Chlorophyll Fluorescence and Fruit Quality Response of Blueberry to Different Mulches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Characteristics of the Study
2.2. Characterization of the Edaphoclimatic Conditions of the Treatments
2.3. Chlorophyll Fluorescence and Stomatal Conductance
2.4. Yield, Fruit Quality and Foliar Indices
2.5. Statistic Analysis
3. Results and Discussion
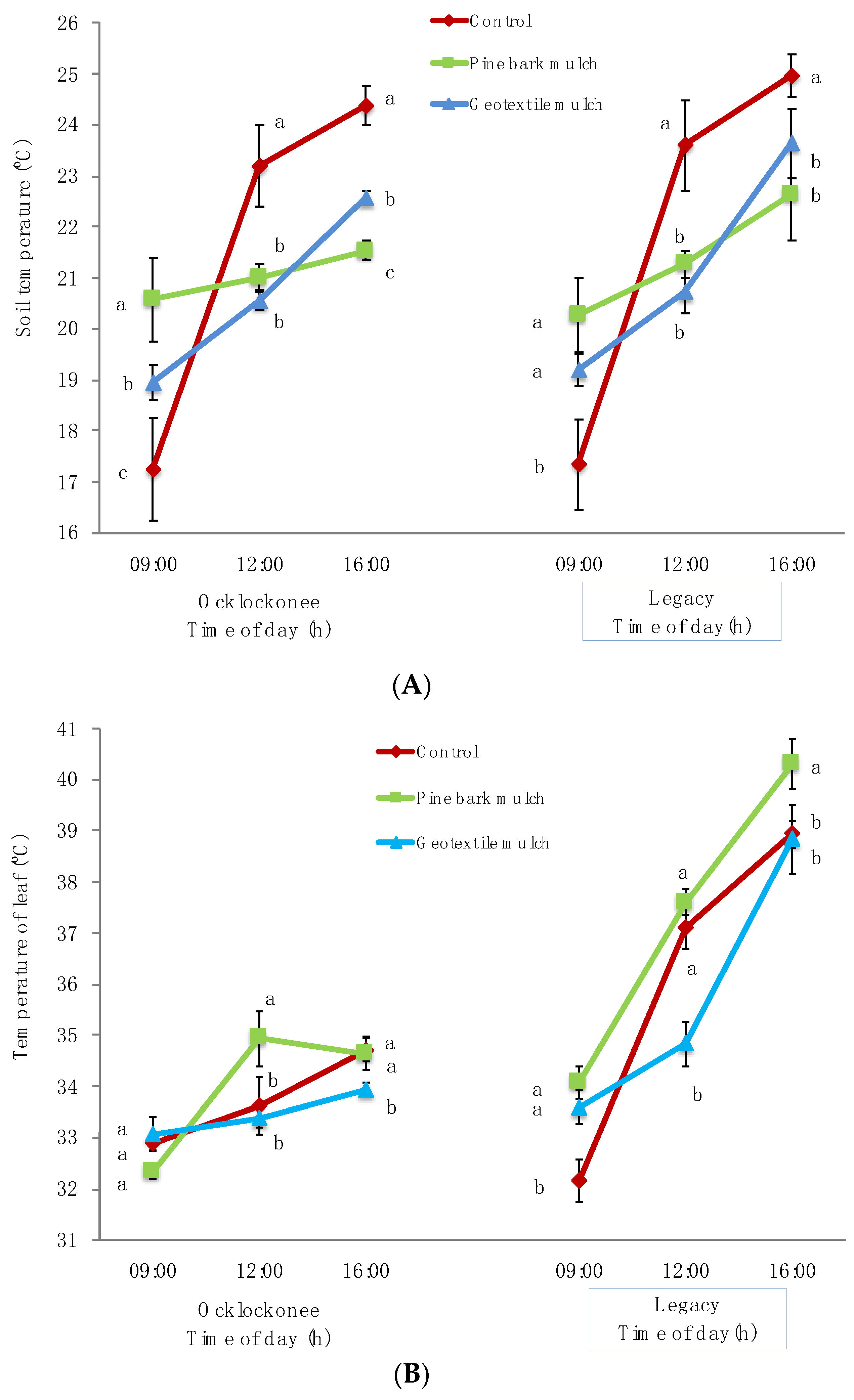
3.1. Edaphoclimatic Variations of the Different Treatments
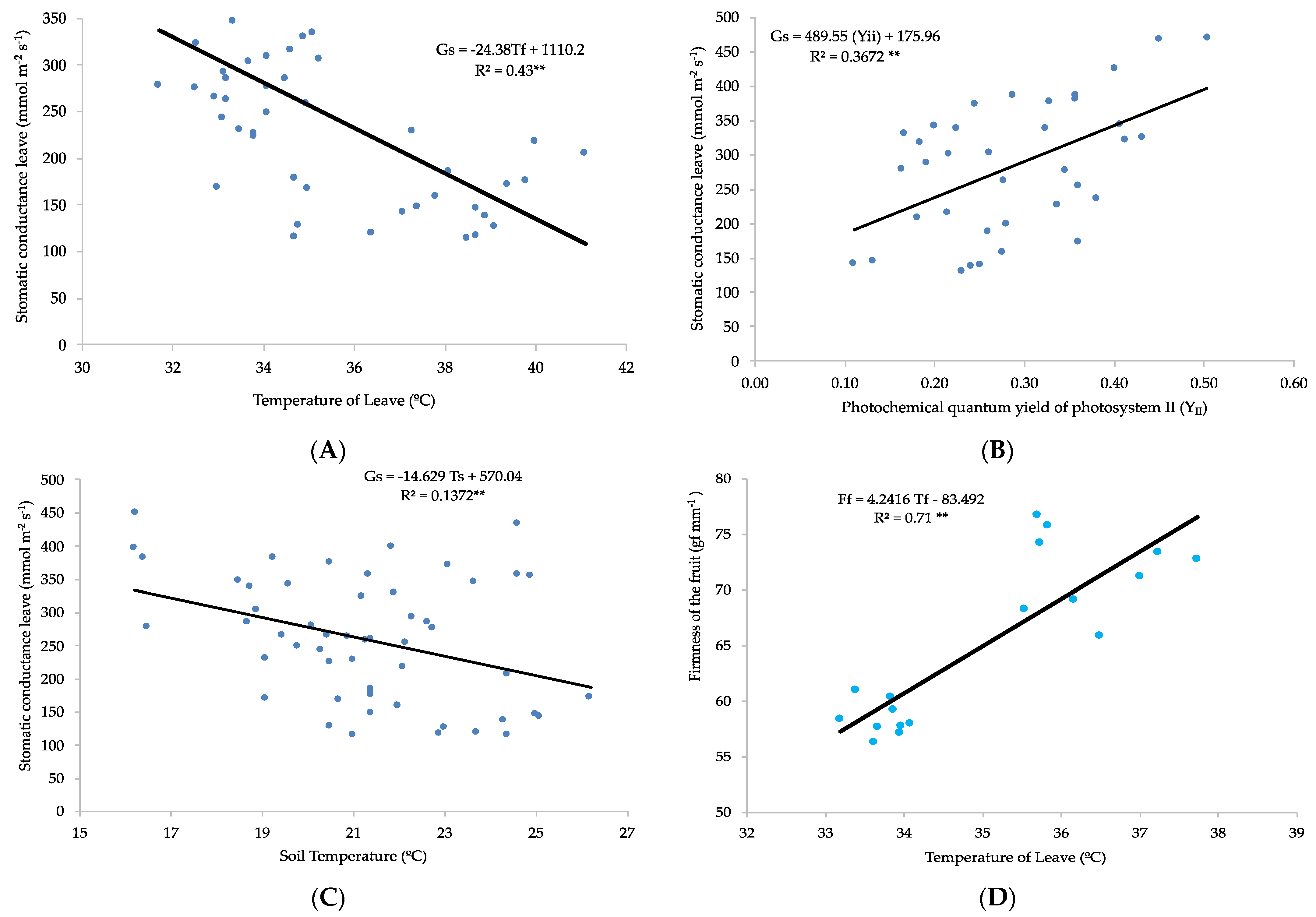
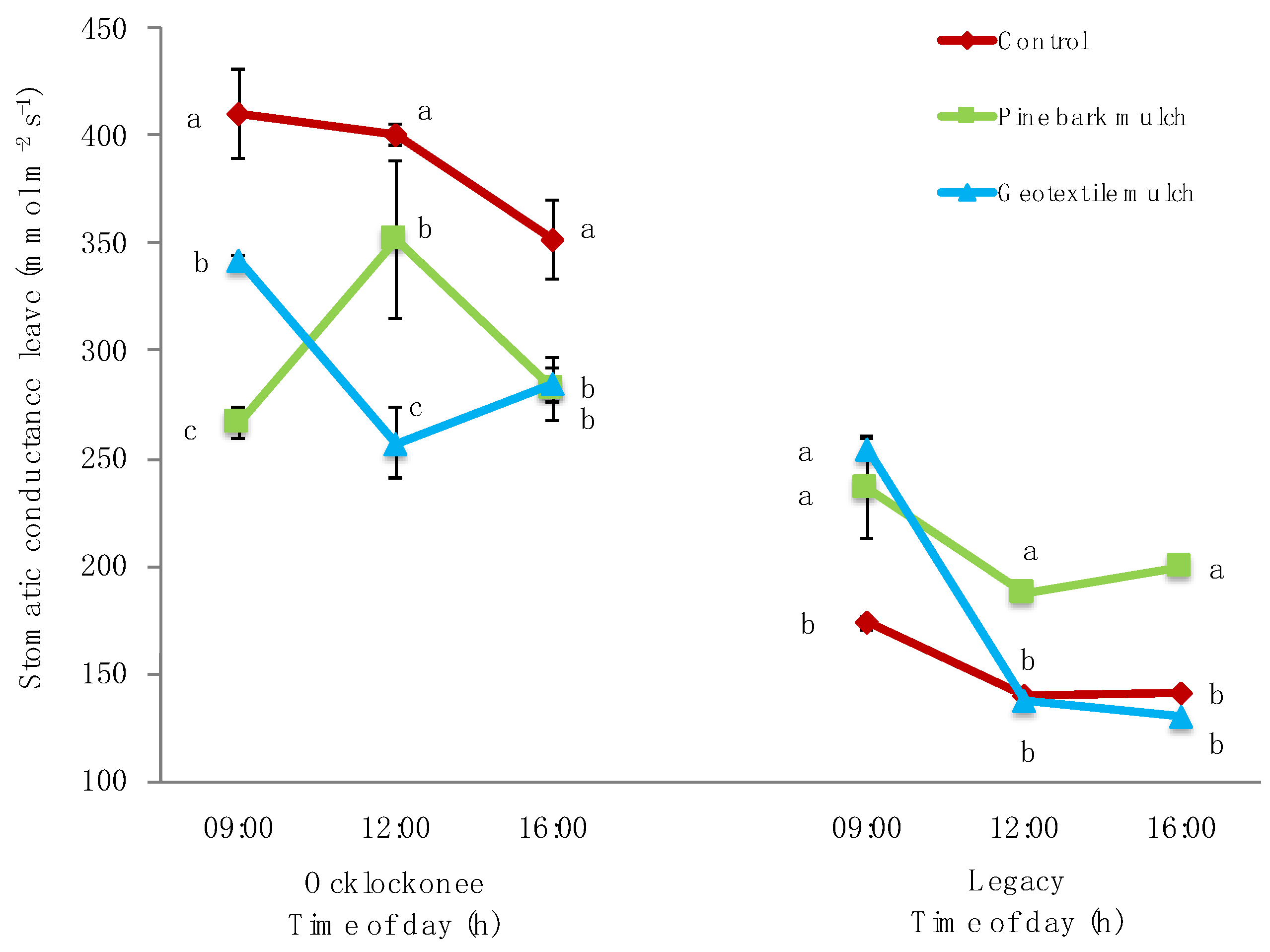
3.2. PSII Chlorophyll Fluorescence Parameters and Stomatal Conductance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strik, B.C.; Vance, A.; Bryla, D.R.; Sullivan, D.M. Organic Production Systems in Northern Highbush Blueberry: I. Impact of Planting Method, Cultivar, Fertilizer, and Mulch on Yield and Fruit Quality from Planting through Maturity. HortScience 2017, 52, 1201–1213. [Google Scholar] [CrossRef] [Green Version]
- Bryla, D.R.; Valenzuela-Estrada, L.R.; Vargas, O.L. Root Production, Distribution, and Turnover in Conventional and Organic Northern Highbush Blueberry Systems. Acta Hortic. 2017, 169–176. [Google Scholar] [CrossRef]
- Clark, J.R.; Moore, J.N. Southern Highbush Blueberry Response to Mulch. HortTechnology 1991, 1, 52–54. [Google Scholar] [CrossRef] [Green Version]
- Abo-Ogiala, A.M.M.E.-N.; Khalafallah, N.E. Effect of Rice Straw Mulching on Water Use Efficiency, Growth, Yield and Quality of King Ruby Grape under Surface Irrigation. Egypt J. Hortic. 2019, 46, 29–39. [Google Scholar] [CrossRef]
- Fan, W.; Wu, J.; Ahmed, S.; Hu, J.; Chen, X.; Li, X.; Zhu, W.; Opoku-Kwanowaa, Y. Short-Term Effects of Different Straw Returning Methods on the Soil Physicochemical Properties and Quality Index in Dryland Farming in NE China. Sustainability 2020, 12, 2631. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Harayama, H.; Uemura, A.; Ito, E.; Utsugi, H.; Kitao, M.; Maruyama, Y. High Biomass Productivity of Short-Rotation Willow Plantation in Boreal Hokkaido Achieved by Mulching and Cutback. Forests 2020, 11, 505. [Google Scholar] [CrossRef]
