1. Introduction
Despite significant achievements in the field of regenerative medicine, cellular technologies and tissue engineering, the problem of adequate restoration of a full-fledged skin remains relevant in combustiology [
1,
2,
3,
4,
5,
6]. Therefore, it is advisable to search and evaluate the effectiveness of new ways to stimulate reparative regeneration of the skin after a burn [
1,
2,
4]. At the same time, the multiplicity of technologies for local treatment of the burned significantly sidelines the elimination of the main cause of the formation and progression of burn disease—the burn wound itself [
1,
3,
4,
7]. Taking into account the fact that one of the most significant mechanisms of post-traumatic tissue damage is oxidative stress [
3,
8], a logical component of the complex treatment of the burned should be a regulator of the state of pro- and antioxidant systems, which can act as reactive oxygen species [
9,
10].
A similar agent, actively used in clinical practice in recent decades, is medical ozone [
11]. It is known that it has a wide range of biological and sanogenetic effects; however, in surgery [
11,
12,
13], combustiology [
4,
10,
14,
15] and the treatment of wounds of various origins [
5,
11,
12,
13,
14,
15,
16], most attention is paid to the antibacterial activity of the compound [
6,
9,
13,
17]. Being a chemically unstable molecule, ozone, when interacting with biological objects, either decomposes rapidly to molecular and atomic oxygen (followed by the generation of other reactive oxygen species) [
4], or reacts with organic compounds (primarily unsaturated fatty acids) [
6]. The first of these ways, when treating biological objects with high concentrations of ozone, ensures the destruction of pathogenic microorganisms on the surface and in wound tissues, which makes it possible to realize an antibacterial effect [
11,
14,
18,
19,
20]. On the other hand, low ozone concentrations, moderately intensifying free radical processes [
4,
6], contribute to the predominant stimulation of the antioxidant system, primarily due to the activation of antioxidant enzymes (in particular, catalase and superoxide dismutase) [
4]. This suggests that the use of low concentrations of ozone will provide correction of oxidative stress arising in wound tissues caused by cell destruction [
7,
13], and, consequently, create conditions for stimulating regenerative processes [
3,
16,
21]. There are separate studies in the literature on the effectiveness of ozone therapy for deep burns [
4,
14,
16,
18,
22], but there is no full-fledged pathogenetic justification for this method of treatment [
17,
23]. This fact fully relates to local ozone therapy, which can be carried out in various ways (using ozonated solutions or oils [
23], direct treatment of the wound surface with an ozone–oxygen mixture in special chambers or without them [
16,
19], pricking wounds with an ozonated saline solution or an ozone-containing gas mixture [
17,
24], etc.). At the same time, a comparative assessment of the effectiveness of these methods of treatment has not been carried out before.
That is why the aim of this work was the comparative estimation of the efficiency of different variants of local ozone therapy in experimental burn wounds.
2. Materials and Methods
The experiments were carried out on 45 white rats of the Wistar line (age: 12–14 months; weight: 200–250 g) obtained from the Stolbovaya branch of the Federal Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency (Moscow), randomized into 3 groups. The experiment was carried out on 30 white male rats. All animals were kept in standard vivarium conditions in cages with free access to food and water on a diet, according to GOST standards “Maintenance of experimental animals in Research Institute nurseries”. Working with animals was in accordance with the rules of the European Convention ET/S 129, 1986.
Under ether anesthesia, all animals were once subjected to thermal trauma with the help of an experimental installation for applying a contact thermal burn. As a result, a contact thermal burn of the III degree was inflicted on the dorsal part, which is 20% of the entire skin surface [
25,
26,
27]. The verification of the depth of the burn was carried out by us in previous studies on the basis of histological examination of wound tissue in the dynamics of the wound process [
25,
26,
27]. Animals’ euthanasia was realized with use of lethal dose of anesthetics.
The animals of the first group (control) were treated with burn wounds daily with Levomecol (liniment, containing Methyluracilum 40 mg/1 g and Chloramphenicol 7.5 mg/1 g [
28]); in the second group, with ozonated oil (OOM) [
4,
14,
17,
18]; in the third group, an ozone–oxygen gas mixture was subcutaneously injected into the edges of the wounds and the wounds were treated with Levomecol.
The nature and features of post-traumatic skin regeneration were studied by histological examination. The preparations were mainly stained with hematoxylin and eosin. For morphological examination, light microscopes with a total magnification of ×50–×200 were used. The experiments were carried out on 45 white Wistar rats weighing 160–180 g, randomized into 3 groups. Under ether anesthesia, thermal trauma was inflicted on all animals—a contact thermal burn of the III degree of the posterior surface of the body on an area of 20% of body square [
25,
26,
27]. The animals of the first group (control) had their burn wounds treated daily with Levomecol; in the second group, with ozonated oil (OOM) [
10,
12]; in the third group, an ozone–oxygen gas mixture was subcutaneously injected into the edges of the wounds and the wounds were treated with Levomecol [
25,
26,
27,
28].
Ozonation of the contact thermal wound was carried out by impregnating the wound with a cotton-gauze bandage.
