Antibiotics in Dairy Production: Where Is the Problem?
Abstract
1. Use of Antibiotics in Dairy Animals
2. Antibiotic Residues in Milk and Dairy Products
2.1. Origin of Antibiotic Residues in Milk
2.2. Antibiotic Residues in Commercial Cow’s Milk Worldwide
2.3. Antibiotic Residues in Sheep and Goats Milk
2.4. Transfer of Antibiotic Residues from Milk to Dairy Products
2.5. Antibiotic Residues in Commercial Dairy Products
3. Effect of Antibiotic Residues in Dairy Products Elaboration
4. The Use of Antibiotics and the Emergence of Antibiotic Resistant Bacteria
5. Other Aspects
5.1. Human Health
5.2. Dairy Farm Environment
6. Conclusions: So, Where Is the Problem?
Author Contributions
Funding
Conflicts of Interest
References
- Treiber, F.M.; Beranek-Knauer, H. Antimicrobial Residues in Food from Animal Origin—A Review of the Literature Focusing on Products Collected in Stores and Markets Worldwide. Antibiotics 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA. EFSA J. 2021, 19, e06712. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque Fernandes, S.A.; Magnavita, A.P.; Ferrao, S.P.; Gualberto, S.A.; Faleiro, A.S.; Figueiredo, A.J.; Matarazzo, S.V. Daily ingestion of tetracycline residue present in pasteurized milk: A public health problem. Environ. Sci. Pollut. Res. Int. 2014, 21, 3427–3434. Available online: https://link.springer.com/article/10.1007/s11356-013-2286-5 (accessed on 10 July 2021). [CrossRef]
- Pengov, A.; Kirbis, A. Risks of antibiotic residues in milk following intramammary and intramuscular treatments in dairy sheep. Anal. Chim. Acta 2009, 637, 13–17. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. Available online: https://journals.sagepub.com/doi/10.1177/003335491212700103 (accessed on 17 September 2021). [CrossRef] [PubMed]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34, S93–S106. Available online: https://academic.oup.com/cid/article/34/Supplement_3/S93/293306?login=true (accessed on 19 April 2022). [CrossRef]
- Rajala-Schultz, P.; Nødtvedt, A.; Halasa, T.; Persson Waller, K. Prudent Use of Antibiotics in Dairy Cows: The Nordic Approach to Udder Health. Front. Vet. Sci. 2021, 8, 623998. Available online: https://www.frontiersin.org/article/10.3389/fvets.2021.623998 (accessed on 17 September 2021). [CrossRef]
- Pol, M.; Ruegg, P.L. Treatment Practices and Quantification of Antimicrobial Drug Usage in Conventional and Organic Dairy Farms in Wisconsin. J. Dairy Sci. 2007, 90, 249–261. [Google Scholar] [CrossRef]
- Linage, B.; Gonzalo, C.; Carriedo, J.A.; Asensio, J.A.; Blanco, M.A.; De La Fuente, L.F.; San Primitivo, F. Performance of Blue-Yellow Screening Test for Antimicrobial Detection in Ovine Milk. J. Dairy Sci. 2007, 90, 5374–5379. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Molina, M.P.; Althaus, R.L.; Gallego, L. Residue Persistence in Sheep Milk Following Antibiotic Therapy. Vet. J. 2003, 165, 84–89. [Google Scholar] [CrossRef]
- Beltrán, M.C.; Althaus, R.L.; Molina, A.; Berruga, M.I.; Molina, M.P. Analytical strategy for the detection of antibiotic residues in sheep and goat’s milk. Span. J. Agric. Res. 2015, 13, e0501. Available online: https://revistas.inia.es/index.php/sjar/article/view/6522 (accessed on 2 December 2021). [CrossRef]
- Beyene, T. Veterinary Drug Residues in Food-animal Products: Its Risk Factors and Potential Effects on Public Health. J. Vet. Sci. Technol. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Quintanilla, P.; Doménech, E.; Escriche, I.; Beltrán, M.C.; Molina, M.P. Food Safety Margin Assessment of Antibiotics: Pasteurized Goat’s Milk and Fresh Cheese. J. Food Prot. 2019, 82, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Sachi, S.; Ferdous, J.; Sikder, M.H.; Hussani, S.M.A.K. Antibiotic residues in milk: Past, present, and future. J. Adv. Vet. Anim. Res. 2019, 6, 315–332. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6760505/ (accessed on 9 September 2021). [CrossRef] [PubMed]
- Pyörälä, S. Treatment of mastitis during lactation. Ir. Vet. J. 2009, 62, S4–S40. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Saluti, G.; Moretti, S.; Diamanti, I.; Giusepponi, D.; Galarini, R. Multiclass methods for the analysis of antibiotic residues in milk by liquid chromatography coupled to mass spectrometry: A review. Food Addit. Contam. Part A 2018, 35, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Fejzic, N.; Begagic, M.; Šerić-Haračić, S.; Smajlovic, M. Beta lactam antibiotics residues in cow’s milk: Comparison of efficacy of three screening tests used in Bosnia and Herzegovina. Bosn. J. Basic Med. Sci. 2014, 14, 155–159. Available online: https://www.bjbms.org/ojs/index.php/bjbms/article/view/155 (accessed on 19 April 2022). [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Ind. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Available online: http://data.europa.eu/eli/reg/2019/6/oj (accessed on 19 April 2022).
- Mitchell, J.M.; Griffiths, M.W.; McEwen, S.A.; McNab, W.B.; Yee, A.J. Antimicrobial drug residues in milk and meat: Causes, concerns, prevalence, regulations, tests, and test performance. J. Food Prot. 1998, 61, 742–756. [Google Scholar] [CrossRef]
- Katz, S.E.; Brady, M.S. Antibiotic residues in food and their significance. Food Biotechnol. 2000, 14, 147–171. [Google Scholar] [CrossRef]
- Shitandi, A.; Sternesjö, A. Factors contributing to the occurrence of antimicrobial drug residues in Kenyan milk. J. Food Prot. 2004, 67, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Orwa, J.D.; Matofari, J.W.; Muliro, P.S.; Lamuka, P. Assessment of sulphonamides and tetracyclines antibiotic residue contaminants in rural and peri urban dairy value chains in Kenya. Int. J. Food Contam. 2017, 4, 5. [Google Scholar] [CrossRef][Green Version]
- Brown, K.; Mugoh, M.; Call, D.R.; Omulo, S. Antibiotic residues and antibiotic-resistant bacteria detected in milk marketed for human consumption in Kibera, Nairobi. PLoS ONE 2020, 15, e0233413. [Google Scholar] [CrossRef] [PubMed]
- Kosgey, A.; Shitandi, A.; Marion, J.W. Antibiotic Residues in Milk from Three Popular Kenyan Milk Vending Machines. Am. J. Trop. Med. Hyg. 2018, 98, 1520–1522. Available online: https://www.ajtmh.org/view/journals/tpmd/98/5/article-p1520.xml (accessed on 14 December 2021). [CrossRef] [PubMed]
- Ouma, J.; Gachanja, A.; Mugo, S.; Gikunju, J. Antibiotic Residues in Milk from Juja and Githurai Markets in Kenya by Liquid Chromatography-Tandem Mass Spectrometry. Chem. Afr. 2021, 4, 769–775. [Google Scholar] [CrossRef]
- Kurwijila, L.R.; Omore, A.; Staal, S.; Mdoe, N.S. Investigation of the risk of exposure to antimicrobial residues present in marketed milk in Tanzania. J. Food Prot. 2006, 69, 2487–2492. [Google Scholar] [CrossRef]
- Mdegela, R.H.; Ryoba, R.; Karimuribo, E.D.; Phiri, E.J.; Løken, T.; Reksen, O.; Mtengeti, E.; Urio, N.A. Prevalence of clinical and subclinical mastitis and quality of milk on smallholder dairy farms in Tanzania. J. S. Afr. Vet. Assoc. 2009, 80, 163–168. Available online: https://journals.jsava.aosis.co.za/index.php/jsava/article/view/195 (accessed on 23 December 2021). [CrossRef]
- Kivaria, F.M.; Noordhuizen, J.P.; Kapaga, A.M. Evaluation of the hygienic quality and associated public health hazards of raw milk marketed by smallholder dairy producers in the Dar es Salaam region, Tanzania. Trop. Anim. Health Prod. 2006, 38, 185–194. Available online: https://link.springer.com/article/10.1007/s11250-006-4339-y (accessed on 23 December 2021). [CrossRef] [PubMed]
