Algal-Mediated Nanoparticles, Phycochar, and Biofertilizers for Mitigating Abiotic Stresses in Plants: A Review
Abstract
1. Introduction
2. Salinity, Drought, and Flooding as Abiotic Stresses
3. Sustainable Solutions to Improve Soil Conditions and Plant Health
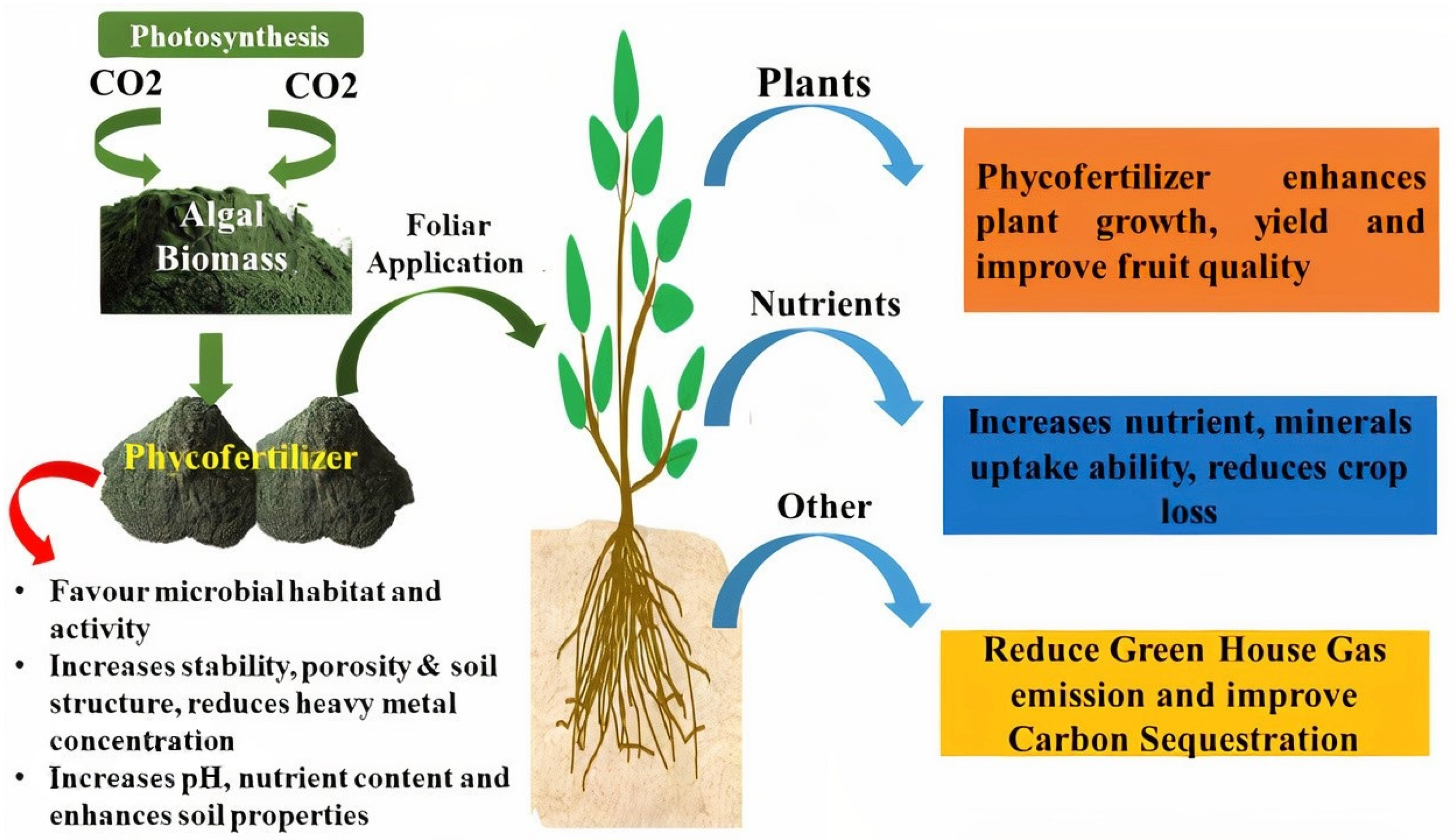
4. Use of Algal Biomass
5. Production of Phycofertilizer from Algae
6. Role of Phycofertilizer in Stress Resistance
7. Role of Phycofertilizer in Drought Resistance
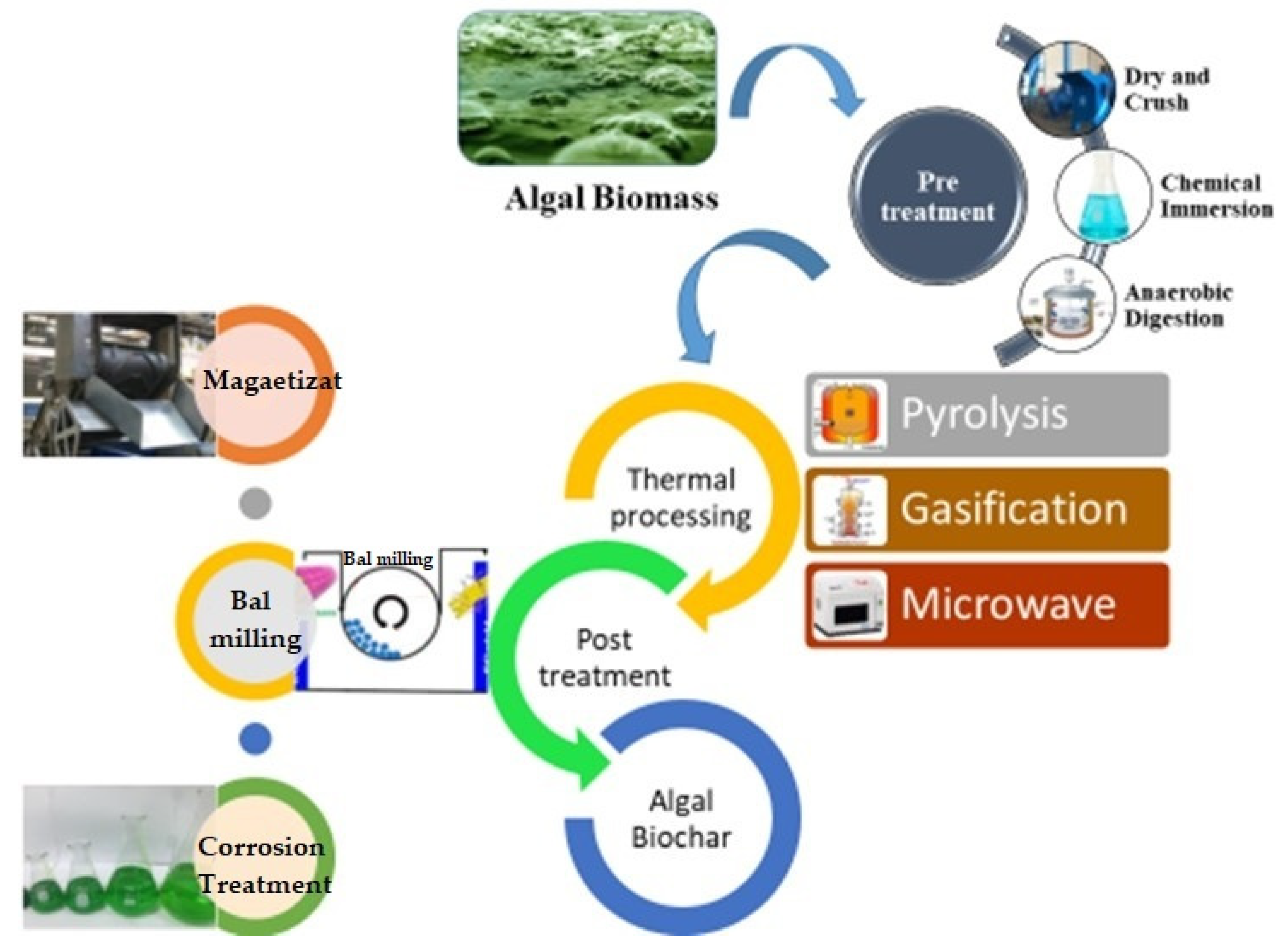
8. Production of Phycochar from Algae
| Algae | Carbon | Nitrogen | Oxygen | Hydrogen | Ash | Reference |
|---|---|---|---|---|---|---|
| C. reinhardtii | 40 | 5 | 10 | 1.5 | 45 | [34] |
| Scenedesmus sp. | 53 | 6.5 | 40 | 8.5 | 44 | [111] |
| Almeriansis sp. | 73 | 5 | 0.8 | 9 | 36 | [112] |
| L. saccharina | 74 | 3 | 14 | 8 | - | [113] |
| Cladophora sp. | 12 | 1.3 | 11 | 0.7 | 75 | [114] |
| Arthrospira sp. | 48 | 11 | - | - | - | [112] |
| Gracilaria sp. | 31 | 3 | 16 | 2.2 | 43 | [34] |
| Spirulina sp. | 46 | 10 | 34 | 7 | 7 | [34] |
| Lyngbya sp. | 70 | 7 | 22 | 2.5 | - | [115] |
| D. tenuissima | 20 | 1 | 22 | 3 | - | [50] |
| Kappaphycus sp. | 31 | 0.7 | 23 | 2 | - | [116] |
| Enteromorpha sp. | 16 | 2 | - | 2 | - | [111] |
| Desmodesmus sp. | 51 | 7 | 14 | 3 | 37 | [115] |
| Oedogonium sp. | 12 | 1 | 6 | 1 | - | [116] |
| Spirulina algae | 69 | 7 | 15 | 9 | - | [117] |
| Ulva | 73 | 7 | 11 | 8 | - | [118] |
| Cyanobacteria sp. | 76 | 6.3 | 7.39 | 9 | - | [119] |
| Chlorella | 51 | 10 | 30 | 8 | - | [113] |
| Ulva fasciata | 26 | 3 | - | 6 | 15.99 | [120] |
| Caulerpa taxifolia | 25 | 2.5 | - | 1.19 | - | [121] |
9. Biochar Alleviates the Negative Effects of Drought Stress
10. Phycochar Improves Plant’s Performance under Combined Drought and Heavy Metals Stress
11. Phycochar Removes Pollutants from Soil and Wastewater
12. Biochar Alleviates Negative Impacts of Salinity Stress
| Environmental Pollution | Role | Reference |
|---|---|---|
| Air | Enhance methane uptake | [115] |
| Absorbs Ammonia | [101] | |
| Decrease N2O emissions | [156] | |
| Water | Remove the synthetic, metallic, phenolic, pesticides pollutants | [143] |
| Soil | Absorb heavy metals such as lead, cadmium, copper & chromium Increase organisms involved in Nitrogen fixation and nitrification | [115,143,146,157] |
13. Benefits and Limitations of Algal Biochar
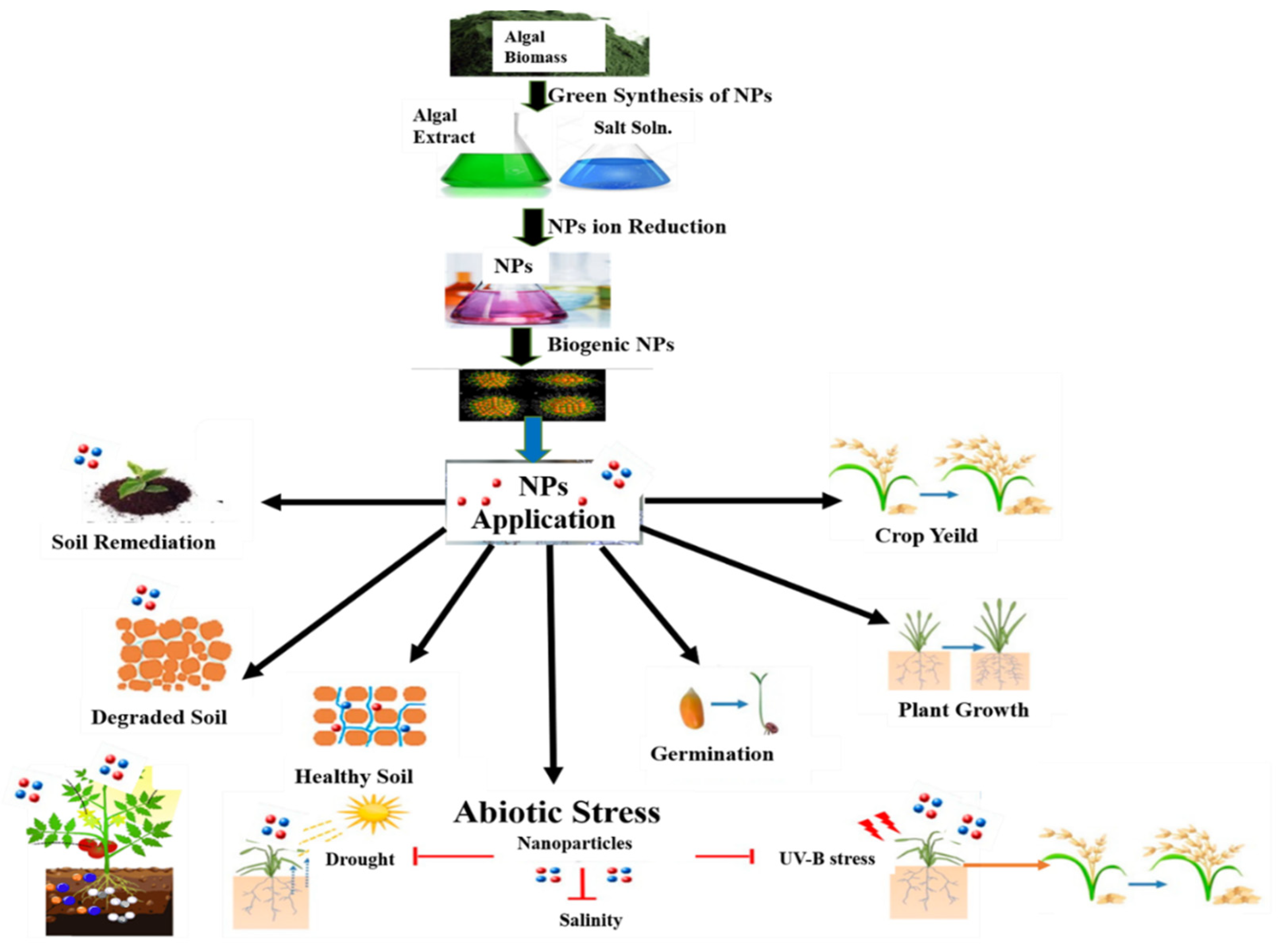
14. Production of Nanoparticles from Algae
15. Role of Algal NPs in Bioremediation
16. Algal Nano Fertilizers
| Species | Nanoparticles | Role of Algal NPs in Bioremediation | References |
|---|---|---|---|
| Chlorella vulgaris | Ag NPs | Shows strong antifungal activity in plant | [185] |
| Haematococcus Candid & chlorella sp. | Ag NPs | Inhibited Penicillium expansum growth, the main cause of loss of quality and quantity of fruit | [186] |
| Turbinaria conoides & Sargassum tenerrimum | (Au) NPs | Act as bioremediation, catalytic reduction efficiency in the degradation of organic dyes | [187] |
| Ulva lactuca | (Ag) NPs | Used for Photocatalytic degradation of methyl orange dye | [175] |
| Cyanobacteria | ZnO NPs | Act as nano fertilizer have antioxidative properties | [187] |
| Cyanobacteria | Ag-NPs | Bactericidal activity | [188] |
| Chaetomorpha linum | Ag NPs | Helps in bioremediation & reduction of silver ions (Ag+) to Ag0 | [189] |
| Chlorella ellipsoidea | AgNPs | Degradation of the hazardous pollutant dyes methylene blue & methyl orange | [190] |
| Scenedesmus obliquus | Lipid cadmium sulphide NPs | For the bioremediation of Cd2+ ions due to its high retention capability | [191] |
17. The Beneficial Role of Algal Nanoparticles in Plant Stress Tolerance
18. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, P.; Tokas, J.; Kumar, N.; Lal, M.; Singal, H.R. Climate change consequences and its impact on agriculture and food security. Int. J. Chem. Stud. 2018, 6, 124–133. [Google Scholar]
- Rasul, G.; Mahmood, A.; Sadiq, A.; Khan, S.I. Vulnerability of the Indus Delta to Climate Change in Pakistan. Pak. J. Meteorol. 2012, 8, 89–107. [Google Scholar]
- Singh, P.; Kumar, N. Impact assessment of climate change on the hydrological response of a snow and glacier melt runoff dominated Himalayan river. J. Hydrol. 1997, 193, 316–350. [Google Scholar] [CrossRef]
- Solomon, S. IPCC 2007: Climate Change the Physical Science Basis; AGU Fall Meeting Abstracts U43D-01; American Geophysical Union: Washington, DC, USA, 2007. [Google Scholar]
- Abideen, Z.; Koyro, H.W.; Huchzermeyer, B.; Ahmed, M.; Zulfiqar, F.; Egan, T.; Khan, M.A. Phragmites karka plants adopt different strategies to regulate photosynthesis and ion flux in saline and water deficit conditions. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 524–534. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Abideen, Z.; Koyro, H.W.; Hussain, T.; Rasheed, A.; Alwahibi, M.S.; Elshikh, M.S.; Hussain, M.I.; Zulfiqar, F.; Mansoor, S.; Abbas, Z. Biomass Production and Predicted Ethanol Yield Are Linked with Optimum Photosynthesis in Phragmites karka under Salinity and Drought Conditions. Plants 2022, 11, 1657. [Google Scholar] [CrossRef]
- Lieber, M.; Chin-Hong, P.; Kelly, K.; Dandu, M.; Weiser, S.D. A systematic review and meta-analysis assessing the impact of droughts, flooding, and climate variability on malnutrition. Glob. Public Health 2022, 17, 68–82. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, B.; Zheng, X.; Liu, G. Plant biomass, soil water content and soil N: P ratio regulating soil microbial functional diversity in a temperate steppe: A regional scale study. Soil Biol. Biochem. 2010, 42, 445–450. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D.J.E. Plant diversity, soil microbial communities, and ecosystem function: Are there any links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Lal, S.; Bagdi, D.L.; Kakralya, B.L.; Jat, M.; Sharma, P.C. Role of brassinolide in alleviating the adverse effect of drought stress on physiology, growth and yield of green gram. Legume Res. 2013, 36, 359–363. [Google Scholar]
