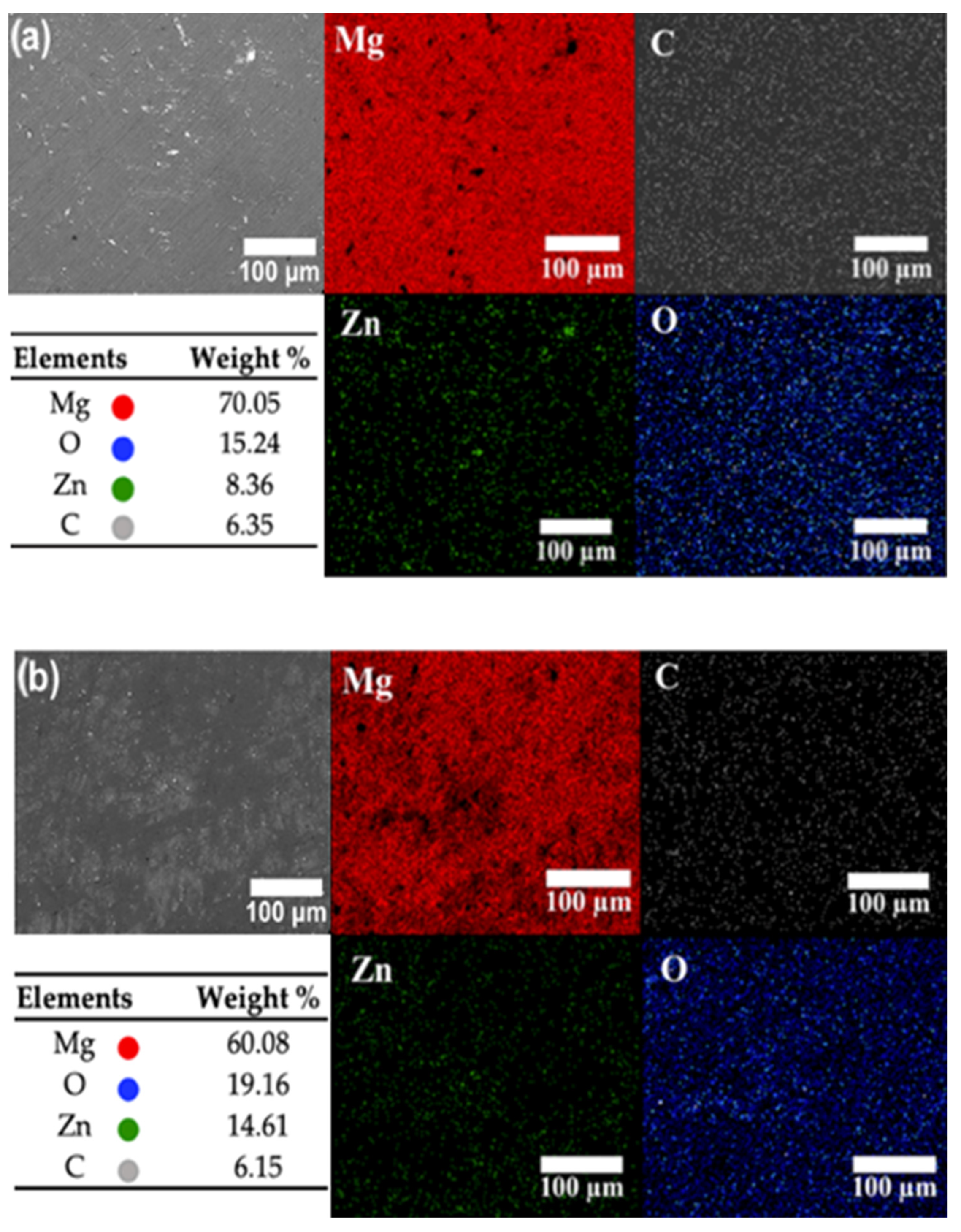
3.1.3. EDS Mapping of the Elements
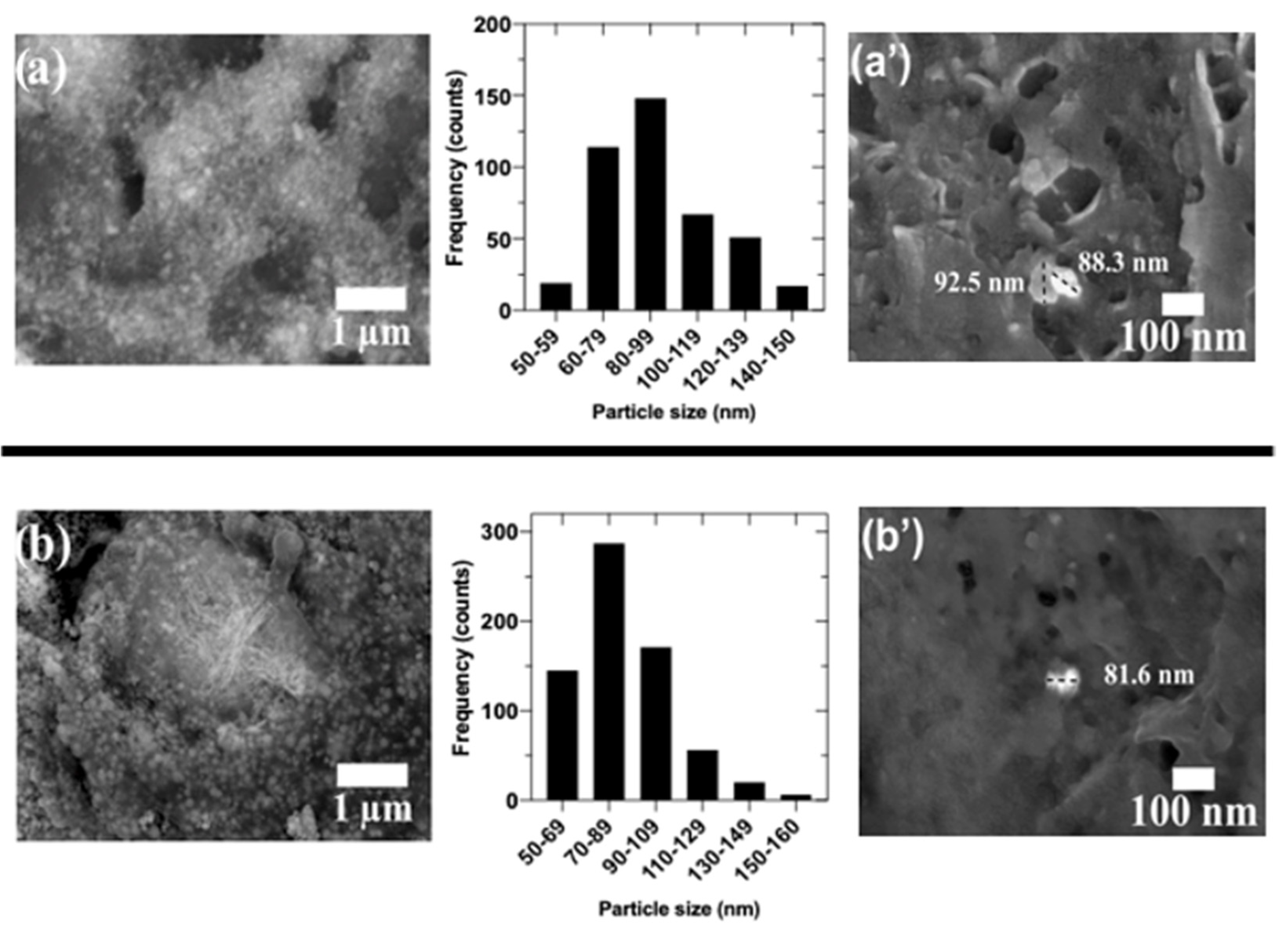
3.1.4. ZnO Nanoparticle Size Distribution
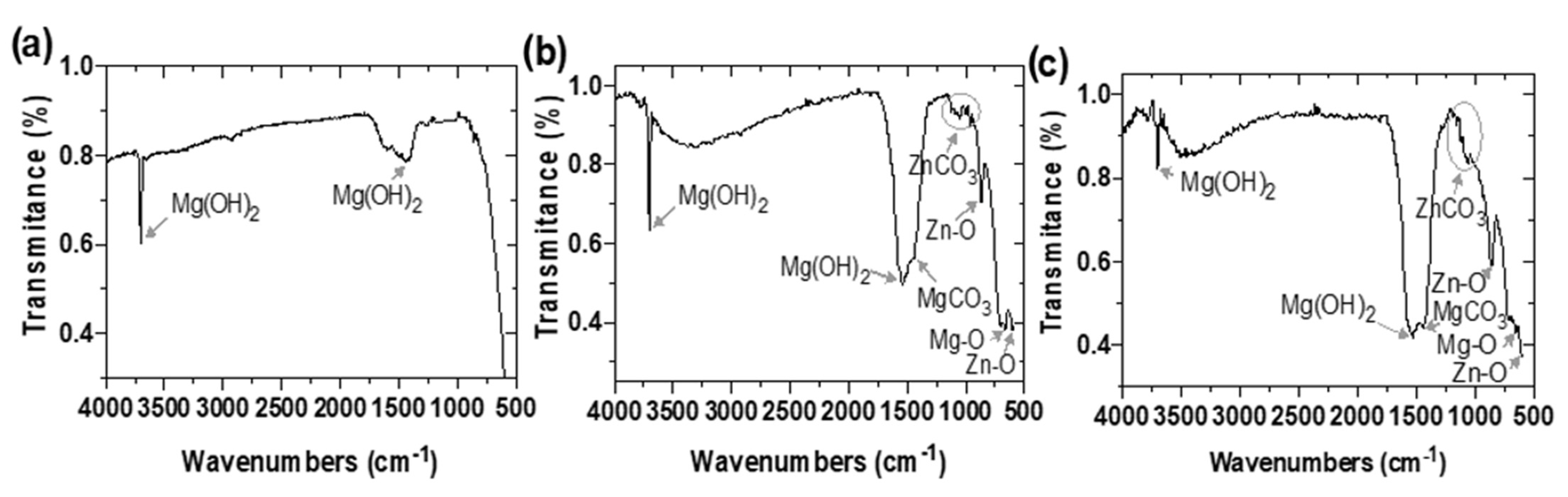
3.1.5. FTIR Analysis
3.1.6. UV-Vis Spectroscopy Analysis
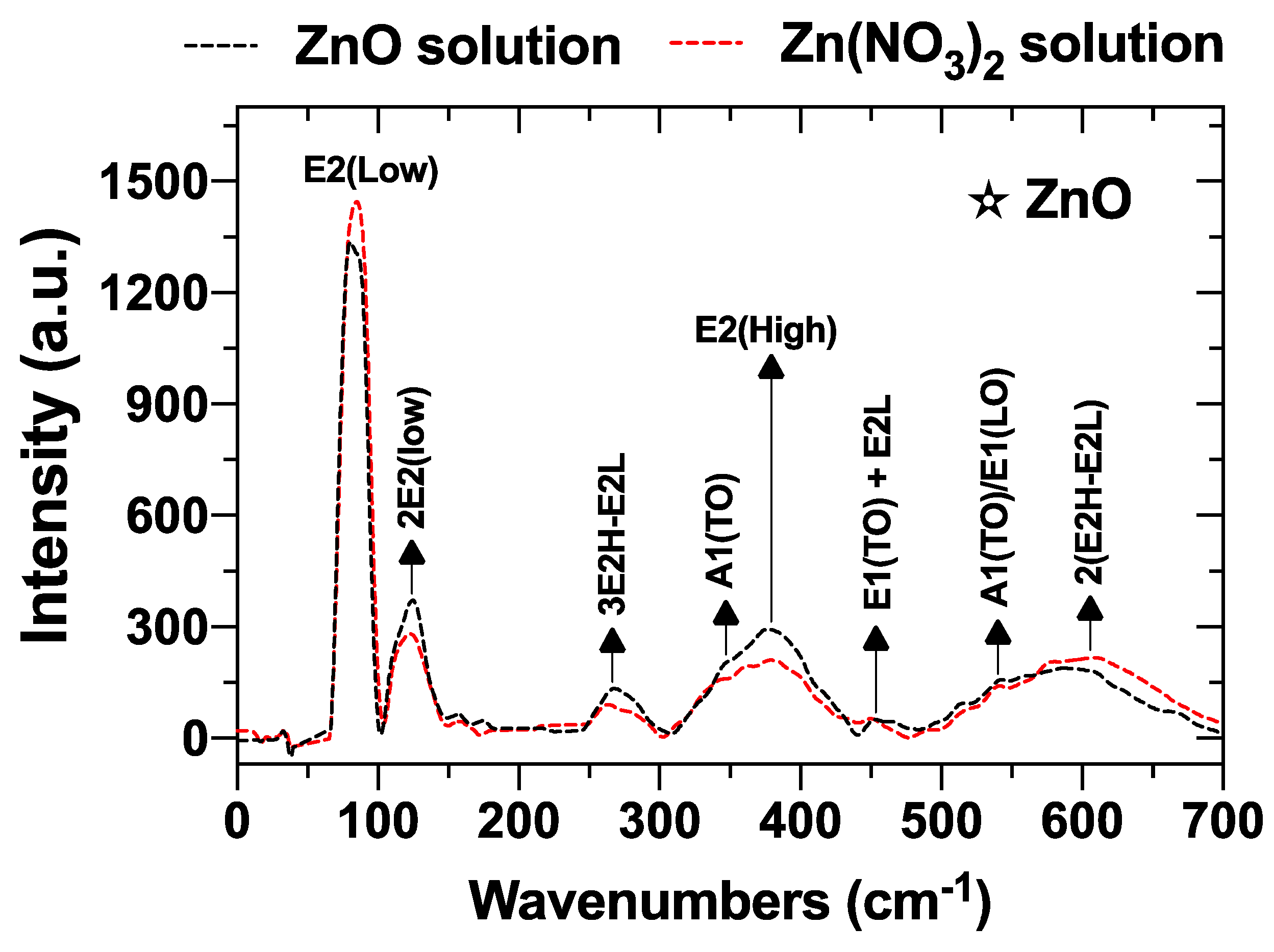
3.1.7. Raman Spectroscopy Analysis
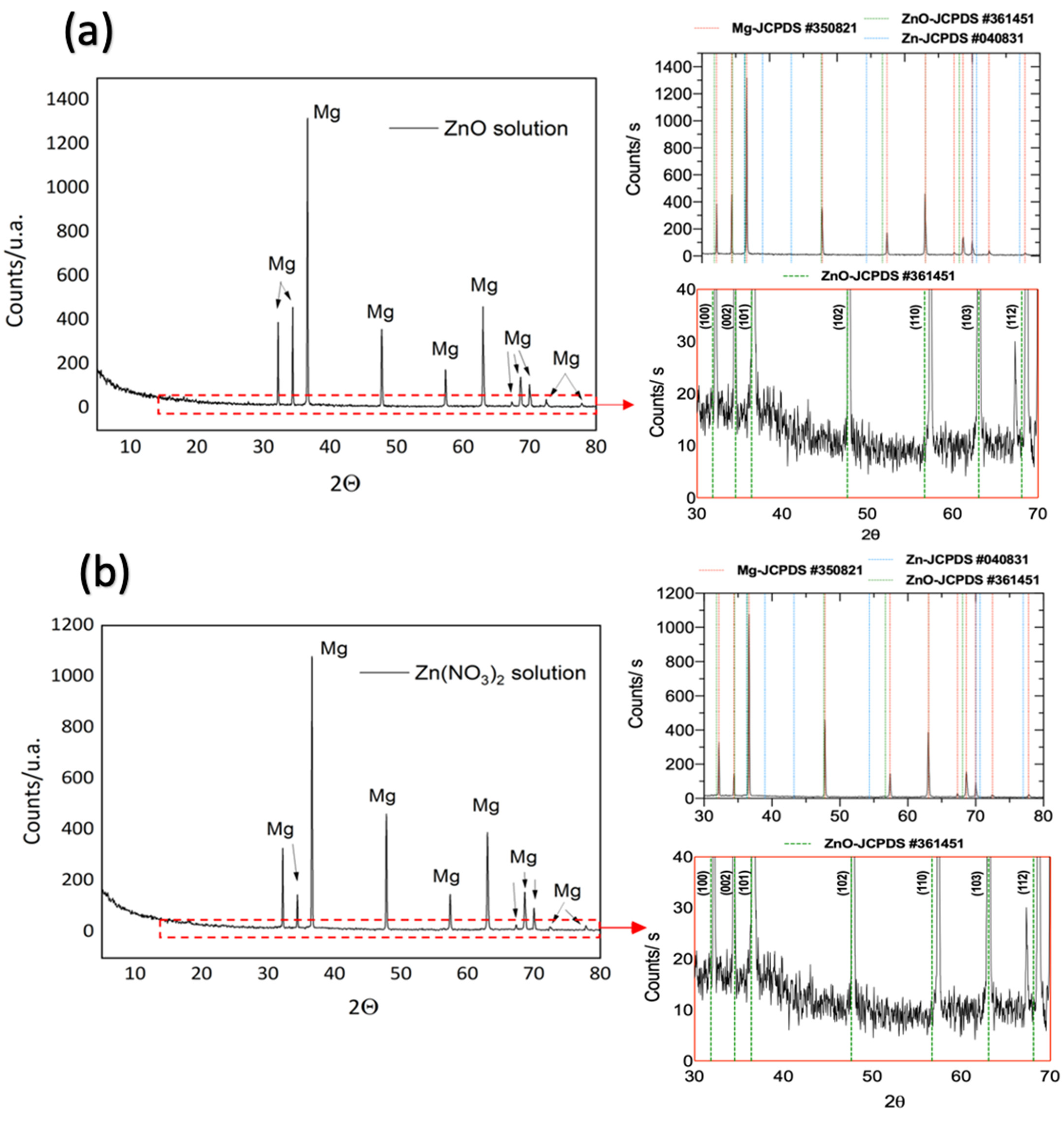
3.1.8. X-ray Diffraction Patterns
3.2. Characterization of the Hybrid ZnO/Ag Electroless Deposits on Mg-Ca0.3
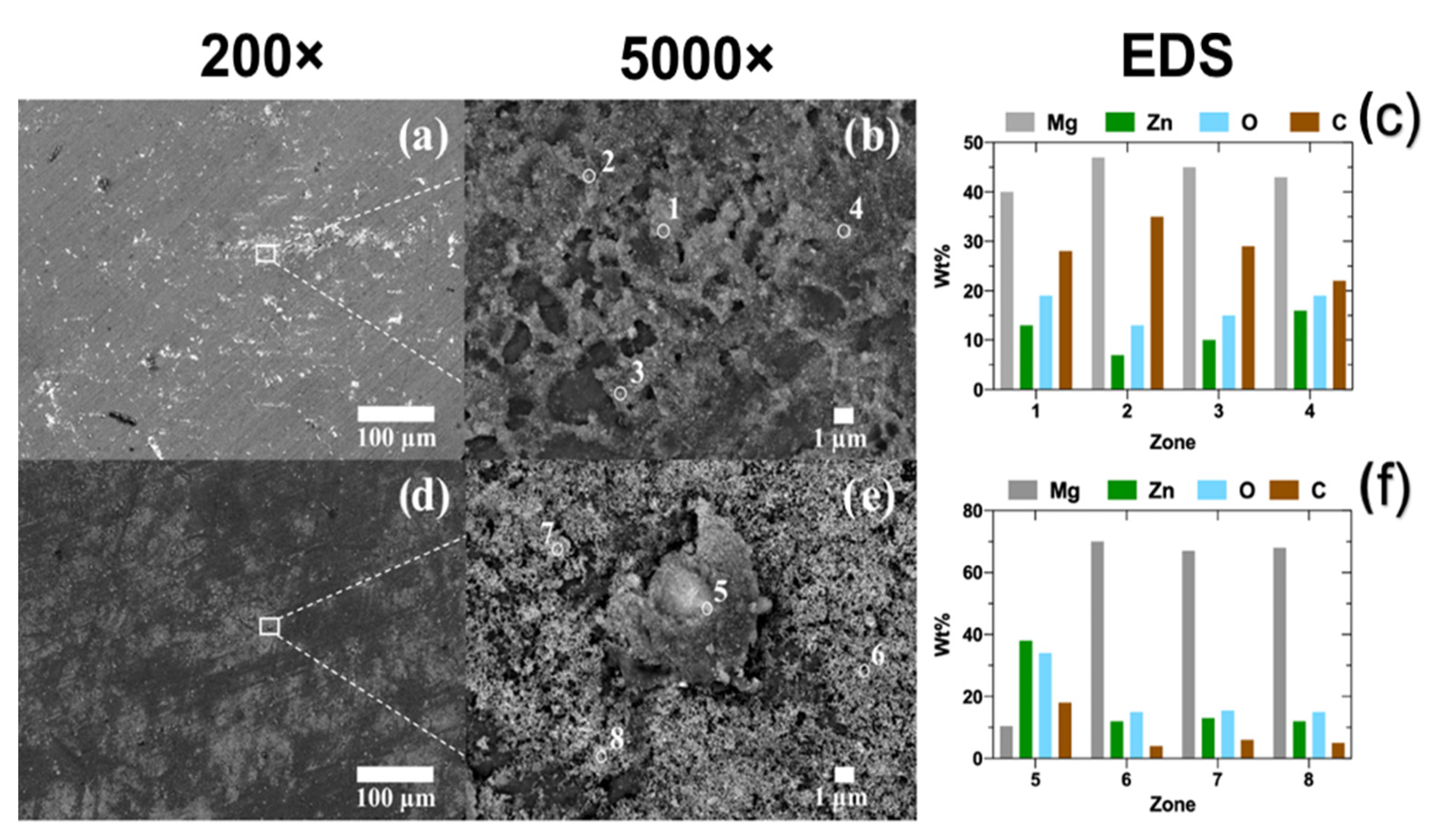
3.2.1. SEM-EDS Analysis
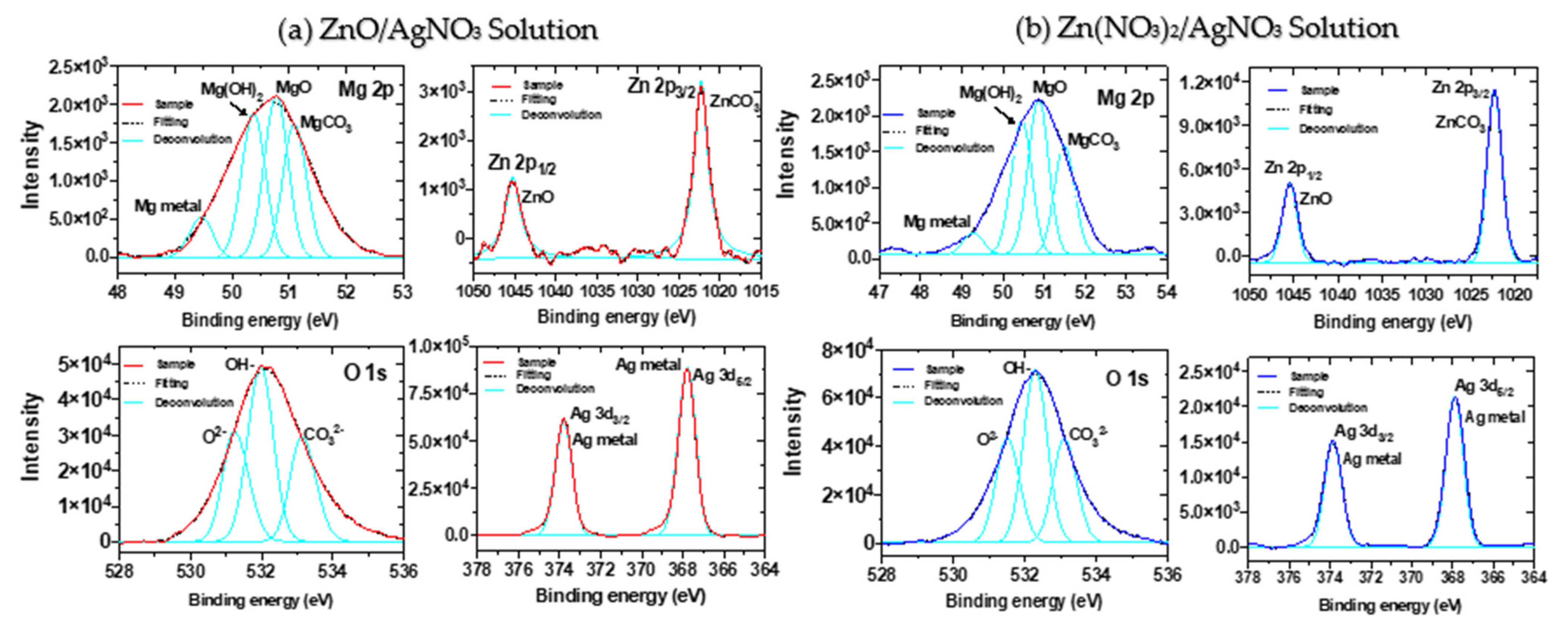
3.2.2. XPS Analysis
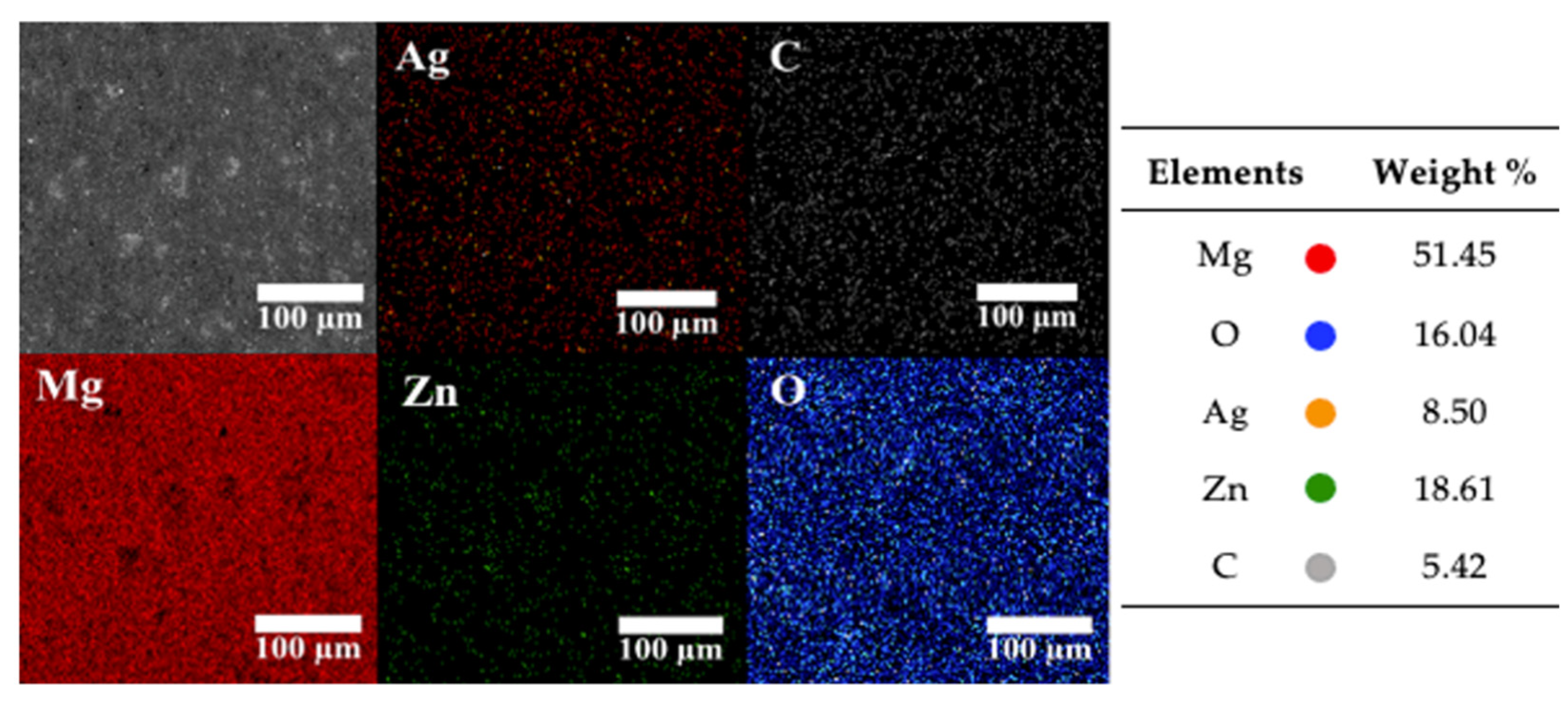
3.2.3. EDS Mapping of the Elements
3.2.4. Ag Nano-Particle Size Distribution
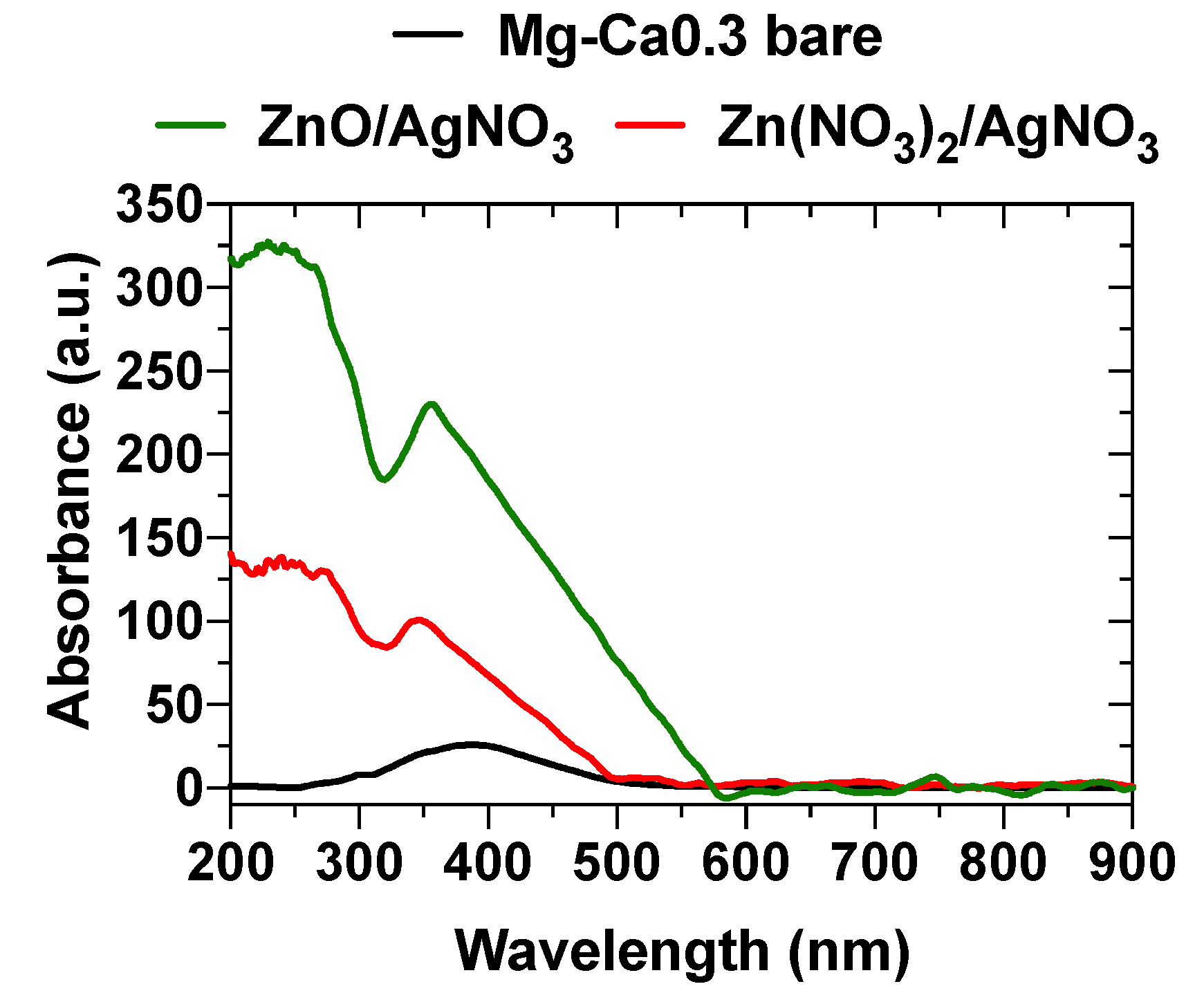
3.2.5. UV-Visible Spectroscopy Analysis
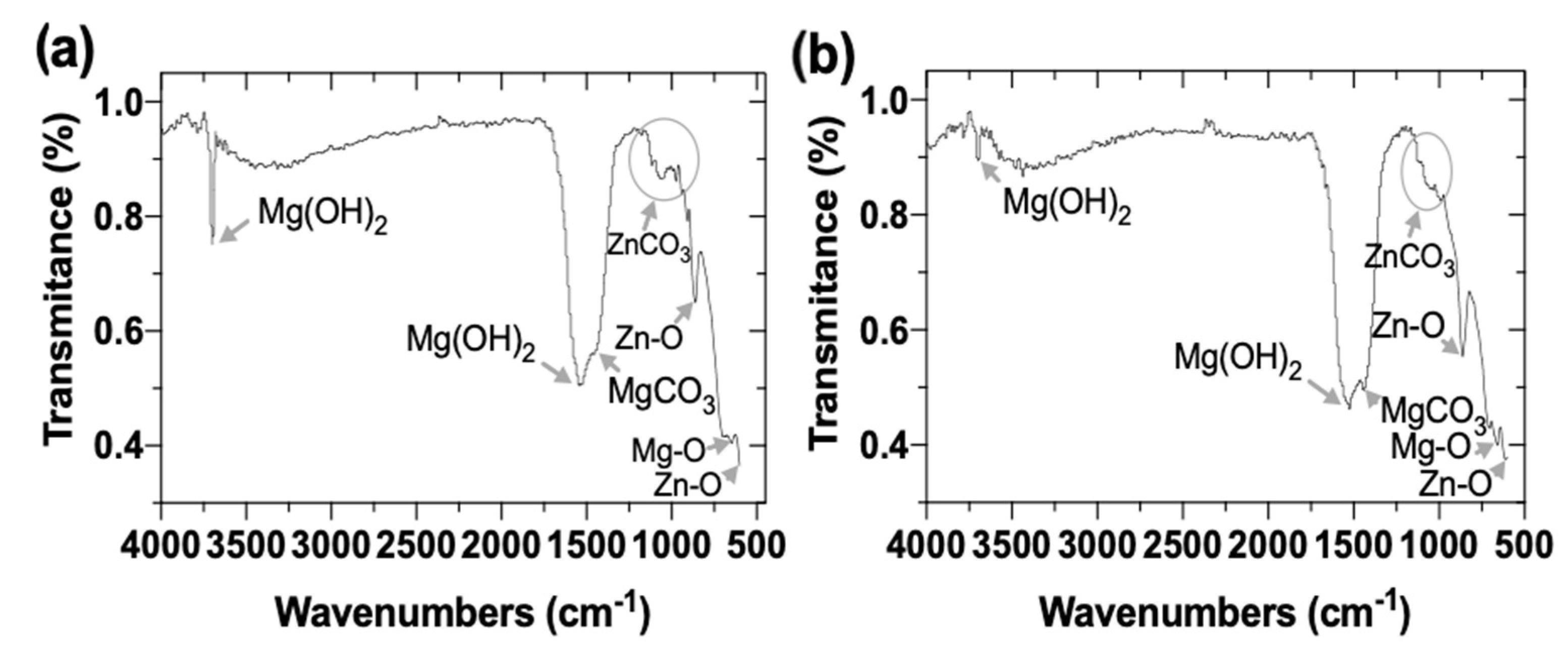
3.2.6. FTIR Analysis
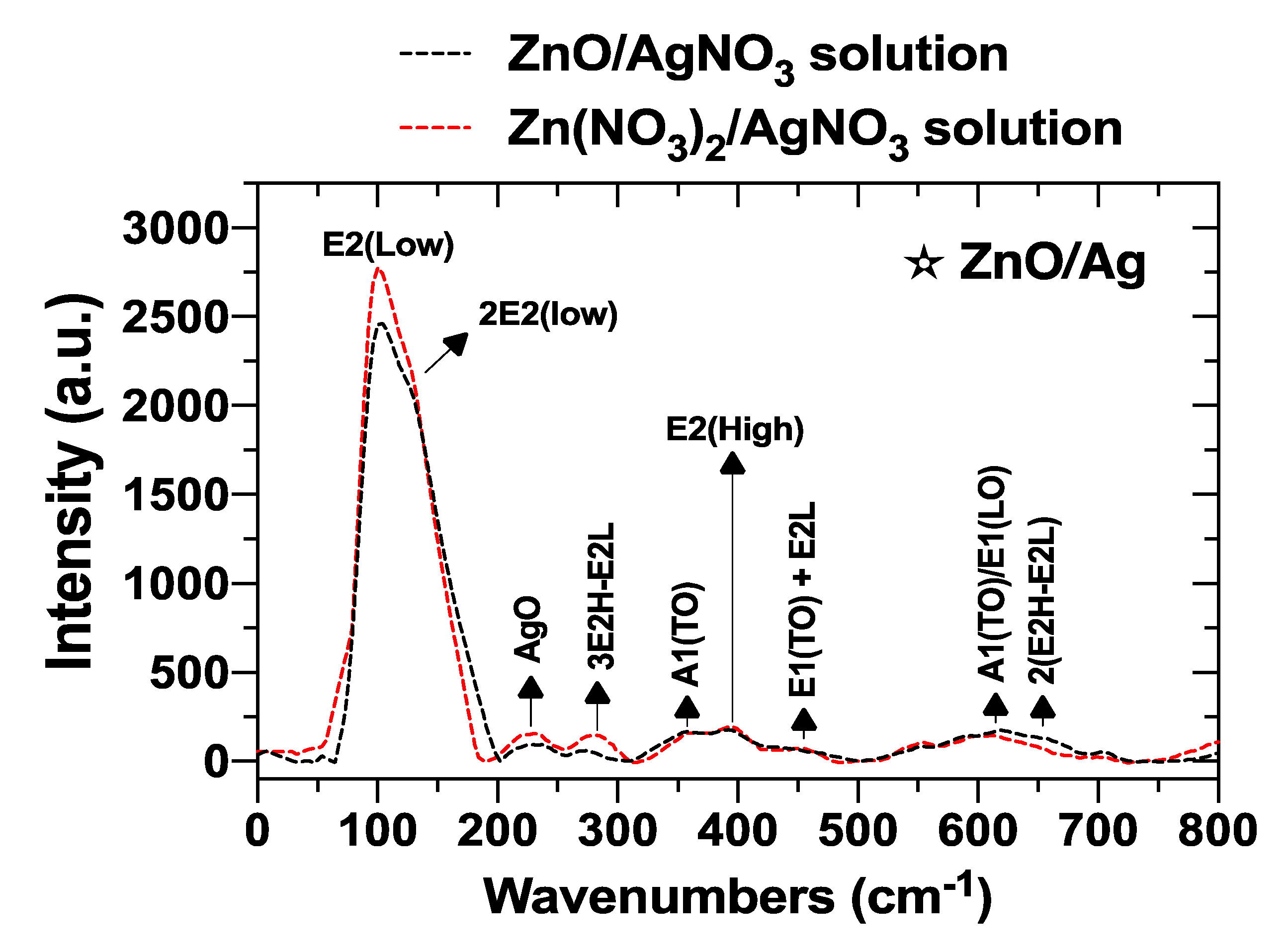
3.2.7. Raman Spectroscopy Analysis
3.2.8. X-ray Diffraction Patterns
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Williams, D. New interest in magnesium. Med. Device Technol. 2006, 17, 9–10. Available online: https://scholar.google.com/scholar_lookup?title=New%20interests%20in%20magnesium&publication_year=2006&author=D.%20Williams (accessed on 15 June 2022). [PubMed]
- Song, G.-L. Corrosion electrochemistry of magnesium (Mg) and its alloys. In Corrosion of Magnesium Alloys; Song, G.-L., Ed.; Woodhead Publishing: Cambridge, MA USA, 2011; pp. 3–65. ISBN 9781845697082. [Google Scholar]
- Kirkland, N.T. Magnesium biomaterials: Past, present and future. Corros. Eng. Sci. Technol. 2012, 47, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Luthringer, B.; Feyerabend, F.; Willumeit, R. Magnesium-based implants: A mini-review. Magnes. Res. 2014, 27, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Tkacz, J.; Slouková, K.; Minda, J.; Drábiková, J.; Fintová, S.; Doležal, P.; Wasserbauer, J. Influence of the Composition of the Hank’s Balanced Salt Solution on the Corrosion Behavior of AZ31 and AZ61 Magnesium Alloys. Metals 2017, 7, 465. [Google Scholar] [CrossRef] [Green Version]
- Riaz, U.; Shabib, I.; Haider, W. The current trends of Mg alloys in biomedical applications—A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 1970–1996. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Sreekala, M.S.; Thomas, S. Fundamental Biomaterials: Metals, 1st ed.; Woodhead Publishing: Cambridge, MA, USA, 2018; pp. 23–24. [Google Scholar]
- Chen, Y.-T.; Hung, F.-Y.; Syu, J.-C. Biodegradable Implantation Material: Mechanical Properties and Surface Corrosion Mechanism of Mg-1Ca-0.5Zr Alloy. Metals 2019, 9, 857. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- La Pleshchitser, A. Biological Role of Magnesium. Clin. Chem. 1958, 4, 429–451. [Google Scholar] [CrossRef]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Revell, P.A.; Damien, E.; Zhang, X.; Evans, P.; Howlett, C.R. The Effect of Magnesium Ions on Bone Bonding to Hydroxyapatite Coating on Titanium Alloy Implants. Key Eng. Mater. 2003, 254–256, 447–450. [Google Scholar] [CrossRef]
