Development and Investigation of High Performance PVA/NiO and PVA/CuO Nanocomposites with Improved Physical, Dielectric and Mechanical Properties
Abstract
:1. Introduction
2. Sample Preparation
3. Instruments and Methods
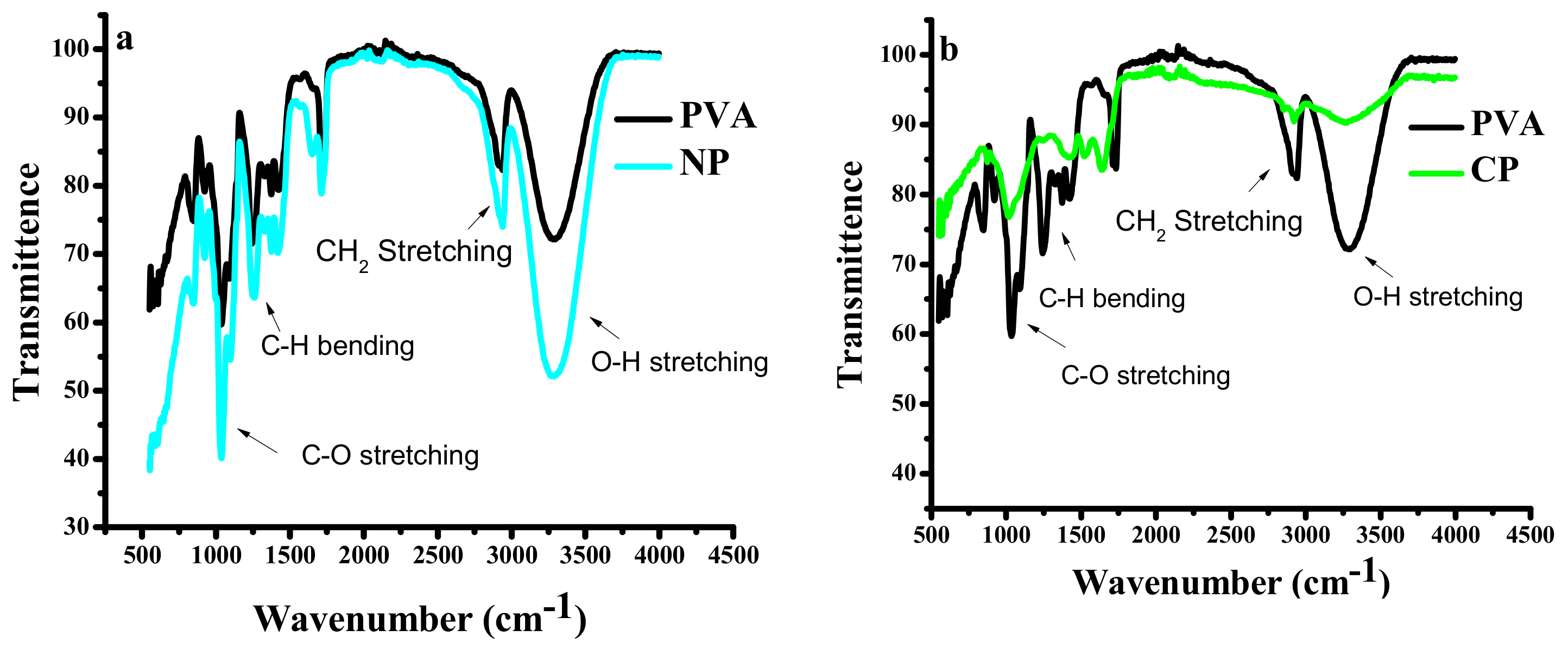
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
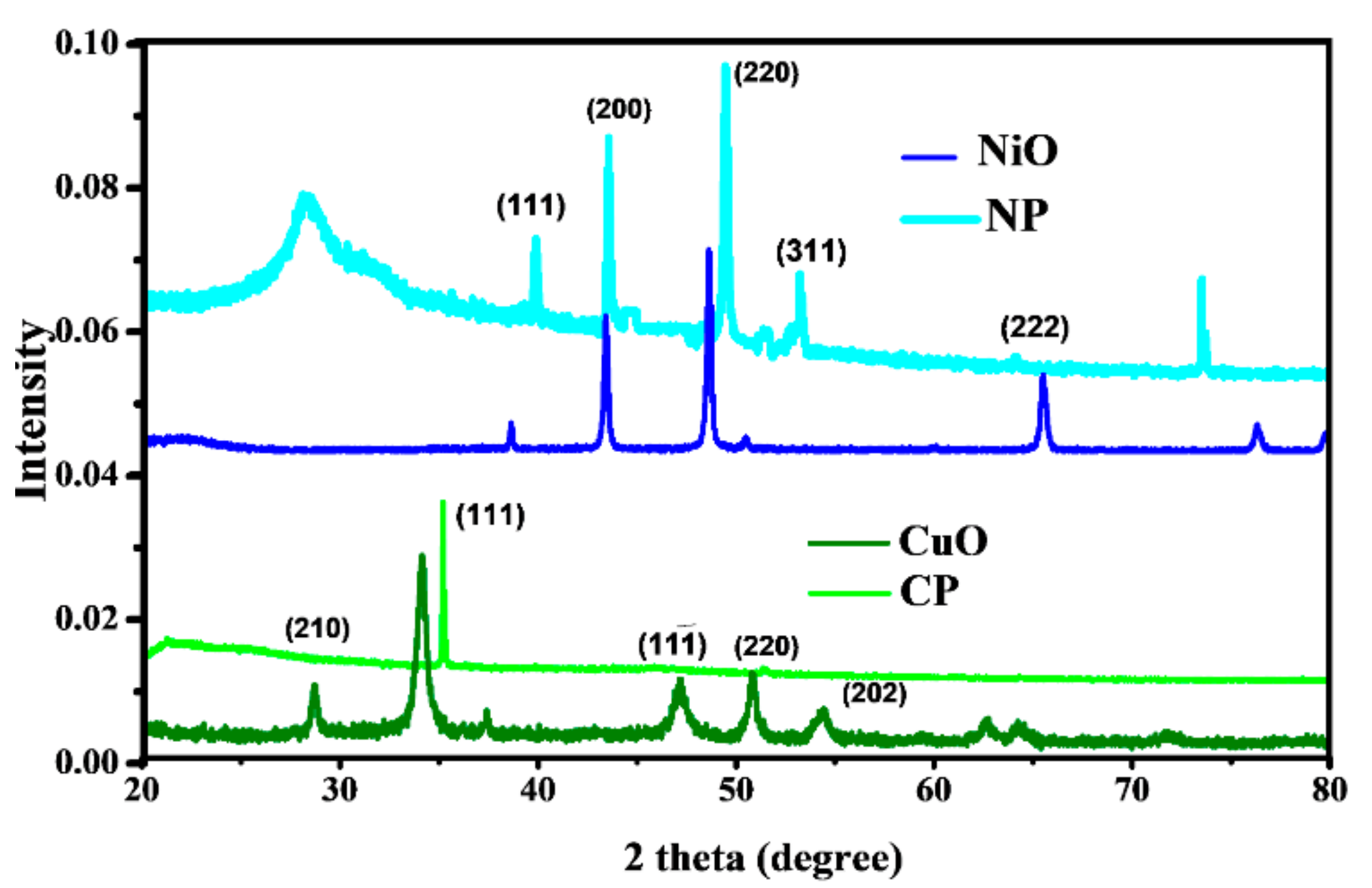
3.2. X-ray Powder Diffraction (XRPD)
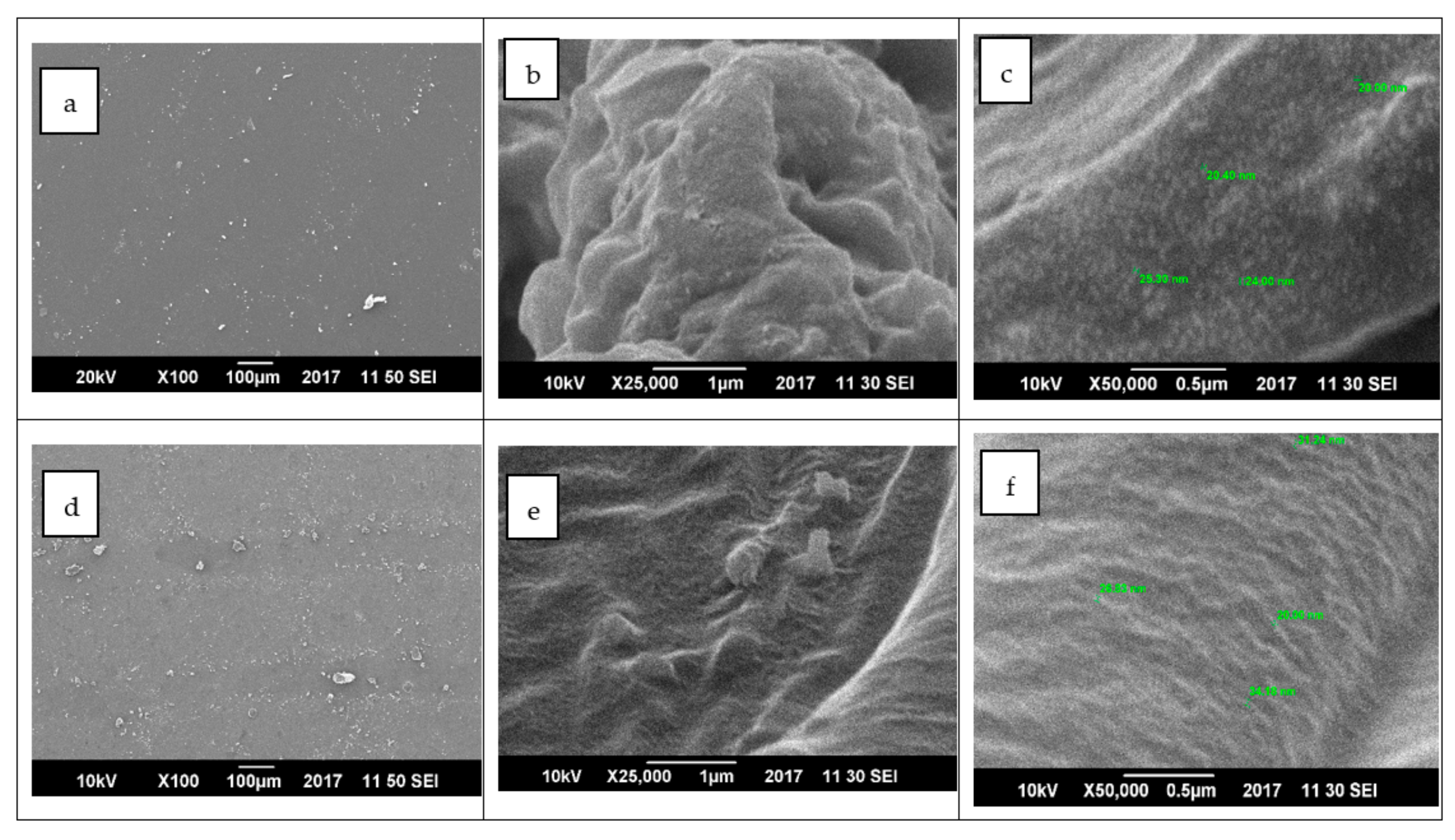
3.3. Field Emission Scanning Electron Microscopy (FESEM)
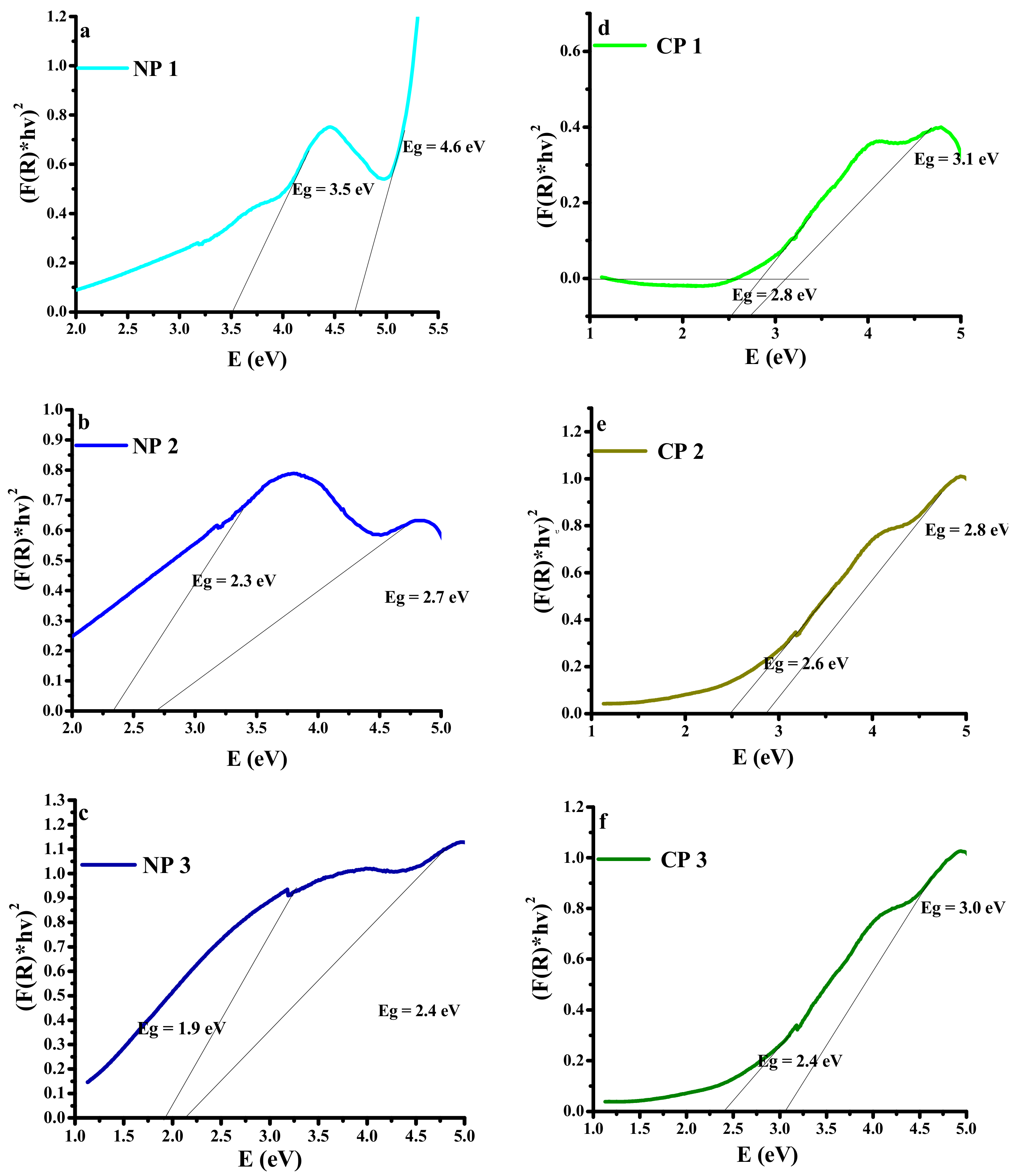
3.4. UV–Vis Diffuses Reflectance Spectroscopy (UV–DRS)
3.5. Dielectric Properties
3.6. Tensile Strength
4. Results and Discussions
4.1. Characterization
4.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
4.1.2. X-ray Powder Diffraction (XRPD)
4.1.3. Field Emission Scanning Electron Microscopy (FESEM)
4.2. Physical Properties
4.2.1. UV–Vis Diffusion Reflectance Spectroscopy (UV–DRS)
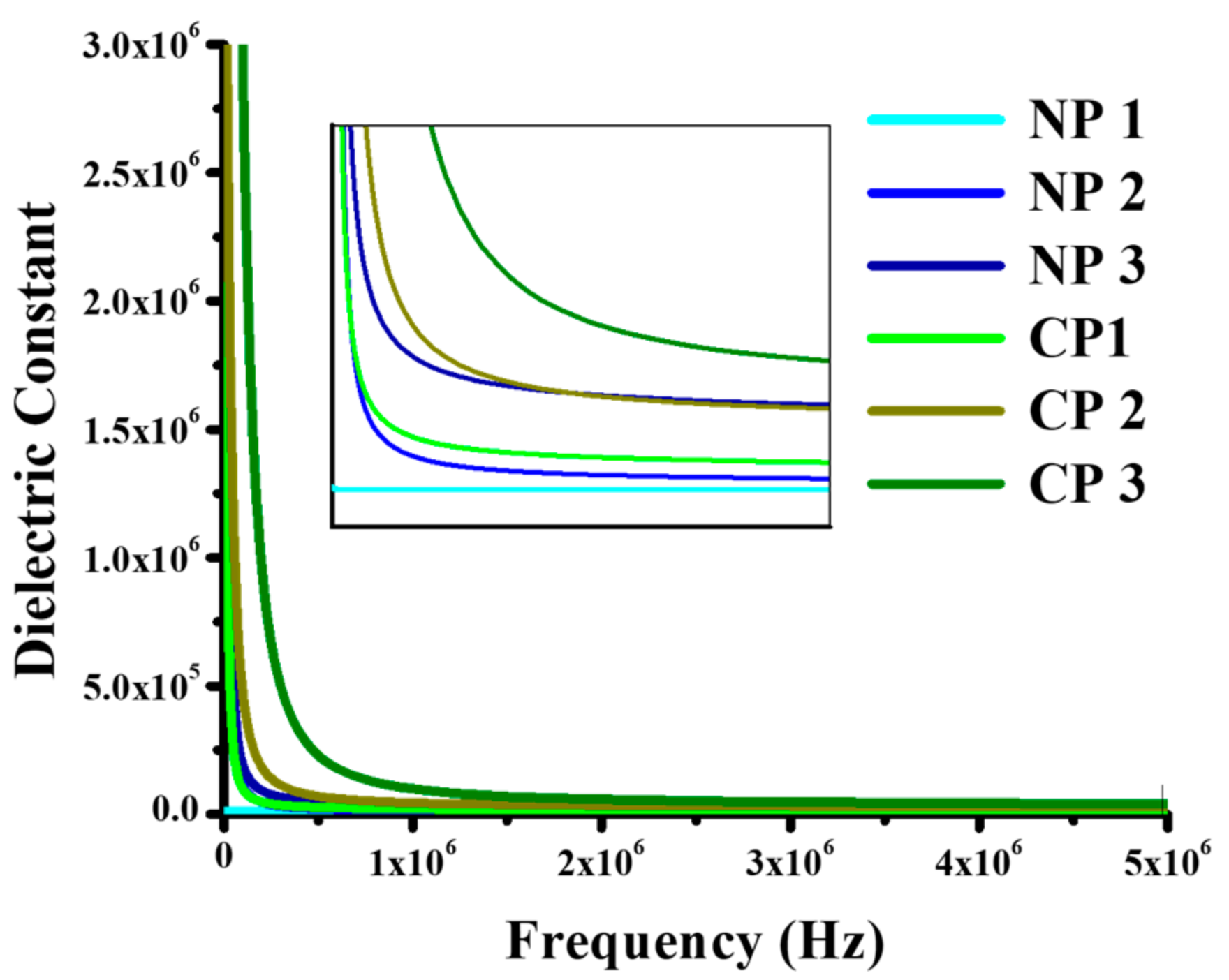
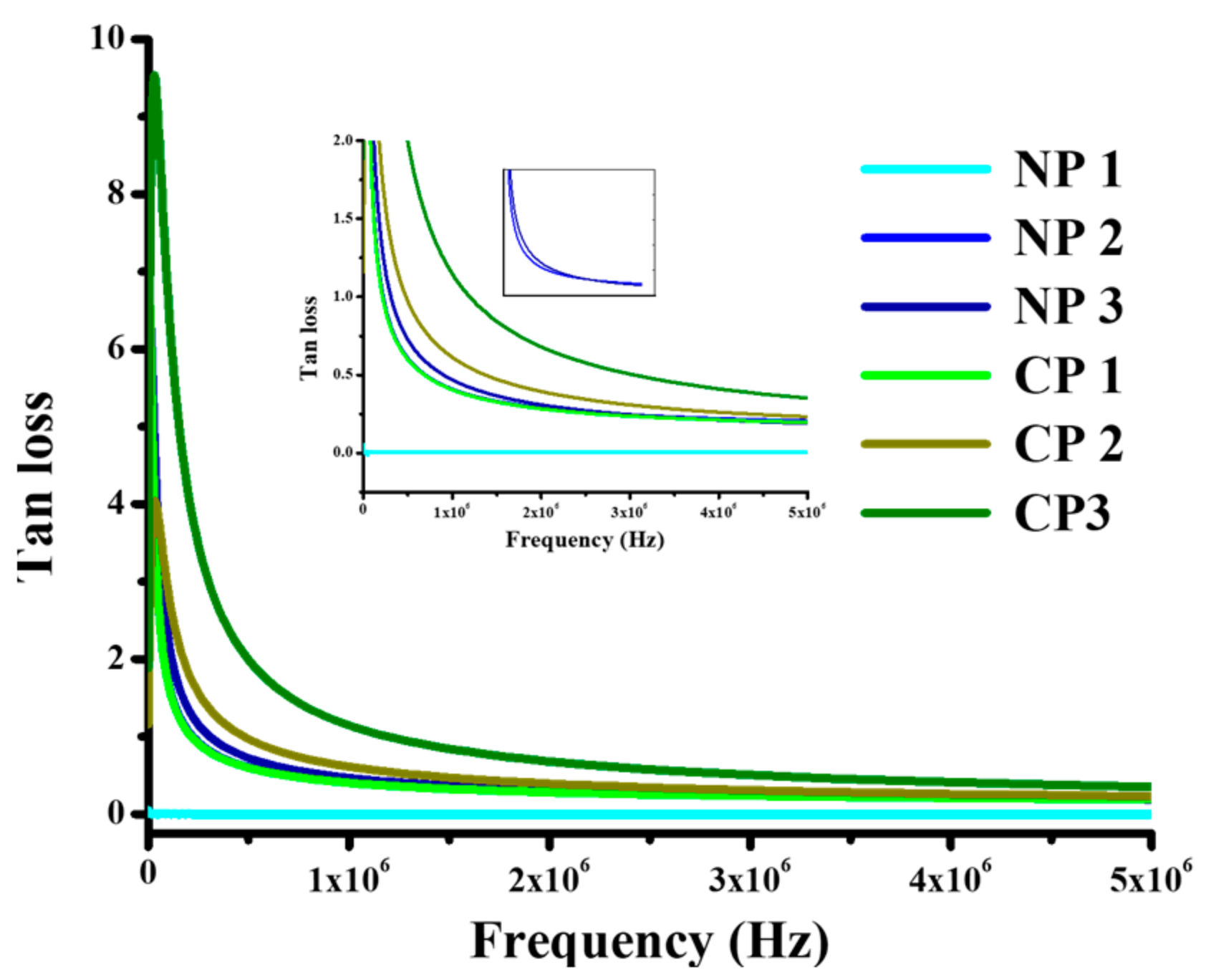
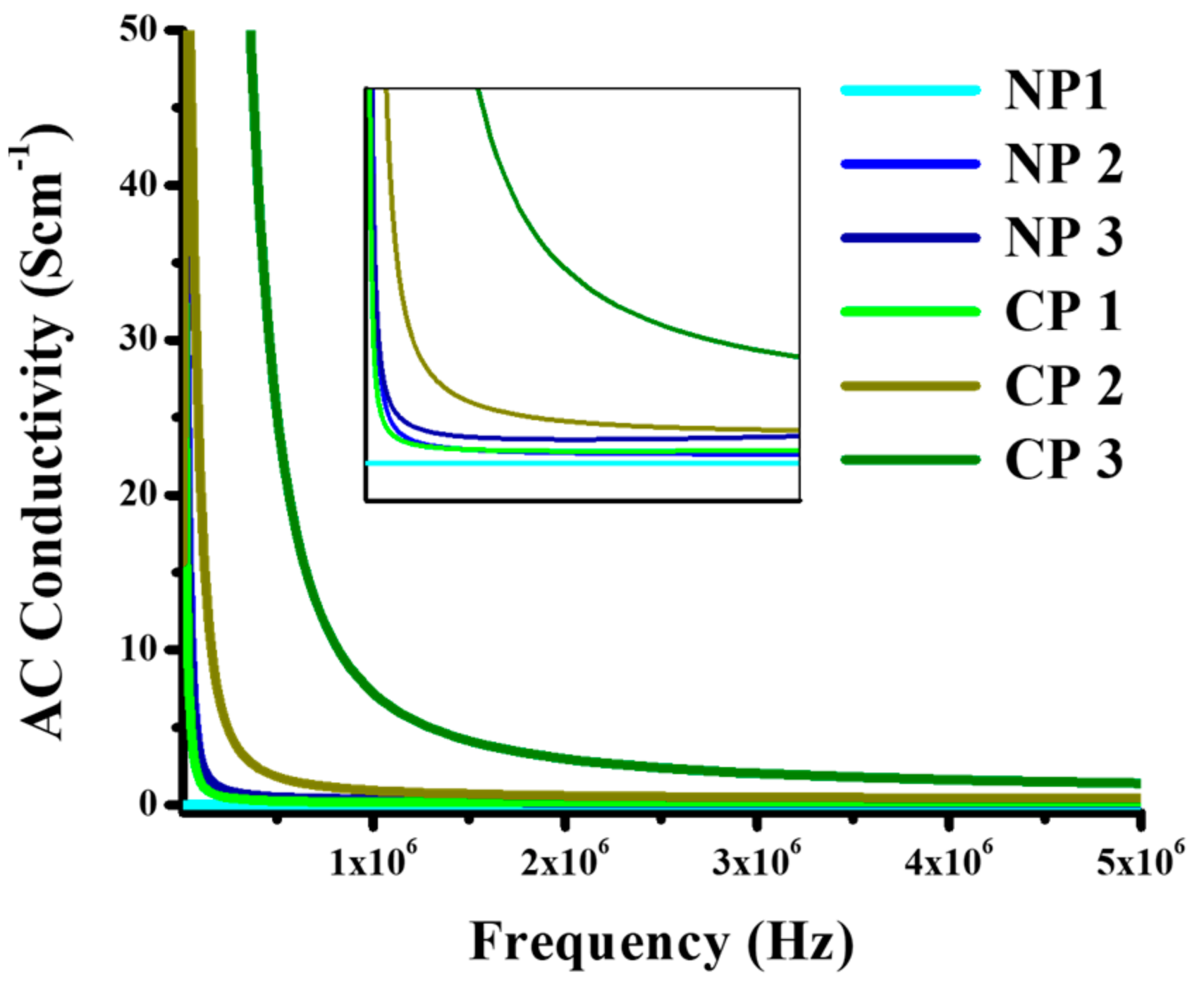
4.2.2. Dielectric Properties
4.2.3. Tensile Strength
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.-H.; Lai, Y.-C.; Lin, C.-W. Enhancement of power generation by microbial fuel cells in treating toluene-contaminated groundwater: Developments of composite anodes with various compositions. Appl. Energy 2018, 233–234, 922–929. [Google Scholar] [CrossRef]