- Geng, S.; Yan, D.; Zhang, T.; Weng, B.; Zhang, Z.; Qin, T.J. Effects of drought stress on agriculture soil. Nat. Hazards 2015, 75, 1997–2011. [Google Scholar] [CrossRef]
- Ali, A.; Bajracharya, R.M.; Koirala, H.L. A review of flood risk assessment. Int. J. Environ. Agric. Biotechnol. 2016, 1, 238636. [Google Scholar] [CrossRef]
- Dilley, M.; Chen, R.S.; Deichmann, U.; Lerner-Lam, A.L.; Arnold, M. Natural Disaster Hotspots: A Global Risk Analysis; World Bank Publications: Washington, DC, USA, 2005; Volume 5. [Google Scholar]
- Cutter, S.L. Vulnerability to environmental hazards. Prog. Hum. Geogr. 1996, 20, 529–539. [Google Scholar] [CrossRef]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial Inoculants for Soil Quality and Plant Health. Int. J. Environ. Agric. Biotechnol. 2017, 27, 281–307. [Google Scholar]
- Gaind, S. Microbial inoculants: An approach to sustainable agriculture. 2011. Available online: https://www.biotecharticles.com/Agriculture-Article/Microbial-Inoculants-an-Approach-to-Sustainable-Agriculture-940.html (accessed on 1 June 2022).
- Carvajal-Muñoz, J.; Carmona-Garcia, C. Benefits and limitations of biofertilization in agricultural practices. Livest. Res. Rural Dev. 2012, 24, 1–8. [Google Scholar]
- Alori, E.T.; Fawole, O. Phytoremediation of soils contaminated with aluminium and manganese by two arbuscular mycorrhizal fungi. J. Agric. Sci. 2012, 4, 246–252. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–320. [Google Scholar] [CrossRef]
- Prasanna, R.; Hossain, F.; Babu, S.; Bidyarani, N.; Adak, A.; Verma, S.; Shivay, Y.S.; Nain, L. Prospecting cyanobacterial formulations as plant-growth-promoting agents for maize hybrids. S. Afr. J. Plant Soil 2015, 32, 199–207. [Google Scholar] [CrossRef]
- Gheda, S.F.; Ahmed, D.A. Improved soil characteristics and wheat germination as influenced by inoculation of Nostoc kihlmani and Anabaena cylindrica. Rend. Lincei 2015, 26, 121–131. [Google Scholar] [CrossRef]
- Bharti, A.; Prasanna, R.; Kumar, G.; Kumar, A.; Nain, L. Co-cultivation of cyanobacteria for raising nursery of chrysanthemum using a hydroponic system. J. Appl. Phycol. 2019, 31, 3625–3635. [Google Scholar] [CrossRef]
- Holajjer, P.; Kamra, A.; Gaur, H.; Manjunath, M. Potential of cyanobacteria for biorational management of plant parasitic nematodes: A review. J. Crop Prot. 2013, 53, 147–151. [Google Scholar] [CrossRef]
- Wang, R.; Peng, B.; Huang, K. The research progress of CO2 sequestration by algal bio-fertilizer in China. J. CO2 Util. 2015, 11, 67–70. [Google Scholar] [CrossRef]
- Yandigeri, M.S.; Yadav, A.K.; Srinivasan, R.; Kashyap, S.; Pabbi, S. Studies on mineral phosphate solubilization by cyanobacteria Westiellopsis and Anabaena. Microbiology 2011, 80, 558–565. [Google Scholar] [CrossRef]
- Rashad, S.; El-Hassanin, A.; Mostafa, S.; El-Chaghaby, G. Cyanobacteria cultivation using olive milling wastewater for bio-fertilization of celery plant. J. Environ. Sci. Manag. 2019, 5, 167–174. [Google Scholar]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Pang, E. Abiotic stress responses in plants: Present and future. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 1–19. [Google Scholar]
- Jaspers, P.; Kangasjärvi, J. Reactive oxygen species in abiotic stress signaling. Physiol. Plant 2010, 138, 405–413. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Ashraful Alam, M.; Syed, M.A.; Islam, T. Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. J. Agron. 2021, 11, 241. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; El-Shershaby, N.A. Algae as Bio-fertilizers: Between current situation and future prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Palakolanu, S.R.; Chopra, P.; Rajurkar, A.B.; Gupta, R.; Iqbal, N.; Maheshwari, C. Improving drought tolerance in rice: Ensuring food security through multi-dimensional approaches. Physiol. Plant. 2021, 172, 645–668. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Younis, A.; Abideen, Z.; Francini, A.; Ferrante, A. Bioregulators can improve biomass production, photosynthetic efficiency, and ornamental quality of Gazania rigens L. Agronomy 2019, 9, 773. [Google Scholar] [CrossRef]
- Munir, N.; Hanif, M.; Dias, D.A.; Abideen, Z. The role of halophytic nano-particles towards the remediation of degraded and saline agricultural lands. Environ. Sci. Pollut. Res. 2021, 28, 60383–60405. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Abideen, Z.; Hanif, M.; Munir, N.; Nielsen, B.L. Impact of Nanomaterials on the Regulation of Gene Expression and Metabolomics of Plants under Salt Stress. Plants 2022, 11, 691. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J. Addition of biochar to a sandy desert soil: Effect on crop growth, water retention and selected properties. Agronomy 2019, 9, 327. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, M.N.; Zhang, K.; Luo, T.; Zhu, K.; Hu, L. The application of biochar alleviated the adverse effects of drought on the growth, physiology, yield and quality of rapeseed through regulation of soil status and nutrients availability. Ind. Crops Prod. 2021, 171, 113878. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.W.; Huchzermeyer, B.; Bilquees, G.U.L.; Khan, M.A. Impact of a biochar or a biochar-compost mixture on water relation, nutrient uptake and photosynthesis of Phragmites karka. Pedosphere 2020, 30, 466–477. [Google Scholar] [CrossRef]
- Duarte, I.J.; Hernández, S.H.Á.; Ibañez, A.L.; Canto, A.R. Macroalgae as soil conditioners or growth promoters of Pisum sativum (L). Annu. Res. Rev. Biol. 2018, 27, 1–8. [Google Scholar] [CrossRef]
- Das, P.; Khan, S.; Chaudhary, A.K.; AbdulQuadir, M.; Thaher, M.I.; Al-Jabri, H. Potential applications of algae-based bio-fertilizer. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 41–65. [Google Scholar]