- Xue, D.; Yun, Y.; Tan, Z.; Dong, Z.; Schulz, M.J. In Vivo and In Vitro Degradation Behavior of Magnesium Alloys as Biomaterials. J. Mater. Sci. Technol. 2012, 28, 261–267. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Tayebi, L.; Vashaee, D. Improvement of in vitro behavior of an Mg alloy using a nanostructured composite bioceramic coating. J. Mater. Sci. Mater. Med. 2018, 29, 159. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Huang, Y. Assessment of magnesium-based biomaterials: From bench to clinic. Biomater. Sci. 2019, 7, 2241–2263. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Wen, C.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Yoshida, Y.; Okazaki, M.; Shimazu, A.; Uchida, T.; Kubo, T.; Akagawa, Y.; Hamada, Y.; Takahashi, J.; Matsuura, N. Synthesis of functionally graded MgCO3 apatite accelerating osteoblast adhesion. J. Biomed. Mater. Res. 2002, 62, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Asp. Med. 2003, 24, 3–9. [Google Scholar] [CrossRef]
- Necula, B.S.; Fratila-Apachitei, L.E.; Berkani, A.; Apachitei, I.; Duszczyk, J. Enrichment of anodic MgO layers with Ag nanoparticles for biomedical applications. J. Mater. Sci. Mater. Electron. 2008, 20, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Cai, S.; Li, Q.; Li, Z.; Xu, G. UV-irradiation induced biological activity and antibacterial activity of ZnO coated magnesium alloy. Mater. Sci. Eng. C 2020, 114, 110997. [Google Scholar] [CrossRef] [PubMed]
- Carra, C.; Dell’Orto, E.; Morandi, V.; Riccardi, C. ZnO Nanostructured Thin Films via Supersonic Plasma Jet Deposition. Coatings 2020, 10, 788. [Google Scholar] [CrossRef]
- Dulski, M.; Gawecki, R.; Sułowicz, S.; Cichomski, M.; Kazek-Kęsik, A.; Wala, M.; Leśniak-Ziółkowska, K.; Simka, W.; Mrozek-Wilczkiewicz, A.; Gawęda, M.; et al. Key Properties of a Bioactive Ag-SiO2/TiO2 Coating on NiTi Shape Memory Alloy as Necessary at the Development of a New Class of Biomedical Materials. Int. J. Mol. Sci. 2021, 22, 507. [Google Scholar] [CrossRef] [PubMed]
- Góral, D.; Góral-Kowalczyk, M. Application of Metal Nanoparticles for Production of Self-Sterilizing Coatings. Coatings 2022, 12, 480. [Google Scholar] [CrossRef]
- Nistor, C.L.; Mihaescu, C.I.; Bala, D.; Gifu, I.C.; Ninciuleanu, C.M.; Burlacu, S.G.; Petcu, C.; Vladu, M.-G.; Ghebaur, A.; Stroea, L.; et al. Novel Hydrophobic Nanostructured Antibacterial Coatings for Metallic Surface Protection. Coatings 2022, 12, 253. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Boil. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in Vitro Assessment of the Antibacterial Properties and Cytotoxicity of Nanoparticulate Silver Bone Cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef]
