The Diagnostic Value of the Added MR Imaging of the Scrotum in the Preoperative Workup of Sonographically Indeterminate Testicular Lesions—A Retrospective Multicenter Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. MRI Criteria for the Characterization of Malignant Tumors
2.3. Terminology
- False positive: incorrect detection of malignant finding via US/MRI despite the presence of a benign finding.
- True positive: correct detection of malignancy via US/MRI.
- False negative: incorrect detection of a benign finding via US/MRI despite the presence of malignancy.
- True negative: correct detection of a benign finding via US/MRI.
2.4. Statistics
2.4.1. Comparisons of Two Subgroups
2.4.2. Comparisons of More Than Two Subgroups
3. Results
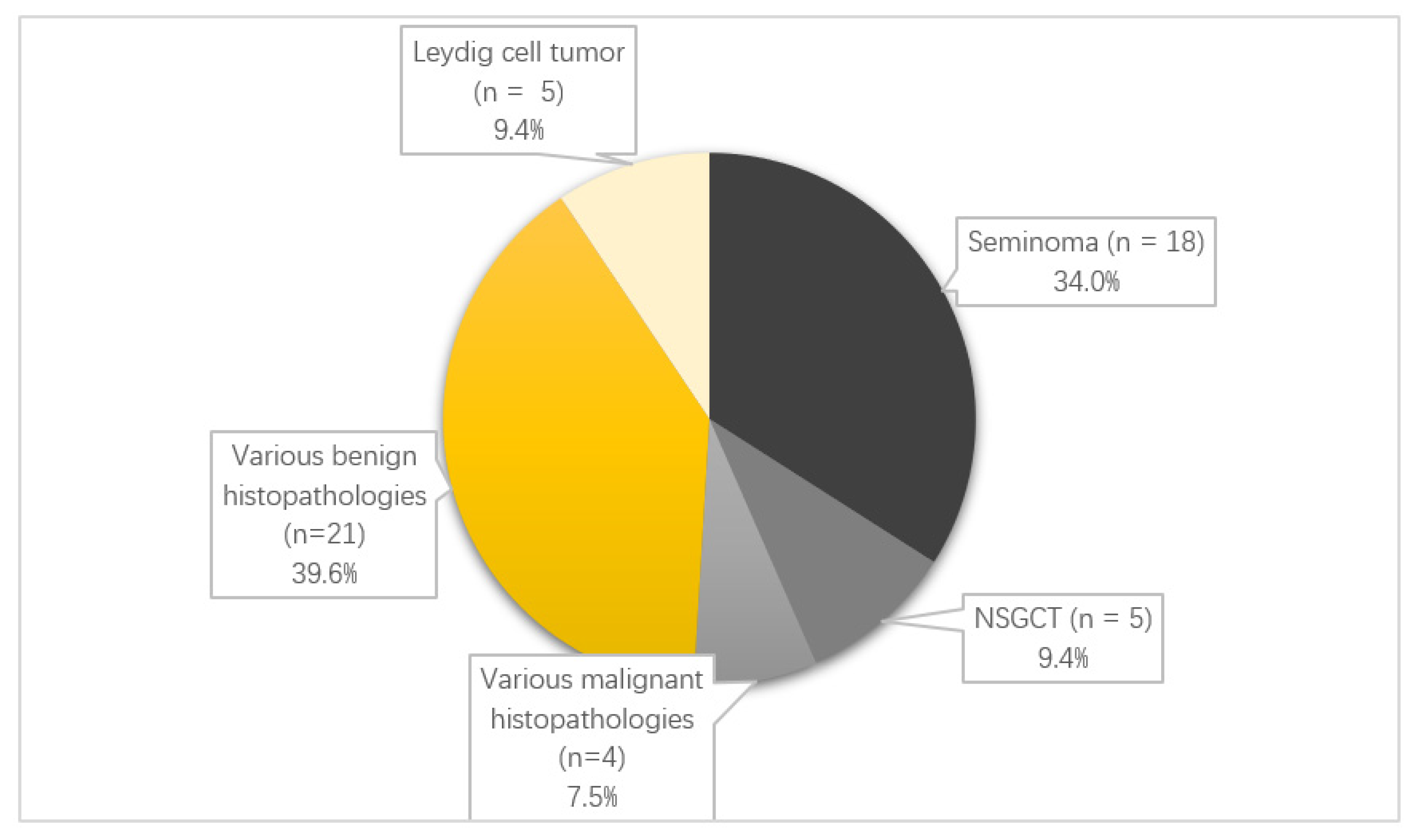
3.1. Epidemiology
3.2. The Rate of True Positive, False Positive, True Negative and False Negative US/MRI Findings
3.3. Sensitivity, Specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) of US and MRI
3.4. Subgroup of Positive Tumor Marker Patients
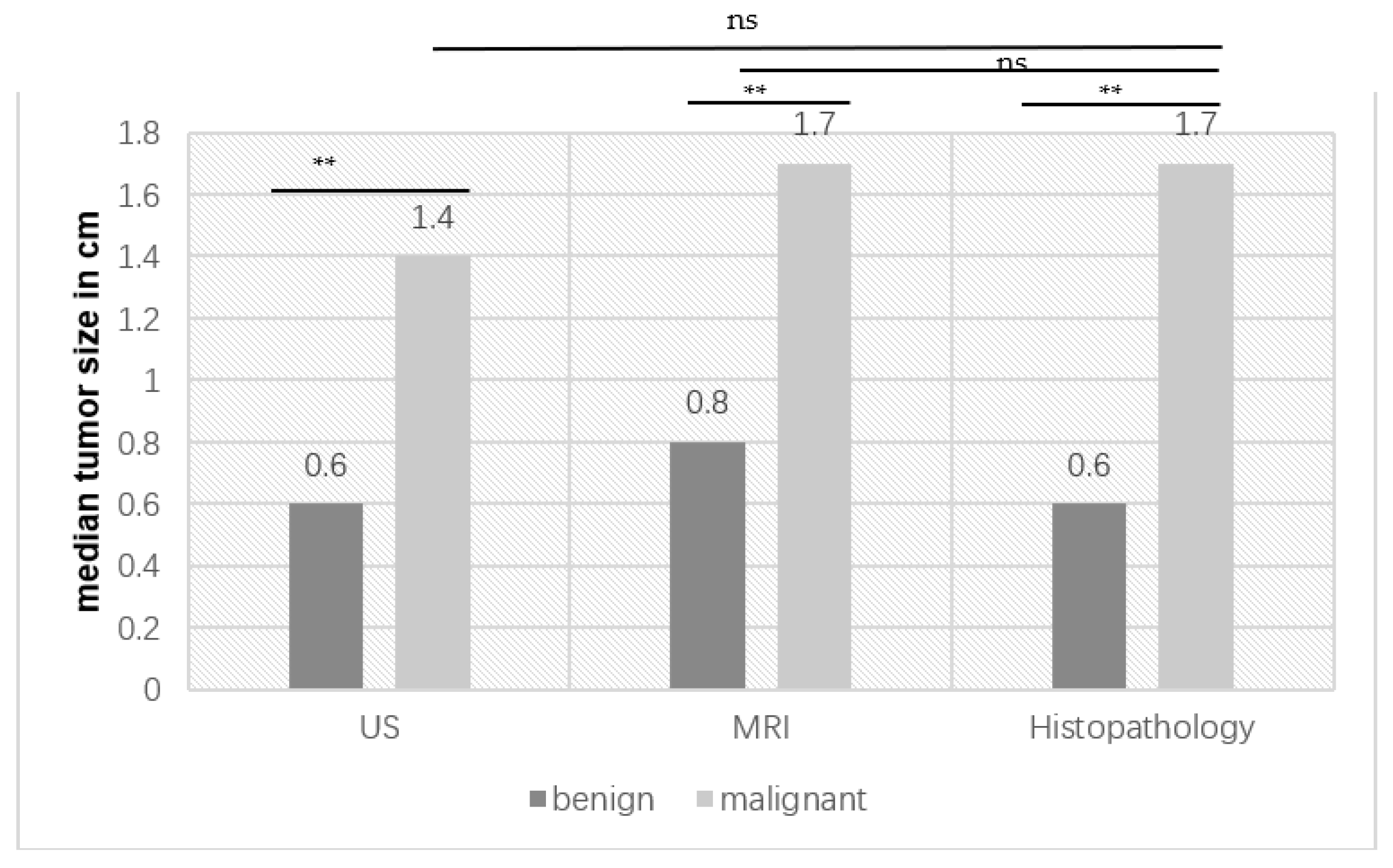
3.5. Subgroup Comparison: Benign Versus Malignant Histopathology
3.6. Analysis of the Performance of the Frozen Section (FFS) Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | alpha-fetoprotein |