Ozonating oil is a concentrate containing some amount of ozone dissolved in olive oil. Ozone enrichment was carried out by passing the resulting gas through an oil tank. The selected filler in the form of an oily liquid promotes optimal moistening of the treated area and physiological adsorption, preventing rapid degradation of the oxygen–ozone mixture. Ozonation of the oil led to formation of endoperoxides from unsaturated fatty acids with double bounds. Common composition of the native oil is saved.
Ozone–oxygen mixture was synthesized with certified ozone generator “Medozons-BM” (“Medozons Ltd.”, Nizhny Novgorod, Russia). For ozone generation, we used pure balloon oxygen (99.5% of purity). Gas mixture contained 3000 mcg/L of ozone; the rest of gas flow was oxygen.
The nature and features of post-traumatic skin regeneration were studied by histological examination. The preparations were mainly stained with hematoxylin and eosin. For morphological examination, light microscopes with a total magnification of ×50–×200 were used. The study was conducted in accordance with the recommendations of the local ethics committee of the University Clinic of the Federal State Educational Institution “PIMU” of the Ministry of Health of the Russian Federation (protocol N 12-04/2007).
3. Results
Visual observation of the animals showed that the rats of the first (control) group were more sluggish than in the experimental groups. The scab in group II of animals after OOM treatment was lighter and more elastic. In animals of group III, which underwent subcutaneous pricking with a gas mixture, the amount of wound discharge was significantly less than in animals of groups I and II. In the experimental groups, by seven days after the injury, signs of wound healing were noted (the edges of the scab began to rise, foci of marginal epithelization appeared). In the control group, such signs appeared only by the 10th day. In animals of group II, active rejection of the scab began three days earlier than the control.
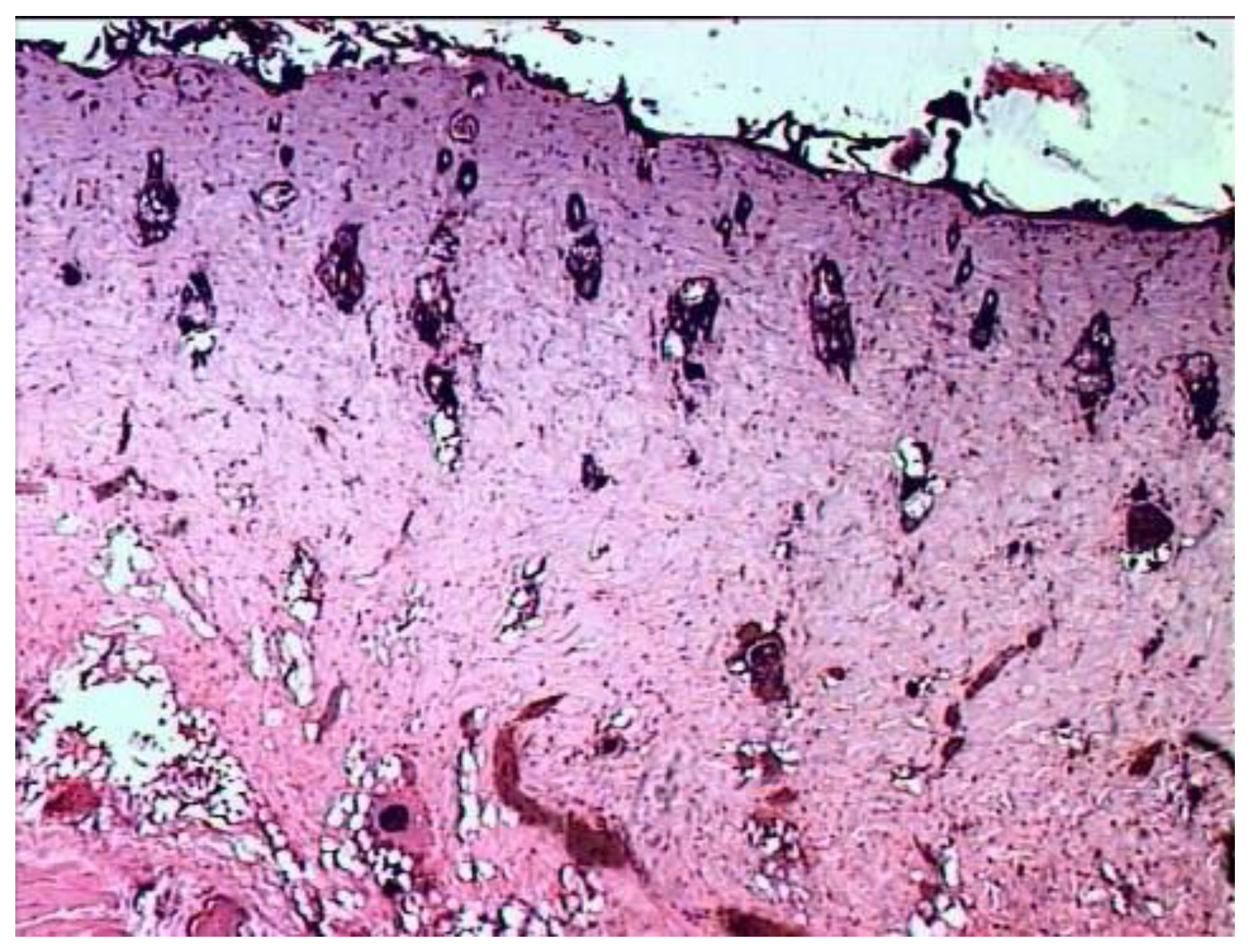
According to the results of histological examination, it was found that the first day after the injury, the histological picture in the edges of wounds in animals of all three groups had no differences (
Figure 1). In all preparations, there is a lesion of the epidermis (necrosis and necrobiosis), dermis (papillary and mesh layers), manifested by the loss of the usual fibrous structure of the connective tissue of the dermis and transformation collagen fibers into a solid homogeneous mass, as well as edema and hemorrhages in loose connective tissue and adipose tissue located above the layer of the skin’s own muscle.