- Velandia, M.; Galinato, S.; Wszelaki, A. Economic Evaluation of Biodegradable Plastic Films in Tennessee Pumpkin Production. Agronomy 2020, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Mirás-Avalos, J.M.; Ramírez-Cuesta, J.M.; Fandiño, M.; Cancela, J.J.; Intrigliolo, D.S. Agronomic Practices for Reducing Soil Erosion in Hillside Vineyards under Atlantic Climatic Conditions (Galicia, Spain). Soil Syst. 2020, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Saha, R.R.; Mian, M.A.K.; Kundu, S.; Sarker, K.K. Performance of Garlic (Allium sativum) Varieties under Zero Tillage Mulch Condition in Southern Coastal Region of Bangladesh. Proceedings 2020, 36, 159. [Google Scholar] [CrossRef] [Green Version]
- Sałata, A.; Pandino, G.; Buczkowska, H.; Lombardo, S. Influence of Catch Crops on Yield and Chemical Composition of Winter Garlic Grown for Bunch Harvesting. Agriculture 2020, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Pinder, R.; Rana, R.; Maan, D.; Kumar, K. Impact of Different Mulching Materials on the Growth and Yield of Tomato (Solanum Lycopersicum) in Dehradun Region of Uttarakhand. Int. J. Environ. Agric. Biotechnol. 2016, 1, 631–636. [Google Scholar] [CrossRef]
- Cozzolino, E.; Giordano, M.; Fiorentino, N.; El-Nakhel, C.; Pannico, A.; Di Mola, I.; Mori, M.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Appraisal of Biodegradable Mulching Films and Vegetal-Derived Biostimulant Application as Eco-Sustainable Practices for Enhancing Lettuce Crop Performance and Nutritive Value. Agronomy 2020, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Sarker, K.K.; Kamar, S.S.A.; Hossain, M.A.; Mainuddin, M.; Bell, R.W.; Barrett-Lennard, E.G.; Gaydon, D.; Glover, M.; Saha, R.R.; Rashid, M.H.; et al. Effect of Straw Mulch and Irrigation on Sunflower and Maize Cultivation in No Tillage Systems of Coastal Heavy Soils. Proceedings 2020, 36, 145. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Niu, W.; Li, Y. Effects of Drip Irrigation with Plastic on Photosynthetic Characteristics and Biomass Distribution of Muskmelon. Agriculture 2020, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, K.; Eldoma, I.M.; Fang, Y.; Zhang, F. Plastic Film Mulching Sustains High Maize (Zea mays L.) Grain Yield and Maintains Soil Water Balance in Semiarid Environment. Agronomy 2020, 10, 600. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Xia, Z.; Fu, Y.; Wang, Q.; Xue, J.; Chu, J. Response of Soil Temperature, Moisture, and Spring Maize (Zea mays L.) Root/Shoot Growth to Different Mulching Materials in Semi-Arid Areas of Northwest China. Agronomy 2020, 10, 453. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Zhu, X.; Sun, R.; Niu, Y.; Yu, Q.; Shen, Y.; Li, S. Effects of Different Mulching and Fertilization on Phosphorus Transformation in Upland Farmland. J. Environ. Manag. 2020, 253, 109717. [Google Scholar] [CrossRef]
- Nwosisi, S.; Nandwani, D.; Hui, D. Mulch Treatment Effect on Weed Biomass and Yields of Organic Sweetpotato Cultivars. Agronomy 2019, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Sarangi, S.K.; Maji, B.; Sharma, P.C.; Digar, S.; Mahanta, K.K.; Burman, D.; Mandal, U.K.; Mandal, S.; Mainuddin, M. Zero Tilled-Paddy Straw Mulched Potato (Solanum tuberosum) Cultivation in the Coastal Saline Soils Reduce Soil Salinity, Increase Yield and Profitability. Proceedings 2020, 36, 147. [Google Scholar] [CrossRef] [Green Version]
- Larco, H.; Strik, B.C.; Bryla, D.R.; Sullivan, D.M. Mulch and Fertilizer Management Practices for Organic Production of Highbush Blueberry. II. Impact on Plant and Soil Nutrients during Establishment. HortScience 2013, 48, 1484–1495. [Google Scholar] [CrossRef] [Green Version]
- Strik, B.C.; Vance, A.; Bryla, D.R.; Sullivan, D.M. Organic Production Systems in Northern Highbush blueberry: II. Impact of Planting Method, Cultivar, Fertilizer, and Mulch on Leaf and Soil Nutrient Concentrations and Relationships with Yield from Planting through Maturity. HortScience 2019, 54, 1777–1794. [Google Scholar] [CrossRef] [Green Version]
- Mgolozeli, S.; Nciizah, A.D.; Wakindiki, I.I.C.; Mudau, F.N. Innovative Pro-Smallholder Farmers’ Permanent Mulch for Better Soil Quality and Food Security Under Conservation Agriculture. Agronomy 2020, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Spiers, J.M. Substrate Temperatures Influence Root and Shoot Growth of Southern Highbush and Rabbiteye blueberries. HortScience 1995, 30, 1029–1030. [Google Scholar] [CrossRef] [Green Version]