- Gwandu, S.H.; Nonga, H.E.; Mdegela, R.H.; Katakweba, A.S.; Suleiman, T.S.; Ryoba, R. Assessment of Raw Cow Milk Quality in Smallholder Dairy Farms in Pemba Island Zanzibar, Tanzania. Vet. Med. Int. 2018, 2018, 1031726. [Google Scholar] [CrossRef] [PubMed]
- Layada, S.; Benouareth, D.; Coucke, W.; Andjelkovic, M. Assessment of antibiotic residues in commercial and farm milk collected in the region of Guelma (Algeria). Int. J. Food Contam. 2016, 3, 19. [Google Scholar] [CrossRef]
- Baazize-Ammi, D.; Dechicha, A.S.; Tassist, A.; Gharbi, I.; Hezil, N.; Kebbal, S.; Morsli, W.; Beldjoudi, S.; Saadaoui, M.R.; Guetarni, D. Screening and quantification of antibiotic residues in broiler chicken meat and milk in the central region of Algeria. Rev. Sci. Tech. 2019, 38, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Madougou, A.M.; Douny, C.; Moula, N.; Scippo, M.L.; Delcenserie, V.; Daube, G.; Hamani, M.; Korsak, N. Survey on the presence of antibiotic residues in raw milk samples from six sites of the dairy pool of Niamey, Niger. Vet. World 2019, 12, 1970–1974. [Google Scholar] [CrossRef] [PubMed]
- Addo, K.K.; Mensah, G.I.; Aning, K.G.; Nartey, N.; Nipah, G.K.; Bonsu, C.; Akyeh, M.L.; Smits, H.L. Microbiological quality and antibiotic residues in informally marketed raw cow milk within the coastal savannah zone of Ghana. Trop. Med. Int. Health 2011, 16, 227–232. [Google Scholar] [CrossRef]
- Bando, E.; Oliveira, R.C.; Ferreira, G.M.; Machinski, M., Jr. Occurrence of antimicrobial residues in pasteurized milk commercialized in the state of Paraná, Brazil. J. Food Prot. 2009, 72, 911–914. [Google Scholar] [CrossRef]
- Zanella, G.N.; Mikcha, J.M.; Bando, E.; Siqueira, V.L.; Machinski, M., Jr. Occurrence and antibiotic resistance of coliform bacteria and antimicrobial residues in pasteurized cow’s milk from Brazil. J. Food Prot. 2010, 73, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.C.; Paschoal, J.A.R.; Reyes, F.G.R. Streptomycin and dihydrostreptomycin residues in bovine milk from the Brazilian retail market. Food Addit. Contam. Part B 2010, 3, 156–162. [Google Scholar] [CrossRef]
- Prado, C.K.; Ferreira, F.D.; Bando, E.; Machinski, M., Jr. Oxytetracycline, tetracycline, chlortetracycline and doxycycline in pasteurised cow’s milk commercialised in Brazil. Food Addit. Contam. Part B 2015, 8, 81–84. [Google Scholar] [CrossRef]
- Valença, L.M.; De Paiva, J.E.; Barbosa, S.B.P.; Pinheiro, I.O.; Batista, A.M.V.; Da Silva, M.J.F.V.; De Medeiros, E.S. Evaluation of residues of β-lactam, sulfonamide, tetracycline, quinolone, fluoroquinolone e pyrimidine in raw milk. Food Sci. Technol. 2021, 41, 603. Available online: http://old.scielo.br/scielo.php?script=sci_arttext&pid=S0101-20612021000300603&nrm=iso (accessed on 9 December 2021). [CrossRef]
- Baynes, R.E.; Lyman, R.; Anderson, K.L.; Brownie, C.F. A Preliminary Survey of Antibiotic Residues and Viable Bacteria in Milk from Three Caribbean Basin Countries. J. Food Prot. 1999, 62, 177–180. [Google Scholar] [CrossRef] [PubMed]
- De Arbo, L.M.; Céspedes, L.G.; Idoyaga, H.; Echeverría, P.; Caballero, E.G.; Arias, M.N.; Bernal, S.S.; Ulke, G.; Desvars, A.B.; Aguirre, F.P. Detección de residuos de antibióticos y micotoxinas en leche vacuna fluida pasteurizada comercializada en Paraguay. Rev. Salud Publica Parag. 2020, 10, 23–29. [Google Scholar] [CrossRef]
- Redding, L.E.; Cubas-Delgado, F.; Sammel, M.D.; Smith, G.; Galligan, D.T.; Levy, M.Z.; Hennessy, S. Antibiotic residues in milk from small dairy farms in rural Peru. Food Addit. Contam. Part A 2014, 31, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi Sani, A.M.; Nikpooyan, H.; Moshiri, R. Aflatoxin M1 contamination and antibiotic residue in milk in Khorasan province, Iran. Food Chem. Toxicol. 2010, 48, 2130–2132. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.M.; Amiri, M.; Riabi, H.R.; Riabi, H.R. Evaluation of Antibiotic Residues in Pasteurized and Raw Milk Distributed in the South of Khorasan-e Razavi Province, Iran. J. Clin. Diagn. Res. 2016, 10, FC31–FC35. [Google Scholar] [CrossRef] [PubMed]
- Rassouli, A.; Zamani, Z.; Bahonar, A.; Shams, G.; Abdolmaleki, A. A trace analysis of oxytetracycline and tetracycline residues in pasteurized milk supplied in Tehran: A one-year study (April 2011–March 2012). Iran. J. Vet. Med. 2014, 8, 119–123. Available online: https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=409506 (accessed on 11 February 2022).
- Mokhtari, A.; Hosseini, B.; Panahi, P. β-Lactams and Tetracyclines Antibiotic Residue Detection in Bulk Tank Milk in Iran. Iran. J. Public Health 2013, 42, 447–448. Available online: https://ijph.tums.ac.ir/index.php/ijph/article/view/4558 (accessed on 11 February 2022).
- Aalipour, F.; Mirlohi, M.; Jalali, M.; Azadbakht, L. Dietary exposure to tetracycline residues through milk consumption in Iran. J. Environ. Health Sci. Eng. 2015, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Panda, A.K.; Sharma, N. Determination of antibiotic residues in bovine milk by HPLC-DAD and assessment of human health risks in Northwestern Himalayan region, India. J. Food Sci. Technol. 2022, 59, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, G.; Leahy, E.; Shome, B.R.; Bandyopadhyay, S.; Deka, R.P.; Shome, R.; Dey, T.K.; Lindahl, J.F. Understanding Antibiotic Usage on Small-Scale Dairy Farms in the Indian States of Assam and Haryana Using a Mixed-Methods Approach—Outcomes and Challenges. Antibiotics 2021, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S. Antibiotic residues and mycotoxins in raw milk in Punjab (India): A rising concern for food safety. J. Food Sci. Technol. 2019, 56, 5146–5151. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Hassan, M.M.; Alam, M.; Sattar, S.; Bari, M.S.; Saifuddin, A.K.; Hoque, M.A. Antibiotic residues in milk and eggs of commercial and local farms at Chittagong, Bangladesh. Vet. World 2015, 8, 467–471. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hassan, M.M.; Chowdhury, S. Determination of antibiotic residues in milk and assessment of human health risk in Bangladesh. Heliyon 2021, 7, e07739. [Google Scholar] [CrossRef]
- Khanal, B.K.S.; Sadiq, M.B.; Singh, M.; Anal, A.K. Screening of antibiotic residues in fresh milk of Kathmandu Valley, Nepal. J. Environ. Sci. Health. Part B 2018, 53, 57–86. [Google Scholar] [CrossRef]
- Wanniatie, V.; Sudarwanto, M.B.; Purnawarman, T.; Jayanegara, A. Chemical compositions, contaminants, and residues of organic and conventional goat milk in Bogor District, Indonesia. Vet. World 2019, 12, 1218–1224. Available online: http://www.veterinaryworld.org/Vol.12/August-2019/7.pdf (accessed on 16 February 2022). [CrossRef] [PubMed]
- Unusan, N. Occurrence of chloramphenicol, streptomycin and tetracycline residues in ultra-heat-treatment milk marketed in Turkey. Int. J Food Sci. Nutr. 2009, 60, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Muji, S.; Mehmedi, B.; Rexhepi, A.; Ramadani, X. Antibiotics residue in raw milk samples from four regions of Kosovo. Bulg. J. Agric. Sci. 2018, 24, 871–874. Available online: https://www.agrojournal.org/24/05-21.pdf (accessed on 29 April 2022).