The blood vessels passing through here are often also necrotic, containing red blood clots. The muscle fibers of the skin’s own muscles are in a state of dystrophy: swollen, often vacuolated. There are no cores in individual fibers. In the loose connective tissue located under the muscle layer, there is fullness, puffiness, as well as an increased number of cells, among which leukocytes and macrophages predominate.
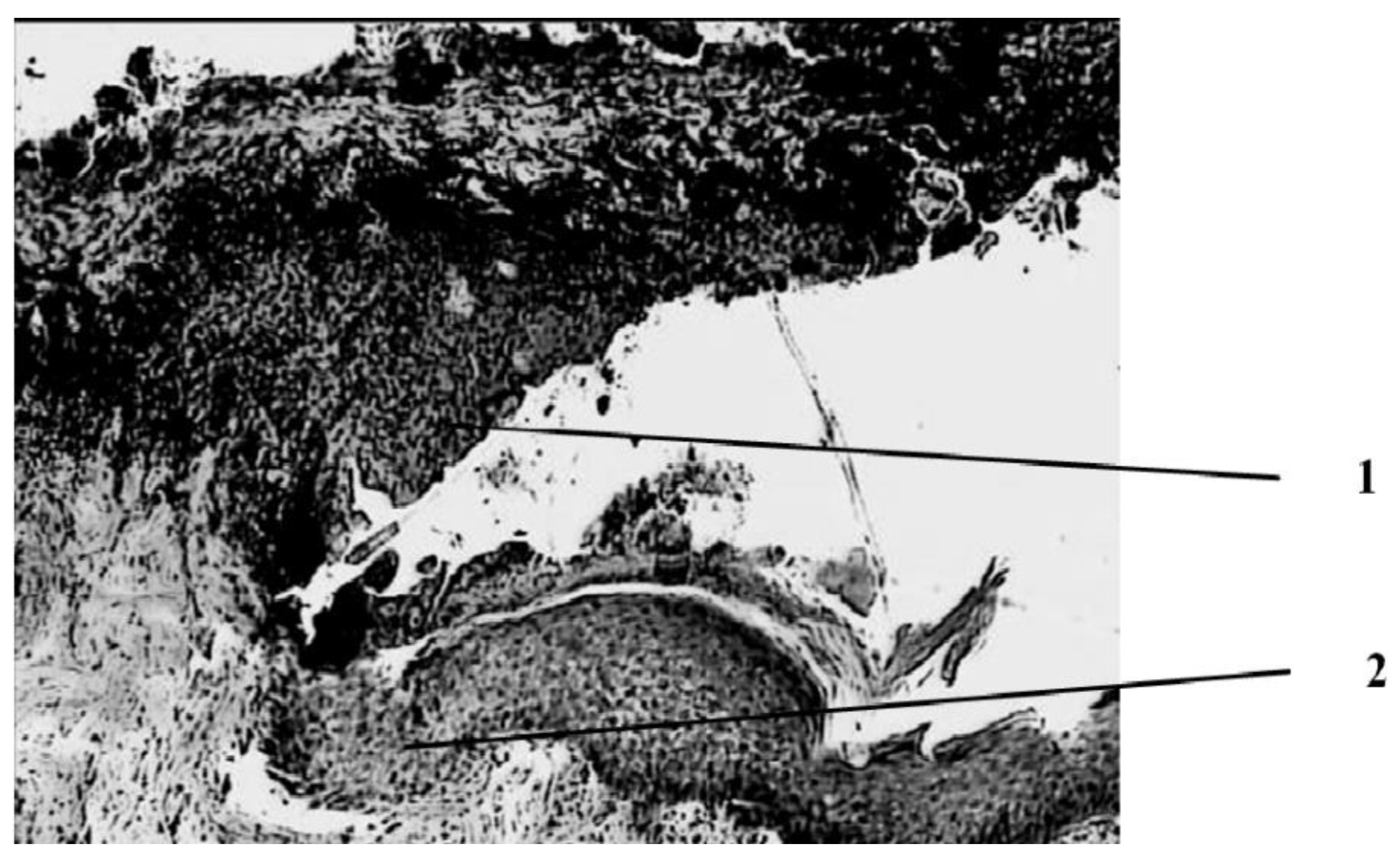
Seven days after the injury, the animals in the wounds of the control group (treatment with Levomecol) showed a picture of inflammation with initial signs of healing (
Figure 2).