- Reddy, P.L.; Deshmukh, K.; Chidambaram, K.; Ali, M.M.N.; Sadasivuni, K.K.; Kumar, Y.R.; Lakshmipathy, R.; Pasha, S.K.K. Dielectric properties of polyvinyl alcohol (PVA) nanocomposites filled with green synthesized zinc sulphide (ZnS) nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 4676–4687. [Google Scholar] [CrossRef]
- Khattak, A.; Amin, M.; Iqbal, M.; Abbas, N. Life estimation and analysis of dielectric strength, hydrocarbon backbone and oxidation of high voltage multi stressed EPDM composites. Mater. Res. Express 2018, 5, 025003. [Google Scholar] [CrossRef]
- El-Molla, S.; Mansour, A.F.; Hammad, A. Enhancement of Fluorescence and Photostability Based on Interaction of Fluorescent Dyes with Silver Nanoparticles for Luminescent Solar Concentrators. J. Nanomater. 2017, 2017, 9701251. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Yu, B. NANO-FILLERS Transistor with Assisted-Gates and Ultra-Shallow “Psuedo” Source and Drain Extensions for Ultra-Large-Scale Integration. U.S. Patent 6,312,995, 6 November 2001. [Google Scholar]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Zhou, L.; Li, C.; Liu, X.; Zhu, Y.; Wu, Y.; van Ree, T. Nano-Fillers in Supercapacitors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–203. [Google Scholar]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Saikia, J.P.; Paul, S.; Konwar, B.K.; Samdarshi, S.K. Nickel oxide nanoparticles: A novel antioxidant. Colloids Surfaces B Biointerfaces 2010, 78, 146–148. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured Carbon–nano-filler Composite Electrodes for Supercapacitors: A Review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Zhu, J.; Kristal, D.J.; Zhao, W.; Lalia, B.S. High-power and High-Energy-Density Flexible Pseudocapacitor Electrodes Made from Porous CuO Nanobelts and Single-walled Carbon Nanotubes. ACS Nano 2011, 5, 2013–2019. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Jiang, D.; Jia, D.; Liu, J. Hierarchical CuO nanorod arrays in situ generated on three-dimensional copper foam via cyclic voltammetry oxidation for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 10474–10483. [Google Scholar] [CrossRef]
- Pradhan, B.; Setyowati, K.; Liu, H.; Waldeck, D.H.; Chen, J. Carbon Nanotube−Polymer Nanocomposite Infrared Sensor. Nano Lett. 2008, 8, 1142–1146. [Google Scholar] [CrossRef]
- Yang, X.; Li, L. Synthesis and characterization of layer-aligned poly(vinyl alcohol)/graphene nanocomposites. Polymer 2010, 51, 3431–3435. [Google Scholar] [CrossRef]
- Duraisamy, N.; Numan, A.; Fatin, S.O.; Ramesh, K.; Ramesh, S. Facile sonochemical synthesis of nanostructured NiO with different particle sizes and its electrochemical properties for supercapacitor application. J. Colloid Interface Sci. 2016, 471, 136–144. [Google Scholar] [CrossRef]
- Zhu, J.; Li, D.; Chen, H.; Yang, X.; Lu, L.; Wang, X. Highly dispersed CuO nanoparticles prepared by a novel quick-precipitation method. Mater. Lett. 2004, 58, 3324–3327. [Google Scholar] [CrossRef]
- Patel, V.B.; Theron, G.; Lenders, L.; Matinyena, B.; Connolly, C.; Singh, R.; Coovadia, Y.; Ndung’U, T.; Dheda, K. Diagnostic Accuracy of Quantitative PCR (Xpert MTB/RIF) for Tuberculous Meningitis in a High Burden Setting: A Prospective Study. PLoS Med. 2013, 10, e1001536. [Google Scholar] [CrossRef]