| BMI | Body Mass Index |
| CM | Contrast median |
| CT | Chemotherapy |
| DWI | Diffusion-Weighted Imaging |
| EAU | European Association of Urology |
| FFS | Fresh frozen section |
| FS | Fatsat |
| FSH | Follicle-stimulating hormone |
| GCNIS | Germ cell neoplasia in situ |
| HPLAP | Human placental alkaline phosphatase |
| LCT | Leydig cell tumor |
| LDH | Lactate dehydrogenase |
| LH | Luteinizing hormone |
| MRI | Magnetic resonance imaging |
| NPV | Negative predictive value |
| ns | Not significant |
| NSGCT | Nonseminomatous germ cell tumor |
| PD | Proton-density weighted |
| PPV | Positive predictive value |
| TIN | Testicular intraepithelial neoplasia |
| TSS | Testis-preserving surgery |
| US | Ultrasound |
| βhCG | Human chorionic gonadotropin |
References
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.P.; Nicolai, N.; Oldenburg, J. Guidelines on Testicular Cancer: 2015 Update. Eur. Urol. 2015, 68, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Aschebrook-Kilfoy, B.; Shikanov, S.; Eggener, S. Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009. World J. Urol. 2015, 33, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, J.; Elghiaty, A.; Ham, W.S. Recent global trends in testicular cancer incidence and mortality. Medicine 2018, 97, e12390. [Google Scholar] [CrossRef] [PubMed]
