DUSP1: Triple-Negative Breast Cancer and Therapeutic Potential
Simple Summary
Abstract
1. Introduction
2. Triple-Negative Breast Cancer (TNBC)
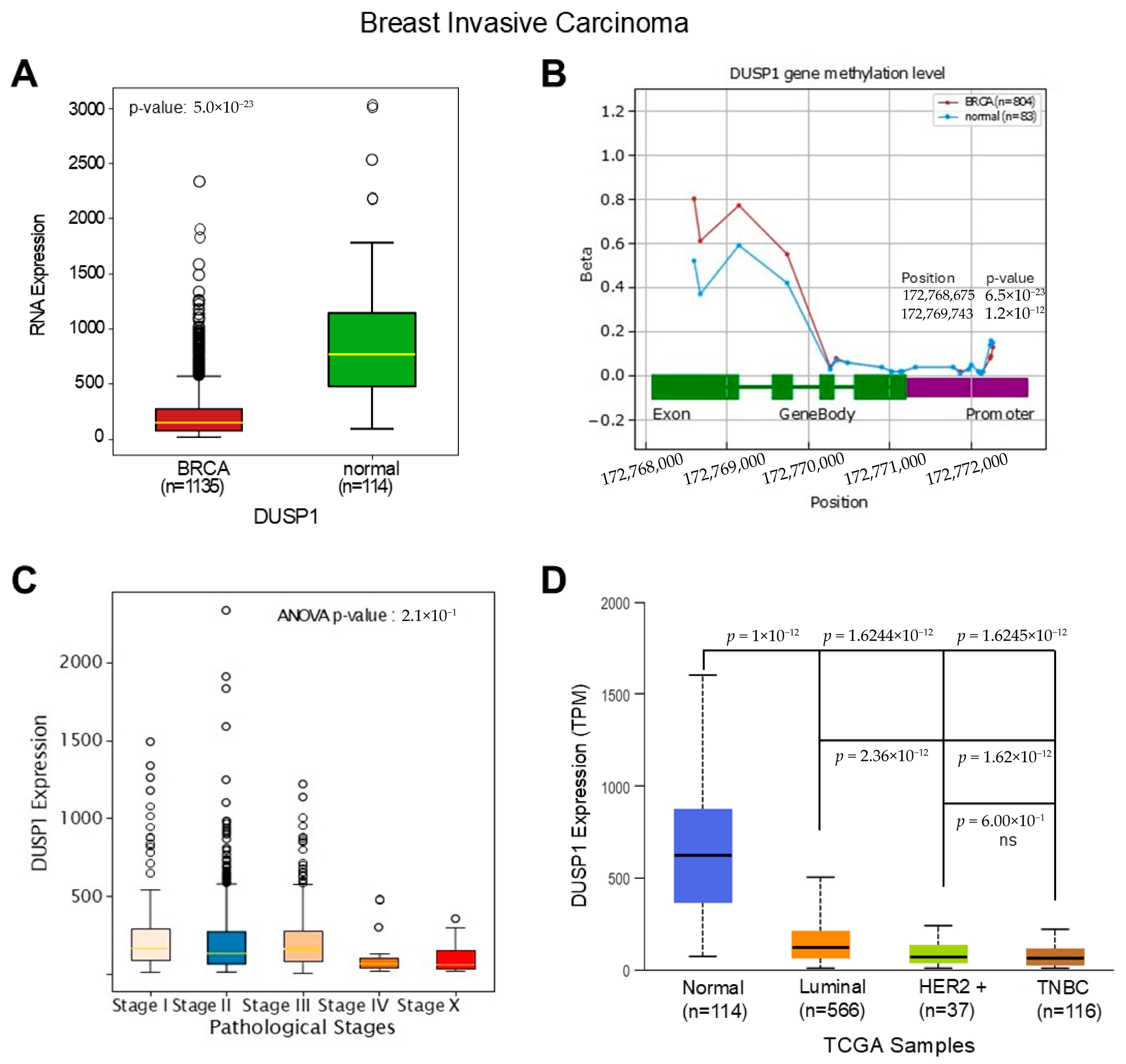
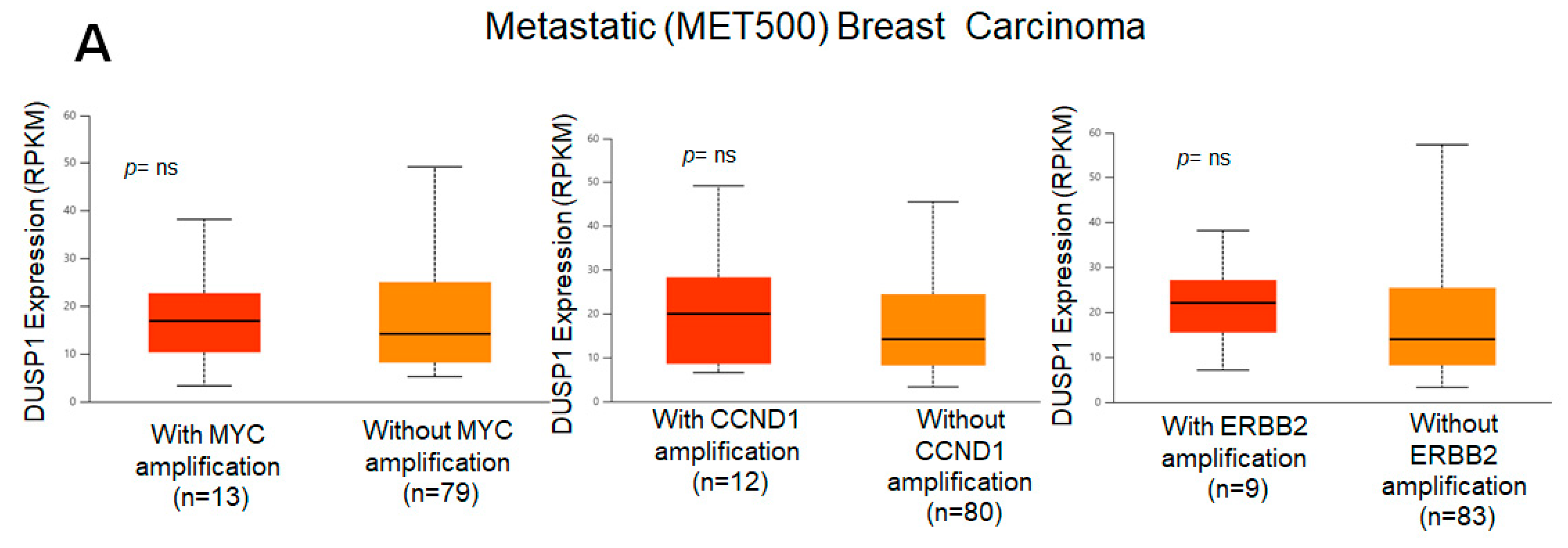
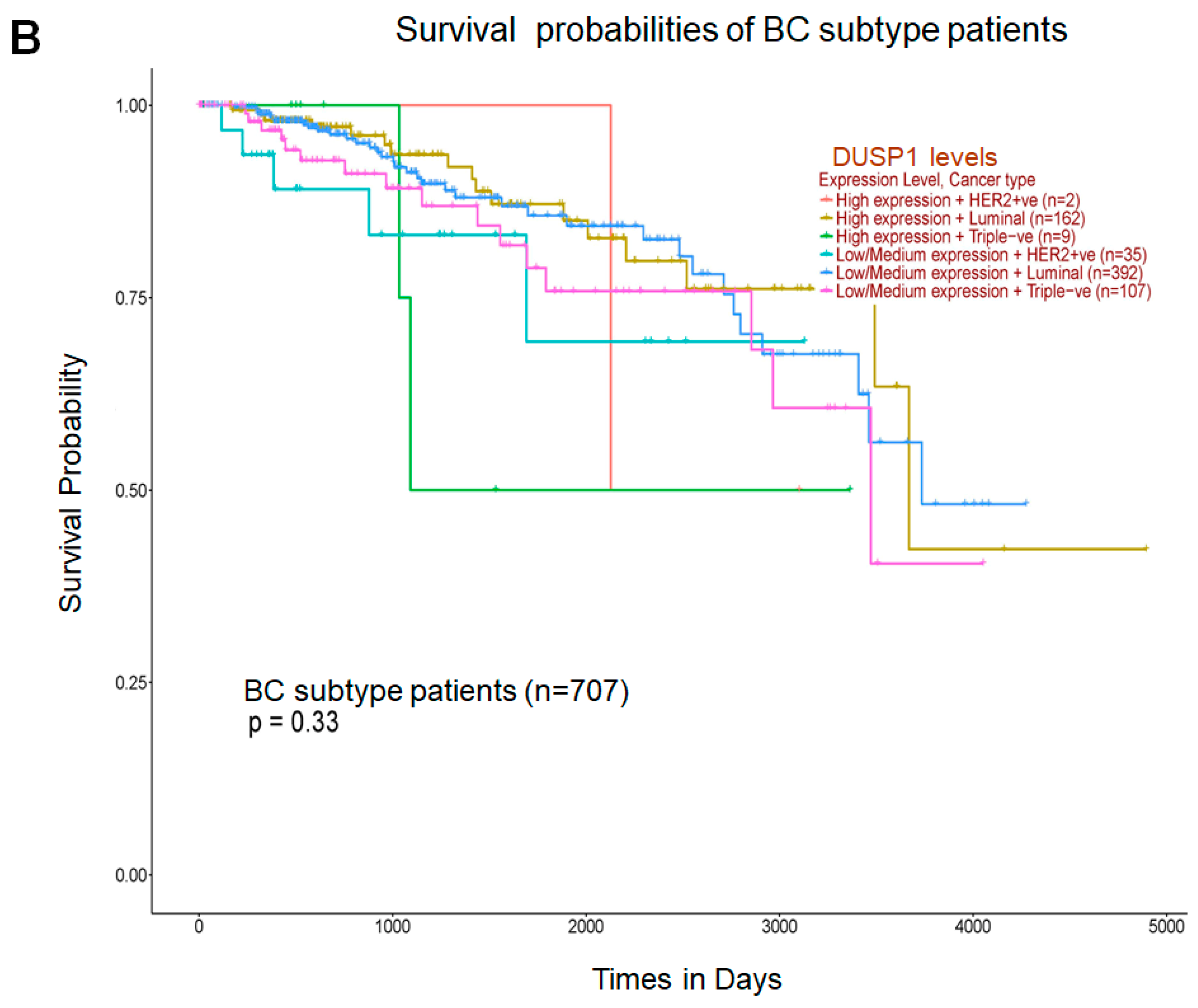
3. DUSP1 Regulation in Breast Cancer (BC) Subtypes
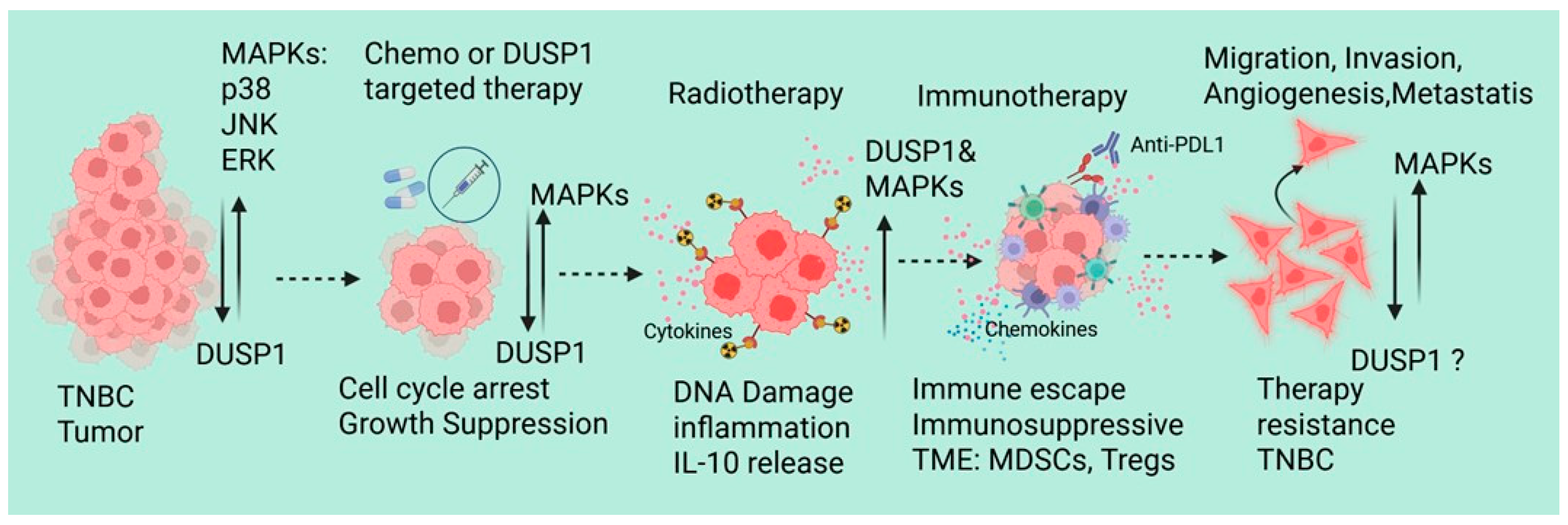
4. DUSP1 Regulation and Its Role in TNBC Chemotherapy
5. DUSP1’s Role in TNBC Radiotherapy
6. DUSP1’s Role in Immunotherapy
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DUSP1 | Dual-specificity protein phosphatase 1 |
| MAPKs | Mitogen-activated protein kinases |
| EMT | Epithelial–mesenchymal transition |
| TNBC | Triple-negative breast cancer |
| HR+ | Hormone receptor positive |
| BRCA1/2 | Breast Cancer gene ½ |
| HER2 | Human epidermal growth factor receptor 2 |
| TP53 | Tumor protein 53 |
| RT | Radiation therapy |
| BC subtypes | Brest cancer subtypes |
| TME | Tumor microenvironment |
| CCND1 | Cyclin D1 gene |
| circRNAs | Circular RNAs |
| BCI | (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one |
| BLBC | Basal-like breast cancer |
| PTX | Paclitaxel |
| CSCs | Cancer stem cells |
References
- Cheang, M.C.; Martin, M.; Nielsen, T.O.; Prat, A.; Voduc, D.; Rodriguez-Lescure, A.; Ruiz, A.; Chia, S.; Shepherd, L.; Ruiz-Borrego, M.; et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncol. 2015, 20, 474–482. [Google Scholar] [CrossRef]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef] [PubMed]
- Aysola, K.; Desai, A.; Welch, C.; Xu, J.; Qin, Y.; Reddy, V.; Matthews, R.; Owens, C.; Okoli, J.; Beech, D.J.; et al. Triple Negative Breast Cancer—An Overview. Hered. Genet. 2013, 2013, 1. [Google Scholar]
- Bai, J.; Gao, Y.; Zhang, G. The treatment of breast cancer in the era of precision medicine. Cancer Biol. Med. 2025, 22, 322–347. [Google Scholar] [CrossRef] [PubMed]