- Guo, S.; Wang, P.; Wang, X.; Zou, M.; Liu, C.; Hao, J. Microalgae as biofertilizer in modern agriculture. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 397–411. [Google Scholar]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Guzmán-Murillo, M.A.; Ascencio, F.; Larrinaga-Mayoral, J.A. Germination and ROS detoxification in bell pepper (Capsicum annuum L.) under NaCl stress and treatment with microalgae extracts. Protoplasma 2013, 250, 33–42. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baz, F.K.; El Baroty, G.S. Enhancing antioxidant availability in wheat grains from plants grown under seawater stress in response to microalgae extract treatments. J. Sci. Food Agric. 2010, 90, 299–303. [Google Scholar] [CrossRef]
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Ng, E.P.; Chang, J.S. Recent developments on algal biochar production and characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar] [CrossRef]
- Salami, R.; Kordi, M.; Bolouri, P.; Delangiz, N.; Asgari Lajayer, B. Algae-based biorefinery as a sustainable renewable resource. Circ. Econ. Environ. Prot. 2021, 1, 1349–1365. [Google Scholar] [CrossRef]
- Hasnain, M.; Munir, N.; Siddiqui, Z.S.; Ali, F.; El-Keblawy, A.; Abideen, Z. Integral Approach for the Evaluation of Sugar Cane Bio-Waste Molasses and Effects on Algal Lipids and Biodiesel Production. Waste Biomass Valorization 2022, 1–20. [Google Scholar] [CrossRef]
- Seltenrich, N. Keeping tabs on HABs: New tools for detecting, monitoring, and preventing harmful algal blooms. Environ. Health Perspect. 2014, 122, A206–A213. [Google Scholar] [CrossRef]
- de Siqueira Castro, J.; Calijuri, M.L.; Ferreira, J.; Assemany, P.P.; Ribeiro, V.J. Microalgae based biofertilizer: A life cycle approach. Sci. Total Environ. 2020, 724, 138138. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Silva-Núñez, A.; García-Perez, J.S.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Castillo-Zacarías, C.; Parra-Saldívar, R. Phyco-remediation of swine wastewater as a sustainable model based on circular economy. J. Environ. Manag. 2021, 278, 111534. [Google Scholar] [CrossRef]
- Sampathkumar, P.; Dineshkumar, R.; Rasheeq, A.A.; Arumugam, A.; Nambi, K.S. Marine microalgal extracts on cultivable crops as a considerable bio-fertilizer: A Review. Indian J. Tradit. Knowl. 2019, 18, 849–854. [Google Scholar]
- Gören-Sağlam, N. Cyanobacteria as Biofertilizer and Their Effect under Biotic Stress. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Springer: Cham, Switzerland, 2021; pp. 485–504. [Google Scholar] [CrossRef]
- Singh, J.S. Plant–Microbe Interactions: A Viable Tool for Agricultural Sustainability Plant Microbes Symbiosis: Applied Facets; Arora, N.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; p. 384. [Google Scholar]
- Mahapatra, D.M.; Chanakya, H.; Joshi, N.; Ramachandra, T.; Murthy, G. Algae-Based Biofertilizers: A Biorefinery Approach; Springer: Berlin/Heidelberg, Germany, 2018; pp. 177–196. [Google Scholar]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Küpper, F.C.; Sunda, W.G.; Carrano, C. Photolysis of iron–siderophore chelates promotes bacterial–algal mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar] [CrossRef]
- Round, F.E. The Biology of the Algae, 2nd ed.; Edward Arnold Publishers: London, UK, 1973. [Google Scholar]
- Gonçalves, A.L. The use of microalgae and cyanobacteria in the improvement of agricultural practices: A review on their biofertilising, biostimulating and biopesticide roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Awasthi, M.; Upadhyay, A.K.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Terpenoids as promising therapeutic molecules against Alzheimer’s disease: Amyloid beta- and acetylcholinesterase-directed pharmacokinetic and molecular docking analyses. Mol. Simul. 2018, 44, 1–11. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine. Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The Impact of Using Microalgae as Biofertilizer in Maize (Zea mays L.). Waste Biomass Valorization 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs. 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Arroussi, H.E.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; Mernissi, N.E.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Cezare-Gomes, E.A.; Mejia-da-Silva, L.d.C.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Lee, K.T. Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef]
- Kusvuran, A.; Kusvuran, S. Using of Microbial Fertilizer as Biostimulant Alleviates Damage from Drought Stress in Guar (Cyamopsis Tetragonoloba (L.) Taub.) Seedlings. Int. Lett. Nat. 2019, 76, 147–157. [Google Scholar] [CrossRef]
- Pan, S.; Jeevanandam, J.; Danquah, M.K. Benefits of Algal Extracts in Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2022; pp. 501–534. [Google Scholar]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology. Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Demirbas, A.; Fatih Demirbas, M. Importance of algae oil as a source of biodiesel. In Energy Conversion and Management; Elsevier: Amsterdam, The Netherlands, 2011; Volume 52, pp. 163–170. [Google Scholar]
- Sakamoto, Y.; Mori, K.; Matsuo, Y.; Mukojima, N.; Watanabe, W.; Sobaru, N.; Tamiya, S.; Nakao, T.; Hayashi, K.; Watanuki, H.J.B. Breeding of a new potato variety ‘nagasaki kogane’ with high eating quality, high carotenoid content, and resistance to diseases and pests. Breed. Sci. 2017, 67, 320–326. [Google Scholar] [CrossRef][Green Version]
- Haggag, W.; Hoballah, M.; Ali, R. Applications of nano biotechnological microalgae product for improve wheat productivity in semi-arid areas. Int. J. Agric. Technol. 2018, 14, 675–692. [Google Scholar]
- Wei, G.; Jia, Q.; Chen, X.; Köllner, T.G.; Bhattacharya, D.; Wong, G.K.S.; Gershenzon, J.; Chen, F. Terpene biosynthesis in red algae is catalyzed by microbial type but not typical plant terpene synthases. Plant Physiol. 2019, 179, 382–390. [Google Scholar] [CrossRef]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Alam, S.; Seth, R.K.; Shukla, D.N. Role of blue green algae in paddy crop. Eur. J. Exp. Biol. 2014, 4, 24–28. [Google Scholar]