- Carmignani, L.; Gadda, F.; Gazzano, G.; Nerva, F.; Mancini, M.; Ferruti, M.; Bulfamante, G.; Bosari, S.; Coggi, G.; Rocco, F.; et al. High incidence of benign testicular neoplasms diagnosed by ultrasound. J. Urol. 2003, 170, 1783–1786. [Google Scholar] [CrossRef]
- Rocher, L.; Ramchandani, P.; Belfield, J.; Bertolotto, M.; Derchi, L.E.; Correas, J.M.; Oyen, R.; Tsili, A.C.; Turgut, A.T.; Dogra, V.; et al. Incidentally detected non-palpable testicular tumours in adults at scrotal ultrasound: Impact of radiological findings on management Radiologic review and recommendations of the ESUR scrotal imaging subcommittee. Eur. Radiol. 2016, 26, 2268–2278. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Rosen, M.A.; Langer, J.E.; Banner, M.P.; Siegelman, E.S.; Ramchandani, P. US MR imaging correlation in pathologic conditions of the scrotum. Radiographics 2007, 27, 1239–1253. [Google Scholar] [CrossRef]
- Stonier, T.; Simson, N.; Challacombe, B. Diagnosing testicular lumps in primary care. Practitioner 2017, 261, 13–17. [Google Scholar]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Milan 2021; EAU Guidelines Office: Arnhem, The Netherlands, 2021; Available online: https://uroweb.org/eau-guidelines/citing-usage-republication (accessed on 30 May 2022).