- Bergamino, M.A.; López-Knowles, E.; Morani, G.; Tovey, H.; Kilburn, L.; Schuster, E.F.; Alataki, A.; Hills, M.; Xiao, H.; Holcombe, C.; et al. HER2-enriched subtype and novel molecular subgroups drive aromatase inhibitor resistance and an increased risk of relapse in early ER+/HER2+ breast cancer. eBioMedicine 2022, 83, 104205. [Google Scholar] [CrossRef]
- Mahvi, D.A.; Liu, R.; Grinstaff, M.W.; Colson, Y.L.; Raut, C.P. Local Cancer Recurrence: The Realities, Challenges, and Opportunities for New Therapies. CA Cancer J. Clin. 2018, 68, 488–505. [Google Scholar] [CrossRef]
- Charpentier, M.; Spada, S.; Van Nest, S.J.; Demaria, S. Radiation therapy-induced remodeling of the tumor immune microenvironment. Semin. Cancer Biol. 2022, 86, 737–747. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.-P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef]
- Wang, X.; Jing, J. Cancer Immunotherapy in Combination with Radiotherapy and/or Chemotherapy: Mechanisms and Clinical Therapy. Medcomm (2020) 2025, 6, e70346. [Google Scholar] [CrossRef]
- Brackstone, M.; Baldassarre, F.G.; Perera, F.E.; Cil, T.; Chavez Mac Gregor, M.; Dayes, I.S.; Engel, J.; Horton, J.K.; King, T.A.; Kornecki, A.; et al. Management of the Axilla in Early-Stage Breast Cancer: Ontario Health (Cancer Care Ontario) and ASCO Guideline. J. Clin. Oncol. 2021, 39, 3056–3082. [Google Scholar] [CrossRef]
- Purrington, K.S.; Slager, S.; Eccles, D.; Yannoukakos, D.; Fasching, P.A.; Miron, P.; Carpenter, J.; Chang-Claude, J.; Martin, N.G.; Montgomery, G.W.; et al. Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis 2014, 35, 1012–1019. [Google Scholar] [CrossRef]
- Stevens, K.N.; Vachon, C.M.; Couch, F.J. Genetic susceptibility to triple-negative breast cancer. Cancer Res. 2013, 73, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Dogra, A.K.; Prakash, A.; Gupta, S.; Gupta, M. Prognostic Significance and Molecular Classification of Triple Negative Breast Cancer: A Systematic Review. Eur. J. Breast Heal 2025, 21, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. npj Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Ovcaricek, T.; Frkovic, S.; Matos, E.; Mozina, B.; Borstnar, S. Triple negative breast cancer—Prognostic factors and survival. Radiol. Oncol. 2011, 45, 46–52. [Google Scholar] [CrossRef]