- Rama, A.; Lucatello, L.; Benetti, C.; Galina, G.; Bajraktari, D. Assessment of antibacterial drug residues in milk for consumption in Kosovo. J. Food Drug Anal. 2017, 25, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Vragović, N.; Bažulić, D.; Njari, B. Risk assessment of streptomycin and tetracycline residues in meat and milk on Croatian market. Food Chem. Toxicol. 2011, 49, 352–355. [Google Scholar] [CrossRef]
- Bilandžić, N.; Kolanović, B.S.; Varenina, I.; Scortichini, G.; Annunziata, L.; Brstilo, M.; Rudan, N. Veterinary drug residues determination in raw milk in Croatia. Food Control 2011, 22, 1941–1948. [Google Scholar] [CrossRef]
- Cerkvenik, V. Analysis and monitoring of chloramphenicol residues in food of animal origin in Slovenia from 1991 to 2000. Food Addit. Contam. 2002, 19, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, M.; Berruga, M.I.; Althaus, R.L.; Molina, M.P.; Molina, A. Occurrence of Antibiotic Residues in Milk from Manchega Ewe Dairy Farms. J. Dairy Sci. 2004, 87, 3132–3137. [Google Scholar] [CrossRef]
- Gonzalo, C.; Carriedo, J.A.; García-Jimeno, M.C.; Pérez-Bilbao, M.; de la Fuente, L.F. Factors influencing variation of bulk milk antibiotic residue occurrence, somatic cell count, and total bacterial count in dairy sheep flocks. J. Dairy Sci. 2010, 93, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Buczkowska, M.; Gorski, M.; Garbicz, J.; Grajek, M.; Buczkowski, K.; Garbowska, D.; Klein, D.; Duda, S. Penicillin and tetracycline residues in selected fresh and UHT milk with different fat contents. Int. Food Res. J. 2021, 28, 780–787. Available online: http://ifrj.upm.edu.my/28%20(04)%202021/DONE%20-%2014%20-%20IFRJ20014.pdf (accessed on 15 February 2022). [CrossRef]
- The National Milk Drug Residue Data Base. Available online: https://www.nmdrd.com/ (accessed on 26 April 2022).
- European Food Safety Authority. Report for 2019 on the Results from the Monitoring of Veterinary Medicinal Product Residues and Other Substances in Live Animals and Animal Products. EFSA Support. Publ. 2021, 18, 1997E. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, J.; Han, R.; Xu, X.; Zhen, Y.; Qu, X.; Sun, P.; Li, S.; Yu, Z. Occurrence of several main antibiotic residues in raw milk in 10 provinces of China. Food Addit. Contam. Part B 2013, 6, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Chen, Q.; Li, Y.; Liu, Y.; Zhang, Y.; Huang, Y.; Zhu, L. Status of antibiotic residues and detection techniques used in Chinese milk: A systematic review based on cross-sectional surveillance data. Food Res. Int. 2021, 147, 110450. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1430/94 of 22 June 1994 Amending Annexes I, II, III and IV of Council Regulation (EEC) No 2377/90 Laying Down a Community Procedure for the Establishment of Maximum Residue Limits of Veterinary Medicinal Products in Foodstuffs of Animal Origin. Available online: http://data.europa.eu/eli/reg/1994/1430/oj (accessed on 7 September 2021).
- Hassan, H.F.; Saidy, L.; Haddad, R.; Hosri, C.; Asmar, S.; Jammoul, A.; Jammoul, R.; Hassan, H.; Serhan, M. Investigation of the effects of some processing conditions on the fate of oxytetracycline and tylosin antibiotics in the making of commonly consumed cheeses from the East Mediterranean. Vet. World 2021, 14, 1644–1649. [Google Scholar] [CrossRef]
- Tian, L.; Khalil, S.; Bayen, S. Effect of thermal treatments on the degradation of antibiotic residues in food. Crit. Rev. Food Sci. Nutr. 2017, 57, 3760–3770. [Google Scholar] [CrossRef]
- Grunwald, L.; Petz, M. Food processing effects on residues: Penicillins in milk and yoghurt. Anal. Chim. Acta 2003, 483, 73–79. [Google Scholar] [CrossRef]
- Quintanilla, P.; Beltrán, M.C.; Molina, A.; Escriche, I.; Molina, M.P. Characteristics of ripened Tronchón cheese from raw goat milk containing legally admissible amounts of antibiotics. J. Dairy Sci. 2019, 102, 2941–2953. [Google Scholar] [CrossRef]
- Gajda, A.; Nowacka-Kozak, E.; Gbylik-Sikorska, M.; Posyniak, A. Tetracycline antibiotics transfer from contaminated milk to dairy products and the effect of the skimming step and pasteurisation process on residue concentrations. Food Addit. Contam. Part A 2018, 35, 66–76. [Google Scholar] [CrossRef]
- Hakk, H.; Shappell, N.W.; Lupton, S.J.; Shelver, W.L.; Fanaselle, W.; Oryang, D.; Yeung, C.Y.; Hoelzer, K.; Ma, Y.; Gaalswyk, D.; et al. Distribution of Animal Drugs between Skim Milk and Milk Fat Fractions in Spiked Whole Milk: Understanding the Potential Impact on Commercial Milk Products. J. Agric. Food Chem. 2016, 64, 326–335. Available online: https://pubs.acs.org/doi/abs/10.1021/acs.jafc.5b04726 (accessed on 25 February 2022). [CrossRef]
- Ziv, G.; Rasmussen, F. Distribution of Labeled Antibiotics in Different Components of Milk Following Intramammary and Intramuscular Administrations®. J. Dairy Sci. 1975, 58, 938–946. [Google Scholar] [CrossRef]
- Sniegocki, T.; Gbylik-Sikorska, M.; Posyniak, A. Transfer of chloramphenicol from milk to commercial dairy products—Experimental proof. Food Control 2015, 57, 411–418. [Google Scholar] [CrossRef]
- Cayle, T.; Guth, J.H.; Hynes, J.T.; Kolen, E.P.; Stern, M.L. Penicillin Distribution during Cheese Manufacture and Membrane Treatment of Whey. J. Food Prot. 1986, 49, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Shappell, N.W.; Shelver, W.L.; Lupton, S.J.; Fanaselle, W.; Van Doren, J.M.; Hakk, H. Distribution of Animal Drugs among Curd, Whey, and Milk Protein Fractions in Spiked Skim Milk and Whey. J. Agric. Food Chem. 2017, 65, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Gbylik-Sikorska, M.; Gajda, A.; Nowacka-Kozak, E.; Posyniak, A. The “force” of cloxacillin residue will be with you in various dairy products—The last experimental evidence. Food Control 2021, 121, 107628. [Google Scholar] [CrossRef]
- Lányi, K.; Darnay, L.; László, N.; Lehel, J.; Friedrich, L.; Győri, R.; Laczay, P. Transfer of certain beta-lactam antibiotics from cow’s milk to fresh cheese and whey. Food Addit. Contam. Part A 2022, 39, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Cabizza, R.; Rubattu, N.; Salis, S.; Pes, M.; Comunian, R.; Paba, A.; Addis, M.; Testa, M.C.; Urgeghe, P.P. Transfer of oxytetracycline from ovine spiked milk to whey and cheese. Int. Dairy J. 2017, 70, 12–17. [Google Scholar] [CrossRef]
- Cabizza, R.; Rubattu, N.; Salis, S.; Pes, M.; Comunian, R.; Paba, A.; Daga, E.; Addis, M.; Testa, M.C.; Urgeghe, P.P. Impact of a thermisation treatment on oxytetracycline spiked ovine milk: Fate of the molecule and technological implications. LWT Food Sci. Technol. 2018, 96, 236–243. [Google Scholar] [CrossRef]
- Giraldo, J.; Althaus, R.L.; Beltrán, M.C.; Molina, M.P. Antimicrobial activity in cheese whey as an indicator of antibiotic drug transfer from goat milk. Int. Dairy J. 2017, 69, 40–44. [Google Scholar] [CrossRef]
- Quintanilla, P.; Beltrán, M.C.; Molina, M.P.; Escriche, I. Enrofloxacin treatment on dairy goats: Presence of antibiotic in milk and impact of residue on technological process and characteristics of mature cheese. Food Control 2021, 123, 107762. [Google Scholar] [CrossRef]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2019, 18, 336–341. Available online: https://www.tandfonline.com/doi/full/10.1080/1828051X.2018.1529544 (accessed on 6 April 2022). [CrossRef]
- Tona, G.O.; Olusola, A.D. Determination of tetracycline antibiotic residue in dairy products sold in Ogbomoso, south-western Nigeria. Int. J. Food Agric. Vet. Sci. 2014, 4, 136–140. Available online: https://www.cibtech.org/J-FOOD-AGRI-VETERINARY-SCIENCES/PUBLICATIONS/2014/Vol_4_No_1/JFAV-04-01-Contents.htm (accessed on 28 February 2022).