The wound is covered with a scab, which consists of necrotized tissues (epidermis, dermis), and in the center of the wound, where the burn was deepest, its own muscle is included in its skin. On the periphery, closer to the edges of the wound, approximately in the middle of the thickness of the dermis, a pronounced leukocyte demarcation shaft is found, which divides the dermis into three parts—upper, necrotized, and lower—with manifestations of regeneration in the form of proliferation of epithelial cells in the course of pre-existing hair follicles and initial manifestations of marginal epithelialization of the wound, foci of fibroblast proliferation in the dermis with the appearance of newly formed collagen fibers and thin-walled blood vessels, and in muscle tissue against the background of dystrophically altered, and even necrotized muscle fibers with the phenomena of sarcoplasm decay are sometimes found as manifestations of regeneration in the form of muscle kidneys. Proliferation of myocyte nuclei is noted along the periphery of a significant part of viable muscle fibers. In addition, fibroblasts proliferate between muscle fibers. In the loose connective tissue under the skin’s own muscle, there is a proliferation of connective tissue cells in these areas.
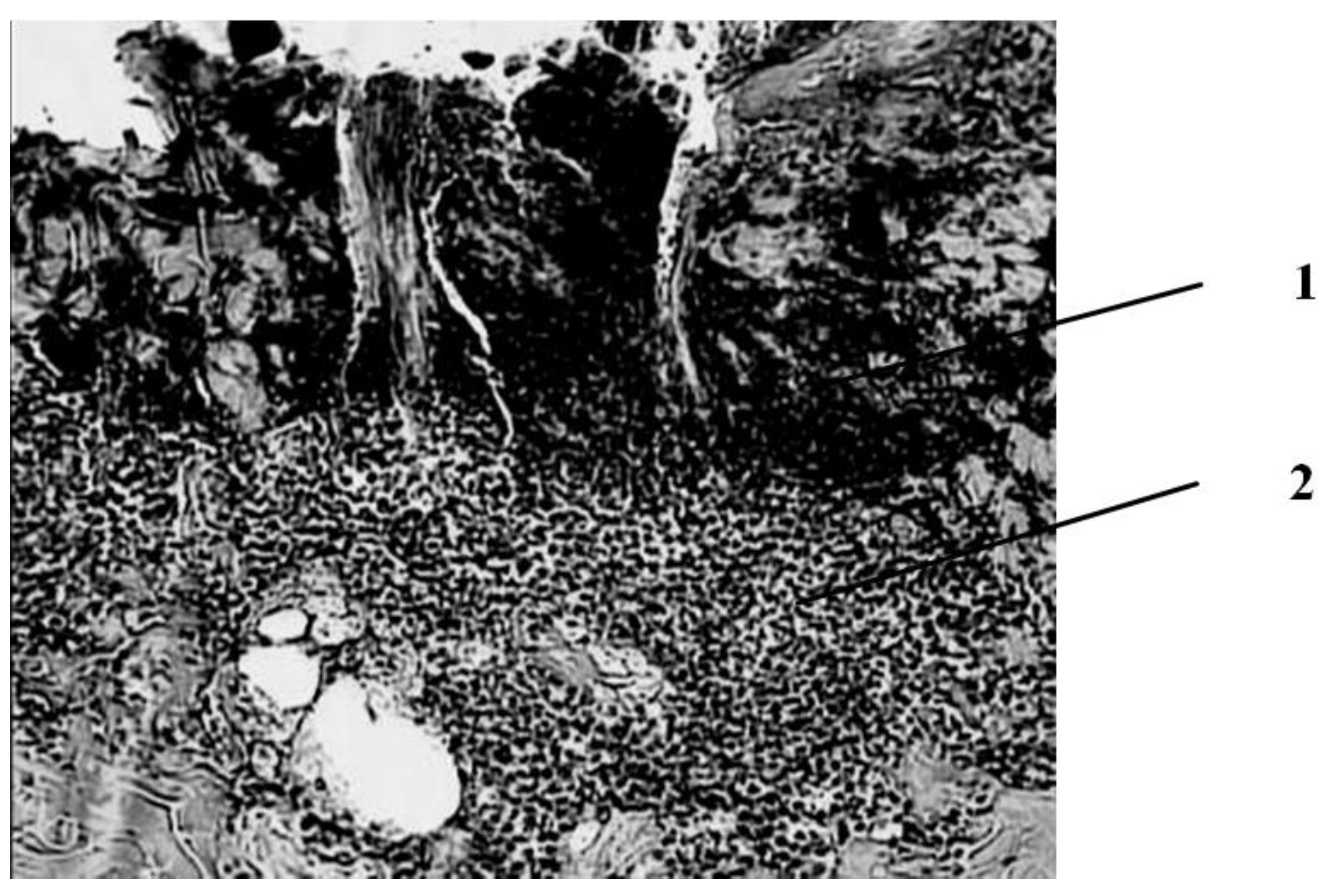
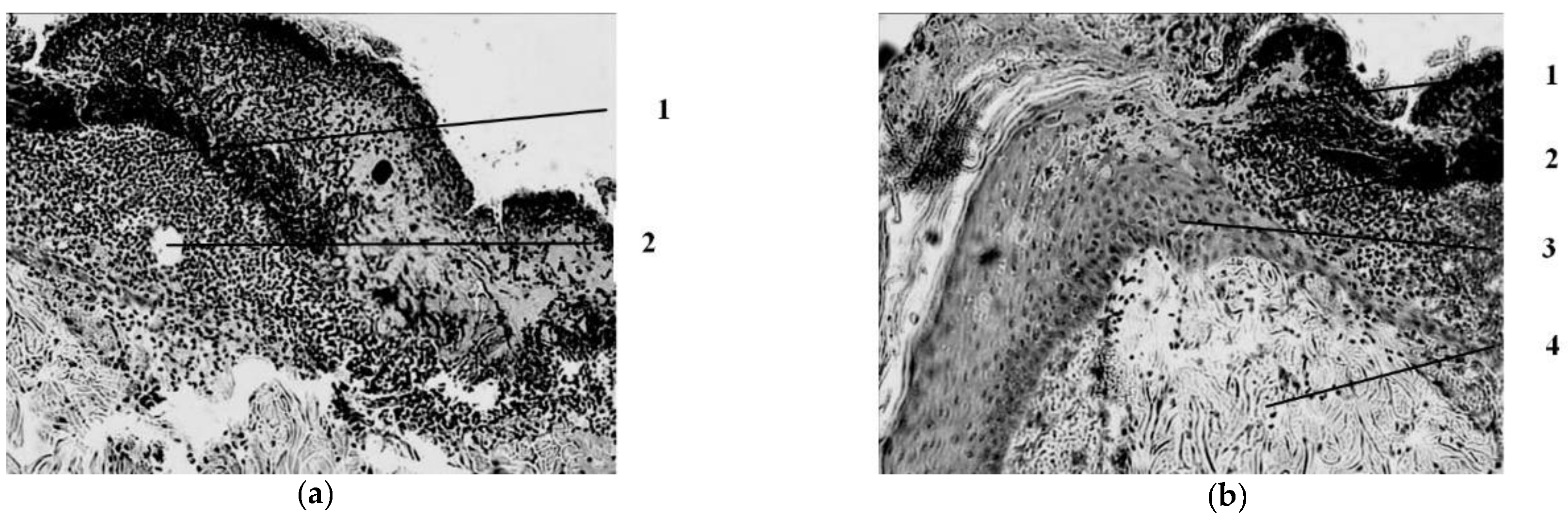
Seven days after the injury, in the group of animals whose wounds were treated with ozonated oil, purulent inflammation prevails, which is noted in the form of purulent exudate on the wound surface among the necrotic epidermis and under it, as well as in the form of focal purulent infiltrates in the necrotic dermis (
Figure 3).
A continuous demarcation shaft is not formed, but, nevertheless, its placement in the dermis is indicated by leukocyte infiltrates located, albeit discretely, but at a short distance from each other. Individual leukocyte