- Davar, F.; Fereshteh, Z.; Salavati-Niasari, M. Nanoparticles Ni and NiO: Synthesis, Characterization and Magnetic Properties. J. Alloys Compd. 2009, 476, 797–801. [Google Scholar] [CrossRef]
- Mansur, H.; Sadahira, C.M.; Souza, A.N.; Mansur, A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Prabhudass, J.M.; Palanikumar, K.; Natarajan, E.; Markandan, K. Enhanced Thermal Stability, Mechanical Properties and Structural Integrity of MWCNT Filled Bamboo/Kenaf Hybrid Polymer Nanocomposites. Materials 2022, 15, 506. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate Nano-fillerre Accurately Nano-crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Kouklin, N.; Tzolov, M.; Straus, D.; Yin, A.; Xu, J.M. Infrared absorption properties of carbon nanotubes synthesized by chemical vapor deposition. Appl. Phys. Lett. 2004, 85, 4463. [Google Scholar] [CrossRef]
- Rao, J.K.; Raizada, A.; Ganguly, D.; Mankad, M.M.; Satyanarayana, S.V.; Madhu, G.M. Enhanced mechanical properties of polyvinyl alcohol composite films containing copper oxide nanoparticles as filler. Polym. Bull. 2015, 72, 2033–2047. [Google Scholar] [CrossRef]
- Yang, L.; Kruse, B. Revised Kubelka–Munk Theory. I. Theory and Application. JOSA A 2004, 21, 1933–1941. [Google Scholar] [CrossRef]
- Abdullah, G.; Aziz, S.B.; Omer, K.M.; Salih, Y.M. Reducing the Optical Bandgap of Polyvinyl alcohol (PVA) Based Nanocomposite. J. Mater. Sci. Mater. Electron. 2015, 26, 5303–5309. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Fabrication of nano-CuO-loaded PVA composite films with enhanced optomechanical properties. Polym. Bull. 2021, 78, 1551–1571. [Google Scholar] [CrossRef]