- Nunnery, S.E.; Mayer, I.A.; Balko, J.M. Triple-Negative Breast Cancer: Breast Tumors With an Identity Crisis. Cancer J. 2021, 27, 2–7. [Google Scholar] [CrossRef]
- Ensenyat-Mendez, M.; Llinàs-Arias, P.; Orozco, J.I.J.; Íñiguez-Muñoz, S.; Salomon, M.P.; Sesé, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front. Oncol. 2021, 11, 681476. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Krasnoselskyi, M.; Starikov, V.; Kartashov, S.; Zhulkevych, I.; Vlasenko, V.; Oleshko, K.; Bilodid, O.; Sadchikova, M.; Vinnyk, Y. Triple-negative breast cancer: Current treatment strategies and factors of negative prognosis. J. Med. Life 2022, 15, 153–161. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.; Al Sendi, M.; Kelly, C.M. Overview of recent advances in metastatic triple negative breast cancer. World J. Clin. Oncol. 2021, 12, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.; Ghosh, S.; Jaboin, J.; Seneviratne, D. Tumor Microenvironment Dynamics of Triple-Negative Breast Cancer Under Radiation Therapy. Int. J. Mol. Sci. 2025, 26, 2795. [Google Scholar] [CrossRef]
- Niture, S.; Mooers, B.H.; Wu, D.H.; Hart, M.; Jaboin, J.; Seneviratne, D. Dual-specificity protein phosphatase 1: A potential therapeutic target in cancer. iScience 2025, 28, 113706. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef]
- Cho, M.; Tang, G.; Rogers, C.S.; Dove, M.; Liu, X.; Li, Y.; Wang, X. OncoDB 2.0: A comprehensive platform for integrated pan-cancer omics analysis. Nucleic Acids Res. 2025, 54, D1537–D1544. [Google Scholar] [CrossRef]
- Lev Maor, G.; Yearim, A.; Ast, G. The alternative role of DNA methylation in splicing regulation. Trends Genet. 2015, 31, 274–280. [Google Scholar] [CrossRef]
