Simple Summary
Pancreatic cancer (PC) is highly lethal, and many patients do not complete postoperative adjuvant chemotherapy (AC). Emerging evidence shows that simple nutritional indices—the prognostic nutritional index (PNI), the geriatric nutritional risk index (GNRI), and the C-reactive protein/albumin ratio (CAR)—predict AC completion and survival across commonly used regimens (modified FOLFIRINOX, gemcitabine–capecitabine, and regionally adopted S-1). In PC, early postoperative declines in nutritional status, driven by systemic inflammation, sarcopenia, and pancreatic exocrine insufficiency, often interrupt treatment. This review focuses on two clinical questions in PC: which nutritional markers predict completing ≥80% of planned AC cycles and when to start nutritional intervention. We highlight recent data showing that interventions within 2–4 weeks after surgery, guided by nutritional and inflammatory markers, may improve chemotherapy adherence and outcomes. Embedding these assessments into standard perioperative care could move nutrition from a supportive role to a proactive, central component of personalized oncology.
Abstract
Pancreatic cancer (PC) remains associated with poor survival despite curative-intent resection. Many patients fail to complete the planned course of adjuvant chemotherapy (AC), most often due to early postoperative nutritional decline. We conducted a narrative review (PubMed, EMBASE, Scopus; Jan 2010–Dec 2024; last update 15 February 2025) focusing on two endpoints: (1) nutritional predictors of completing ≥80% of planned AC cycles and (2) optimal timing of perioperative nutritional interventions. Preoperative GNRI and PNI, early postoperative albumin and CAR, and sarcopenia are robust predictors of AC adherence and survival. Nutritional decline is frequently accelerated by systemic inflammation and pancreatic exocrine insufficiency. Observational data indicate that interventions initiated within 2–4 weeks after surgery—before starting AC—improve chemotherapy completion rates, especially when guided by dynamic monitoring of nutritional and inflammatory markers. Neoadjuvant chemotherapy (NAC) offers a potential window for metabolic optimization, but validated nutrition-based predictors of NAC adherence remain limited. Readily measurable nutritional indices such as PNI, GNRI, and CAR should be integrated into early perioperative assessments to identify high-risk patients. Timely, phase-specific interventions, including enzyme replacement and anti-inflammatory nutritional strategies, can enhance AC adherence and improve prognoses. These findings support the transition from a supportive to a proactive, personalized nutritional oncology model in resectable PC.
1. Introduction
In this review, we define “malnutrition” according to the Global Leadership Initiative on Malnutrition (GLIM) criteria (phenotypic and etiologic components) and “cachexia” as a multifactorial syndrome characterized by progressive skeletal muscle loss—unresponsive to conventional nutrition—and systemic inflammation (e.g., >5% weight loss over 6 months or body mass index (BMI) < 20 kg/m2 with weight loss > 2%, plus elevated inflammatory markers) [1,2,3]. Pancreatic cancer (PC) remains one of the most lethal malignancies, with an estimated 66,440 new cases and 51,750 deaths projected in the United States for 2024 [4]. Global data show similarly poor outcomes, with 5-year survival rates generally below 12% in Western countries and only modestly higher in regions where adjuvant chemotherapy (AC) including modified FOLFIRINOX (folinic acid [leucovorin], 5-fluorouracil, irinotecan, and oxaliplatin), gemcitabine–capecitabine, and S-1, is widely adopted. Nearly 70% of patients are already malnourished at diagnosis, driven by tumor-induced inflammation, pancreatic exocrine insufficiency, and treatment-related gastrointestinal toxicity [5,6,7]. These nutritional deficits impair chemotherapy tolerance, compromise immune competence, and accelerate functional decline. Consequently, malnutrition has been consistently linked to postoperative complications (POCs), early discontinuation of adjuvant chemotherapy (AC), and poor prognosis [8,9,10,11].
Recognizing this, the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma (Version 2.2025) recommend routine perioperative nutritional screening using validated assessment tools, alongside institution-wide implementation of tailored interventions such as high-protein diets, pancreatic enzyme replacement therapy (PERT), and structured physical activity programs [12]. While the guidelines do not specify indices, tools such as the Geriatric Nutritional Risk Index (GNRI) and Prognostic Nutritional Index (PNI) are widely used in clinical research to stratify nutritional risk in pancreatic cancer patients. Similarly, the 2023 European Society for Clinical Nutrition and Metabolism (ESPEN) consensus statement emphasizes that nutritional care should start at diagnosis, be maintained throughout the perioperative course, and be adapted according to ongoing assessments of nutritional risk and functional status [13].