- Olatoye, I.O.; Daniel, O.F.; Ishola, S.A. Screening of antibiotics and chemical analysis of penicillin residue in fresh milk and traditional dairy products in Oyo state, Nigeria. Vet. World 2016, 9, 948–954. [Google Scholar] [CrossRef]
- Bagré, T.S.; Samandoulougou, S.; Traore, S.M.; Illy, D.; Bsadjo-Tchamba, G.; Bawa-Ibrahim, H.; Bouda, S.C.; Traore, A.S.; Barro, N. Détection biologique des résidus d’antibiotiques dans le lait et produits laitiers de vache consommés à Ouagadougou, Burkina Faso. J. Appl. Biosci. 2015, 87, 8105–8112. Available online: https://www.ajol.info/index.php/jab/article/view/116531 (accessed on 11 February 2022). [CrossRef]
- Srimulyati, A.; Purnawarman, T.; Latif, H. Tetracycline residue detection on cheese imported trough Tanjung Priok seaport, Jakarta. Glob. Vet. 2015, 14, 819–823. Available online: https://www.idosi.org/gv/gv14(6)15/5.pdf (accessed on 21 February 2022).
- Adetunji, V.O. Effects of processing on antibiotic residues (streptomycin, penicillin-G and tetracycline) in soft cheese and yoghurt processing lines. Pak. J. Nutr. 2011, 10, 792–795. [Google Scholar] [CrossRef][Green Version]
- Marth, E.H.; Ellickson, B.E. Problems Created by the Presence of Antibiotics in Milk—A Review. J. Milk Food Technol. 1959, 22, 266–272. [Google Scholar] [CrossRef]
- Caplice, E.; Fitzgerald, G.F. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- Chiesa, L.M.; DeCastelli, L.; Nobile, M.; Martucci, F.; Mosconi, G.; Fontana, M.; Castrica, M.; Arioli, F.; Panseri, S. Analysis of antibiotic residues in raw bovine milk and their impact toward food safety and on milk starter cultures in cheese-making process. LWT Food Sci. Technol. 2020, 131, 109783. [Google Scholar] [CrossRef]
- Berruga, M.I.; Molina, A.; Althaus, R.L.; Molina, M.P. Control and prevention of antibiotic residues and contaminants in sheep and goat’s milk. Small Rumin. Res. 2016, 142, 38–43. [Google Scholar] [CrossRef]
- Cogan, T.M. Susceptibility of Cheese and Yogurt Starter Bacteria to Antibiotics. Appl. Microbiol. 1972, 23, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, P.; Cornacchini, M.; Hernando, M.I.; Molina, M.P.; Escriche, I. Impact of the presence of oxytetracycline residues in milk destined for the elaboration of dairy products: The specific case of mature goat cheese. Int. Dairy J. 2020, 101, 104595. [Google Scholar] [CrossRef]
- Berruga, I.; Battacone, G.; Molina, M.P.; Román, M.; Molina, A. Influence of β-lactams on Manchego cheese manufacture. In Proceedings of the 5th International Symposium on the Challenge to Sheep and Goats Milk Sectors, Alghero, Italy, 18–20 April 2007; Special Issue IDF 0801, Part 1/3. pp. 222–224. [Google Scholar]
- Berruga, M.I.; Molina, M.P.; Noves, B.; Roman, M.; Molina, A. “In vitro” study about the effect of several penicillins during the fermentation of yogurt made from ewe’s milk. Milchwiss. Milk Sci. Int. 2007, 62, 303–305. [Google Scholar]
- Berruga, M.I.; Noves, B.; Molina, M.P.; Roman, M.; Molina, A. Influence of cephalosporins on the coagulation time of yogurt made from ewes’ milk. Int. J. Dairy Technol. 2008, 61, 372–378. [Google Scholar] [CrossRef]
- Novés, B.; Libran, C.; Licon, C.C.; Molina, M.P.; Molina, A.; Berruga, M.I. Technological failures caused by cephalexin in set-type sheep’s milk yogurt. CyTA-J. Food 2015, 13, 408–414. [Google Scholar] [CrossRef]
- Beltran, M.C.; Morari-Pirlog, A.; Quintanilla, P.; Escriche, I.; Molina, M.P. Influence of enrofloxacin on the coagulation time and the quality parameters of goat’s milk yoghurt. Int. J. Dairy Technol. 2018, 71, 105–111. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Foodborne antimicrobial resistance as a biological hazard—Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2008, 765, 4–87. [Google Scholar] [CrossRef]
- Aarestrup, F.M. Association between the consumption of antimicrobial agents in animal husbandry and the occurrence of resistant bacteria among food animals. Int. J. Antimicrob. Agents 1999, 12, 279–285. [Google Scholar] [CrossRef]
- Mathur, S.; Singh, R. Antibiotic resistance in food lactic acid bacteria—A review. Int. J. Food Microbiol. 2005, 105, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microb. New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Manuzon, M.; Lehman, M.; Wan, K.; Luo, H.; Wittum, T.E.; Yousef, A.; Bakaletz, L.O. Food commensal microbes as a potentially important avenue in transmitting antibiotic resistance genes. FEMS Microbiol. Lett. 2006, 254, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Jamet, E.; Akary, E.; Poisson, M.; Chamba, J.; Bertrand, X.; Serror, P. Prevalence and characterization of antibiotic resistant Enterococcus faecalis in French cheeses. Food Microbiol. 2012, 31, 191–198. [Google Scholar] [CrossRef]
- Hammad, A.M.; Hassan, H.A.; Shimamoto, T. Prevalence, antibiotic resistance and virulence of Enterococcus spp. in Egyptian fresh raw milk cheese. Food Control 2015, 50, 815–820. [Google Scholar] [CrossRef]
- Výrostková, J.; Regecova, I.; Dudrikova, E.; Marcincak, S.; Vargova, M.; Kovacova, M.; Mal’ova, J. Antimicrobial Resistance of Enterococcus sp. Isolated from Sheep and Goat Cheeses. Foods 2021, 10, 1844. [Google Scholar] [CrossRef] [PubMed]
- Devirgiliis, C.; Barile, S.; Perozzi, G. Antibiotic resistance determinants in the interplay between food and gut microbiota. Genes Nutr. 2011, 6, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Abriouel, H.; Knapp, C.W.; Gálvez, A.; Benomar, N. Chapter 29—Antibiotic Resistance Profile of Microbes from Traditional Fermented Foods. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 675–704. ISBN 9780128023099. [Google Scholar] [CrossRef]
- Guo, H.; Pan, L.; Li, L.; Lu, J.; Kwok, L.; Menghe, B.; Zhang, H.; Zhang, W. Characterization of Antibiotic Resistance Genes from Lactobacillus Isolated from Traditional Dairy Products. J. Food Sci. 2017, 82, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.A.; Mateus, A.; Marshall, L.; Pfeiffer, D.U.; Lubroth, J.; Ormel, H.J.; Otto, P.; Patriarchi, A. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production; FAO: Rome, Italy, 2016; ISBN 978-92-5-109441-9. Available online: https://www.fao.org/publications/card/en/c/d5f6d40d-ef08-4fcc-866b-5e5a92a12dbf/ (accessed on 24 March 2022).