- Mitlin, D.; Ding, J. Hydrogel Derived Carbon for Energy Storage Devices. U.S. Patent 10707026-B2, 7 July 2020. [Google Scholar]
- Tiuri, M.; Sihvola, A.; Nyfors, E.; Hallikaiken, M. The complex dielectric constant of snow at microwave frequencies. IEEE J. Ocean. Eng. 1984, 9, 377–382. [Google Scholar] [CrossRef]
- Da Silva, A.B.; Arjmand, M.; Sundararaj, U.; Bretas, R.E.S. Novel composites of copper nanowire/PVDF with superior dielectric properties. Polymer 2014, 55, 226–234. [Google Scholar] [CrossRef]
- Rao, J.K.; Raizada, A.; Ganguly, D.; Mankad, M.M.; Satayanarayana, S.V.; Madhu, G.M. Investigation of structural and electrical properties of novel CuO–PVA nanocomposite films. J. Mater. Sci. 2015, 50, 7064–7074. [Google Scholar] [CrossRef]
- Long, Y.; Shanks, R.A. PP–elastomer–filler hybrids. I. Processing, microstructure, and mechanical properties. J. Appl. Polym. Sci. 1996, 61, 1877–1885. [Google Scholar] [CrossRef]
- Bartczak, Z.; Argon, S.A.; Cohen, R.E.; Weinber, M. Toughness mechanism in semi-crystalline polymer blends: II. High-density polyethylene toughened with calcium carbonate filler particles. Polymer 1999, 40, 2347–2365. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Nerikar, P.; Bertrand, K.; Murphy, D. Some aspects of surface deformation and fracture of 5–20% calcium carbonate-reinforced polyethylene composites. Mater. Sci. Eng. 2004, 384, 284–298. [Google Scholar] [CrossRef]