- Singh, N.K.; Dhar, D.W. Cyanobacterial reclamation of salt-affected soil. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Springer: Dordrecht, The Netherlands, 2010; pp. 243–275. [Google Scholar]
- Abideen, Z.; Qasim, M.; Hussain, T.; Rasheed, A.; Gul, B.; Koyro, H.W.; Ansari, R.; Khan, M.A. Salinity improves growth, photosynthesis and bioenergy characteristics of Phragmites karka. Crop Pasture Sci. 2018, 69, 944–953. [Google Scholar] [CrossRef]
- Aljasmi, M.; El-Keblawy, A.; Mosa, K.A. Abiotic factors controllinggermination of the multipurpose invasive Prosopis pallida: Towards afor-estation of salt-afected lands in the subtropical arid Arabian desert. Trop. Ecol. 2021, 62, 116–125. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Aljasmi, M.; Gairola, S.; Mosa, K.A.; Hameed, A. Provenance determines salinity tolerance and germination requirements of the multipurpose tree Prosopis juliflora seeds. Arid Land Res. Manag. 2021, 35, 446–462. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Stella, A.M.; Storni, M.M.; Zulpa, G.; Zaccaro, M.C. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006, 2, 7. [Google Scholar] [CrossRef]
- Saadatnia, H.; Riahi, H. Cyanobacteria from paddy fields in Iran as a biofertilizer in rice plants. Plant Soil Environ. 2009, 55, 207–212. [Google Scholar] [CrossRef]
- Chatterjee, A.; Singh, S.; Agrawal, C.; Yadav, S.; Rai, R.; Rai, L.C. Role of algae as a biofertilizer. In Algal Green Chem; Elsevier: Amsterdam, The Netherlands, 2017; pp. 189–200. [Google Scholar]
- Chen, M.; Yang, Z.; Liu, J.; Zhu, T.; Wei, X.; Fan, H.; Wang, B. Adaptation mechanism of salt excluders under saline conditions and its applications. Int. J. Mol. Sci. 2018, 19, 3668. [Google Scholar] [CrossRef]
- El Semary, N.A.; Alouane, M.H.H.; Nasr, O.; Aldayel, M.F.; Alhaweti, F.H.; Ahmed, F. Salinity Stress Mitigation Using Encapsulated Biofertilizers for Sustainable Agriculture. Sustainability 2020, 12, 9218. [Google Scholar] [CrossRef]
- Kannaiyan, S.; Aruna, S.; Kumari, S.M.P.; Hall, D.O. Immobilized cyanobacteria as a biofertilizer for rice crops; Intl. Conference on Applied Algology, Knysna, South Africa, April 1996. J. Appl. Phycol. 1997, 9, 167–174. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1. [Google Scholar] [CrossRef]
- Benjamin, J.; Nielsen, D. Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crops Res. 2006, 97, 248–253. [Google Scholar] [CrossRef]
- Praba, M.L.; Cairns, J.E.; Babu, R.C.; Lafitte, H.R. Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J. Agron. Crop Sci. 2009, 195, 30–46. [Google Scholar] [CrossRef]
- Sotelo-Cuitiva, Y.M.; Restrepo-Díaz, H.; García-Castro, A.; Ramírez-Godoy, A.; Flórez-Roncancio, V.J. Effect of Kaolin Film Particle Applications (Surround WP®) and Water Deficit on Physiological Characteristics in Rose Cut Plants (Rose spp L.). Am. J. Plant Sci. 2011, 2, 354–358. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. J. Sustain. Agric. 2009, 29, 153–188. [Google Scholar]
- Bello, A.S.; Saadaoui, I.; Ben-Hamadou, R. “Beyond the Source of Bioenergy”: Microalgae in Modern Agriculture as a Biostimulant, Biofertilizer, and Anti-Abiotic Stress. J. Agron. 2021, 11, 1610. [Google Scholar] [CrossRef]
- Duo, L.A.; Liu, C.X.; Zhao, S.L. Alleviation of drought stress in turfgrass by the combined application of nano-compost and microbes from compost. Russ. J. Plant Physiol. 2018, 65, 419–426. [Google Scholar] [CrossRef]
- Kusvuran, S. Microalgae (Chlorella vulgaris Beijerinck) alleviates drought stress of broccoli plants by improving nutrient uptake, secondary metabolites, and antioxidative defense system. Hortic. Plant J. 2021, 7, 221–231. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Chaiwong, C.; Koottatep, T.; Polprasert, C. Effects of specific wavelengths of lights on performance of biofilm photobioreactor for treating septic tank effluent. J. Water Process Eng. 2021, 40, 101907. [Google Scholar] [CrossRef]
- Chaiwong, K.; Kiatsiriroat, T.; Vorayos, N.; Thararax, C. Biochar production from freshwater algae by slow pyrolysis. Maejo Int. J. Sci. Technol. 2012, 6, 186–195. [Google Scholar]
- Yao, C.; Wu, P.; Pan, Y.; Lu, H.; Chi, L.; Meng, Y.; Cao, X.; Xue, S.; Yang, X. Evaluation of the integrated hydrothermal carbonization-algal cultivation process for enhanced nitrogen utilization in Arthrospira platensis production. Bioresour. Technol. 2016, 216, 381–390. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, W.H.; Lin, B.J.; Chang, J.S.; Ong, H.C. Impact of torrefaction on the composition, structure, and reactivity of a microalga residue. Appl. Energy 2016, 181, 110–119. [Google Scholar] [CrossRef]
- Das, P.; Chandramohan, V.P.; Mathimani, T.; Pugazhendhi, A. Recent advances in thermochemical methods for the conversion of algal biomass to energy. Sci. Total Environ. 2021, 766, 144–608. [Google Scholar] [CrossRef]
- Yu, K.L.; Chen, W.H.; Sheen, H.K.; Chang, J.-S.; Lin, C.S.; Ong, H.C.; Show, P.L.; Ng, E.P.; Ling, T.C. Production of microalgal biochar and reducing sugar using wet torrefaction with microwave-assisted heating and acid hydrolysis pretreatment. Renew. Energy 2020, 156, 349–360. [Google Scholar] [CrossRef]
- Gan, Y.Y.; Ong, H.C.; Chen, W.H.; Sheen, H.K.; Chang, J.S.; Chong, C.T.; Ling, T.C. Microwave-assisted wet torrefaction of microalgae under various acids for coproduction of biochar and sugar. J. Clean. Prod. 2020, 253, 119–944. [Google Scholar] [CrossRef]
- Norouzi, O.; Jafarian, S.; Safari, F.; Tavasoli, A.; Nejati, B. Promotion of hydrogen-rich gas and phenolic-rich bio-oil production from green macroalgae Cladophora glomerata via pyrolysis over its bio-char. Bioresour. Technol. 2016, 219, 643–651. [Google Scholar] [CrossRef]