- Cassidy, F.H.; Ishioka, K.M.; McMahon, C.J.; Chu, P.; Sakamoto, K.; Lee, K.; Aganovic, L. MR imaging of scrotal tumors and pseudotumors. Radiographics 2010, 30, 665–683. [Google Scholar] [CrossRef]
- Manganaro, L.; Vinci, V.; Pozza, C.; Saldari, M.; Gianfrilli, D.; Pofi, R.; Bernardo, S.; Cantisani, V.; Lenzi, A.; Scialpi, M.; et al. A prospective study on contrast-enhanced magnetic resonance imaging of testicular lesions: Distinctive features of Leydig cell tumours. Eur. Radiol. 2015, 25, 3586–3595. [Google Scholar] [CrossRef]
- Tsili, A.C.; Sofikitis, N.; Stiliara, E.; Argyropoulou, M.I. MRI of testicular malignancies. Abdom. Radiol. 2019, 44, 1070–1082. [Google Scholar] [CrossRef]
- Tsili, A.C.; Bertolotto, M.; Turgut, A.T.; Dogra, V.; Freeman, S.; Rocher, L.; Belfield, J.; Studniarek, M.; Ntorkou, A.; Derchi, L.E.; et al. MRI of the scrotum: Recommendations of the ESUR Scrotal and Penile Imaging Working Group. Eur. Radiol. 2018, 28, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Tsili, A.C.; Argyropoulou, M.I.; Giannakis, D.; Sofikitis, N.; Tsampoulas, K. MRI in the characterization and local staging of testicular neoplasms. AJR Am. J. Roentgenol. 2010, 194, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.O.; Mattrey, R.F.; Phillipson, J. Differentiation of seminomatous from nonseminomatous testicular tumors with MR imaging. AJR Am. J. Roentgenol. 1990, 154, 539–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsili, A.C.; Tsampoulas, C.; Giannakopoulos, X.; Stefanou, D.; Alamanos, Y.; Sofikitis, N.; Efremidis, S.C. MRI in the histologic characterization of testicular neoplasms. AJR Am. J. Roentgenol. 2007, 189, W331–W337. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Yilmaz, F.; Turkay, R.; Özel, S.; Bilgiç, B.; Velioglu, A.; Saka, B.; Salmaslioglu, A. Role of diffusion-weighted MR imaging in the differentiation of benign retroperitoneal fibrosis from malignant neoplasm: Preliminary study. Radiology 2014, 272, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, E.; Gilbert, B.R. Office ultrasound for the urologist. Curr. Urol. Rep. 2012, 13, 460–466. [Google Scholar] [CrossRef]
- Tsili, A.C.; Argyropoulou, M.I.; Dolciami, M.; Ercolani, G.; Catalano, C.; Manganaro, L. When to ask for an MRI of the scrotum. Andrology 2021, 9, 1395–1409. [Google Scholar] [CrossRef]
- Guthrie, J.A.; Fowler, R.C. Ultrasound diagnosis of testicular tumours presenting as epididymal disease. Clin. Radiol. 1992, 46, 397–400. [Google Scholar] [CrossRef]
- Isidori, A.M.; Pozza, C.; Gianfrilli, D.; Giannetta, E.; Lemma, A.; Pofi, R.; Barbagallo, F.; Manganaro, L.; Martino, G.; Lombardo, F.; et al. Differential diagnosis of nonpalpable testicular lesions: Qualitative and quantitative contrast-enhanced US of benign and malignant testicular tumors. Radiology 2014, 273, 606–618. [Google Scholar] [CrossRef]
- Reginelli, A.; D’Andrea, A.; Clemente, A.; Izzo, A.; Urraro, F.; Scala, F.; Nardone, V.; Guida, C.; Scialpi, M.; Cappabianca, S. Does multiparametric US improve diagnostic accuracy in the characterization of small testicular masses? Gland Surg. 2019, 8, S136–S141. [Google Scholar] [CrossRef]