- Ohira, T.; Yamamoto, O. Correlation between antibacterial activity and crystallite size on ceramics. Chem. Eng. Sci. 2011, 68, 355–361. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, L.; Wen, D.; Ding, Y. Role of physical and chemical interactions in the antibacterial behavior of ZnO nanoparticles against E. coli. Mater. Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Thirumoorthy, G.S.; Balasubramaniam, O.; Kumaresan, P.; Muthusamy, P.; Subramani, K. Tetraselmis indica Mediated Green Synthesis of Zinc Oxide (ZnO) Nanoparticles and Evaluating Its Antibacterial, Antioxidant, and Hemolytic Activity. BioNanoScience 2021, 11, 172–181. [Google Scholar] [CrossRef]
- Coman, A.N.; Mare, A.; Tanase, C.; Bud, E.; Rusu, A. Silver-Deposited Nanoparticles on the Titanium Nanotubes Surface as a Promising Antibacterial Material into Implants. Metals 2021, 11, 92. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuadra, J.G.; Scalschi, L.; Vicedo, B.; Guc, M.; Izquierdo-Roca, V.; Porcar, S.; Fraga, D.; Carda, J.B. ZnO/Ag Nanocomposites with Enhanced Antimicrobial Activity. Appl. Sci. 2022, 12, 5023. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Reyes-Vidal, Y.; Suarez-Rojas, R.; Ruiz, C.; Torres, J.; Ţălu, Ş.; Méndez, A.; Trejo, G. Electrodeposition, characterization, and antibacterial activity of zinc/silver particle composite coatings. Appl. Surf. Sci. 2015, 342, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Kasiri-Asgarani, M.; Ghayour, H.; Razzaghi, M.; Hadisi, Z. In vitro corrosion behavior, bioactivity, and antibacterial performance of the silver-doped zinc oxide coating on magnesium alloy. Mater. Corros. 2017, 68, 1228–1236. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Kasiri-Asgarani, M.; Akbari, E.; Jabbarzare, S.; Najafinezhad, A.; Hadisi, Z. Synthesis of a novel nanostructured zinc oxide/baghdadite coating on Mg alloy for biomedical application: In-vitro degradation behavior and antibacterial activities. Ceram. Int. 2017, 43, 14842–14850. [Google Scholar] [CrossRef]
- Coelho, P.G.; Jimbo, R. Osseointegration of metallic devices: Current trends based on implant hardware design. Arch. Biochem. Biophys. 2014, 561, 99–108. [Google Scholar] [CrossRef]
- González-Murguía, J.L.; Veleva, L.; Rodríguez-Gattorno, G.; Figueroa-Torres, M.Z.; Feliu, S. Mg-Ca0.3 Electrochemical Activity Exposed to Hank’s Physiological Solution and Properties of Ag-Nano-Particles Deposits. Metals 2021, 11, 1357. [Google Scholar] [CrossRef]
- Barker, B. Electroless deposition of metals. Surf. Technol. 1981, 12, 77–88. [Google Scholar] [CrossRef]
- Koura, N.; Kubota, A. Electroless plating of silver. J. Met. Finish. Soc. Jpn. 1985, 36, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Kerr, C.; Barker, D.; Walsh, F. Electroless Deposition of Metals. Trans. IMF 2001, 79, 41–46. [Google Scholar] [CrossRef]
- Staňo, M.; Fruchart, O. Magnetic Nanowires and Nanotubes. Handb. Magn. Mater. 2018, 27, 155–267. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, A.; Pulletikurthi, G.; Endres, F. A Review on the Electroless Deposition of Functional Materials in Ionic Liquids for Batteries and Catalysis. Front. Chem. 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Dang, S.; Liu, H.; Zhang, Z.; Yu, C.; Liu, X.; Xu, B. Evidence of the formation mechanism of ZnO in aqueous solution. Mater. Lett. 2012, 82, 99–101. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.; Lu, B.; Wei, Y. Formation Mechanism of 1D ZnO Nanowhiskers in Aqueous Solution. J. Phys. Chem. C 2010, 114, 21132–21137. [Google Scholar] [CrossRef]