- Firth, C.L.; Käsbohrer, A.; Pless, P.; Koeberl-Jelovcan, S.; Obritzhauser, W. Analysis of Antimicrobial Use and the Presence of Antimicrobial-Resistant Bacteria on Austrian Dairy Farms—A Pilot Study. Antibiotics 2022, 11, 124. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Wegener, H.C.; Collignon, P. Resistance in bacteria of the food chain: Epidemiology and control strategies. Expert Rev. Anti-Infect. Ther. 2008, 6, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Zeina, K.; Pamela, A.K.; Fawwak, S. Quantification of Antibiotic Residues and Determination of Antimicrobial Resistance Profiles of Microorganisms Isolated from Bovine Milk in Lebanon. Food Nutr. Sci. 2013, 4, 9. Available online: www.scirp.org/journal/paperinformation.aspx?paperid=33574 (accessed on 24 March 2022). [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2013, 69, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Manie, T.; Brözel, V.S.; Veith, W.J.; Gouws, P.A. Antimicrobial resistance of bacterial flora associated with bovine products in South Africa. J. Food Prot. 1999, 62, 615–618. [Google Scholar] [CrossRef]
- Tempini, P.N.; Aly, S.S.; Karle, B.M.; Pereira, R.V. Multidrug residues and antimicrobial resistance patterns in waste milk from dairy farms in Central California. J. Dairy Sci. 2018, 101, 8110–8122. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.V.R.; Lima, S.; Siler, J.D.; Foditsch, C.; Warnick, L.D.; Bicalho, R.C. Ingestion of Milk Containing Very Low Concentration of Antimicrobials: Longitudinal Effect on Fecal Microbiota Composition in Preweaned Calves. PLoS ONE 2016, 11, e0147525. [Google Scholar] [CrossRef]
- Maynou, G.; Migura-Garcia, L.; Chester-Jones, H.; Ziegler, D.; Bach, A.; Terré, M. Effects of feeding pasteurized waste milk to dairy calves on phenotypes and genotypes of antimicrobial resistance in fecal Escherichia coli isolates before and after weaning. J. Dairy Sci. 2017, 100, 7967–7979. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; et al. Risk for the development of Antimicrobial Resistance (AMR) due to feeding of calves with milk containing residues of antibiotics. EFSA J. 2017, 15, e04665. [Google Scholar] [CrossRef] [PubMed]
- El Zubeir, I.E.; El Owni, O. Antimicrobial resistance of bacteria associated with raw milk contaminated by chemical preservatives. World J. Dairy Food Sci. 2009, 4, 65–69. Available online: https://idosi.org/wjdfs/wjdfs4(1)/11.pdf (accessed on 24 March 2022).
- Cerniglia, C.E.; Pineiro, S.A.; Kotarski, S.F. An update discussion on the current assessment of the safety of veterinary antimicrobial drug residues in food with regard to their impact on the human intestinal microbiome. Drug Test. Anal. 2016, 8, 539–548. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2015.01543 (accessed on 31 March 2022). [CrossRef] [PubMed]
- Piñeiro, S.A.; Cerniglia, C.E. Antimicrobial drug residues in animal-derived foods: Potential impact on the human intestinal microbiome. J. Vet. Pharmacol. Ther. 2021, 44, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.S.; Finch, R.; Wegener, H.C.; Bywater, R.; Walters, J.; Lipsitch, M. Antibiotic resistance—The interplay between antibiotic use in animals and human beings. Lancet Infect. Dis. 2003, 3, 47–51. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J. Antibiotic resistance in the food supply chain: Where can sequencing and metagenomics aid risk assessment? Curr. Opin. Food Sci. 2017, 14, 66–71. [Google Scholar] [CrossRef]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; Van der Zwaluw, K.; et al. Extended-Spectrum β-Lactamase Genes of Escherichia coli in Chicken Meat and Humans, the Netherlands. Emerg. Infect. Dis. 2011, 17, 1216. Available online: https://wwwnc.cdc.gov/eid/article/17/7/11-0209_article (accessed on 27 April 2022). [CrossRef] [PubMed]