- Velandia, M.; Rejesus, R.; Clark, C.; DeLong, K.L.; Wszelaki, A.; Schexnayder, S.; Jensen, K. Evaluating the Relationship between Fruit and Vegetable Growers Use of Plastic Biodegradable Mulches, and Environmental Stewardship and Labor Savings: The Case of Tennessee Fruit and Vegetable Farmers. Sustainability 2020, 12, 2075. [Google Scholar] [CrossRef] [Green Version]
- Cowan, J.S.; Miles, C.A.; Andrews, P.K.; Inglis, D.A. Biodegradable Mulch Performed Comparably to Polyethylene in High Tunnel Tomato (Solanum lycopersicum L.) Production. J. Sci. Food Agric. 2014, 94, 1854–1864. [Google Scholar] [CrossRef]
- Williamson, J.G.; Mejia, L.; Ferguson, B.; Miller, P.; Haman, D.Z. Seasonal Water Use of Southern Highbush Blueberry Plants in a Subtropical Climate. HortTechnology 2015, 25, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Neilsen, G.H.; Hogue, E.J.; Forge, T.; Neilsen, D. Mulches and Biosolids Affect Vigor, Yield and Leaf Nutrition of Fertigated High Density Apple. HortScience 2003, 38, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Forge, T.; Temple, W.; Bomke, A. Using Compost as Mulch for Highbush Blueberry. Acta Hortic. 2013, 1001, 369–374. [Google Scholar] [CrossRef]
- Burkhard, N.; Lynch, D.; Percival, D.; Sharifi, M. Organic Mulch Impact on Vegetation Dynamics and Productivity of Highbush Blueberry Under Organic Production. HortScience 2009, 44, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Rodríguez, G. Efecto de La Cobertura Del Suelo Con Cascarilla de Arroz En El Crecimiento y Rendimiento Del Tomate de Ramillete. Cienc. E Investig. Agrar. 2007, 34, 225–230. [Google Scholar] [CrossRef]
- Stagnari, F.; Galieni, A.; Speca, S.; Cafiero, G.; Pisante, M. Effects of Straw Mulch on Growth and Yield of Durum Wheat during Transition to Conservation Agriculture in Mediterranean Environment. Field Crops Res. 2014, 167, 51–63. [Google Scholar] [CrossRef]
- Zribi, W.; Faci, J.M.; Aragüés, R. Mulching effects on moisture, temperature, structure and salinity of agricultural soils. Inf. Técnica Económica Agrar. 2011, 107, 148–162. [Google Scholar]
- Stolpe, N. Descripción de Los Principales Suelos de la Octava Región de Chile; Universidad de Concepcion: Chillan, Chile, 2006. [Google Scholar]