- El Sanharawi, I.; Correas, J.M.; Glas, L.; Ferlicot, S.; Izard, V.; Ducot, B.; Bellin, M.-F.; Benoît, G.; Rocher, L. Non-palpable incidentally found testicular tumors: Differentiation between benign, malignant, and burned-out tumors using dynamic contrast-enhanced MRI. Eur. J. Radiol. 2016, 85, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Ramos, M.; Gupta, M.; Cheaib, J.G.; Sharma, R.; Zhang, A.; Bass, E.B.; Pierorazio, P.M. Magnetic Resonance Imaging to Differentiate the Histology of Testicular Masses: A Systematic Review of Studies With Pathologic Confirmation. Urology 2020, 135, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Sun, Z.; Chen, Y.; Zhao, F.; Yu, H.; Guo, X.; Shi, K. Testicular tumors: Discriminative value of conventional MRI and diffusion weighted imaging. Medicine 2021, 100, e27799. [Google Scholar] [CrossRef] [PubMed]
- Shilo, Y.; Zisman, A.; Lindner, A.; Raz, O.; Strauss, S.; Siegel, Y.I.; Segal, M.; Sandbank, J.; Leibovici, D. The predominance of benign histology in small testicular masses. Urol. Oncol. 2012, 30, 719–722. [Google Scholar] [CrossRef]
- Richie, J.P. Detection and treatment of testicular cancer. CA Cancer J. Clin. 1993, 43, 151–175. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Richter-Simonsen, H.; Kulejewski, M.; Ikogho, R.; Zecha, H.; Anheuser, P.; Pichlmeier, U.; Isbarn, H. Testicular Germ-Cell Tumours: A Descriptive Analysis of Clinical Characteristics at First Presentation. Urol. Int. 2018, 100, 409–419. [Google Scholar] [CrossRef]
- Abboudi, H.; Malde, S.; Mchaourab, A.; Eddy, B.; Shrotri, N. Nonpalpable Testicular Masses—Should We Be Worried? Open J. Urol. 2013, 3, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Bieniek, J.M.; Juvet, T.; Margolis, M.; Grober, E.D.; Lo, K.C.; Jarvi, K.A. Prevalence and Management of Incidental Small Testicular Masses Discovered on Ultrasonographic Evaluation of Male Infertility. J. Urol. 2018, 199, 481–486. [Google Scholar] [CrossRef]
- Yagil, Y.; Naroditsky, I.; Milhem, J.; Leiba, R.; Leiderman, M.; Badaan, S.; Gaitini, D. Role of Doppler ultrasonography in the triage of acute scrotum in the emergency department. J. Ultrasound. Med. 2010, 29, 11–21. [Google Scholar] [CrossRef]
- Shtricker, A.; Silver, D.; Sorin, E.; Schreiber, L.; Katlowitz, N.; Tsivian, A.; Katlowitz, K.; Benjamin, S.; Sidi, A.A. The value of testicular ultrasound in the prediction of the type and size of testicular tumors. Int. Braz J. Urol. 2015, 41, 655–660. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Lei, Z.; Li, A.; Jiang, Y.; Ji, J. Differentiation of testicular seminoma and nonseminomatous germ cell tumor on magnetic resonance imaging. Medicine 2019, 98, e17937. [Google Scholar] [CrossRef] [PubMed]
- Elert, A.; Olbert, P.; Hegele, A.; Barth, P.; Hofmann, R.; Heidenreich, A. Accuracy of frozen section examination of testicular tumors of uncertain origin. Eur. Urol. 2002, 41, 290–293. [Google Scholar] [CrossRef]
- Krebserkrankungen in Österreich 2020. Available online: https://www.statistik.at/fileadmin/publications/Krebserkrankungen_in_OEsterreich_2020.pdf (accessed on 7 February 2022).