- Toomey, N.; Monaghan, Á.; Fanning, S.; Bolton, D.J. Assessment of Antimicrobial Resistance Transfer between Lactic Acid Bacteria and Potential Foodborne Pathogens Using In Vitro Methods and Mating in a Food Matrix. Foodborne Pathog. Dis. 2009, 6, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Van Reenen, C.A.; Dicks, L.M.T. Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: What are the possibilities? A review. Arch. Microbiol. 2011, 193, 157–168. [Google Scholar] [CrossRef]
- De Paula, A.C.L.; Medeiros, J.D.; De Azevedo, A.C.; De Assis Chagas, J.M.; Da Silva, V.L.; Diniz, C.G. Antibiotic Resistance Genetic Markers and Integrons in White Soft Cheese: Aspects of Clinical Resistome and Potentiality of Horizontal Gene Transfer. Genes 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Gao, J.; Guo, J.; Wang, H.; Zhang, E.N.; Lin, Y.; Chen, Z.; Li, S.; Tao, S. Characterization of Bacteria and Antibiotic Resistance in Commercially Produced Cheeses Sold in China. J. Food Prot. 2022, 85, 484–493. [Google Scholar] [CrossRef]
- Dolliver, H.; Kumar, K.; Gupta, S. Sulfamethazine uptake by plants from manure-amended soil. J. Environ. Qual. 2007, 36, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Kyselková, M.; Jirout, J.; Vrchotová, N.; Schmitt, H.; Elhottová, D. Spread of tetracycline resistance genes at a conventional dairy farm. Front. Microbiol. 2015, 6, 536. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2015.00536 (accessed on 24 March 2022). [CrossRef] [PubMed]
- Oliver, J.P.; Gooch, C.A.; Lansing, S.; Schueler, J.; Hurst, J.J.; Sassoubre, L.; Crossette, E.M.; Aga, D.S. Invited review: Fate of antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes in US dairy manure management systems. J. Dairy Sci. 2020, 103, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Akram, S.; Stedtfeld, R.; Johnson, M.; Chabrelie, A.; Yin, D.; Mitchell, J. Distribution of antimicrobial resistance across the overall environment of dairy farms—A case study. Sci. Total Environ. 2021, 788, 147489. [Google Scholar] [CrossRef] [PubMed]
- Pitta, D.W.; Dou, Z.; Kumar, S.; Indugu, N.; Toth, J.D.; Vecchiarelli, B.; Bhukya, B. Metagenomic Evidence of the Prevalence and Distribution Patterns of Antimicrobial Resistance Genes in Dairy Agroecosystems. Foodborne Pathog. Dis. 2016, 13, 296–302. [Google Scholar] [CrossRef] [PubMed]
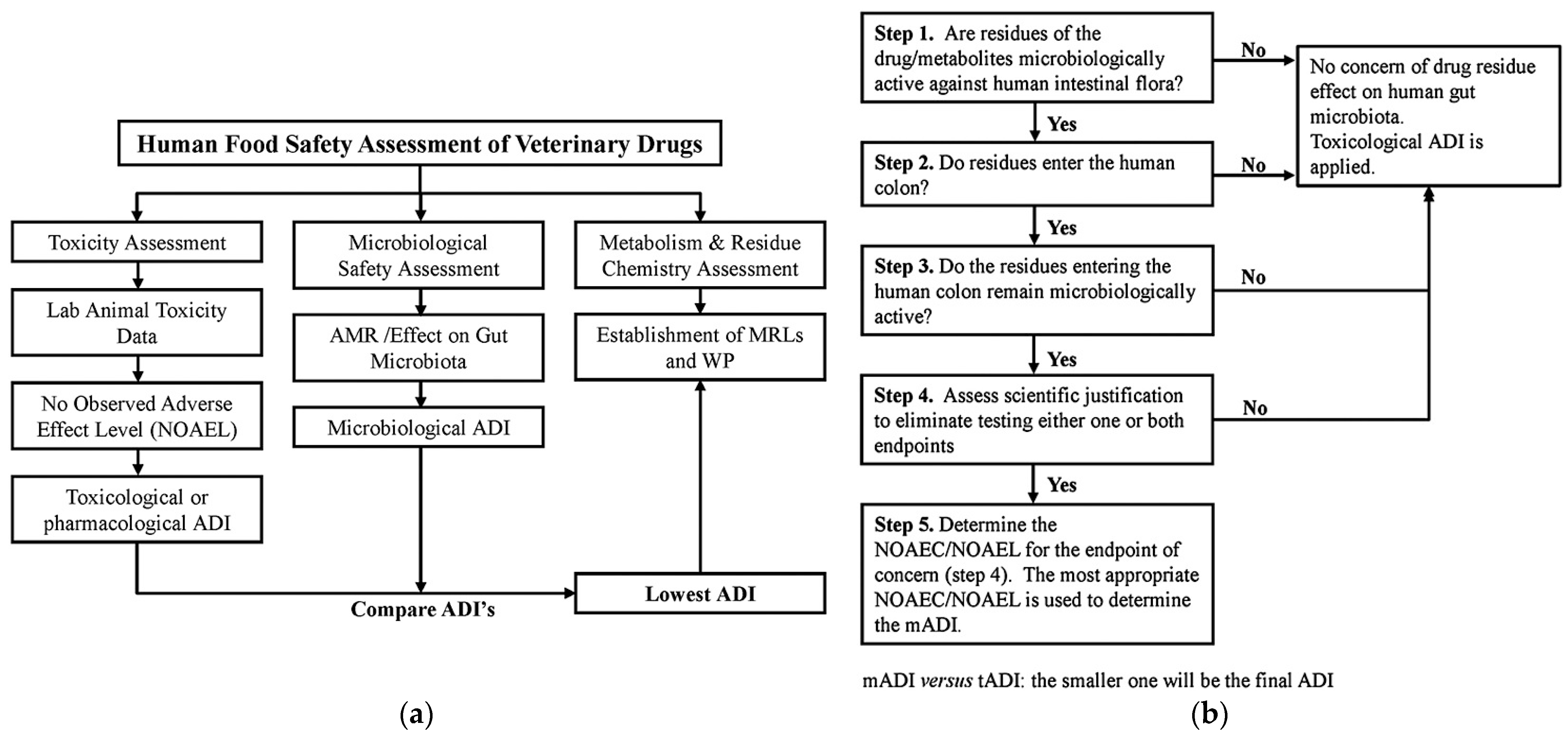
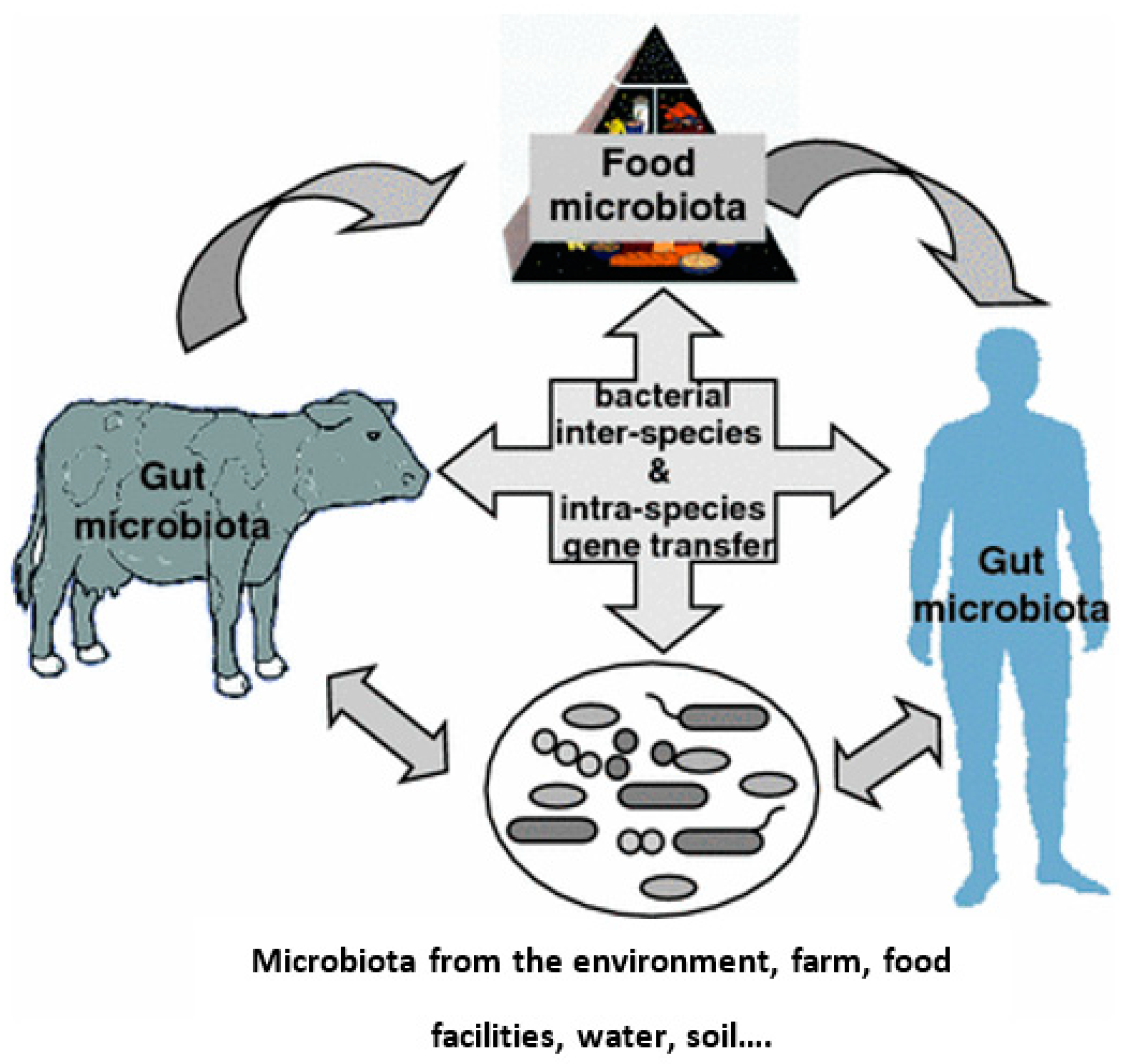
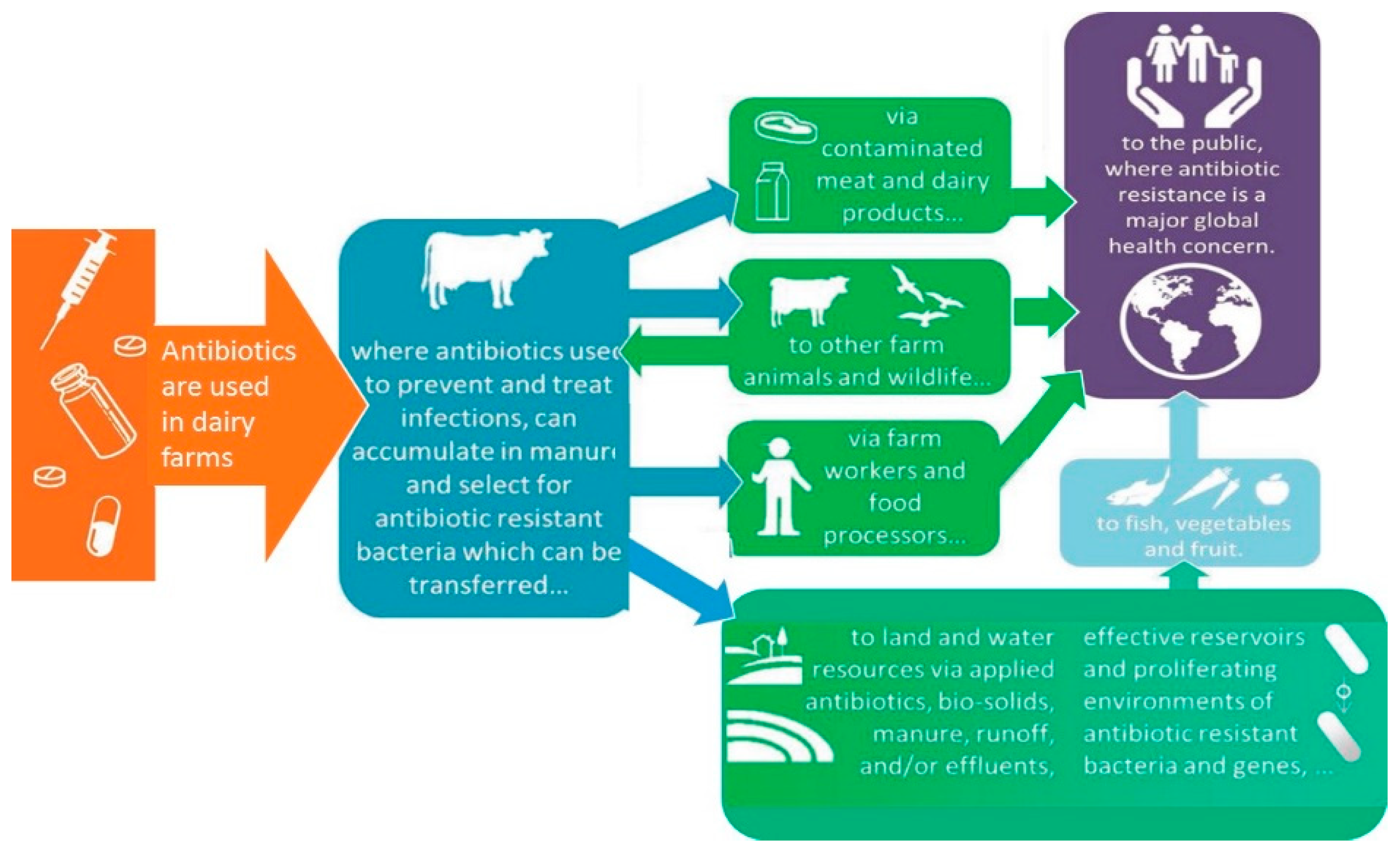
| Chemical Class | Compound | MRL (μg kg−1) * | Primary Use |
|---|---|---|---|
| Aminoglycosides | Gentamycin | 100 | All animals, humans |
| Kanamycin | 150 | Dogs, pigs, cattle, horses | |
| Neomycin | 1500 | All animals | |
| Spectinomycin | 500 | Dogs, pigs, cattle, horses | |
| Streptomycin | 200 | Obsolete | |
| Quinolones | Enrofloxacin | 100 | All animals |
| Ciprofloxacin | 100 | Humans | |
| Difloxacin | Not for use in animals from which milk is produced for human consumption | ||
| Danofloxacin | 30 | ||
| Marbofloxacin | 75 | All animals | |
| Flumequine | 50 | Humans | |
| Oxolinic acid | Not for use in animals from which milk is produced for human consumption | ||
| β-Lactams (penicillins) | Amoxicillin | 4 | All animals |
| Ampicillin | 4 | All animals | |
| Benzylpenicillin (Pen G) | 4 | All animals | |
| Cloxacillin | 30 | Cattle | |
| Dicloxacillin | 30 | Cattle | |
| Nafcillin | 30 | Humans | |
| Oxacillin | 30 | Cattle | |
| β-Lactams (Cefalosporins) | Cefalonium | 20 | Dogs, cats and cattle |
| Cefazolin | 50 | Humans | |
| Cefoperazone | 50 | Humans, cattle | |
| Cefquinome | 20 | Cattle, pigs | |
| Cefapirin | 60 | Cattle, sheep, goat and pigs | |
| Ceftiofur | 100 | Cattle, pigs | |
| Desacetylcefapirin | 60 | Metabolite of cefapirin | |
| Desfuroylceftiofur | 100 | Metabolite of ceftiofur | |
| Macrolides | Tylosin | 50 | Animals only |
| Tilmicosin | 50 | Sheep, cattle | |
| Spiramycin | 200 | All animals | |
| Neospiramycin | 20 | Metabolite of spiramycin | |
| Erythromicyn | 40 | Humans, cattle, chicken | |
| Sulfonamides | Sulfadiazine | 100 | Humans |
| Sulfadimethoxine | 100 | Cattle, pigs, chicken | |
| Sulfadimidine | 100 | Cattle, sheep, chicken | |