- Nagaya, S.; Nishikiori, H.; Mizusaki, H.; Wagata, H.; Teshima, K. Formation Process of Eosin Y-Adsorbing ZnO Particles by Electroless Deposition and Their Photoelectric Conversion Properties. ACS Appl. Mater. Interfaces 2015, 7, 11592–11598. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, T.; Otomo, S.; Katayama, J.-I.; Izaki, M. Electroless deposition of transparent conducting and 〈0001〉-oriented ZnO films from aqueous solutions. Electrochimica Acta 2007, 53, 1170–1174. [Google Scholar] [CrossRef]
- Donia, D.; Bauer, E.; Missori, M.; Roselli, L.; Cecchetti, D.; Tagliatesta, P.; Gontrani, L.; Carbone, M. Room Temperature Syntheses of ZnO and Their Structures. Symmetry 2021, 13, 733. [Google Scholar] [CrossRef]
- Montazer, M.; Allahyarzadeh, V. Electroless Plating of Silver Nanoparticles/Nanolayer on Polyester Fabric Using Ag-NO3/NaOH and Ammonia. Ind. Eng. Chem. Res. 2013, 52, 8436–8444. [Google Scholar] [CrossRef]
- Brejna, P.R.; Griffiths, P.R. Electroless Deposition of Silver onto Silicon as a Method of Preparation of Reproducible Surface-Enhanced Raman Spectroscopy Substrates and Tip-Enhanced Raman Spectroscopy Tips. Appl. Spectrosc. 2010, 64, 493–499. [Google Scholar] [CrossRef]
- Formanek, F.; Takeyasu, N.; Tanaka, T.; Chiyoda, K.; Ishikawa, A.; Kawata, S. Selective electroless plating to fabricate complex three-dimensional metallic micro/nanostructures. Appl. Phys. Lett. 2006, 88, 083110. [Google Scholar] [CrossRef] [Green Version]
- Basuki, M.A.; Suryanto, H.; Larasati, A.; Puspitasari, P.; Mujiono. The effect of ZnO addition against crystallinity and water absorption capacity of biofoam based cassava starch reinforced bacterial cellulose. AIP Conf. Proc. 2019, 2120, 050016. [Google Scholar] [CrossRef]
- Rezende, C.; Da Silva, J.; Mohallem, N. Influence of drying on the characteristics of zinc oxide nanoparticles. Braz. J. Phys. 2009, 39, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Kesim, Y.E.; Battal, E.; Okyay, A.K. Plasmonic materials based on ZnO films and their potential for developing broadband middle-infrared absorbers. AIP Adv. 2014, 4, 077106. [Google Scholar] [CrossRef] [Green Version]
- Rad, H.R.B.; Idris, M.H.; Kadir, M.R.A.; Farahany, S. Microstructure analysis and corrosion behavior of biodegradable Mg-Ca implant alloys. Mater. Des. 2012, 33, 88–97. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhang, Z.; Farrell, N.; Chen, D.; Zheng, Y. Comparative, real-time in situ monitoring of galvanic corrosion in Mg-Mg2Ca and Mg-MgZn2 couples in Hank’s solution. Corros. Sci. 2019, 161, 108185. [Google Scholar] [CrossRef]
- Ding, Z.-Y.; Cui, L.-Y.; Chen, X.-B.; Zeng, R.-C.; Guan, S.-K.; Li, S.-Q.; Zhang, F.; Zou, Y.-H.; Liu, Q.-Y. In vitro corrosion of micro-arc oxidation coating on Mg-1Li-1Ca alloy—The influence of intermetallic compound Mg2Ca. J. Alloys Compd. 2018, 764, 250–260. [Google Scholar] [CrossRef]