- Kim, D.; Shivakumar, M.; Han, S.; Sinclair, M.S.; Lee, Y.-J.; Zheng, Y.; Olopade, O.I.; Kim, D.; Lee, Y. Population-dependent Intron Retention and DNA Methylation in Breast Cancer. Mol. Cancer Res. 2018, 16, 461–469. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Olopade, O.I. MYC and Breast Cancer. Genes Cancer 2010, 1, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, S.A.; Becker, T.M.; Khan, S.; Soon, P.; Neubauer, H.; de Souza, P.; Powter, B. Prognostic and Predictive Value of CCND1/Cyclin D1 Amplification in Breast Cancer With a Focus on Postmenopausal Patients: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 895729. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurti, U.; Silverman, J.F. HER2 in breast cancer: A review and update. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; Wu, Y.-M.; Lonigro, R.J.; Vats, P.; Cobain, E.; Everett, J.; Cao, X.; Rabban, E.; Kumar-Sinha, C.; Raymond, V.; et al. Integrative clinical genomics of metastatic cancer. Nature 2017, 548, 297–303. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; Chen, W.; Wu, H.; Yuan, Z.; Wang, K.; Li, G.; Sun, J.; Yu, L. Molecular Features of Triple Negative Breast Cancer: Microarray Evidence and Further Integrated Analysis. PLoS ONE 2015, 10, e0129842. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Yu, H.; Tian, J.; Yuan, F.; Fan, J.; Liu, Y.; Zhu, L.; Wang, F.; Zhao, Y.; et al. DUSP1 promoter methylation in peripheral blood leukocyte is associated with triple-negative breast cancer risk. Sci. Rep. 2017, 7, srep43011. [Google Scholar] [CrossRef]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target. Ther. 2021, 6, 185. [Google Scholar] [CrossRef]
- Jian, C.; Tian, X.; Luo, S.; Zeng, J.; Li, J.; Xu, T.; Wang, J. The circDUSP1/miR-429/DLC1 regulatory network affects proliferation, migration, and invasion of triple-negative breast cancer cells. Sci. Rep. 2025, 15, 26300. [Google Scholar] [CrossRef]
- Skor, M.N.; Wonder, E.L.; Kocherginsky, M.; Goyal, A.; Hall, B.A.; Cai, Y.; Conzen, S.D. Glucocorticoid Receptor Antagonism as a Novel Therapy for Triple-Negative Breast Cancer. Clin. Cancer Res. 2013, 19, 6163–6172. [Google Scholar] [CrossRef]