| Sulfamerazine | 100 | Humans and animals | |
| Sulfamethoxazole | 100 | Human | |
| Sulfamonomethoxine | 100 | Humans | |
| Sulfatiazole | 100 | Humans | |
| Trimethoprim | 100 | In combination with sulfonamides | |
| Tetracyclines | Chlorotetracycline | 100 | Cattle, pigs |
| Oxytetracycline | 100 | Humans, cattle, sheep, pigs | |
| Tetracycline | 100 | Humans, cattle, sheep, pigs | |
| Doxyicycline | Not for use in animals from which milk is produced for human consumption | ||
| Lincosamides | Lincomycin | 150 | Pigs, cats, dogs, cattle |
| Amphenicols | Tiamphenicol | 50 | All animals |
| Florfenicol | Not for use in animals from which milk is produced for human consumption | ||
| Florfenicol amine | Not for use in animals from which milk is produced for human consumption | ||
| Nitroimidazoles | Dimetridazole | Prohibited | |
| Ronidazole | Prohibited | ||
| Metronidazole | Prohibited |
| Territory | Sample Type | No. of Samples | Year 1 | Detection Method | Antimicrobials | % Positive Samples 2 | Concentration | Ref. |
|---|---|---|---|---|---|---|---|---|
| Kenya | Raw cow milk | 1600 | 2001–2002 | Two-tube diffusion Penicillinase βL plate assay | βL | 13% | >4 µg/kg | [24] |
| Cow milk | 229 | 2014–2015 | Charm II Blue-Yellow HPLC-UV | SAM TC | 31.4% 0% | 66.14–8979.51 μg/ kg | [25] | |
| Cow milk | 95 | 2015 | IDEXX SNAP® | 7.4% βL, 3.2% TC | [26] | |||
| Cow milk | 55 | 2015–2016 | IDEXX SNAP® | βL, SMZ,TC, GM | 24% in at least one antibiotic | [27] | ||
| Cow milk | 65 | 2020 | HPLC | AO, CO,TC, SMX,TriM | 10.8% above MRL 20% detectable residues | 6.7, 53.3, 30.6, 5.0, 6.2 μg/L, respectively | [28] | |
| Tanzania | Cow milk | 982 | 1999–2000 | Charm AIM | βL, TC, AMG, ML, SAM | 36% | [29] | |
| Raw cow milk | 91 | 2006 | Delvotest® | 4.5% | [30] | |||
| Raw cow milk | 128 | 2006 | IDF Method and Delvotest SP® | 7% | [31] | |||
| Raw cow milk From dairy farms | 98 | 2010–2011 | Delvotest SP® | 83% | [32] | |||
| Algeria | Cow milk | 194 | 2013–2014 | Delvotest SP-NT® LC-MS/MS | βL, ML, SAM, QN, TC | 25% (Delvotest®) 65% detectable | [33] | |
| Cow milk Goats milk | 117 33 | 2019 | Delvotest SP® BetaStar® Combo | βL, TC | 12.67% (Delvo) 2.5% (cow βL), 1.7% (cow TC) 6.1% (goat βL), 3.0% (goat TC) | [34] | ||
| Nigeria | Cow milk | 192 | 2015 | Delvotest T® | 9.9% | [35] | ||
| Ghana | Cow raw milk | 224 | 2007 | Charm Blue-Yellow | 3.1% | [36] | ||
| Brazil | Pasteurized cow milk | 151 | 2005–2006 | SNAP tests ELISA kits | TC, βL, GM, CHA, StM, NM | 41.3% 4 positive in CHA | dStM 260 µg/kg NM 69.8–110.2 µg/kg CHA 0.157–0.402 µg/kg | [37] |
| Pasteurized cow milk | 260 | 2006–2007 | SNAP tests Ridascreen | TC, βL, GM, CHA, StM-dStM, NM | TC 18.5%, βL 3.5% GM 2.3%, CHA 1.5%, StM-dStM 0.4%, NM 17.4% | 0.16–9.23 µg/kg, 25.86 µg/kg 60.08–278.42 µg/kg | [38] | |
| Pasteurized cow milk | 299 | 2009 | ELISA kit and LC–APCI–MS/MS QToF | StM, dStM | 2 samples (ELISA) 0 LC-MS | 213 and 290 µg/kg | [39] | |
| Pasteurized cow milk | 252 | 2010–2011 | Delvotest SP-NT® HPLC-DAD | TC OTC | 8% positive 10% dubious | 107–2297 μg/L 125–2782 μg/L | [5] | |
| Pasteurized milk | 100 | 2010–2013 | HPLC-UV/Vis | OTC, TC, cTC, dOC | 3% | Average, 42.3 μg/kg | [40] | |
| Raw cow milk | 184 | 2020 | LC-MS/MS | βL, SAM, TC, QN, fQN, PY | CO (1), PNG (3) TC (11) | 464 μg/L,, 0.2–4.0 μg/L 7.1–49.7 μg/L | [41] | |
| Puerto Rico, Barbados, Jamaica | UHT cow milk | 80 | 1996–1997 | Delvotest-P® HPLC-UV | βL | Puerto Rico 0% Barbados 8% Jamaica 10% | APC 1.8–18.4 μg/L CF 15 μg/mL CO 61–358 μg/L PNG 6.6–11.8 μg/L | [42] |
| Paraguay | Cow milk | 450 | 2015 | 4Sensor and Gentasensor | GM, βL, StM, CHA, TC | 0 | [43] | |
| Peru | Cow milk | 156 | 2013 | Snap Duo™ Beta-Tetra test | 0–4.2% | [44] | ||
| Iran | Cow milk | 196 | 2008 | Copan test kit | βL,TC, SAM, AMG, ML | 40.8% | [45] | |
| Pasteurized/raw cow milk | 251 | 2009–2013 | Copan test kit | βL,TC, SAM, AMG, ML | 24.8% | [46] | ||
| Pasteurized cow milk | 432 | 2011–2012 | HPLC-UV | TC | 1.62% | 274–1270 μg/L | [47] | |