- Torri, C.; Samorì, C.; Adamiano, A.; Fabbri, D.; Faraloni, C.; Torzillo, G. Preliminary investigation on the production of fuels and biochar from Chlamydomonas reinhardtii biomass residue after bio-hydrogen production. Bioresour. Technol. 2011, 102, 8707–8713. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Sharma, N.R.; Singh, J.; Kumar, A. A comprehensive review on hydrothermal carbonization of biomass and its applications. Afr. J. Chem. 2020, 3, 1–19. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Huang, M.Y.; Chang, J.S. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Ok, Y.S. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Kuroki, A.; Loh, K.C.; Wang, C.H.; Dai, Y.; Tong, Y.W. Methane yield enhancement of mesophilic and thermophilic anaerobic co-digestion of algal biomass and food waste using algal biochar: Semi-continuous operation and microbial community analysis. Bioresour. Technol. 2020, 302, 122–892. [Google Scholar] [CrossRef]
- Gollakota, A.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Poo, K.M.; Son, E.B.; Chang, J.S.; Ren, X.; Choi, Y.J.; Chae, K.J. Biochars derived from wasted marine macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. J. Environ. Manag. 2018, 206, 364–372. [Google Scholar] [CrossRef]
- Michalak, I.; Messyasz, B. Concise review of Cladophora spp. macroalgae of commercial interest. J. Appl. Phycol. 2021, 33, 133–166. [Google Scholar] [CrossRef]
- Mona, S.; Malyan, S.K.; Saini, N.; Deepak, B.; Pugazhendhi, A.; Kumar, S.S. Towards sustainable agriculture with carbon sequestration, and greenhouse gas mitigation using algal biochar. Chemosphere 2021, 275, 129–856. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A review on the pyrolysis of algal biomass for biochar and bio-oil bottlenecks and scope. Fuel 2021, 283, 119–190. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Zhang, Y.; Strathmann, T.J. Chemical properties of biocrude oil from the hydrothermal liquefaction of Spirulina algae, swine manure, and digested anaerobic sludge. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef] [PubMed]
- Neveux, N.; Yuen, A.; Jazrawi, C.; Magnusson, M.; Haynes, B.; Masters, A.; Montoya, A.; Paul, N.; Maschmeyer, T.; De Nys, R. Biocrude yield and productivity from the hydrothermal liquefaction of marine and freshwater green macroalgae. Bioresour. Technol. 2014, 155, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Y.; Xie, J.; Liu, H.; Yin, X.; Wu, C. Bio-oil production from hydrothermal liquefaction of high-protein high-ash microalgae including wild Cyanobacteria sp. and cultivated Bacillariophyta sp. Fuel 2016, 183, 9–19. [Google Scholar] [CrossRef]
- Trivedi, N.; Gupta, V.; Reddy, C.; Jha, B. Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour. Technol. 2013, 150, 106–112. [Google Scholar] [CrossRef]
- Bird, M.I.; Wurster, C.M.; de Paula Silva, P.H.; Bass, A.M.; De Nys, R. Algal biochar production and properties. Bioresour. Technol. 2011, 102, 1886–1891. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009; pp. 1–12. [Google Scholar]
- Matovic, D. Biochar as a viable carbon sequestration option: Global and Canadian perspective. Energy J. 2011, 36, 2011–2016. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Mohd, A.; da Silva, G.; Bachmann, R.T.; Taufiq-Yap, Y.H.; Rashid, U. Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: Chemical and physical characterization. Ind. Crops Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties, and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.; Nazir, U.; Anjum, S.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.W.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B.J.P.B. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 2020, 22, 259–266. [Google Scholar] [CrossRef]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agron. Crop Sci. 2021, 42, 770–781. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Ullah, N.; Ditta, A.; Khalid, A.; Mehmood, S.; Rizwan, M.S.; Ashraf, M.; Mubeen, F.; Imtiaz, M.; Iqbal, M.M. Integrated effect of algal biochar and plant growth promoting Rhizobacteria on physiology and growth of maize under deficit irrigations. J. Soil Sci. Plant Nutr. 2020, 20, 346–356. [Google Scholar] [CrossRef]
- Haider, G.; Koyro, H.W.; Azam, F.; Steffens, D.; Müller, C.; Kammann, C. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil 2015, 395, 141–157. [Google Scholar] [CrossRef]
- Olmo, M.; Alburquerque, J.A.; Barrón, V.; Del Campillo, M.C.; Gallardo, A.; Fuentes, M.; Villar, R. Wheat growth and yield responses to biochar addition under Mediterranean climate conditions. Biol. Fertil. Soils 2014, 50, 1177–1187. [Google Scholar] [CrossRef]
- Haider, I.; Raza, M.A.S.; Iqbal, R.; Aslam, M.U.; Habib-ur-Rahman, M.; Raja, S.; Ahmad, S. Potential effects of biochar application on mitigating the drought stress implications on wheat (Triticum aestivum L.) under various growth stages. J. Saudi Chem. Soc. 2020, 24, 974–981. [Google Scholar] [CrossRef]
- Keshavarz Afshar, R.; Hashemi, M.; Da Costa, M.; Spargo, J.; Sadeghpour, A. Biochar application and drought stress effects on physiological characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 2016, 47, 743–752. [Google Scholar] [CrossRef]
- Tayyab, M.; Islam, W.; Khalil, F.; Ziqin, P.; Caifang, Z.; Arafat, Y.; Hua, Z. Biochar: An efficient way to manage low water availability in plants. Appl. Ecol. Environ. Res. 2018, 16, 2565–2583. [Google Scholar] [CrossRef]
- De Silva, N.D.G.; Cholewa, E.; Ryser, P. Effects of combined drought and heavy metal stresses on xylem structure and hydraulic conductivity in red maple (Acer rubrum L.). J. Exp. Bot. 2012, 63, 5957–5966. [Google Scholar] [CrossRef]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Ahmad, P. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged, contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Karthik, A.; Hussainy, S.A.H.; Rajasekar, M. Comprehensive study on biochar and its effect on Soil properties: A review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 459–477. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. J. Chem. Eng. 2012, 200, 673–680. [Google Scholar]