- INIA Red Agrometeorológica de INIA. Available online: http://agromet.inia.cl (accessed on 27 April 2020).
- Juan, H.C. (Ed.) Diagnóstico Nutricional y Principios de Fertilización en Frutales y Vides. Segunda Edición Aumentada y Corregida, 2nd ed.; No 33; Instituto de Investigaciones Agropecuarias: Chillan, Chile, 2014. [Google Scholar]
- Al-Helal, I.M.; Abdel-Ghany, A.M. Responses of Plastic Shading Nets to Global and Diffuse PAR Transfer: Optical Properties and Evaluation. NJAS-Wagening. J. Life Sci. 2010, 57, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Cordon, G.; Lagorio, M.G.; Paruelo, J.M. Chlorophyll Fluorescence, Photochemical Reflective Index and Normalized Difference Vegetative Index during Plant Senescence. J. Plant Physiol. 2016, 199, 100–110. [Google Scholar] [CrossRef] [Green Version]
- van Kooten, O.; Snel, J.H. The Use of Chlorophyll Fluorescence Nomenclature in Plant Stress Physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Reyes-Diaz, M.; Alberdi, M.; de la Luz Mora, M. Short-Term Aluminum Stress Differentially Affects the Photochemical Efficiency of Photosystem II in Highbush Blueberry Genotypes. J. Am. Soc. Hortic. Sci. 2009, 134, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Retamal-Salgado, J.; Bastías, R.M.; Wilckens, R.; Paulino, L. Influence of Microclimatic Conditions under High Tunnels on the Physiological and Productive Responses in Blueberry Cv. O´Neal. Chil. J. Agric. Res. 2015, 75, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Vásquez, R.; Fischer, S.; Hirzel, J.; Zapata, N. Decrease in Artificial Radiation with Netting Reduces Stress and Improves Rabbit-Eye Blueberry (Vaccinium virgatum Aiton) Cv. Ochlockonee Productivity. Chil. J. Agric. Res. 2017, 77, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, A.R.; Katz, L.; Sousa, A.d.P.; Martinez Uribe, R.A. Indice SPAD En El Crecimiento y Desarrollo de Plantas de Lisianthus En Función de Diferentes Dosis de Nitrógeno En Ambiente Protegido. Idesia (Arica) 2015, 33, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Sonnentag, O.; Talbot, J.; Chen, J.M.; Roulet, N.T. Using Direct and Indirect Measurements of Leaf Area Index to Characterize the Shrub Canopy in an Ombrotrophic Peatland. Agric. For. Meteorol. 2007, 144, 200–212. [Google Scholar] [CrossRef]