| Cow tank milk | 79 | 2012 | HPLC-UV | βL | 32.9 % | [48] | ||
| Commercial cow milk | 187 | 2012 | Eclipse 100-kit HPLC-UV-vis | TC | 19.8% | 197–2452 μg/kg | [49] | |
| India | Cow milk | 491 | 2016–2017 | DPA test and Charm ROSA | βL, TC, NV, EM, SMA | 0.6%, 0.8%, 3.5%, 2.4%, 1% | [50] | |
| Raw/pasteurized cow milk | 128/45 | 2018–2019 | HPLC-DAD | AO, TC | 1.7%, 1.2% | 67.9 ± 40.9, 11.3 ± 1.5 µg/kg | [51] | |
| Cow milk | 168 | 2019 | MaxSignal (ELISA) | EF, OTC, PNG, SMX | 1.7%, 1.2%, 0.6%, 0% | 87.9 ± 44.0, 70.7 ± 45.9, 2.2 ± 1.5 μg/mL, nd | [52] | |
| Bangladesh | Local/commercial cow milk | 200 | 2011–2012 | MIT, TLC HPLC | AO, TC, CPF | AO 14%, 38% local/commer TC 11%, 23% CPF 8%, 17% | AO 9.84, 56.16 μg/mL | [53] |
| Cow milk | 100 | 2019 | TLC UHPLC | AO, OTC, StM, GM, CTX | 2%, 3.33%, 1.33%, 0.6% 0.6 % | AO 124 μg/mL OTC 61.3 μg/mL | [54] | |
| Nepal | Cow milk | 140 | 2018 | Agar diffusion HPLC | PN, SAM | 23% | PN 0–16 µg/kg (2 samples 128, 256 µg/kg) SAM 0–64 µg/kg (in 9 samples 128–256 mg/kg) | [55] |
| Indonesia | Goat milk | 36 | 2018 | Triple bio screening test | TC, ML | TC 2.8%, ML 3.6% | [56] | |
| Turkey | UHT cow milk | 60 | 2005 | Ridascreen | CHA, StM, TC | 46.8% (CHA 30%) | 806, 360, 602 ng/L | [57] |
| Kosovo | Milk from collection points and farms | 1734 | 2009–2010 | Delvotest P® SNAP tests, HPLC | βL, TC, SAM | 6.11% | AO, PNG, and CO between 2.1 and 1973 mg/kg | [58] |
| Cow milk | 1055 | 2015–2016 | Delvotest SP, SNAP test | 10% | [59] | |||
| Croatia | Cow milk | 90 | 2009 | ELISA | StM, TC | 0–73.82, 0–4.26 µg/kg | [60] | |
| Raw cow milk | 1259 | 2008–2010 | Delvotest® SP-NT Immunoassay (EIA) HPLC-DAD | PN, CPh, TC, SAM, AMG, ML | 0.69% | 12 µg/kg PNG 19 µg/kg AO, 1671 µg/kg TC | [61] | |
| Slovenia | Raw cow milk | 286 | 1991–2000 | GC-ECD | CHA | 1 sample | 4.6 µg/ kg | [62] |
| Spain | Ewes raw milk | 2686 | 2004 | Delvotest® SP | 1.7% positive, 2.1% “doubtful” | βL or SAM n.d. | [63] | |
| Ewes raw milk | 71,228 | 2004–2008 | Eclipse 100ov | 1.36% (2004)–0.30% (2008) | [64] | |||
| Poland | Fresh and UHT cow milk | 36 and 48 | 2019 | PN ELISA Ridascreen | PN TC | 1.15% below MRL 28.57% below MRL | 0.040 to 0.804 μg/L 0.450 to 2.520 μg/L | [65] |
| Territory | Sample Type | No. of Samples | Year 1 | Method Detection | Antibiotics | % Positive Samples 2 | Concentration | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nigeria | cow milk, goat milk, butterfat, soft cheese, yoghurt | 8 of each | 2014 | HPLC-fluorimeter | TC | All below MRL | 3.2 ± 1.8, 4.0 ± 1.1, 2.0 ± 0.8 8.0 ± 3.4, 1.9 ± 0.8 μg/L, respectively | [88] |
| fresh milk local cheese fermented milk | 328 180 90 | 2016 | Premi® test HPLC | PNG | 40.8% 24.4% 62.3% | 15.22 ± 0.61 μg/L 8.24 ± 0.50 μg/L 7.6 ± 0.60 μg/L | [89] | |
| Burkina Faso | Raw milk Curd Pasteurized milk Yogurt | 29 40 42 90 | 2014 | Microbial test | βL, SAM, TC | 59.7% of samples positive for some antibiotic | [90] | |
| Indonesia | Imported cheese (Cheddar, Mozzarella) | 51 | 2015 | Ridascreen | TC | 13.7% | 2.47 μg/L to 11.99 μg/L | [91] |
| Pakistan | Cheese Yogurt | 40, 18 | 2011 | HPLC | PNG, StM, TC | 6.2, 4.0, 2.3 μg/L 1.7, 1.4, 1.1 μg/L | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virto, M.; Santamarina-García, G.; Amores, G.; Hernández, I. Antibiotics in Dairy Production: Where Is the Problem? Dairy 2022, 3, 541-564. https://doi.org/10.3390/dairy3030039
Virto M, Santamarina-García G, Amores G, Hernández I. Antibiotics in Dairy Production: Where Is the Problem? Dairy. 2022; 3(3):541-564. https://doi.org/10.3390/dairy3030039
Chicago/Turabian StyleVirto, Mailo, Gorka Santamarina-García, Gustavo Amores, and Igor Hernández. 2022. "Antibiotics in Dairy Production: Where Is the Problem?" Dairy 3, no. 3: 541-564. https://doi.org/10.3390/dairy3030039
APA StyleVirto, M., Santamarina-García, G., Amores, G., & Hernández, I. (2022). Antibiotics in Dairy Production: Where Is the Problem? Dairy, 3(3), 541-564. https://doi.org/10.3390/dairy3030039