- Popovics, S. A numerical approach to the complete stress-strain curve of concrete. Cem. Concr. Res. 1973, 3, 583–599. [Google Scholar] [CrossRef]
- Luyt, A.S.; Dramićanin, M.D.; Antić, Ž.; Djoković, V. Morphology, mechanical and thermal properties of composites of polypropylene and nanostructured wollastonite filler. Polym. Test. 2009, 28, 348–356. [Google Scholar] [CrossRef]
- Musto, P.; Ragosta, G.; Scarinzi, S.; Mascia, L. Microstructural features, diffusion and molecular relaxations in polyimide/silica hybrids. Polymer 2004, 45, 1697. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, H.; Chan, M.C. Nucleating effect of calcium stearate coated CaCO3 nanoparticles on polypropylene. J. Colloid Interface Sci. 2011, 354, 570–576. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@ MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Petrovic, Z.S.; Javni, I.; Waddon, A.; Banhegyi, G. Properties of bulk-polymerized thermoplastic polyurethane nanocomposites. Polymer 2000, 76, 133. [Google Scholar]
- Siegel, R.; Chang, S.; Ash, B.; Stone, J.; Ajayan, P.; Doremus, R.; Schadler, L. Mechanical behavior of polymer and ceramic matrix nanocomposites. Scr. Mater. 2001, 44, 2061–2064. [Google Scholar] [CrossRef]
- Ash, B.J.; Rogers, D.F.; Wiegand, C.J.; Schadler, L.S.; Siegel, R.W.; Benicewicz, B.C.; Apple, T. Preparation and characterization of poly (lactic acid)-grafted TiO2 nanoparticles with improved dispersions. Appl. Surf. Sci. 2002, 23, 1014. [Google Scholar]