- Callejas Rodríguez, R.; Brayovic Piñones, M.; Peppi Aronowsky, C.; Kania Kuhl, E. Categorías de Firmeza de Bayas En Diferentes Variedades de Uva de Mesa (Vitis vinifera L.). Rev. Fac. Cienc. Agrar. 2011, 43, 127–141. [Google Scholar]
- SAS Institute. Usage and Reference; SAS Institute Inc.: Cary, CA, USA, 1989. [Google Scholar]
- Retamales, J.B.; Hancock, J.F. Blueberries. Crop Production Science in Horticulture Series, 2nd ed.; CABI: Boston, MA, USA, 2018; ISBN 978-1-78064-726-5. [Google Scholar]
- Cox, J. Comparison of Plastic Weedmat and Woodchip Mulch on Low Chill Blueberry Soil in New South Wales, Australia. Acta Hortic. 2009, 810, 475–482. [Google Scholar] [CrossRef]
- Namaghi, M.N.; Davarynejad, G.H.; Ansary, H.; Nemati, H.; Feyzabady, A.Z. Effects of Mulching on Soil Temperature and Moisture Variations, Leaf Nutrient Status, Growth and Yield of Pistachio Trees (Pistacia vera. L.). Scientia Horticult. 2018, 241, 115–123. [Google Scholar] [CrossRef]
- Losciale, P.; Hendrickson, L.; Grappadelli, L.C.; Chow, W.S. Quenching Partitioning through Light-Modulated Chlorophyll Fluorescence: A Quantitative Analysis to Assess the Fate of the Absorbed Light in the Field. Environ. Exp. Bot. 2011, 73, 73–79. [Google Scholar] [CrossRef]
- Li, T.; Heuvelink, E.; Dueck, T.A.; Janse, J.; Gort, G.; Marcelis, L.F.M. Enhancement of Crop Photosynthesis by Diffuse Light: Quantifying the Contributing Factors. Ann. Bot. 2014, 114, 145–156. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, K.E. Pollination Requirements of Three Highbush Blueberry (Vaccinium corymbosum L.) Cultivars. J. Am. Soc. Hortic. Sci. 1997, 122, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Brevis, P.A.; NeSmith, D.S.; Wetzstein, H.Y. Flower Age Affects Fruit Set and Stigmatic Receptivity in Rabbiteye blueberry. HortScience 2006, 41, 1537–1540. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, R.; Sun, Y.; Xu, M.; Zhang, H.; Huang, L.; Zhu, Y.; Wang, H.; Li, G.; Liu, L.; et al. The Optimal Temperature for the Growth of Blueberry (Vaccinium corymbosum L.). Pak. J. Bot. 2017, 49, 965–979. [Google Scholar]