infiltrates occur at different depths of the necrotic dermis up to the skin’s own muscle. Against this background, the initial phenomena of marginal epithelialization of the skin are noted. The tongues of the epidermis proliferating from the edges of the wound are embedded under the scab, separating it from the rest of the dermis mass. Under the skin’s own muscle, there is also a pronounced proliferation of cells with large nuclei and basophilic cytoplasm. The predominant part of them has a fusiform shape (fibroblasts). There are newly formed thin-walled vessels containing red blood cells. There are single muscle buds in the muscle tissue.
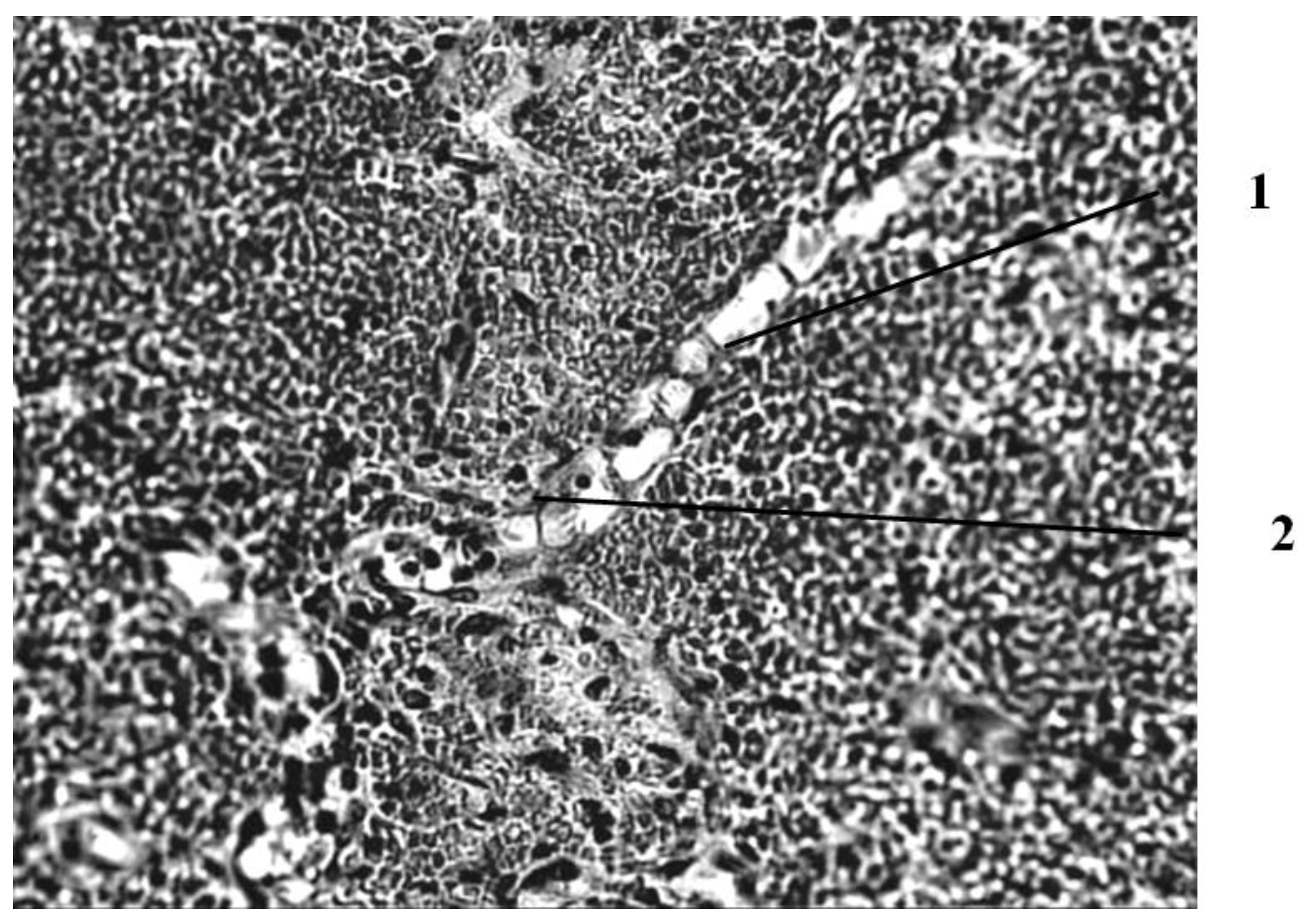
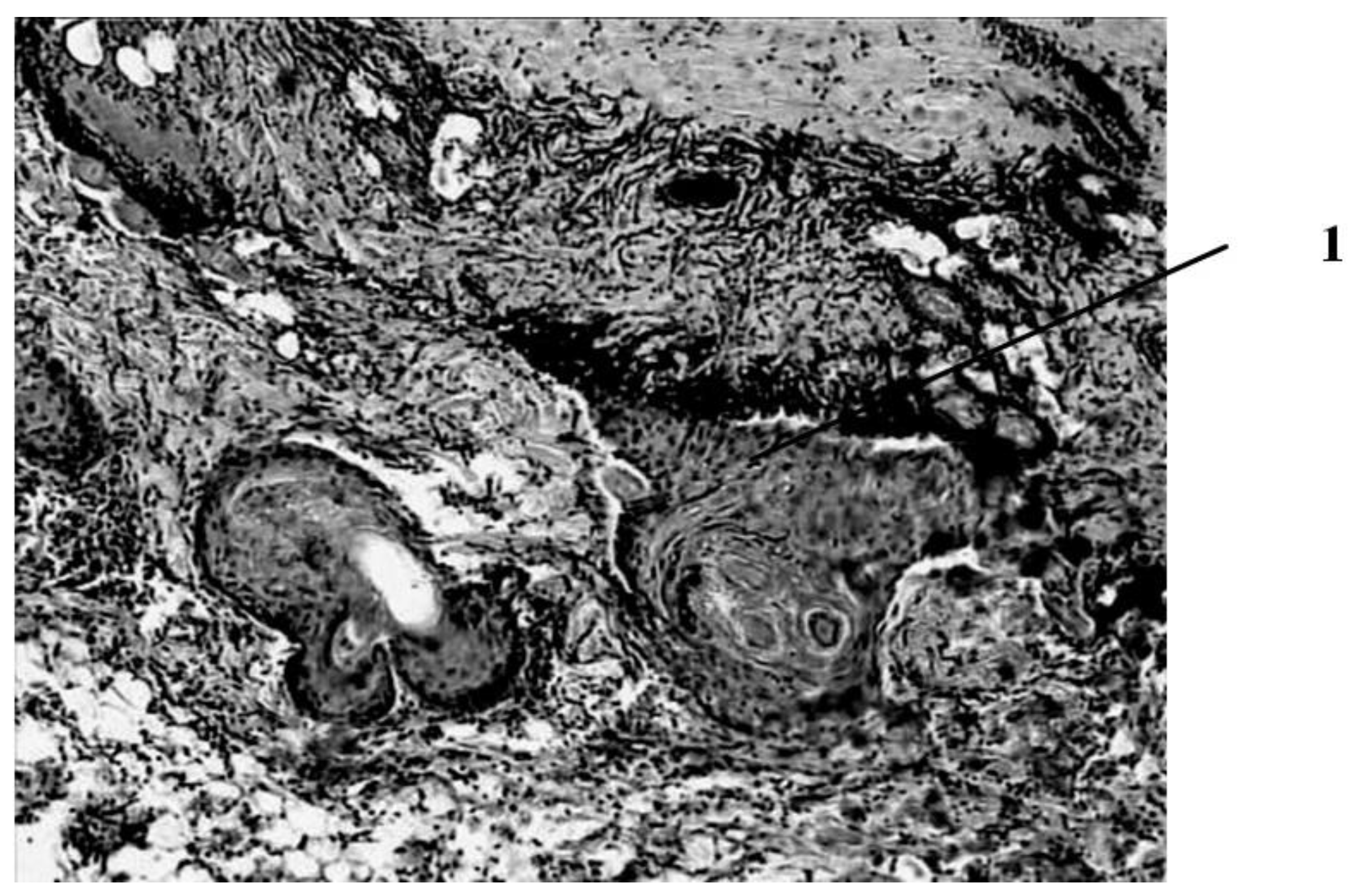
Seven days after the burn, in animals that had a wound punctured daily with a gas mixture with ozone, the scab partially separates from the wound surface as a result of penetration of a growing layer of epidermis under it (
Figure 4).