The survival benefits of adjuvant therapy are well established internationally. Randomized trials, including CONKO-001 [14], PRODIGE 24/CCTG PA.6 and ESPAC-4, have established modified FOLFIRINOX and gemcitabine–capecitabine as widely used standards [15,16], while S-1 is adopted in certain East Asian settings based on JASPAC-01 [17]. Our focus in this review is not to compare regimens but to synthesize how nutritional status influences the completion of adjuvant chemotherapy across regimens. In the Prep-02/JSAP-05 trial, neoadjuvant chemotherapy (NAC) with gemcitabine plus S-1 before surgery yielded a 28% reduction in the risk of death and superior 2-year survival compared with upfront surgery [18]. Nevertheless, postoperative nutritional decline—manifested by hypoalbuminemia, elevated C-reactive protein-to-albumin ratio (CAR), and marked weight loss—remains a major barrier to AC completion [19]. Accordingly, AC should be initiated as early as clinically feasible once patients are medically fit; exact timing varies across guidelines and regimens. We therefore frame the “pre-AC nutritional optimization” window pragmatically rather than anchoring it to a single trial. This timeframe serves as the target for pre-AC nutritional optimization discussed in this review [17]. Large-scale observational cohorts and meta-analyses confirm that low GNRI or PNI and high CAR predict chemotherapy non-adherence and inferior survival [20,21,22,23,24]. Despite this, nearly half of malnourished patients receive no structured nutritional support [25], often due to limited oncologic awareness, insufficient dietetic staffing, and lack of multidisciplinary coordination. A range of interventions has demonstrated potential benefit. PERT mitigates malabsorption and weight loss [26], and has been associated with improved overall survival in observational cohorts [27,28] in patients with pancreatic exocrine insufficiency. Prehabilitation and rehabilitation programs that combine individualized nutrition counseling, resistance exercise, and anti-inflammatory dietary components (such as ω-3 fatty acids) can improve inflammatory–nutritional markers and functional capacity [29,30,31]. Expert panels, including the International Study Group on Pancreatic Surgery, emphasize the importance of longitudinal monitoring of weight, skeletal muscle mass, and overall nutritional status throughout the perioperative period [32].
Mechanistic insights provide further rationale for targeted nutritional strategies. Pro-inflammatory cytokines, particularly IL-6 and TNF-α, mediate muscle proteolysis and adipose tissue browning via the JAK/STAT3 and NF-κB pathways, thereby exacerbating cachexia and reducing chemotherapy tolerance [33,34]. In parallel, gut microbiota dysbiosis diminishes production of beneficial short-chain fatty acids (SCFAs), compromising intestinal barrier function and amplifying systemic inflammation. Butyrate, a key SCFA, has been shown to inhibit histone deacetylase 8 (HDAC8), suppress NF-κB–driven pro-inflammatory genes, and up-regulate tight junction proteins, leading to improved nutrient absorption and reduced chemotherapy-induced mucositis [35]. These microbiota–nutrition–inflammation interactions, illustrated in Figure 1, underscore the potential of integrating biomarker-guided nutritional support into standard perioperative care (Figure 1).
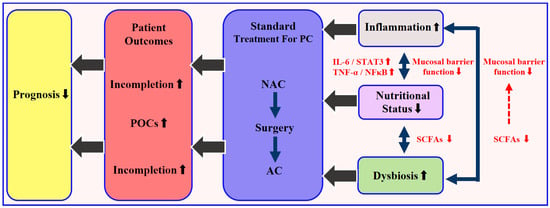
Figure 1.
Schematic of the microbiota–nutrition–inflammation axis in resectable pancreatic cancer and its effect on the treatment sequence (NAC → surgery → AC). Dysbiosis reduces short-chain fatty acid (SCFA) production and impairs the barrier function, leading to malabsorption and malnutrition. This triggers excess IL-6 and TNF-α, activating JAK/STAT3 and NF-κB pathways, which worsen systemic inflammation and muscle loss. The cycle increases postoperative complications and lowers chemotherapy completion rates, leading to poorer prognosis. Note: For resectable PDAC, neoadjuvant chemotherapy (NAC) is not universally standard and remains under investigation; the sequence shown is a schematic for discussing nutrition and does not imply guideline uniformity.
Collectively, these data highlight a dual challenge: identifying patients at highest nutritional risk before and after surgery, and delivering timely, evidence-based interventions to maintain or restore their capacity to complete planned chemotherapy. This review focuses on two clinically actionable endpoints: (1) nutritional determinants of completing ≥80% of planned AC cycles, and (2) optimal timing of nutritional intervention. In doing so, we aim to provide a practical framework that synthesizes current evidence, acknowledges existing limitations, and outlines priorities for future clinical trials in personalized nutritional oncology. Accordingly, when regimen-specific studies are cited—many of which come from East Asia using S-1—the biological rationale and observed associations (e.g., with GNRI, PNI, CAR, sarcopenia) are interpreted as regimen-agnostic unless otherwise specified.
2. Materials and Methods
We performed a comprehensive literature search in PubMed, EMBASE, Scopus, and the Cochrane Library (January 2010–December 2024; last update 15 February 2025) using both controlled vocabulary (e.g., MeSH) and free-text terms for “pancreatic adenocarcinoma” or “resectable pancreatic cancer” and nutritional parameters (GNRI, PNI, CAR, sarcopenia, serum albumin). Boolean operators and filters for English language and human studies were applied. The PubMed search string was: (“pancreatic adenocarcinoma” [MeSH] OR “resectable pancreatic cancer”) AND (“Geriatric Nutritional Risk Index” OR “GNRI” OR “Prognostic Nutritional Index” OR “PNI” OR “C-reactive protein-to-albumin ratio” OR “CAR” OR “sarcopenia” OR “serum albumin”). Database-specific adaptations were used for EMBASE, Scopus, and Cochrane Library.
We included adult patients (≥18 years) with resectable PC receiving NAC and/or AC and reporting at least one nutritional index or intervention, with outcomes related to chemotherapy completion, tolerance, or survival. We a priori defined “chemotherapy completion” as maintaining a relative dose intensity (RDI) ≥ 80% over the planned 6-month AC course; when only cycle counts were reported, completion of ≥80% of planned cycles was considered an approximate equivalent endpoint. Exclusion criteria comprised case reports, editorials, letters, conference abstracts, non-original works, non-English publications, and studies without full text.
Screening followed a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-style flow: of 5793 records retrieved, 5525 remained after deduplication; 1014 full texts were reviewed, and 212 original studies plus 53 review articles were included. Discrepancies between two independent reviewers were resolved by consensus (Figure 2).