- Chen, Y.D.; Liu, F.; Ren, N.Q.; Ho, S.H. Revolutions in algal biochar for different applications: State-of-the-art techniques and future scenarios. Chin. Chem. Lett. 2020, 31, 2591–2602. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Cho, H.J.; Baek, K.; Jeon, J.K.; Park, S.H.; Suh, D.J.; Park, Y.K. Removal characteristics of copper by marine macro-algae-derived chars. J. Chem. Eng. 2013, 217, 205–211. [Google Scholar]
- Singh, A.; Sharma, R.; Pant, D.; Malaviya, P. Engineered algal biochar for contaminant remediation and electrochemical applications. Sci. Total Environ. 2021, 774, 145–676. [Google Scholar]
- Kim, B.S.; Lee, H.W.; Park, S.H. Removal of Cu2+ by biochars derived from green macroalgae. Environ. Sci. Pollut. Res. 2016, 23, 985–994. [Google Scholar] [CrossRef]
- Son, E.B.; Poo, K.M.; Mohamed, H.O.; Choi, Y.J.; Cho, W.C.; Chae, K.J. A novel approach to developing a reusable marine macro-algae adsorbent with chitosan and ferric oxide for simultaneous efficient heavy metal removal and easy magnetic separation. Bioresour. Technol. 2018, 259, 381–387. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Usman, M.; Tariq, W.; Ullah, Z.; Shareef, M.; Ditta, A. Biochar induced modifications in soil properties and its impacts on crop growth and production. J. Plant Nutr. 2021, 44, 1677–1691. [Google Scholar] [CrossRef]
- Thomas, S.C.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Melamed, S.; Murray, J.; Petroff, A.; Winsborough, C. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manag. 2013, 129, 62–68. [Google Scholar] [CrossRef]
- Mulcahy, D.N.; Mulcahy, D.L.; Dietz, D. Biochar soil amendment increases tomato seedling resistance to drought in sandy soils. J. Arid Environ. 2013, 88, 222–225. [Google Scholar] [CrossRef]
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Mohan, B.C.; Yao, Z.; Prabhakar, A.K.; Lin, X.H.; et al. Biochar industry to circular economy. Sci. Total Environ. 2021, 757, 143820. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770–781. [Google Scholar] [CrossRef]
- Nehela, Y.; Mazrou, Y.S.; Alshaal, T.; Rady, A.; El-Sherif, A.; Omara, A.E.D.; Hafez, E.M. The Integrated Amendment of Sodic-Saline Soils Using Biochar and Plant Growth-Promoting Rhizobacteria Enhances Maize (Zea mays L.) Resilience to Water Salinity. Plants 2021, 10, 1960. [Google Scholar] [CrossRef]
- Fazal, A.; Bano, A. Role of plant growth-promoting rhizobacteria (PGPR), biochar, and chemical fertilizer under salinity stress. Commun. Soil Sci. Plant Anal. 2016, 47, 1985–1993. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Wenga, T.; Mtisi, M. Biochars as media for air pollution control systems: Contaminant removal, applications and future research directions. Sci. Total Environ. 2020, 753, 142–249. [Google Scholar] [CrossRef]
- Li, H.; Watson, J.; Zhang, Y.; Lu, H.; Liu, Z. Environment-enhancing process for algal wastewater treatment, heavy metal control and hydrothermal biofuel production: A critical review. Bioresour. Technol. 2020, 298, 122–421. [Google Scholar] [CrossRef]
- Ross, A.; Jones, J.; Kubacki, M.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef]
- Maraseni, T.N. Biochar: Maximising the benefits. Int. J. Environ. Sci. 2010, 67, 319–327. [Google Scholar] [CrossRef]
- Mathimani, T.; Mallick, N. A comprehensive review on harvesting of microalgae for biodiesel–key challenges and future directions. Renew. Sustain. Energy Rev. 2018, 91, 1103–1120. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Agronomic and remedial benefits and risks of applying biochar to soil: Current knowledge and future research directions. Environ. Int. 2016, 87, 1–12. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef]
- Kavitha, M.; Raja, M.; Perumal, P. Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822). Aquac. Rep. 2018, 11, 59–69. [Google Scholar] [CrossRef]
- Yuan, Y.; Bolan, N.; Prévoteau, A.; Vithanage, M.; Biswas, J.K.; Ok, Y.S.; Wang, H. Applications of biochar in redox-mediated reactions. Bioresour. Technol. 2017, 246, 271–281. [Google Scholar] [CrossRef]
- Jing, F.; Chen, C.; Chen, X.; Liu, W.; Wen, X.; Hu, S.; Yang, Z.; Guo, B.; Xu, Y.; Yu, Q. Effects of wheat straw derived biochar on cadmium availability in a paddy soil and its accumulation in rice. Environ. Pollut. 2020, 257, 113592. [Google Scholar] [CrossRef]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Fatima Rana, N.; Menaa, F. Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. J. Bioeng. 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bhargava, A. Green Nanoparticles: The Future of Nanobiotechnology; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Arya, A.; Mishra, V.; Chundawat, T.S. Green synthesis of silver nanoparticles from green algae (Botryococcus braunii) and its catalytic behavior for the synthesis of benzimidazoles. Chem. Data Collect. 2019, 20, 100190. [Google Scholar] [CrossRef]
- Korbekandi, H.; Ashari, Z.; Iravani, S.; Abbasi, S. Optimization of biological synthesis of silver nanoparticles using Fusarium oxysporum. Iran. J. Pharm. Sci. 2013, 12, 289. [Google Scholar]
- Kannan, R.R.R.; Arumugam, R.; Ramya, D.; Manivannan, K.; Anantharaman, P. Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Appl. Nanosci. 2013, 3, 229–233. [Google Scholar] [CrossRef]
- Asmathunisha, N.; Kathiresan, K.J.C.; Biointerfaces, S.B. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf. B Biointerfaces 2013, 103, 283–287. [Google Scholar] [CrossRef]
- AlNadhari, S.; Al-Enazi, N.M.; Alshehrei, F.; Ameen, F. A review on biogenic synthesis of metal nanoparticles using marine algae and its applications. Environ. Res. 2021, 194, 110672. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Rajesh Babu, D.; Gengan, R.M.; Chandra, S.; Nageswara Rao, G. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum Muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef]