This is the marginal epithelization of the wound, in which the proliferating epithelium of the deep sections of the hair follicles preserved in the lower layers of the dermis also takes part. The separation of the scab follows the line of a clearly expressed solid leukocyte demarcation shaft. In the center of the wound bottom, necrosis of the skin’s own muscle is noted for its entire thickness and infiltration of necrotized muscle fibers by leukocytes. In the muscle layer located to the periphery of its necrosis zone, there are phenomena of muscle fiber regeneration in the form of proliferation of myocyte nuclei and neoplasm of thin muscle fibers with basophilic cytoplasm. Under the layer of the skin’s own muscle, a hemorrhage site containing nonhemolysed erythrocytes is visible, and along its periphery there is fibroblast proliferation and manifestations of neoangiogenesis. Individual newly formed capillary-type vessels grow into the hemorrhage zone. There is also a pronounced proliferation of epithelium in the hair follicles located in the deep layers of the dermis. Thus, seven days after the burn, under conditions of pricking wounds with an ozone-containing gas mixture, more pronounced (compared to animals of other series) reparative processes are noted in the wounds in the form of more intense marginal epithelization, the formation of granulation tissue and the appearance of not only muscle kidneys, but also newly formed muscle fibers in the damaged own muscle layer of the skin (
Figure 5).
Fourteen days after the burn, distinct signs of regeneration were found in the wounds of animals of the control group (treatment of the wound with Levomecol ointment) against the background of inflammation (
Figure 6).
Thus, under the epidermis, as well as on the non-epithelized surface of the wound, there is a significant layer of young scar tissue containing fibroblasts, the number of which decreases when approaching the edge of the wound, and the cells themselves gradually differentiate into fibrocytes. Among the cells are thin-walled newly formed vessels of the capillary type. Cells and vessels are located parallel to the surface of the wound. Among the collagen fibers of the preserved part of the underlying dermis, separate foci of fibroblast proliferation are noted, as well as the formation of single capillary-type vessels located perpendicular to the surface. There is practically no fibroblastic proliferation in the area between the dermis and the underlying layer of the skin’s own muscle, among the muscle fibers. The muscle layer in a small area corresponding to the center of the wound partially loses its continuity, obviously due to the death of the muscle fibers located here. In their place, fibroblasts proliferate, and a neoplasm of collagen fibers occurs.
The preserved muscle fibers have signs of regeneration: proliferation of myocyte nuclei is noted, as well as the formation of muscle kidneys and even individual thin muscle fibers. Thus, when treating a wound with Levomecol ointment, 14 days after the burn, the scab is separated from the wound surface, where a scar is formed, covered with regenerated epidermis.
Fourteen days after the burn in the group of animals treated with ozonated oil, the scab on most of the wound surface is not separated from it (
Figure 7). Purulent exudate is found under the scab. There is also a purulent discharge and fragments of necrotic tissues on the open (scab-free) surface of the wound.
The demarcation shaft in the part of the preparation (the center of the burn wound) passes directly over the layer of the skin’s own muscle, the muscle fibers of which are in a state of necrosis, necrobiosis or dystrophic changes. Part of the vessels passing over the muscle layer here is necrotic. Along the periphery of this zone, in the lower parts of the preserved dermis, in the layer of the skin’s own muscle and under it, there is a pronounced cellular reaction in the form of an accumulation of leukocytes (lymphocytes, neutrophils, eosinophils), macrophages, proliferating fibroblasts, which in some areas form young connective (granulation) tissue with single newly formed vessels. The muscle layer near the necrosis zone is in a state of rarefaction due to the death of muscle fibers and the proliferation of connective tissue cells and with the neoplasm of collagen fibers, but at the same time, muscle fibers with signs of regeneration (proliferation of nuclei, formation of muscle kidneys) are determined. Scarring of the wound is not pronounced; its epithelization is in its initial state. Thus, the use of ozonated oil for the treatment of a burn wound is accompanied by purulent inflammation, which is not limited to the formation of a demarcation leukocyte shaft, but also extends to the underlying tissues. The processes of scarring of the wound and its epithelization are weakened.