- Tuglu, M.M.; Bostanabad, S.Y.; Ozyon, G.; Dalkiliç, B.; Gurdal, H. The role of dual-specificity phosphatase 1 and protein phosphatase 1 in β2-adrenergic receptor-mediated inhibition of extracellular signal regulated kinase 1/2 in triple negative breast cancer cell lines. Mol. Med. Rep. 2018, 17, 2033–2043. [Google Scholar] [CrossRef]
- Liu, T.; Sun, H.; Liu, S.; Yang, Z.; Li, L.; Yao, N.; Cheng, S.; Dong, X.; Liang, X.; Chen, C.; et al. The suppression of DUSP5 expression correlates with paclitaxel resistance and poor prognosis in basal-like breast cancer. Int. J. Med. Sci. 2018, 15, 738–747. [Google Scholar] [CrossRef]
- Shen, J.-J.; Yang, X.; Yu, M.; Li, Q.-C.; Wang, R.-Y.; Yu, W.-Y.; Zhang, J.-L.; Chen, Y.-L.; Zhu, W.-T.; Li, J.; et al. Discovery of aurovertin B as a potent metastasis inhibitor against triple-negative breast cancer: Elucidating the complex role of the ATF3-DUSP1 axis. J. Pharmacol. Exp. Ther. 2025, 392, 100005. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Shi, Y.Y.; Higgins, L.S.; Orlowski, R.Z. Mitogen-Activated Protein Kinase Phosphatase-1 Is a Mediator of Breast Cancer Chemoresistance. Cancer Res. 2007, 67, 4459–4466. [Google Scholar] [CrossRef] [PubMed]
- Metin, S.; Altan, H.; Tercan, E.; Dedeoglu, B.G.; Gurdal, H. DUSP1 protein’s impact on breast cancer: Anticancer response and sensitivity to cisplatin. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2025, 1868, 195103. [Google Scholar] [CrossRef] [PubMed]
- Błaszczak, E.; Miziak, P.; Odrzywolski, A.; Baran, M.; Gumbarewicz, E.; Stepulak, A. Triple-Negative Breast Cancer Progression and Drug Resistance in the Context of Epithelial–Mesenchymal Transition. Cancers 2025, 17, 228. [Google Scholar] [CrossRef]
- Boulding, T.; Wu, F.; McCuaig, R.; Dunn, J.; Sutton, C.R.; Hardy, K.; Tu, W.; Bullman, A.; Yip, D.; Dahlstrom, J.E.; et al. Differential Roles for DUSP Family Members in Epithelial-to-Mesenchymal Transition and Cancer Stem Cell Regulation in Breast Cancer. PLoS ONE 2016, 11, e0148065. [Google Scholar] [CrossRef]