- Prasad, A.R.; Garvasis, J.; Oruvil, S.K.; Joseph, A. Bio-inspired green synthesis of zinc oxide nanoparticles using Abelmoschus esculentus mucilage and selective degradation of cationic dye pollutants. J. Phys. Chem. Solids 2019, 127, 265–274. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Kassas, H.Y.; Shams El-Din, N.G.; Eissa, D.I.; El-Sherbiny, B.A. Green synthesis, characterization applications of iron oxide nanoparticles for antialgal and wastewater bioremediation using three brown algae. Int. J. Phytoremediat. 2021, 23, 1538–1552. [Google Scholar] [CrossRef]
- Madhavi, V.; Prasad, T.N.; Reddy, A.V.; Ravindra Reddy, B.; Madhavi, G. Application of phytogenic zero valent iron nanoparticles in the adsorption of hexavalent chromium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 17–25. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Madkour, F.F.; El-Kassas, H.Y.; Mohamed, A.A.; Elgarahy, A.M. Green synthesis of recyclable iron oxide nanoparticles using Spirulina platensis microalgae for adsorptive removal of cationic and anionic dyes. Environ. Sci. Pollut. Res. 2021, 28, 65549–65572. [Google Scholar] [CrossRef]
- Subramaniyam, V.; Subashchandrabose, S.R.; Thavamani, P.; Megharaj, M.; Chen, Z.; Naidu, R. Chlorococcum sp. MM11—A novel phyco-nanofactory for the synthesis of iron nanoparticles. J. Appl. Phycol. 2015, 27, 1861–1869. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Sharma, G.; Pandey, S.; Ghatak, S.; Watal, G.; Rai, P.K. Potential of Spectroscopic Techniques in the Characterization of “Green Nanomaterials”; Elsevier: Amsterdam, The Netherlands, 2018; pp. 59–77. [Google Scholar]
- Thakkar, K.N.; Mhatre, S.S. Parikh, biology and medicine. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- El-Moslamy, S.; Kabeil, S.; Hafez, E. Bioprocess Development for Chlorella vulgaris cultivation and biosynthesis of anti phytopathogens silver nanoparticles. J. Nanomater. Mol. Nanotechnol. 2016, 5, 2. [Google Scholar] [CrossRef]
- Terra, A.L.M.; Kosinski, R.d.C.; Moreira, J.B.; Costa, J.A.V.; Morais, M.G.d. Microalgae biosynthesis of silver nanoparticles for application in the control of agricultural pathogens. J. Environ. Sci. Health Part B 2019, 54, 709–716. [Google Scholar] [CrossRef]
- Annamalai, J.; Shanmugam, J.; Nallamuthu, T. Salt stress enhancing the production of Phytochemicals in Chlorella vulgaris and Chlamydomonas reinhardtii. J. Algal Biomass Util. 2016, 7, 37–44. [Google Scholar]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.; Bin-Meferij, M.M. Cyanobacteria—A promising platform in green nanotechnology: A review on nanoparticles fabrication and their prospective applications. Int. J. Nanomed. 2020, 15, 6033–6066. [Google Scholar] [CrossRef]
- Paulkumar, K.; Rajeshkumar, S.; Gnanajobitha, G.; Vanaja, M.; Malarkodi, C.; Annadurai, G. Eco-friendly synthesis of silver chloride nanoparticles using Klebsiella planticola (MTCC 2277). International. Int. J. Green Chem. Bioprocess 2013, 3, 12–16. [Google Scholar]
- Priyadharshini, R.I.; Prasannaraj, G.; Geetha, N.; Venkatachalam, P. Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Appl. Biochem. Biotechnol. 2014, 174, 2777–2790. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Aishvarya, V.; Nayak, R.R.; Dash, B.P.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Biological sequestration and retention of cadmium as CdS nanoparticles by the microalga Scenedesmus-24J. J. Appl. Phycol. 2015, 27, 2251–2260. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; Restic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomásková, I.; Struve, D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. Leśne Pr. Badaw. 2015, 76, 350–359. [Google Scholar] [CrossRef]
- Sarraf, M.; Vishwakarma, K.; Kumar, V.; Arif, N.; Das, S.; Johnson, R.; Hasanuzzaman, M. Metal/metalloid-based nanomaterials for plant abiotic stress tolerance: An overview of the mechanisms. Plants 2022, 11, 316. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron. Sustain. Dev. 2017, 37, 5. [Google Scholar] [CrossRef]
- Jaberzadeh, A.; Moaveni, P.; Moghadam, H.R.; Zahedi, H. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 201–207. [Google Scholar]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Regul. 2022, 41, 364–375. [Google Scholar] [CrossRef]
- Hashimoto, T.; Mustafa, G.; Nishiuchi, T.; Komatsu, S. Comparative analysis of the effect of inorganic and organic chemicals with silver nanoparticles on soybean under flooding stress. Int. J. Mol. Sci. 2020, 21, 1300. [Google Scholar] [CrossRef] [PubMed]
- Faraji, J.; Sepehri, A. Exogenous nitric oxide improves the protective effects of TiO2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J. Soil. Sci. Plant Nutr. 2020, 20, 703–714. [Google Scholar] [CrossRef]
- Das, A.; Ray, R.; Mandal, N.; Chakrabarti, K. An analysis of transcripts and enzyme profiles in drought stressed jute (Corchorus capsularis) and rice (Oryza sativa) seedlings treated with CaCl2, hydroxyapatite nano-particle and β-amino butyric acid. Plant Growth Regul. 2016, 79, 401–412. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Mohamed, A.K.S.H.; Qayyum, M.F.; Abdel-Hadi, A.; Rehman, R.A.; Ali, S.; Rizwan, M. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Nair, R.; Giraldo, J.P.; Prasad, P.V.V. Cerium oxide nanoparticles decrease drought-induced oxidative damage in sorghum leading to higher photosynthesis and grain yield. ACS Omega 2018, 3, 14406–14416. [Google Scholar] [CrossRef]
- Haghighi, M.; Abolghasemi, R.; da Silva, J.A.T. Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Sci. Hortic. 2014, 178, 231–240. [Google Scholar]
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc oxide nanoparticles alleviate chilling stress in rice (Oryza Sativa L.) by regulating antioxidative system and chilling response transcription factors. Molecules 2021, 26, 2196. [Google Scholar]