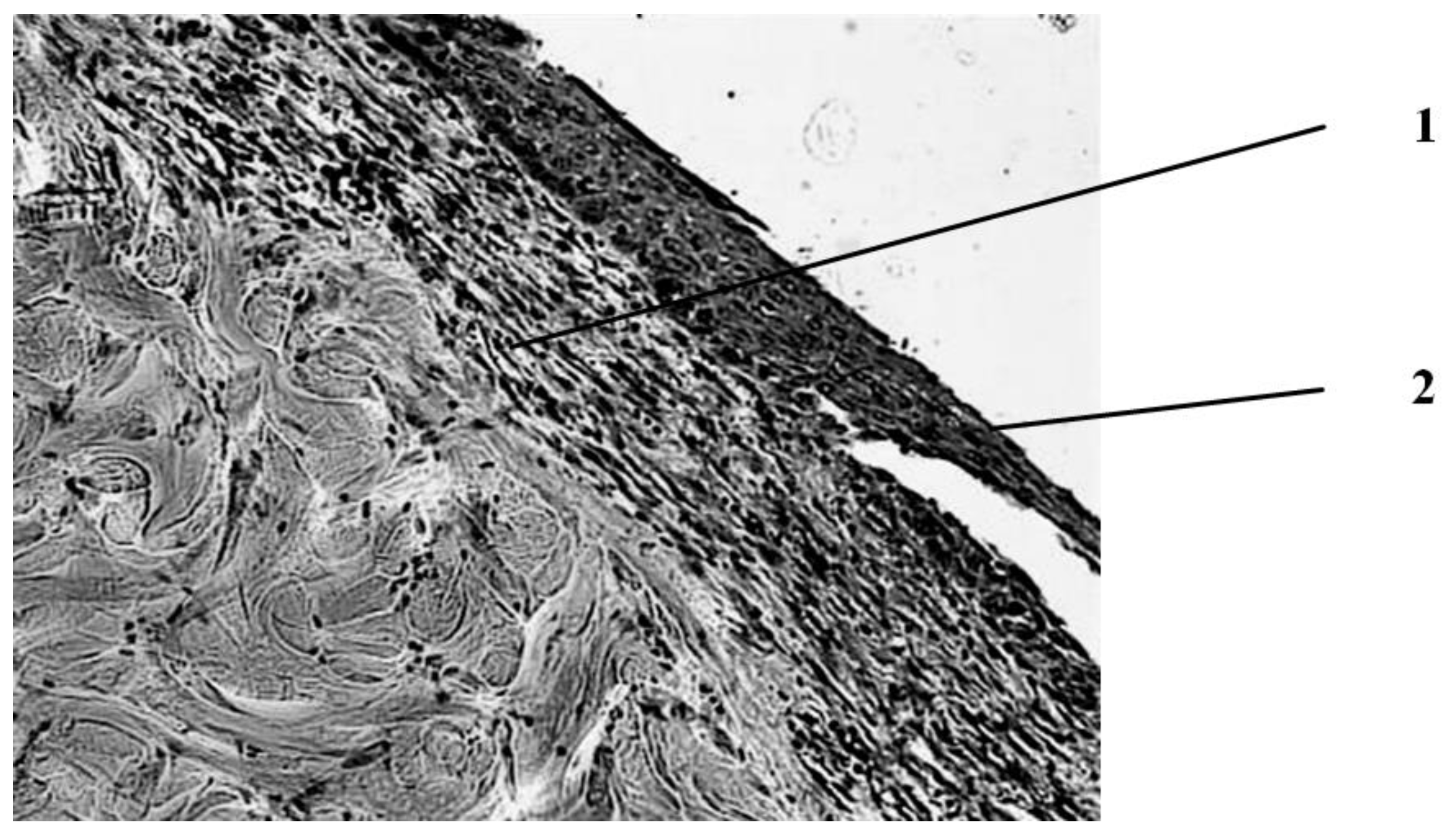
Fourteen days after the burn, the wound healing pattern is determined in a group of animals that were pierced with a gas mixture containing ozone (
Figure 8).
The scab is separated from the wound surface. In part of the preparations, complete epithelization of the wound surface is noted both due to marginal epithelization and due to the proliferation of the epithelium of the hair follicles that have remained viable in the deep parts of the dermis. Under the epidermis, there is a layer of young scar tissue with a horizontal (parallel to the skin surface) arrangement of cells and a relatively small number of newly formed vessels. In the underlying dermis, there is a slightly increased number of connective tissue cells. There is a layer of fatty tissue above the muscle layer. The thickness of the scar layer varies: from the maximum, occupying almost the entire thickness of the former dermis in the center of the wound, to a narrow subepidermal layer at the edges of the wound. The layer of the skin’s own muscle closer to the center of the wound is represented by thin, sometimes wavy newly formed muscle fibers with basophilic cytoplasm and a large number of juicy nuclei, sometimes arranged in a chain. However, in the center of the wound, the muscle layer is interrupted. Simultaneously with newly formed muscle fibers, there are also fibers that are in a state of dystrophy—swollen, losing their tinctorial properties, sometimes in a state of discoid cleavage and local lysis.
Thus, the local application of reactive oxygen species in the form of wound pricking with an ozone–oxygen mixture is accompanied 14 days after the burn by a picture of pronounced reparative processes in the form of complete scarring and epithelization.