- Rhodes, L.V.; Martin, E.C.; Segar, H.C.; Miller, D.F.B.; Buechlein, A.; Rusch, D.B.; Nephew, K.P.; Burow, M.E.; Collins-Burow, B.M. Dual regulation by microRNA-200b-3p and microRNA-200b-5p in the inhibition of epithelial-to-mesenchymal transition in triple-negative breast cancer. Oncotarget 2015, 6, 16638–16652. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, J.; Bao, L.; Qu, H.; Xiang, J.; Sun, P. Upregulation of the ferroptosis-related STEAP3 gene is a specific predictor of poor triple-negative breast cancer patient outcomes. Front. Oncol. 2023, 13, 1032364. [Google Scholar] [CrossRef]
- Jiang, J.; Li, H.; Ma, Q.; Liu, J.; Ren, F.; Song, Y.; Wang, T.; Li, K.; Li, N. Synergies between radiotherapy and immunotherapy: A systematic review from mechanism to clinical application. Front. Immunol. 2025, 16, 1554499. [Google Scholar] [CrossRef]
- Galeaz, C.; Totis, C.; Bisio, A. Radiation Resistance: A Matter of Transcription Factors. Front. Oncol. 2021, 11, 662840. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Y.; Yu, H.; Shen, B.; Liang, Y.; Jin, R.; Liu, X.; Shi, L.; Cai, X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016, 5, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.C.; Srikanth, S.; Kraft, A.S. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 3014–3019. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.J.; Owens, D.M.; Maier, J.V.; Cato, A.C.B.; Keyse, S.M. Cross-talk between the p38α and JNK MAPK Pathways Mediated by MAP Kinase Phosphatase-1 Determines Cellular Sensitivity to UV Radiation. J. Biol. Chem. 2010, 285, 25928–25940. [Google Scholar] [CrossRef] [PubMed]
- Candas, D.; Lu, C.-L.; Fan, M.; Chuang, F.Y.; Sweeney, C.; Borowsky, A.D.; Li, J.J. Mitochondrial MKP1 Is a Target for Therapy-Resistant HER2-Positive Breast Cancer Cells. Cancer Res. 2014, 74, 7498–7509. [Google Scholar] [CrossRef]