- Khorasani, F.; Emamy, M.; Malekan, M.; Mirzadeh, H.; Pourbahari, B.; Krajnák, T.; Minárik, P. Enhancement of the microstructure and elevated temperature mechanical properties of as-cast Mg-Al2Ca-Mg2Ca in-situ composite by hot extrusion. Mater. Charact. 2018, 147, 155–164. [Google Scholar] [CrossRef]
- Haycock, D.E.; Kasrai, M.; Nicholls, C.J.; Urch, D.S. The electronic structure of magnesium hydroxide (brucite) using X-ray emission, X-ray photoelectron, and auger spectroscopy. J. Chem. Soc. Dalton Trans. 1978, 12, 1791–1796. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.E.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastian, J., Ed.; Perkin—Elmer-Corp: Lansing, MI, USA, 1992. [Google Scholar]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database, Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 15 June 2022).
- Saleh, H.; Weling, T.; Seidel, J.; Schmidtchen, M.; Kawalla, R.; Mertens, F.O.R.L.; Vogt, H.-P. An XPS Study of Native Oxide and Isothermal Oxidation Kinetics at 300 °C of AZ31 Twin Roll Cast Magnesium Alloy. Oxid. Met. 2013, 81, 529–548. [Google Scholar] [CrossRef]
- Huang, C.-H.; Jan, Y.-L.; Lu, P.-T. Investigation of Approaches to Control the Compositions of Zn(Se,OH) Buffers Prepared by Chemical Bath Deposition Process for Cu(In,Ga)Se2 (CIGS) Solar Cells. Crystals 2018, 8, 343. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Li, Y.; Wee, A. An XPS investigation of the oxidation/corrosion of melt-spun Mg. Appl. Surf. Sci. 2000, 158, 112–119. [Google Scholar] [CrossRef]
- Fournier, V.; Marcus, P.; Olefjord, I. Oxidation of magnesium. Surf. Interface Anal. 2002, 34, 494–497. [Google Scholar] [CrossRef]
- Lindsay, R.; Thornton, G. Structure of Atomic and Molecular Adsorbates on Low-Miller-Index ZnO Surfaces Using X-ray Absorption Spectroscopy. Top. Catal. 2002, 18, 15–19. [Google Scholar] [CrossRef]
- Rössler, N.; Kotsis, K.; Staemmler, V. Ab initio calculations for the Zn 2s and 2p core level binding energies in Zn oxo compounds and ZnO. Phys. Chem. Chem. Phys. 2006, 8, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Ballerini, G.; Ogle, K.; Barthés-Labrousse, M.-G. The acid–base properties of the surface of native zinc oxide layers: An XPS study of adsorption of 1,2-diaminoethane. Appl. Surf. Sci. 2007, 253, 6860–6867. [Google Scholar] [CrossRef]
- Hoogewijs, R.; Fiermans, L.; Vennik, J. Electronic relaxation processes in the KLL′ auger spectra of the free magnesium atom, solid magnesium and MgO. J. Electron Spectrosc. Relat. Phenom. 1977, 11, 171–183. [Google Scholar] [CrossRef]
- Corneille, J.S.; He, J.-W.; Goodman, D. XPS characterization of ultra-thin MgO films on a Mo(100) surface. Surf. Sci. 1994, 306, 269–278. [Google Scholar] [CrossRef]