- Ash, B.J.; Siegel, R.W.; Schadler, L.S. Polymer nanocomposites: A small part of the story. Macromolecules 2004, 37, 1358. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, F.; Yu, M.; Zhuang, X.; Feng, X. Two-dimensional materials for miniaturized energy storage devices: From individual devices to smart integrated systems. Chem. Soc. Rev. 2018, 47, 7426–7451. [Google Scholar] [CrossRef]
- Kumar, Y.R.; Deshmukh, K.; Ali, M.M.N.; Abhijay, G.; Al-Onazi, W.A.; Al-Mohaimeed, A.M.; Pasha, S.K. Structure, morphology and modelling studies of polyvinylalcohol nanocomposites reinforced with nickel oxide nanoparticles and graphene quantum dots. Environ. Res. 2021, 203, 111842. [Google Scholar] [CrossRef]



| Sr. # | Sample Code | NiO (wt%) | CuO (wt%) |
|---|---|---|---|
| 1 | CP 1 | - | 0.2 |
| 2 | CP 2 | - | 0.4 |
| 3 | CP 3 | - | 0.6 |
| 4 | NP 1 | 0.2 | - |
| 5 | NP 2 | 0.4 | - |
| 6 | NP 3 | 0.6 | - |
| Sr. No. | Sample | Bandgap (eV) | |
|---|---|---|---|
| Nano-Filler | PVA | ||
| 1 | NP 1 | 3.5 | 4.6 |
| 2 | NP 2 | 2.3 | 2.7 |
| 3 | NP 3 | 1.9 | 2.4 |
| 4 | CP 1 | 2.8 | 3.1 |
| 5 | CP 2 | 2.6 | 2.8 |
| 6 | CP 3 | 2.4 | 3.0 |
| Code | Sample Compositions | Young’s Modulus (E) | Elastic Limit (Sy) MPa | * Ultimate Tensile Strength (UTS) MPa | Total Strain (etotal) | Elastic Strain (eelastic) | |
|---|---|---|---|---|---|---|---|
| MPa | Difference | ||||||
| 0 | PVA@0 wt% NiO | 71.5 | - | 34.4 | 95.4 | 0.37 | 0.32 |
| NP 1 | PVA@0.2 wt% NiO | 22.7 | 68% | 2.1 | 4.9 | 0.34 | 0.26 |
| NP 2 | PVA@0.4 wt% NiO | 25.7 | 64% | 1.6 | 2.5 | 0.26 | 0.13 |
| NP 3 | PVA@0.6 wt% NiO | 30.9 | 56.7% | 1.2 | 5.14 | 0.5 | 0.4 |