- Candas, D.; Li, J.J. MKP1 mediates resistance to therapy in HER2-positive breast tumors. Mol. Cell. Oncol. 2015, 2, e997518. [Google Scholar] [CrossRef][Green Version]
- Perez-Anorve, I.X.; Gonzalez-De la Rosa, C.H.; Soto-Reyes, E.; Beltran-Anaya, F.O.; Del Moral-Hernandez, O.; Salgado-Albarran, M.; Angeles-Zaragoza, O.; Gonzalez-Barrios, J.A.; Landero-Huerta, D.A.; Chavez-Saldana, M.; et al. New insights into radioresistance in breast cancer identify a dual function of miR-122 as a tumor suppressor and oncomiR. Mol. Oncol. 2019, 13, 1249–1267. [Google Scholar] [CrossRef]
- Qi, X.S.; Pajonk, F.; McCloskey, S.; Low, D.A.; Kupelian, P.; Steinberg, M.; Sheng, K. Radioresistance of the breast tumor is highly correlated to its level of cancer stem cell and its clinical implication for breast irradiation. Radiother. Oncol. 2017, 124, 455–461. [Google Scholar] [CrossRef]
- Gray, M.; Turnbull, A.K.; Ward, C.; Meehan, J.; Martínez-Pérez, C.; Bonello, M.; Pang, L.Y.; Langdon, S.P.; Kunkler, I.H.; Murray, A.; et al. Development and characterisation of acquired radioresistant breast cancer cell lines. Radiat. Oncol. 2019, 14, 64. [Google Scholar] [CrossRef]
- Wang, C.; Xie, D.; Bai, C.; Mao, C.; Wang, F.; Guo, X. Serum dual-specificity phosphatase 1 reflects decreased exacerbation risk, correlates with less advanced exacerbation severity and lower inflammatory cytokines in children with asthma. Allergol. Immunopathol. 2022, 50, 60–67. [Google Scholar] [CrossRef]
- Pemmari, A.; Paukkeri, E.; Hämäläinen, M.; Leppänen, T.; Korhonen, R.; Moilanen, E. MKP-1 promotes anti-inflammatory M(IL-4/IL-13) macrophage phenotype and mediates the anti-inflammatory effects of glucocorticoids. Basic. Clin. Pharmacol. Toxicol. 2019, 124, 404–415. [Google Scholar] [CrossRef]
- Khadir, A.; Kavalakatt, S.; Dehbi, M.; Alarouj, M.; Bennakhi, A.; Tiss, A.; Elkum, N. DUSP1 Is a Potential Marker of Chronic Inflammation in Arabs with Cardiovascular Diseases. Dis. Markers 2018, 2018, 9529621. [Google Scholar] [CrossRef]