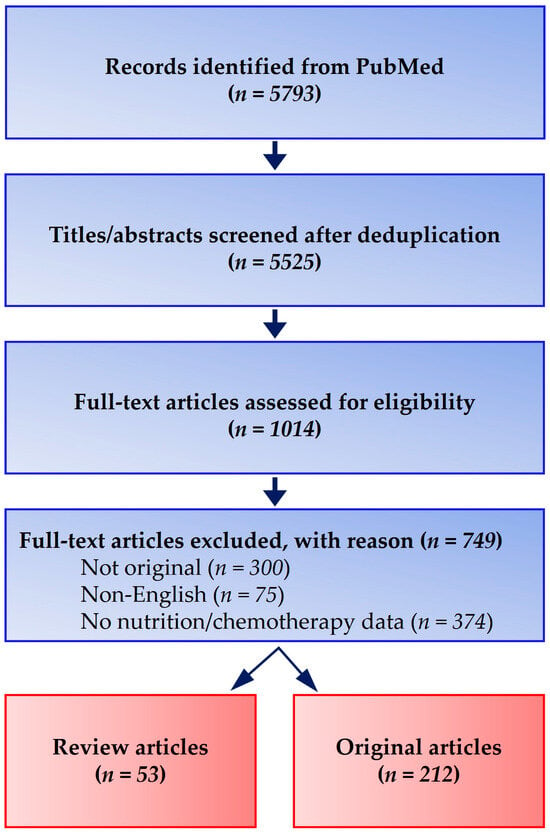
Figure 2.
PRISMA-style flow diagram of literature identification and selection. A comprehensive search (January 2010–December 2024; final update 15 February 2025) in PubMed, EMBASE, Scopus, and the Cochrane Library retrieved 5793 records. After deduplication (n = 5525) and screening, 1014 full-text articles were assessed, with 212 original studies and 53 review articles meeting inclusion criteria.
Limitations include the narrative nature of this review, potential selection and publication bias, and reliance primarily on PubMed, although supplemental searches in other databases were conducted to minimize omissions. Greater weight was given to prospective, multicenter studies with external validation.
Evidence appraisal. We assigned study-level designs to the Oxford Centre for Evidence-Based Medicine (OCEBM) 2011 Levels of Evidence according to the clinical question (therapy vs. prognosis) [36]. We then rated outcome-level certainty using GRADE (high, moderate, low, very low), considering risk of bias, inconsistency, indirectness, imprecision, and publication bias; upgrading criteria were applied when appropriate (large effect, dose–response, residual confounding) [37,38]. Two reviewers assessed each item independently; discrepancies were resolved by consensus.
3. Results
3.1. Nutritional Predictors of AC Completion
Although several cohorts evaluated S-1 in East Asia, these indices have also been associated with adherence and outcomes in studies of modified FOLFIRINOX and gemcitabine-based AC; thus we consider them broadly applicable across regimens. Multiple clinical parameters reflecting nutritional and inflammatory status have been evaluated as predictors of AC completion in resectable PC. The most consistently validated are the GNRI, PNI, CAR, and sarcopenia.
- GNRI: Low preoperative GNRI is associated with early discontinuation of S-1 AC and reduced survival in multiple retrospective and prospective cohorts, including studies with external validation [20,21,39,40].
- PNI: Lower PNI values—calculated from serum albumin and lymphocyte count—predict incomplete AC and poorer survival [41,42,43,44].
- CAR: Elevated CAR, reflecting combined systemic inflammation and malnutrition, independently forecasts poor S-1 tolerability and inferior recurrence-free and overall survival [22,23,45,46].
- Sarcopenia: Skeletal muscle depletion predicts reduced chemotherapy tolerance and completion, as well as worse overall survival [47,48,49,50,51,52,53].
These indices outperform single variables such as BMI or albumin alone, as they integrate both nutritional and inflammatory dimensions. Table 1 summarizes the main indices and their clinical characteristics.

Table 1.
Summary of nutritional assessment indices in pancreatic cancer care.
3.2. Neoadjuvant Chemotherapy (NAC) vs. AC: Nutritional Vulnerability
In contrast to AC, NAC is administered before postoperative nutritional decline and therefore generally achieves higher treatment completion rates. The Japanese randomized Prep-02/JSAP-05 trial, along with complementary cohort studies, demonstrated that gemcitabine plus S-1 NAC preserved nutritional status and improved resection outcomes [54,55]. While multi-agent regimens such as FOLFIRINOX are widely adopted in international practice, our nutritional framework is intentionally regimen-agnostic. By contrast, AC has demonstrated unequivocal survival benefits in landmark randomized controlled trials, including CONKO-001, ESPAC-4, and PRODIGE 24 [14,15,16,17]. However, AC delivery is particularly vulnerable to postoperative weight loss, sarcopenia, and hypoalbuminemia, which have been consistently associated with premature discontinuation of therapy [10,11]. These observations underscore the central role of nutritional status as a determinant of successful chemotherapy completion.
3.3. Timing of Nutritional Intervention
Evidence, though mostly observational, suggests that early postoperative nutritional intervention (within 2–4 weeks after surgery) improves AC completion and functional recovery compared with interventions starting at AC initiation. AC is ideally initiated once the patient is medically fit—commonly within ~12 weeks after surgery, per prevailing guidance—though trial-specific windows vary (e.g., ≤10 weeks in JASPAC-01 [17]). Therefore, we consider the “pre-AC nutritional optimization” period pragmatically, without anchoring it to any single study.