| Averaged values forPVA@ NiO nano-filler | 26.43 | 63.03% | 1.63 | 4.18 | 0.366 | 0.26 | |
| CP 1 | PVA@0.2 wt% CuO | 8.2 | 88.53% | 1.09 | 5.13 | 0.60 | 0.44 |
| CP 2 | VA@0.4 wt% CuO | 18.9 | 73.56% | 0.37 | 1.7 | 0.62 | 0.53 |
| CP 3 | PVA@0.6 wt% CuO | 27.2 | 61.95% | 1.53 | 5.06 | 0.47 | 0.39 |
| Averaged values forPVA@ CuO nano-filler | 18.1 | 74.68% | 3.96 | 0.55 | 0.44 | 1.16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faiza; Malik, Z.; Khattak, A.; Alahmadi, A.A.; Butt, S.U. Development and Investigation of High Performance PVA/NiO and PVA/CuO Nanocomposites with Improved Physical, Dielectric and Mechanical Properties. Materials 2022, 15, 5154. https://doi.org/10.3390/ma15155154
Faiza, Malik Z, Khattak A, Alahmadi AA, Butt SU. Development and Investigation of High Performance PVA/NiO and PVA/CuO Nanocomposites with Improved Physical, Dielectric and Mechanical Properties. Materials. 2022; 15(15):5154. https://doi.org/10.3390/ma15155154
Chicago/Turabian StyleFaiza, Zahida Malik, Abraiz Khattak, Ahmad Aziz Alahmadi, and Safi Ullah Butt. 2022. "Development and Investigation of High Performance PVA/NiO and PVA/CuO Nanocomposites with Improved Physical, Dielectric and Mechanical Properties" Materials 15, no. 15: 5154. https://doi.org/10.3390/ma15155154
APA StyleFaiza, Malik, Z., Khattak, A., Alahmadi, A. A., & Butt, S. U. (2022). Development and Investigation of High Performance PVA/NiO and PVA/CuO Nanocomposites with Improved Physical, Dielectric and Mechanical Properties. Materials, 15(15), 5154. https://doi.org/10.3390/ma15155154