- Yang, F.-H.; Bryla, D.R.; Strik, B.C. Critical Temperatures and Heating Times for Fruit Damage in Northern Highbush Blueberry. HortScience 2019, 54, 2231–2239. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Díaz, M.; Meriño-Gergichevich, C.; Alarcón, E.; Alberdi, M.; Horst, W.J. Calcium Sulfate Ameliorates the Effect of Aluminum Toxicity Differentially in Genotypes of Highbush Blueberry (Vaccinium corymbosum L.). J. Soil Sci. Plant Nutr. 2011, 11, 59–78. [Google Scholar] [CrossRef] [Green Version]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Cobo, N.; del Pozo, A. Spectral Irradiance, Gas Exchange Characteristics and Leaf Traits of Vaccinium Corymbosum L. ‘Elliott’ Grown under Photo-Selective Nets. Environ. Exp. Bot. 2012, 75, 142–149. [Google Scholar] [CrossRef]
- Rho, H.; Yu, D.J.; Kim, S.J.; Lee, H.J. Limitation Factors for Photosynthesis in ‘Bluecrop’ Highbush Blueberry (Vaccinium corymbosum) Leaves in Response to Moderate Water Stress. J. Plant Biol. 2012, 55, 450–457. [Google Scholar] [CrossRef]
- Kim, S.J.; Yu, D.J.; Kim, T.-C.; Lee, H.J. Growth and Photosynthetic Characteristics of Blueberry (Vaccinium corymbosum cv. Bluecrop) under Various Shade Levels. Sci. Hortic. 2011, 129, 486–492. [Google Scholar] [CrossRef]
- Da Cunha-Chiamolera, T.P.L.; Filho, A.B.C.; dos Santos, D.M.M.; Cruz, F.J.R. Gas Exchange, Photosynthetic Pigments, and Growth in Tomato: Lettuce Intercropping. Chil. J. Agric. Res. 2017, 77, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.-H.; DeVetter, L.W.; Strik, B.C.; Bryla, D.R. Stomatal Functioning and Its Influence on Fruit Calcium Accumulation in Northern Highbush Blueberry. HortScience 2020, 55, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Vega, P.; Paillán, H.; Serri, H.; Donnay, D.; Sanhueza, C.; Merino, E.; Hirzel, J. Effects of organic fertilizers on the vegetative, nutritional, and productive parameters of blueberries ‘Corona’, ‘Legacy’, and ‘Liberty’. Chil. J. Agric. Res. 2016, 76, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Bakesh, P.; Kumar, R.; Wali, V.K.; Bhushan, B. Influence of Mulching on Fruit Quality of Aonla (Emblica officinalis Gaertn.) Cv. Na-7. Ecol. Environ. Conserv. 2015, 21, 263–268. [Google Scholar]