- PERT: Timely initiation after pancreaticoduodenectomy prevents malabsorption, limits weight loss, and has been associated with improved survival [26,27,28].
- High-protein, high-calorie diets and immunonutrition: Use of omega-3 fatty acids and other immunonutrition components during the early postoperative period may help preserve lean mass and reduce inflammation [56,57,58,59,60,61].
- Prehabilitation programs: During NAC, combined nutritional counseling and exercise interventions have demonstrated improvements in PNI and inflammatory ratios before surgery [31,62,63].
Table 2 presents interventions stratified by treatment phase (NAC, early postoperative, AC). Figure 3 outlines the potential impact of intervention timing across the perioperative course.

Table 2.
Nutritional interventions during different treatment phases in pancreatic cancer and their intended clinical outcomes.
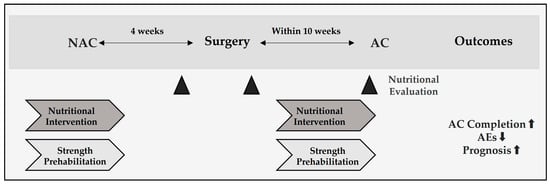
Figure 3.
Perioperative timeline and recommended windows for nutritional evaluation and intervention in resectable pancreatic cancer. Neoadjuvant chemotherapy (NAC) is followed by surgery at approximately 4 weeks, and adjuvant chemotherapy (AC) should begin early after surgery once patients are clinically ready, in accordance with prevailing guidelines and institutional practice. Nutrition checkpoints at the end of NAC, postoperative day 7–14, and pre-AC assess GNRI/PNI, albumin/CAR, and sarcopenia to prompt a dietitian-led high-protein diet with or without oral nutritional supplements (ONS), pancreatic enzyme replacement (PERT), and resistance training—aiming to maintain AC relative dose intensity (RDI) at ≥80% for 6 months.
3.4. Limitations of Current Evidence
Most studies are retrospective single-center analyses; randomized controlled trials specifically designed to test nutritional optimization for AC completion in PC are lacking. Predictors of NAC adherence are less well-validated than those for AC. Multicenter prospective trials are needed to confirm optimal intervention timing and content.
Interventions include nutritional counseling, pancreatic enzyme replacement therapy (PERT; for pancreatic exocrine insufficiency [PEI]), immunonutrition, exercise therapy, and emerging options such as vitamin D analogs. Their timing and impact vary depending on the treatment context—neoadjuvant, postoperative, or adjuvant; where pancreatic-specific trials are limited, evidence from gastrointestinal (GI) cancers is cited.
4. Discussion
4.1. Clinical Evidence Linking Nutritional Status and Chemotherapy Outcomes with Nutritional Indices and Sarcopenia
Historically, composite nutritional–inflammatory indices, such as GNRI, CAR, and PNI, together with sarcopenia, were primarily validated as predictors of postoperative outcomes and survival after pancreatic resection [65,66], with additional risk conveyed by the combined effect of frailty and sarcopenia [67,68]. More recently, multi-institutional cohorts with external validation have demonstrated that these indices also predict adherence to AC and early discontinuation, refining pre-AC risk appraisal alongside sarcopenia [20,22,67]. Taken together, because these indices capture inflammation-nutrition biology rather than drug-specific effects, their predictive relevance extends to commonly used international adjuvant regimens (e.g., modified FOLFIRINOX, gemcitabine–capecitabine)
4.1.1. GNRI (Geriatric Nutritional Risk Index)
A lower preoperative GNRI predicts POCs after resection and early discontinuation of S-1 AC after curative surgery in a multi-institutional study with external validation, supporting GNRI as a practical pre-AC triage tool [19,20,69].
4.1.2. CAR (C-Reactive Protein-to-Albumin Ratio)
A higher preoperative CAR is associated with POCs after resection, and poor tolerability and early cessation of S-1 AC after curative resection, shown in a development cohort and confirmed by external validation [21,22,70].
4.1.3. PNI (Prognostic Nutritional Index)
In patients who received NAC followed by curative surgery, PNI < 45 independently predicted failure to continue S-1 AC, and meta-analytic data link low preoperative PNI with worse post-resection survival—supporting its use alongside GNRI/CAR for pre-AC risk appraisal [8,42,43].
4.1.4. Sarcopenia
Sarcopenia. CT-defined sarcopenia (low skeletal muscle index at L3) has been associated with treatment modifications and failure to complete S-1 AC in PC cohorts; moreover, in patients receiving adjuvant modified FOLFIRINOX or gemcitabine, baseline sarcopenia was linked to markedly shorter overall survival irrespective of regimen, underscoring its prognostic and tolerance implications during AC [71]. Sarcopenic obesity and skeletal muscle depletion strongly predict outcomes independent of BMI, aligning with our emphasis on body-composition–aware indices [72,73,74].
4.2. Nutritional Interventions and Their Protective Effects
Targeted nutritional interventions have the potential to modify treatment trajectories in resectable PC. In clinical settings, early, structured nutritional support during chemotherapy has been associated with better treatment adherence and survival in PC cohorts [75]. Clinically, individualized nutritional counseling and oral nutritional supplements (ONS) have been associated with improved treatment tolerance, quality of life, and AC completion [76,77]. Perioperative or chemotherapy-concurrent omega-3–enriched nutrition has shown reductions in CRP and signals for improved tolerance in clinical studies of GI/pancreatic cancer, including a randomized phase II trial in advanced PC (gemcitabine ± EPA); however, effects on lean mass, survival, and AC completion remain heterogeneous across trials [57,59,60,61].