- Rößler, N.; Staemmler, V. Ab initio calculations for the 2s and 2p core level binding energies of atomic Zn, Zn metal, and Zn containing molecules. Phys. Chem. Chem. Phys. 2003, 5, 3580–3586. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S. Formation of zinc sulfide species on smithsonite surfaces and its response to flotation performance. J. Alloys Compd. 2017, 709, 602–608. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurements of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Zachara, J.; Kittrick, J.; Dake, L.; Harsh, J. Solubility and surface spectroscopy of zinc precipitates on calcite. Geochim. et Cosmochim. Acta 1989, 53, 9–19. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Ghosh, S.; Islam, S.M. Flower-like AgNPs@m-MgO as an excellent catalyst for CO2 fixation and acylation reactions under ambient conditions. New J. Chem. 2018, 42, 14194–14202. [Google Scholar] [CrossRef]
- Farias, S.A.S.; Longo, E.; Gargano, R.; Martins, J.B.L. CO2 adsorption on polar surfaces of ZnO. J. Mol. Model. 2012, 19, 2069–2078. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Kumar, R.T.; Kennedy, L.J.; Vijaya, J.J. Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J. Alloys Compd. 2011, 509, 9809–9815. [Google Scholar] [CrossRef]
- Zahir, M.H.; Rahman, M.M.; Irshad, K. Shape-Stabilized Phase Change Materials for Solar Energy Storage: MgO and Mg(OH)2 Mixed with Polyethylene Glycol. Nanomaterials 2019, 9, 1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. Microwave-assisted MgO NP catalyzed one-pot multicomponent synthesis of polysubstituted steroidal pyridines. New J. Chem. 2017, 42, 184–197. [Google Scholar] [CrossRef]
- John, M. Low Temperature Synthesis of Nano Crystalline Zero-Valent Phases and (Doped) Metal Oxides as AxB3−xO4 (Ferrite), ABO2 (Delafossite), A2O and AO. A New Process to Treat Industrial Wastewaters? Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2015. [Google Scholar] [CrossRef]
- Sharma, V.; Basak, S.; Rishabh, K.; Umaria, H.; Ali, S.W. Synthesis of zinc carbonate nanoneedles, a potential flame retardant for cotton textiles. Cellulose 2018, 25, 6191–6205. [Google Scholar] [CrossRef]
- Mancarella, F.; D’Elia, M.; Longo, G.M.; Longo, S.; Orofino, V. Kinetics of Thermal Decomposition of Particulate Samples of MgCO3: Experiments and Models. Chemistry 2022, 4, 548–559. [Google Scholar] [CrossRef]
- Maddahi, P.; Shahtahmasebi, N.; Kompany, A.; Mashreghi, M.; Safaee, S.; Roozban, F. Effect of doping on structural and optical properties of ZnO nanoparticles: Study of antibacterial properties. Mater. Sci. 2014, 32, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Zamiri, R.; Rebelo, A.; Zamiri, G.; Adnani, A.; Kuashal, A.; Belsley, M.S.; Ferreira, J.M.F. Far-infrared optical constants of ZnO and ZnO/Ag nanostructures. RSC Adv. 2014, 4, 20902–20908. [Google Scholar] [CrossRef]
- Saoud, K.; Alsoubaihi, R.; Bensalah, N.; Bora, T.; Bertino, M.; Dutta, J. Synthesis of supported silver nano-spheres on zinc oxide nanorods for visible light photocatalytic applications. Mater. Res. Bull. 2015, 63, 134–140. [Google Scholar] [CrossRef]
- Hoseinpour, V.; Souri, M.; Ghaemi, N.; Shakeri, A. Optimization of green synthesis of ZnO nanoparticles by Dittrichia grave-olens (L.) aqueous extract. HBB 2017, 2, 39–49. [Google Scholar] [CrossRef]