| US Finding | MRI Finding | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n Benign (%) | n Malignant (%) | n Indeterminate (%) | n Benign (%) | n Malignant (%) | n Indeterminate (%) | ||||
| Histology | n benign (%) | 2 (4.1) | 12 (25.0) | 11 (22.9) | 25 (52.1) | 5 (9.1) | 14 (25.5) | 8 (14.5) | 27 (49.1) |
| n malignant (%) | 1 (2.1) | 18 (37.5) | 4 (8.3) | 23 (47.9) | 1 (1.8) | 24 (43.6) | 3 (5.5) | 28 (50.9) | |
| 3 (6.2) | 30 (62.5) | 15 (31.3) | 48 (100) | 6 (10.9) | 38 (69.1) | 11 (20.0) | 55 (100) | ||
| US | MRI | |
|---|---|---|
| Sensitivity in % (95%-CI) | 94.7 (74.0–99.9) | 85.7 (67.3–96.0) |
| Specificity in % (95%-CI) | 20.0 (9.1–35.7) | 72.8 (61.8–82.1) |
| PPV in % (95%-CI) | 36.0 (22.9–50.8) | 52.1 (37.0–67.1) |
| NPV I % (95%-CI) | 88.9 (51.8–99.7) | 93.7 (84.5–98.2) |
| Patient Nr. | LDH (135–225 U/L) | HCG (<1 U/L) | AFP (0.5–10 µg/L) | Indication for MRI |
|---|---|---|---|---|
| 1 | 150 | 18 | 2.2 | Exclusion of testicular lesion in the case of cervical lymphadenopathy (Choriocarinoma) |
| 2 | 162 | <1 | 12.9 | Unclear increase in AFP |
| 3 | 190 | <1 | 79.2 | Indeterminate testicular lesion |
| 4 | NA | 25 | 47.5 | Indeterminate testicular lesion, mental retardation |
| 5 | 178 | 4 | 14.3 | Lymphadenopathy; MRI was performed during upfront chemotherapy for metastatic tumors |
| 6 | 379 | >2.0 | 2465 | Mediastinal primum; search for the origin |
| 7 | 254 | 11.67 | 4.3 | Indeterminate testicular lesion |
| 8 | 115 | <2.3 | 10.85 | Indeterminate testicular lesion |
| Parameter | p-Value |
|---|---|
| Age | 0.207 |
| History of smoking | 0.161 |
| History of testicular tumors | 0.252 |
| History of undescended testis | 0.661 |
| Presence of symptoms | 0.128 |
| Median volume of the affected testicle in MRI or ultrasound | 0.676 |
| Tumor size for US | 0.001 ** |
| Tumor size in histopathology | 0.001 ** |
| Positive tumor markers (AFP, βHCG, HPLAP, LDH) | 0.014 * |
| Presence of testosterone deficiency | 0.541 |
| Tumor size in MRI | 0.004 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deininger, S.; Lusuardi, L.; Pallauf, M.; Hecht, S.; Forstner, R.; Meissnitzer, M.; Distler, F.A.; Erne, E.; Graf, S.; Lenart, S.; et al. The Diagnostic Value of the Added MR Imaging of the Scrotum in the Preoperative Workup of Sonographically Indeterminate Testicular Lesions—A Retrospective Multicenter Analysis. Cancers 2022, 14, 3594. https://doi.org/10.3390/cancers14153594
Deininger S, Lusuardi L, Pallauf M, Hecht S, Forstner R, Meissnitzer M, Distler FA, Erne E, Graf S, Lenart S, et al. The Diagnostic Value of the Added MR Imaging of the Scrotum in the Preoperative Workup of Sonographically Indeterminate Testicular Lesions—A Retrospective Multicenter Analysis. Cancers. 2022; 14(15):3594. https://doi.org/10.3390/cancers14153594
Chicago/Turabian StyleDeininger, Susanne, Lukas Lusuardi, Maximilian Pallauf, Stefan Hecht, Rosemarie Forstner, Matthias Meissnitzer, Florian A. Distler, Eva Erne, Sebastian Graf, Sebastian Lenart, and et al. 2022. "The Diagnostic Value of the Added MR Imaging of the Scrotum in the Preoperative Workup of Sonographically Indeterminate Testicular Lesions—A Retrospective Multicenter Analysis" Cancers 14, no. 15: 3594. https://doi.org/10.3390/cancers14153594
APA StyleDeininger, S., Lusuardi, L., Pallauf, M., Hecht, S., Forstner, R., Meissnitzer, M., Distler, F. A., Erne, E., Graf, S., Lenart, S., Putz, J., Deininger, C., & Törzsök, P. (2022). The Diagnostic Value of the Added MR Imaging of the Scrotum in the Preoperative Workup of Sonographically Indeterminate Testicular Lesions—A Retrospective Multicenter Analysis. Cancers, 14(15), 3594. https://doi.org/10.3390/cancers14153594