Despite these data, malnutrition and cachexia are often underdiagnosed, and nutritional therapy remains underutilized in oncology practice. Early and sustained implementation of evidence-based nutritional support—tailored to individual inflammatory and metabolic profiles—should be viewed as a proactive, modifiable component of perioperative care. In metastatic PDAC, early nutritional support has been associated with improved treatment tolerance and patient-centered outcomes, although prospective evidence remains limited [25,78].
4.3. Mechanistic Insights: Inflammation, Muscle Proteolysis, and Immune Modulation
Preclinical studies have elucidated the molecular basis by which nutritional decline compromises treatment tolerance. Interleukin-6 (IL-6) family cytokines activate the JAK/STAT3 pathway via the IL-6Rα/gp130 receptor complex, thereby inducing acute-phase protein synthesis, skeletal muscle proteolysis, and browning of adipose tissue—core phenotypic features of cancer cachexia [33]. These processes lead to muscle weakness and diminished physiological reserve, ultimately reducing tolerance to cytotoxic therapies. In parallel, tumor necrosis factor-α (TNF-α) engages TNFR1/2 to activate NF-κB signaling, which upregulates muscle-specific E3 ubiquitin ligases such as MuRF1 and Atrogin-1, accelerating protein degradation through the ubiquitin–proteasome system [79]. These inflammatory cascades also contribute to immunosuppression within the tumor microenvironment, further attenuating therapeutic efficacy [80]. Consistently, PC harbors a tumor microbiome that shapes antitumor immunity and disease trajectory, with specific bacterial consortia associating with outcome and immune reprogramming in preclinical and translational studies [81,82], and intracellular, tumor–type–specific bacteria documented across malignancies [83].
From a translational perspective, mechanism-guided interventions that temper IL-6/JAK/STAT3 activity are being explored. Although direct evidence in pancreatic cancer is limited, observational data in other malignancies suggest that IL-6 receptor blockade with tocilizumab can reduce CRP, increase serum albumin, maintain body weight, improve symptoms, and prolong median overall survival in cachectic patients with advanced non-small-cell lung cancer [84], while tocilizumab plus corticosteroids yielded short-term benefits in cancer cachexia with systemic hyperinflammation [85]. Direct STAT3 inhibition (TTI-101) has also shown favorable safety and pharmacodynamic activity in a phase I study of advanced solid tumors [86]. These observations support a multimodal approach coupling anti-inflammatory pharmacotherapy with nutrition and exercise to bridge mechanistic insights and clinical outcomes.
4.4. Gaps Between Evidence and Clinical Implementation
International guidance already specifies what “good practice” should look like: the 2017 ESPEN expert recommendations and the 2021 ESPEN practical guideline call for routine screening with validated tools, early dietitian involvement, and protocolized nutrition pathways embedded across oncology services [13,87]. Despite this, malnutrition remains common and undertreated in real-world oncology: a multicentre audit of French comprehensive cancer centers documented high prevalence at presentation, while in pancreatic/periampullary cancer, early dietetic consultation correlated with improved survival [88,89]. These data frame the current gap between recommendations and practice in perioperative PC care. Despite strong evidence, the translation of nutritional screening and intervention into routine perioperative PC care remains inconsistent. International guidelines, including NCCN v2.2025, recommend early screening; indices such as GNRI and PNI are widely used in research and in this review [12], yet observational studies indicate that nearly half of malnourished patients receive no structured nutritional support [90]. Barriers include limited oncologic awareness, resource constraints, and the absence of standardized care pathways linking surgical, oncologic, and nutrition teams.
Pancreatic exocrine insufficiency (PEI) after pancreaticoduodenectomy further exacerbates postoperative malnutrition through fat malabsorption. Although PERT improves nutrient absorption, mitigates weight loss, and has been associated with survival benefits [27,28], it remains underprescribed in many centers. Recent evidence indicates that dose optimization of PERT is essential to mitigate muscle loss in advanced PC patients with PEI [91], and systematic reviews confirm the high prevalence of PEI after pancreatic resection, supporting the need for routine postoperative screening and treatment [92]. Bridging this evidence–practice gap requires institutional protocols that embed nutritional and enzymatic support into standard perioperative oncology workflows. Formalizing a ‘nutritional oncology board’ has been proposed to close implementation gaps between surgical, medical, and nutrition teams [93].
4.5. Cancer Cachexia as a Therapeutic Target
Cachexia in PC represents a systemic metabolic derangement driven by both tumor-derived and host inflammatory signals. Cytokines such as IL-6 and TGF-β1 activate muscle proteolysis and lipolysis, while modulating immune responses in ways that impair therapeutic efficacy [34,94]. Recent mechanistic work has shown that TGF-β1 induces a KLF10-dependent transcriptional program promoting muscle atrophy, and IL-6–mediated STAT3 activation further amplifies systemic inflammation [95,96].
In PC-specific models, tumor organoid–derived factors from cachectic patients have been shown to disrupt contractile smooth muscle cells, providing a mechanistic link between tumor-secreted mediators and gastrointestinal motility impairment [97]. Clinically, cachexia-associated inflammation, captured by indices such as the CAR and GNRI, predicts survival more accurately than conventional nutritional assessments in PC and other gastrointestinal tumors [98].