- Sams, C.E. Preharvest Factors Affecting Postharvest Texture. Postharvest Biol. Technol. 1999, 15, 249–254. [Google Scholar] [CrossRef]
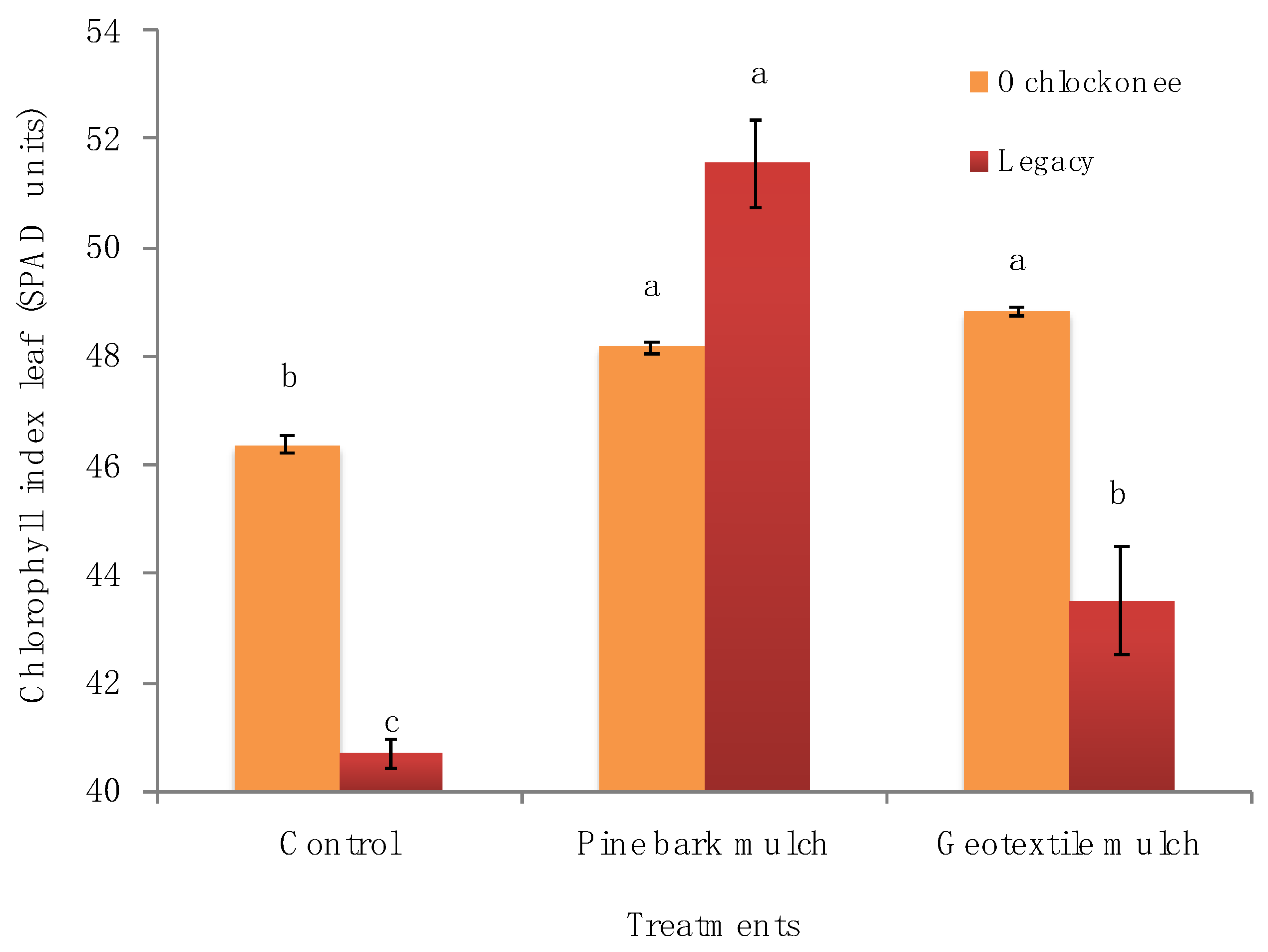
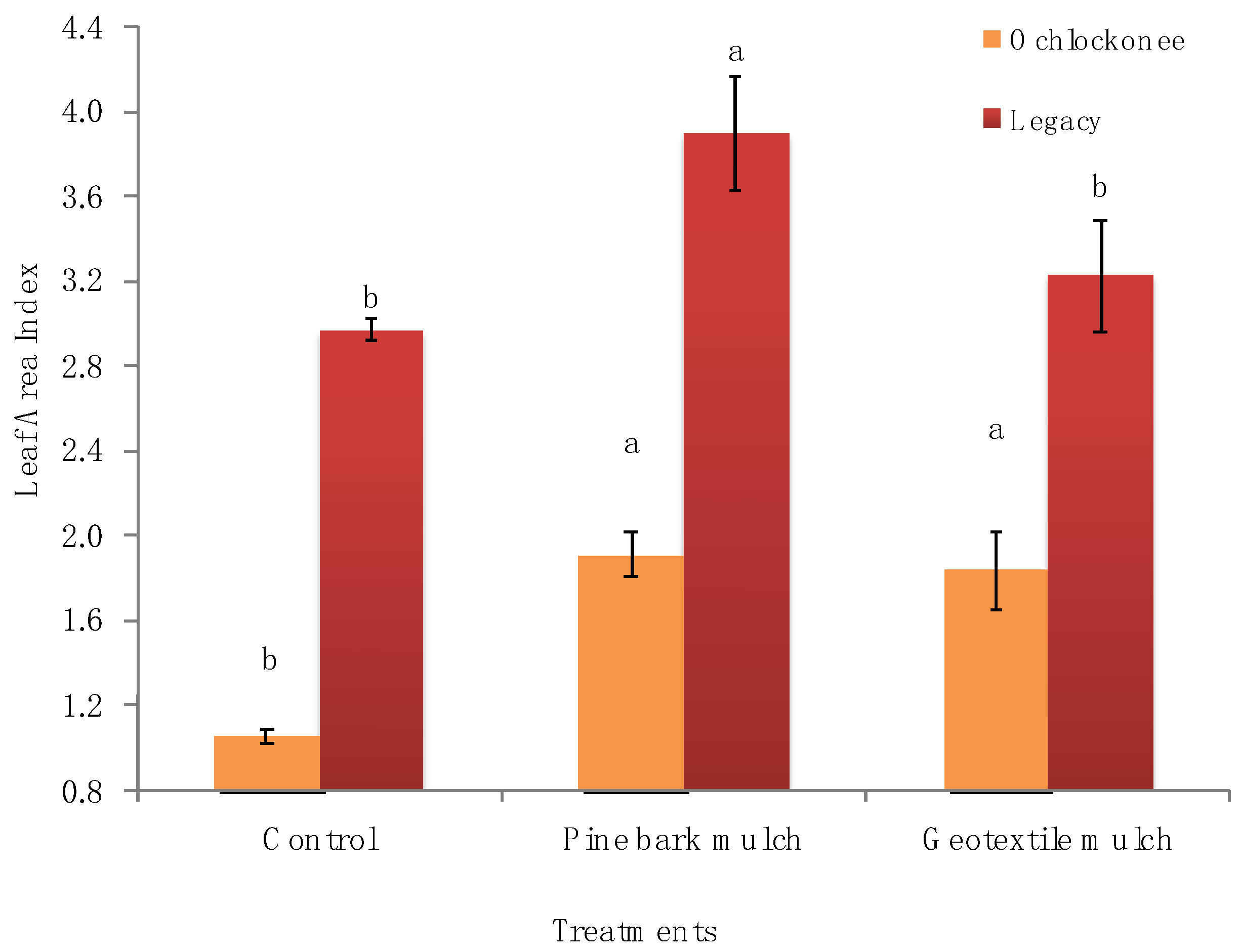
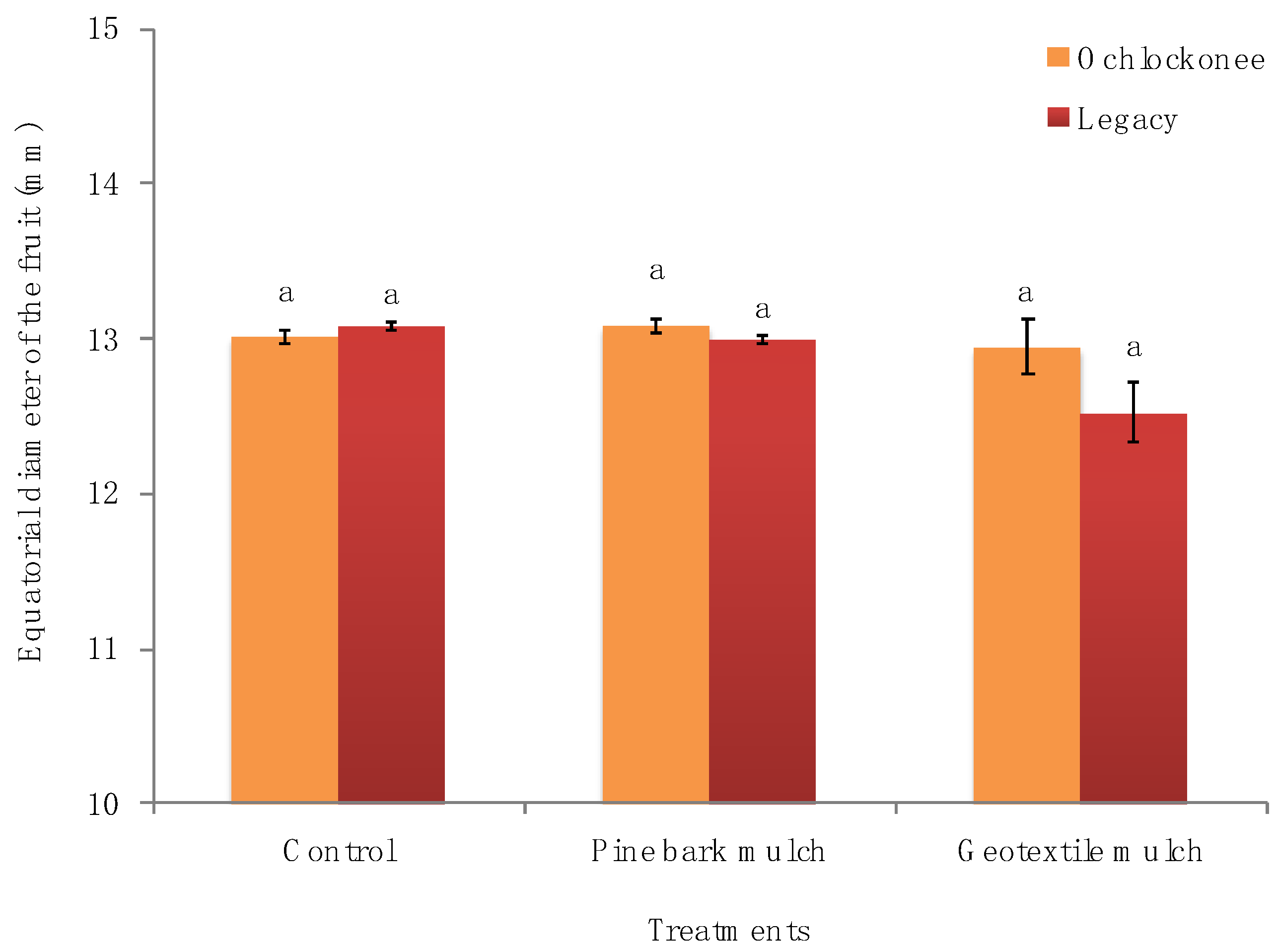
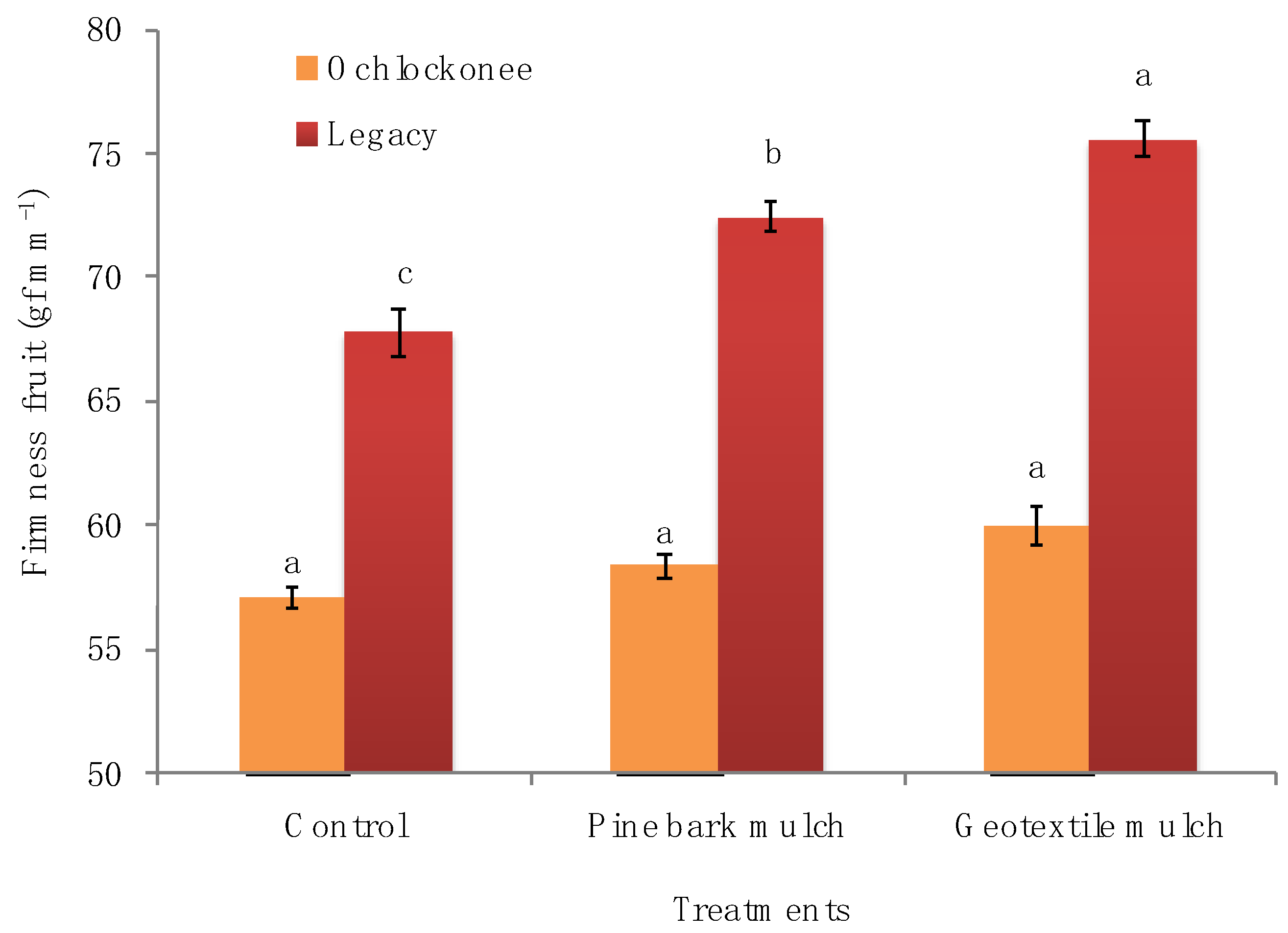

| Parameter | Treatments | Ochlockonee Time of Day (h) | Legacy Time of Day (h) | |||||
|---|---|---|---|---|---|---|---|---|
| 9:00 | 12:00 | 16:00 | 9:00 | 12:00 | 16:00 | |||
| Control | 0.35 a | 0.22 a | 0.18 c | 0.36 a | 0.25 a | 0.11 c | ||
| (A) | YII | Pine bark mulch | 0.39 a | 0.21 a | 0.33 a | 0.38 a | 0.26 a | 0.28 a |
| Geotextile mulch | 0.39 a | 0.20 a | 0.26 b | 0.36 a | 0.24 a | 0.23 b | ||
| Control | 0.79 a | 0.77 a | 0.74 b | 0.79 a | 0.75 a | 0.73 c | ||
| (B) | Fv/Fm | Pine bark mulch | 0.79 a | 0.77 a | 0.78 a | 0.80 a | 0.76 a | 0.77 a |
| Geotextile mulch | 0.78 a | 0.76 a | 0.75 b | 0.80 a | 0.76 a | 0.76 a | ||
| Control | 98.82 b | 91.30 a | 8.87 b | 89.42 b | 78.65 a | 6.47 b | ||
| (C) | ETR | Pine bark mulch | 82.20 b | 84.62 a | 53.17 a | 83.32 b | 88.23 a | 43.74 a |
| Geotextile mulch | 127.27 a | 87.77 a | 12.82 b | 110.46 a | 85.12 a | 11.27 b | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retamal-Salgado, J.; Loor, B.; Hirzel, J.; López, M.D.; Undurraga, P.; Zapata, N.; Vergara-Retamales, R.; Olivares-Soto, H. Chlorophyll Fluorescence and Fruit Quality Response of Blueberry to Different Mulches. Agronomy 2022, 12, 1702. https://doi.org/10.3390/agronomy12071702
Retamal-Salgado J, Loor B, Hirzel J, López MD, Undurraga P, Zapata N, Vergara-Retamales R, Olivares-Soto H. Chlorophyll Fluorescence and Fruit Quality Response of Blueberry to Different Mulches. Agronomy. 2022; 12(7):1702. https://doi.org/10.3390/agronomy12071702
Chicago/Turabian StyleRetamal-Salgado, Jorge, Beder Loor, Juan Hirzel, María Dolores López, Pablo Undurraga, Nelson Zapata, Rosa Vergara-Retamales, and Héctor Olivares-Soto. 2022. "Chlorophyll Fluorescence and Fruit Quality Response of Blueberry to Different Mulches" Agronomy 12, no. 7: 1702. https://doi.org/10.3390/agronomy12071702