- Nagaraju, G.; Udayabhanu; Shivaraj; Prashanth, S.; Shastri, M.; Yathish, K.; Anupama, C.; Rangappa, D. Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of Ag–ZnO nanomaterial. Mater. Res. Bull. 2017, 94, 54–63. [Google Scholar] [CrossRef]
- Abdullah, E.A.; Anber, A.A.; Edan, F.F.; Fraih, A.J. Synthesis of ZnO Nanoparticles by Using an Atmospheric-Pressure Plasma Jet. OALib 2018, 5, e4755. [Google Scholar] [CrossRef]
- Zak, A.K.; Razali, R.; Majid, W.H.B.A.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Gurgur, E.; Oluyamo, S.S.; Adetuyi, A.O.; Omotunde, O.I.; Okoronkwo, A.E. Green synthesis of zinc oxide nanoparticles and zinc oxide–silver, zinc oxide–copper nanocomposites using Bridelia ferruginea as biotemplate. SN Appl. Sci. 2020, 2, 911. [Google Scholar] [CrossRef] [Green Version]
- Spanhel, L.; Anderson, M.A. Semiconductor clusters in the sol-gel process: Quantized aggregation, gelation, and crystal growth in concentrated zinc oxide colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Kormann, C.; Hoffmann, M.R. Preparation and characterization of quantum size zinc oxide: A detailed spectroscopic study. J. Phys. Chem. 1987, 91, 3789–3798. [Google Scholar] [CrossRef]
- Monticone, S.; Tufeu, R.; Kanaev, A.V. Complex Nature of the UV and Visible Fluorescence of Colloidal ZnO Nanoparticles. J. Phys. Chem. B 1998, 102, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gattorno, G.; Santiago-Jacinto, P.; Rendon-Vázquez, L.; Németh, J.; Dékány, I.; Díaz, D. Novel Synthesis Pathway of ZnO Nanoparticles from the Spontaneous Hydrolysis of Zinc Carboxylate Salts. J. Phys. Chem. B 2003, 107, 12597–12604. [Google Scholar] [CrossRef]
- Németh, J.; Rodríguez-Gattorno, G.; Diaz, D.; Vázquez-Olmos, A.R.; Dékány, I. Synthesis of ZnO Nanoparticles on a Clay Mineral Surface in Dimethyl Sulfoxide Medium. Langmuir 2004, 20, 2855–2860. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Karunagaran, B.; Suh, E.-K.; Hahn, Y.B. Structural and optical properties of single-crystalline ZnO nanorods grown on silicon by thermal evaporation. Nanotechnology 2006, 17, 4072–4077. [Google Scholar] [CrossRef]
- Khan, A.; Kordesch, M.E. Synthesis of novel zinc oxide microphone-like microstructures. Mater. Lett. 2008, 62, 230–234. [Google Scholar] [CrossRef]
- Aurangzeb, K. Raman Spectroscopic Study of The ZnO Nanostructures. J. Pak Mater. Soc. 2010, 4, 5–9. Available online: https://scholar.google.com/scholar_lookup?title=Raman%20spectroscopic%20study%20of%20the%20ZnO%20nanostructures&publication_year=2010&author=A.%20Khan (accessed on 15 June 2022).
- Silambarasan, M.; Saravanan, S.; Soga, T. Raman and Photoluminescence Studies of Ag and Fe-doped ZnO Nanoparticles. Int. J. Chemtech. Res. 2015, 7, 1644–1650. Available online: https://scholar.google.com/scholar_lookup?title=Raman+and+photoluminescence+studies+of+Ag+and+Fe-doped+ZnO+nanoparticles&author=Silambarasan,+M.&author=Saravanan,+S.&author=Soga,+T.&publication_year=2015&journal=Int.+J.+Chem.+Technol.+Res.&volume=7&pages=1644%E2%80%931650 (accessed on 15 June 2022).