- Liu, Z.; He, S.; Huang, Z.; Liu, J.; Gong, Y.; Yao, Y.; Zhang, X. Regulation of ferroptosis-related genes in CD8+ NKT cells and classical monocytes may affect the immunotherapy response after combined treatment in triple negative breast cancer. Thorac. Cancer 2023, 14, 3369–3380. [Google Scholar] [CrossRef]
- Patysheva, M.R.; Iamshchikov, P.S.; Fedorenko, A.A.; Bragina, O.D.; Vostrikova, M.A.; Garbukov, E.Y.; Cherdyntseva, N.V.; Denisov, E.V.; Gerashchenko, T.S. Single-cell transcriptomic profiling of immune landscape in triple-negative breast cancer during neoadjuvant chemotherapy. npj Syst. Biol. Appl. 2025, 11, 72. [Google Scholar] [CrossRef]
- Mangiola, S.; Brown, R.; Zhan, C.; Berthelet, J.; Guleria, S.; Liyanage, C.; Ostrouska, S.; Wilcox, J.; Merdas, M.; Fuge-Larsen, P.; et al. Circulating immune cells exhibit distinct traits linked to metastatic burden in breast cancer. Breast Cancer Res. 2025, 27, 73. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, Q. Immunotherapy resistance in triple-negative breast cancer: Molecular mechanisms, tumor microenvironment, and therapeutic implications. Front. Oncol. 2025, 15, 1630464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Niture, S.; Thotala, D.; Jaboin, J.; Seneviratne, D. DUSP1: Triple-Negative Breast Cancer and Therapeutic Potential. Curr. Oncol. 2026, 33, 82. https://doi.org/10.3390/curroncol33020082
Niture S, Thotala D, Jaboin J, Seneviratne D. DUSP1: Triple-Negative Breast Cancer and Therapeutic Potential. Current Oncology. 2026; 33(2):82. https://doi.org/10.3390/curroncol33020082
Chicago/Turabian StyleNiture, Suryakant, Dinesh Thotala, Jerry Jaboin, and Danushka Seneviratne. 2026. "DUSP1: Triple-Negative Breast Cancer and Therapeutic Potential" Current Oncology 33, no. 2: 82. https://doi.org/10.3390/curroncol33020082
APA StyleNiture, S., Thotala, D., Jaboin, J., & Seneviratne, D. (2026). DUSP1: Triple-Negative Breast Cancer and Therapeutic Potential. Current Oncology, 33(2), 82. https://doi.org/10.3390/curroncol33020082