These mechanisms underscore why cachexia is not merely a symptom but a direct mediator of poor prognosis. Given its high prevalence and predictive value for AC intolerance, cachexia should be targeted as aggressively as other modifiable risk factors, combining anti-inflammatory nutritional strategies (e.g., omega-3 PUFAs, immunonutrition) with structured exercise and metabolic support. Narrative and mechanistic overviews emphasize cachexia as a systemic, treatable driver of poor outcomes, not merely a comorbidity [99,100,101].
4.6. Toward Personalized Nutritional Oncology
While indices such as the GNRI, PNI, CAR, and sarcopenia are valuable for risk stratification, they do not fully capture the multidimensional determinants of treatment response. Integrating molecular and microenvironmental factors—such as microRNA profiles, epithelial–mesenchymal transition (EMT) status, and stromal activation markers—could refine predictive accuracy. For example, microRNAs such as miR-301b and miR-200b have been implicated in gemcitabine resistance through EMT promotion and tumor microenvironment modulation [102,103,104,105,106]. Experimental evidence also shows that deregulation of microRNA networks following key tumor suppressor loss, such as Ink4a/Arf, can further drive oncogenic transformation and treatment resistance [107]. Nutritional factors, notably vitamin D signaling, can counteract these resistance mechanisms by reprogramming pancreatic stellate cells, normalizing stromal architecture, and modulating immunosuppressive niches [64]. Such interactions suggest that future predictive models should combine inflammatory–nutritional indices with molecular resistance markers to guide both systemic therapy and supportive care.
4.7. Future Research Directions
Host–microbiome–tumor interactions are increasingly recognized as modifiers of both efficacy and toxicity of systemic cancer therapies beyond immune checkpoint inhibitors [108,109,110]. Mechanistically, SCFAs—particularly butyrate—can influence epithelial barrier integrity and inflammatory signaling through HDAC/NF-κB axes [35], providing a biologic rationale for integrating microbial metabolites into perioperative supportive care for PC.
Emerging synthesis work specific to PC indicates that microbially derived metabolites may mitigate chemotherapy resistance by rewiring tumor–stromal crosstalk and inflammatory pathways [111]. Complementing this metabolite-centric view, intratumoral microbiota can directly alter drug pharmacology: Geller et al. demonstrated that tumor-resident bacteria possessing cytidine deaminase activity can inactivate gemcitabine, thereby diminishing cytotoxicity [112]. Together, these observations support a development strategy that (i) profiles gut and intratumoral microbiomes and metabolites before NAC/AC, (ii) pilots selective antimicrobial, pre/pro/synbiotic, or dietary interventions, and (iii) quantifies tumor microbial drug-modifying enzymes (e.g., cytidine deaminase) in resection specimens.
For fluoropyrimidines, the S-1 combination (tegafur/gimeracil/oteracil) controls systemic 5-FU exposure chiefly via gimeracil-mediated inhibition of host dihydropyrimidine dehydrogenase (DPD). The gut microbiome may modulate fluoropyrimidine pharmacodynamics and mucosal toxicity through pyrimidine metabolism, bile-acid/SCFA signaling, and inflammation [108]. These considerations motivate an integrative monitoring framework—host DPD activity × microbiome/metabolites × inflammatory–nutritional indices—to personalize S-1 dosing and supportive care. Practically, the early postoperative “window” (2–4 weeks after resection) is well suited to implement: (a) concurrent assessment of SCFAs, bile acids, CRP/CAR, and GNRI/PNI; (b) small, adaptive trials of targeted pre/pro/synbiotics [27] with metabolite-response readouts; and (c) longitudinal tracking after S-1 initiation to sustain RDI ≥ 80% across the planned 6-month course.
Key design elements for future perioperative trials include:
- Stratification: Baseline GNRI/PNI/CAR combined with microbial diversity and metabolite panels (SCFAs, TMAO).
- Endpoints: Primary—maintenance of RDI ≥ 80% (6 months); secondary—completion of ≥80% of planned cycles, CTCAE-graded toxicities, QoL.
- Testable interventions: Optimization of PERT, ω-3–enriched immunonutrition, and pre/pro/synbiotics—alone and in combination.
- Intratumoral microbiota readouts: 16S/shotgun metagenomics with quantification of drug-modifying genes (e.g., cytidine deaminase) in resection tissue; exploratory circulating microbial DNA/metabolites as non-invasive biomarkers.
- Methodologic rigor: Contamination control, pre-registered protocols, multiplicity-adjusted analyses.
By overlaying microbiome and metabolite features onto established inflammatory–nutritional indices (GNRI, PNI, CAR), the field can move toward a feasible, biomarker-guided model that personalizes both anticancer pharmacology and supportive nutrition to maximize adjuvant chemotherapy completion and clinical benefit in resectable PC.
5. Conclusions
This narrative review consolidates current evidence demonstrating that readily obtainable nutritional indices—including the GNRI, CAR, PNI, and sarcopenia—consistently predict completion of perioperative chemotherapy and long-term outcomes in resectable PC. These metrics, which integrate dimensions of nutritional reserve and systemic inflammation, capture key biological determinants of treatment tolerance and survival and are further modulated by gut microbiota–host interactions. The weight of both mechanistic and clinical data supports embedding early, standardized nutritional risk assessments into routine perioperative oncology workflows, followed by phase-specific, targeted interventions to preserve metabolic and functional capacity.
Despite robust retrospective evidence, prospective multicenter validation of these indices, ideally coupled with biomarker-guided stratification (e.g., inflammatory cytokine profiles and microbiota composition), is urgently needed. Such integration could transform nutritional management from a supportive adjunct to a proactive, central pillar of personalized oncologic care—enhancing chemotherapy adherence, reducing postoperative morbidity, and ultimately improving survival. By reframing perioperative nutrition as a modifiable, prognostically relevant domain, clinicians can leverage it as a therapeutic target rather than a secondary consideration.