- Xu, C.X.; Sun, X.; Zhang, X.H.; Ke, L.; Chua, S.J. Photoluminescent properties of copper-doped zinc oxide nanowires. Nanotechnology 2004, 15, 856–861. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Luévano-Hipólito, E.; Torres-Martínez, L.M.; Torres-Sánchez, A. Photocatalytic H2 production and CO2 reduction on Cu, Ni-doped ZnO: Effect of metal doping and oxygen vacancies. J. Mater. Sci. Mater. Electron. 2019, 30, 18506–18518. [Google Scholar] [CrossRef]
- Russo, V.; Ghidelli, M.; Gondoni, P.; Casari, C.S.; Bassi, A.L. Multi-wavelength Raman scattering of nanostructured Al-doped zinc oxide. J. Appl. Phys. 2014, 115, 073508. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, H.; Uchida, H.; Funakubo, H.; Katoda, T.; Nishida, K. Evaluation of oxygen vacancies in ZnO single crystals and powders by micro-Raman spectroscopy. J. Ceram. Soc. Jpn. 2017, 125, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Débarre, A.; Jaffiol, R.; Julien, C.; Tchénio, P.; Mostafavi, M. Raman scattering from single Ag aggregates in presence of EDTA. Chem. Phys. Lett. 2004, 386, 244–247. [Google Scholar] [CrossRef]
- Van Der Horst, C.; Silwana, B.; Iwuoha, E.; Somerset, V.S. Synthesis and Characterization of Bismuth-Silver Nanoparticles for Electrochemical Sensor Applications. Anal. Lett. 2014, 48, 1311–1332. [Google Scholar] [CrossRef]
- Lai, C.-H.; Wang, G.-A.; Ling, T.-K.; Wang, T.-J.; Chiu, P.-K.; Chau, Y.-F.C.; Huang, C.-C.; Chiang, H.-P. Near infrared surface-enhanced Raman scattering based on star-shaped gold/silver nanoparticles and hyperbolic metamaterial. Sci. Rep. 2017, 7, 5446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, N.; Jain, N.; Pathak, A.; Singh, J.; Prasad, R.; Upadhyaya, C.P. Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J. Sol-Gel Sci. Technol. 2018, 86, 682–689. [Google Scholar] [CrossRef]




| Element | Mg | Ca | Al | Zn | Fe | Cu | Ni |
|---|---|---|---|---|---|---|---|
| Composition (wt.%) | Bal. | 0.23 | 0.016 | 0.006 | 0.0019 | 0.0019 | 0.0014 |
| Solution (10−3 M) | Concentration (mg L−1) | Zn2+ Ions (mg L−1) | Ag+ Ions (mg L−1) | pH |
|---|---|---|---|---|
| ZnO | 83.38 | 1.05 ± 0.32 | --- | 7.11 ± 0.05 |
| Zn(NO3)2 | 297.48 | 66.7 ± 1.21 | --- | 6.45 ± 0.03 |
| AgNO3 | 169.87 | --- | 108.8 ± 1.72 | 6.22 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Murguía, J.L.; Lucien, V.; Alpuche-Avilés, M. Electroless Deposits of ZnO and Hybrid ZnO/Ag Nanoparticles on Mg-Ca0.3 Alloy Surface: Multiscale Characterization. Coatings 2022, 12, 1109. https://doi.org/10.3390/coatings12081109
González-Murguía JL, Lucien V, Alpuche-Avilés M. Electroless Deposits of ZnO and Hybrid ZnO/Ag Nanoparticles on Mg-Ca0.3 Alloy Surface: Multiscale Characterization. Coatings. 2022; 12(8):1109. https://doi.org/10.3390/coatings12081109
Chicago/Turabian StyleGonzález-Murguía, José Luis, Veleva Lucien, and Mario Alpuche-Avilés. 2022. "Electroless Deposits of ZnO and Hybrid ZnO/Ag Nanoparticles on Mg-Ca0.3 Alloy Surface: Multiscale Characterization" Coatings 12, no. 8: 1109. https://doi.org/10.3390/coatings12081109