- Mustafa, G.; Sakata, K.; Hossain, Z.; Komatsu, S. Proteomic study on the effects of silver nanoparticles on soybean under flooding stress. J. Proteom. 2015, 122, 100–118. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017, 110, 70–81. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdelaziz, S.M.; El-Mogy, M.M.; Abdeldaym, E.A. Effect of foliar ZnO and FeO nanoparticles application on growth and nutritional quality of red radish and assessment of their accumulation on human health. Agriculture 2019, 65, 16–29. [Google Scholar] [CrossRef]
- Molnár, Á.; Papp, M.; Kovács, D.Z.; Bélteky, P.; Oláh, D.; Feigl, G.; Kolbert, Z. Nitro-oxidative signalling induced by chemically synthetized zinc oxide nanoparticles (ZnO NPs) in Brassica species. Chemosphere 2020, 251, 126419. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Srivastava, A.K.; El-sadek, M.S.A.; Kordrostami, M.; Tran, L.S.P. Titanium Dioxide Nanoparticles Improve Growth and Enhance Tolerance of Broad Bean Plants under Saline Soil Conditions. Land Degrad. Dev. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Abbas, Q.; Liu, G.; Yousaf, B.; Ali, M.U.; Ullah, H.; Munir, M.A.M.; Rehman, A. Biochar-assisted transformation of engineered-cerium oxide nanoparticles: Effect on wheat growth, photosynthetic traits and cerium accumulation. Ecotoxicol. Environ. Saf. 2020, 187, 109845. [Google Scholar] [CrossRef]

| Algal Species | Metabolites | Biological Activities | Tested Crop | Observed Improvement | References |
|---|---|---|---|---|---|
| Chlorella vulgaris | Polyphenols flavonoids; phenolic acids | Anti-fungal, anti-oxidant, and anti-microbial | Maize Crop | Plant protection against pathogens, viuses, or other biotic and abiotic stress conditions. Enhances germination rate and crop yield | [57,58,59] |
| Nostoc sp. | Structural polysaccharides, extra cellular Polysaccharides, and energy-storage poly-saccharides | Anti-bacterial, anti-cancer, anti-oxidant, and anti-coagulant | Pepper | Improves the quality of soil, plant growth stimulation, and plants protection against abiotic and biotic stresses | [60,61] |
| Dunaliella salina | Alpha-Beta carotene lycopene, astaxanthin, and zeaxanthin | Anticancer, anti-inflammatory, and antioxidant | Wheat Crop | Soil fertilization and bioremediation, plant crop protection against bacteria, viruses, and other abiotic and biotic stress conditions. Plant’s fortification, improved germination rate, and seedling growth | [62,63] |
| Anabaena sp. | Fatty acids, saturated and unsaturated | Antifungal, antiviral, and antioxidant | Rice Crop | Plant’s protection against pathogens or other biotic and abiotic stress conditions, increase in germination rate, the height of the plant, increased soil moisture and porosity | [61,62,64,65] |
| Arthrospira platensis | Auxins, cytokinins, abscisic acid, ethylene, and gibberellins | Chemical messengers | Lettuce Crop | Plant growth stimulation. Regulation of cellular activities in crops plant’s response to stress conditions enhances seedling growth | [66,67] |
| Spirulina platensis | Saturated & unsaturated fatty acids | Antibiotic, antifungal, antioxidant, and antiviral | Maize Crop | Protection against pathogens or other biotic and abiotic stress conditions increased plant yield and shoot length, and no of plant leaves | [68,69] |
| Oscillatoria sp. | Hemiterpenes, Mono, di tri & poly terpenes | Antibacterial, anticarcinogenic, and antioxidant | Wheat Crop | Plant’s protection against bacteria, and viruses. Stimulation of primary growth of plants, increase in activity of plant’s defense enzymes, alleviation of drought stress | [70,71] |
| Algae | Biochar Production | Pyrolysis (°C) | Biochar Yield (%) | Application | References |
|---|---|---|---|---|---|
| Chlorella vulgaris | Fast pyrolysis | 500 | 30 | Biofertilizer | [95] |
| Spirogyra sp. | Slow pyrolysis | 900 | 18.6 | Fuel & soil amendment | [96] |
| Cladophora sp. | Slow pyrolysis | 900 | 32 | Fuel & soil amendment | [97] |
| Oedogonium intermedium | Slow pyrolysis | 450 | 29 | Biofertilizer | [98] |
| Chlamydomans sp. | Torrefaction | 200 to 300 | 93.8 | Fuel | [99] |
| Scenedesmus sp. | Pyrolysis | 600 | 36 | Soil amendment | [100] |
| Chlorella vulgaris | Wet Torrefaction | 180 | 53 | Fuel | [101] |
| Chlorella vulgaris | Fast pyrolysis | 500 | 26 | Soil fertility | [102] |
| Laminaria japonica Brown Macroalgae | Slow Pyrolysis | 200–800 | 78 | Metal removal efficiency, Soil amendment | [34] |
| Cladophora glomerata Green macroalgae | Fixed-bed pyrolysis | 400–600 | 43.98 | Fuel, Biofertilizer | [103] |
| Chlamydomans reinhardtti | Slow pyrolysis | 350 | 44 | Nitrogen releasing fertilizer | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abideen, Z.; Waqif, H.; Munir, N.; El-Keblawy, A.; Hasnain, M.; Radicetti, E.; Mancinelli, R.; Nielsen, B.L.; Haider, G. Algal-Mediated Nanoparticles, Phycochar, and Biofertilizers for Mitigating Abiotic Stresses in Plants: A Review. Agronomy 2022, 12, 1788. https://doi.org/10.3390/agronomy12081788
Abideen Z, Waqif H, Munir N, El-Keblawy A, Hasnain M, Radicetti E, Mancinelli R, Nielsen BL, Haider G. Algal-Mediated Nanoparticles, Phycochar, and Biofertilizers for Mitigating Abiotic Stresses in Plants: A Review. Agronomy. 2022; 12(8):1788. https://doi.org/10.3390/agronomy12081788
Chicago/Turabian StyleAbideen, Zainul, Huma Waqif, Neelma Munir, Ali El-Keblawy, Maria Hasnain, Emanuele Radicetti, Roberto Mancinelli, Brent L. Nielsen, and Ghulam Haider. 2022. "Algal-Mediated Nanoparticles, Phycochar, and Biofertilizers for Mitigating Abiotic Stresses in Plants: A Review" Agronomy 12, no. 8: 1788. https://doi.org/10.3390/agronomy12081788
APA StyleAbideen, Z., Waqif, H., Munir, N., El-Keblawy, A., Hasnain, M., Radicetti, E., Mancinelli, R., Nielsen, B. L., & Haider, G. (2022). Algal-Mediated Nanoparticles, Phycochar, and Biofertilizers for Mitigating Abiotic Stresses in Plants: A Review. Agronomy, 12(8), 1788. https://doi.org/10.3390/agronomy12081788