Author Contributions
Conceptualization, N.F. and Y.K.; methodology, N.F.; validation, M.U. and C.I.; investigation, N.F.; resources, A.S.; data curation, M.I.; writing—original draft preparation, N.F.; writing—review and editing, Y.U.; visualization, M.I.; supervision, Y.K.; project administration, Y.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the review article.
Informed Consent Statement
Patient consent was waived due to a narrative review.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Acknowledgments
The authors thank Noriko Funamizu (Department of Internal Medicine, Hirose Hospital, Ehime, Japan) for the invaluable advice and discussions regarding the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AC | Adjuvant Chemotherapy |
| AE(s) | Adverse Event(s) |
| BMI | Body Mass Index |
| BTC | Biliary Tract Cancer |
| BW | Body Weight |
| CAR | C-reactive Protein-to-Albumin Ratio |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| GLIM | Global Leadership Initiative on Malnutrition |
| GNRI | Geriatric Nutritional Risk Index |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluations |
| HDAC | Histone deacetylase |
| IL-6 | interleukin-6 |
| JAK | Janus kinase |
| NAC | Neoadjuvant Chemotherapy |
| NCCN | National Comprehensive Cancer Network |
| NF-κB | nuclear factor-κB |
| OCEBM | Oxford Centre for Evidence-Based Medicine |
| ONS(s) | Oral Nutritional Supplement(s) |
| OS | Overall Survival |
| PC | Pancreatic Cancer |
| PEI | Pancreatic Exocrine Insufficiency |
| PERT | Pancreatic Enzyme Replacement Therapy |
| PNI | Prognostic Nutritional Index |
| POC | Postoperative Complication |
| RDI | Relative Dose Intensity |
| SCFA | short-chain fatty acid |
| SMI | skeletal muscle index |
| STAT3 | signal transducer and activator of transcription 3 |
| TNF-α | tumor necrosis factor-α |
References
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Ballesteros-Pomar, M.D.; Barazzoni, R.; Compher, C.; Correia, M.I.T.D.; Cruz-Jentoft, A.J.; García de Lorenzo, A.; Higashiguchi, T.; Keller, H.; et al. Guidance for assessment of the inflammation etiologic criterion for the GLIM diagnosis of malnutrition: A modified Delphi approach. Clin. Nutr. 2024, 43, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Tumas, J.; Tumiene, B.; Jurkeviciene, J.; Gruslys, V.; Šileikis, A.; Strupas, K.; Venskutonis, D.; Barauskas, G.; Pundzius, J.; Gulbinas, A.; et al. Nutritional and immune impairments and their effects on outcomes in early pancreatic cancer patients undergoing pancreatoduodenectomy. Clin. Nutr. 2020, 39, 3385–3394. [Google Scholar] [CrossRef]
- Mękal, D.; Sobocki, J.; Badowska-Kozakiewicz, A.; Ciseł, B.; Rucińska, M.; Kokoszka-Bargieł, I.; Cybulski, M.; Guzińska-Ustymowicz, K.; Kraj, L.; Kaźmierczak-Siedlecka, K.; et al. Evaluation of Nutritional Status and the Impact of Nutritional Treatment in Patients with Pancreatic Cancer. Cancers 2023, 15, 3816. [Google Scholar] [CrossRef]
- Menozzi, R.; Valoriani, F.; Ballarin, R.; Fontana, A.; Giacobazzi, P.; Spaggiari, M.; Brunocilla, E.; Gafà, R.; Faccioli, N.; D’Amico, G.; et al. Impact of nutritional status on postoperative outcomes in cancer patients following elective pancreatic surgery. Nutrients 2023, 15, 1958. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, Z.; Wang, Z.; Feng, J.; Chen, J.; Li, Y.; Zhou, Y.; Xu, L.; Liu, Y.; Huang, L.; et al. Prognostic role of the prognostic nutritional index in patients with pancreatic cancer who underwent curative resection without preoperative neoadjuvant treatment: A systematic review and meta-analysis. Front. Surg. 2022, 9, 992641. [Google Scholar] [CrossRef]
- Grinstead, C.; Yoon, S.L. Geriatric Nutritional Risk Index (GNRI) and Survival in Pancreatic Cancer: A Retrospective Study. Nutrients 2025, 17, 509. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Tanaka, M.; Shirakawa, S.; Shinzeki, M.; Toyama, H.; Asari, S.; Goto, T.; Yamashita, H.; Ishida, J.; Ajiki, T.; et al. Postoperative serum albumin level is a marker of incomplete adjuvant chemotherapy in patients with pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2015, 22, 2408–2415. [Google Scholar]
- Morita, Y.; Sakaguchi, T.; Kitajima, R.; Furuhashi, S.; Kiuchi, R.; Takeda, M.; Hiraide, T.; Shibasaki, Y.; Kikuchi, H.; Konno, H.; et al. Body weight loss after surgery affects the continuity of adjuvant chemotherapy for pancreatic cancer. BMC Cancer 2019, 19, 416. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Pancreatic Adenocarcinoma. Version 2.2025. National Comprehensive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 9 August 2025).
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zülke, C.; Burkart, C.; et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007, 297, 267–277. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; François, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomized, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase III, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef]
- Sakamoto, A.; Funamizu, N.; Shine, M.; Uraoka, M.; Nagaoka, T.; Honjo, M.; Tamura, K.; Sakamoto, K.; Ogawa, K.; Takada, Y. Geriatric nutritional risk index predicts tolerability of S-1 as adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Pancreas 2023, 52, e196–e202. [Google Scholar]
- Funamizu, N.; Sakamoto, A.; Mori, S.; Iwata, M.; Shine, M.; Ito, C.; Uraoka, M.; Ueno, Y.; Tamura, K.; Kamei, Y.; et al. Postoperative geriatric nutritional risk index as a determinant of tolerance to S-1 adjuvant chemotherapy after curative surgery for pancreatic ductal adenocarcinoma: A cohort study with external validation. Cancers 2025, 17, 1448. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Sakamoto, A.; Hikida, T.; Ito, C.; Shine, M.; Nishi, Y.; Uraoka, M.; Nagaoka, T.; Honjo, M.; Tamura, K.; et al. C-reactive protein-to-albumin ratio to predict tolerability of S-1 as an adjuvant chemotherapy in pancreatic cancer. Cancers 2024, 16, 922. [Google Scholar]
- Funamizu, N.; Mori, S.; Sakamoto, A.; Iwata, M.; Shine, M.; Ito, C.; Uraoka, M.; Ueno, Y.; Tamura, K.; Umeda, Y.; et al. C-reactive protein-to-albumin ratio as a predictive indicator for evaluating tolerability in S-1 adjuvant chemotherapy after curative surgery for pancreatic cancer: An external validation cohort study. Cancers 2024, 16, 3372. [Google Scholar]
- Yamada, D.; Takeda, Y.; Takahashi, H.; Sasaki, K.; Iwagami, Y.; Tomimaru, Y.; Noda, T.; Kobayashi, S.; Asaoka, T.; Shimizu, J.; et al. Preoperative nutritional status is a useful predictor of the feasibility of postoperative treatment in octogenarian-plus pancreatic ductal adenocarcinoma patients. Eur. J. Surg. Oncol. 2024, 50, 108650. [Google Scholar]
- Funamizu, N.; Sakamoto, A.; Utsunomiya, T.; Uraoka, M.; Nagaoka, T.; Iwata, M.; Ito, C.; Tamura, K.; Sakamoto, K.; Ogawa, K.; et al. Geriatric nutritional risk index as a potential prognostic marker for patients with resectable pancreatic cancer: A single-center, retrospective cohort study. Sci. Rep. 2022, 12, 13644. [Google Scholar] [CrossRef]
- Trestini, I.; Carbognin, L.; Sperduti, I.; Bonaiuto, C.; Auriemma, A.; Melisi, D.; Salvatore, L.; Bria, E.; Tortora, G. Prognostic impact of early nutritional support in patients affected by locally advanced and metastatic pancreatic ductal adenocarcinoma undergoing chemotherapy. Eur. J. Clin. Nutr. 2018, 72, 772–779. [Google Scholar] [CrossRef]
- Roberts, K.J.; Bannister, C.A.; Schrem, H. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology 2019, 19, 114–121. [Google Scholar] [CrossRef]
- Bruno, M.; Haverkort, E.; Tijssen, G.; Tytgat, G.; van Leeuwen, D.J. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 1998, 42, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.V.; Scoggins, C.R.; Philips, P.; Egger, M.E.; Martin, R.C.G., II. Optimization of exocrine pancreatic insufficiency in pancreatic adenocarcinoma patients. Nutrients 2024, 16, 3499. [Google Scholar] [CrossRef]
- Tsukagoshi, M.; Harimoto, N.; Araki, K.; Kubo, N.; Watanabe, A.; Igarashi, T.; Ishii, N.; Yamanaka, T.; Hagiwara, K.; Hoshino, K.; et al. Impact of Preoperative Nutritional Support and Rehabilitation Therapy in Patients Undergoing Pancreaticoduodenectomy. Int. J. Clin. Oncol. 2021, 26, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, K.; Ohno, E.; Kuramitsu, K.; Kuzuya, T.; Funasaka, K.; Tochio, T.; Fujii, T.; Takahashi, H.; Kondo, N.; Miyahara, R.; et al. Efficacy of 1-Kestose Supplementation in Patients with Pancreatic Ductal Adenocarcinoma: A Randomized Controlled Pilot Study. Nutrients 2024, 16, 2889. [Google Scholar] [CrossRef]
- Mizuno, Y.; Yokoyama, Y.; Nakajima, H.; Inoue, T.; Tanaka, S.; Nagaya, M.; Inokawa, Y.; Ando, M.; Nishida, Y.; Ebata, T.; et al. The Impact of Goal-Directed Prehabilitation Therapy on Functional Capacity in Patients Undergoing Hepatobiliary and Pancreatic Surgery: A Randomized Clinical Trial. Surgery 2024, 176, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, L.; Besselink, M.G.; Sandini, M.; Hackert, T.; Conlon, K.; Gerritsen, A.; Griffin, O.; Fingerhut, A.; Probst, P.; Abu Hilal, M.; et al. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2018, 164, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Agca, S.; Kir, S. The Role of Interleukin-6 Family Cytokines in Cancer Cachexia. FEBS J. 2024, 291, 4009–4023. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef]
- Peng, K.; Xiao, S.; Xia, S.; Li, C.; Yu, H.; Yu, Q. Butyrate inhibits the HDAC8/NF-κB pathway to enhance Slc26a3 expression and improve the intestinal epithelial barrier to relieve colitis. J. Agric. Food Chem. 2024, 72, 24400–24416. [Google Scholar] [CrossRef] [PubMed]
- OCEBM Working Group. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine. 2011. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence (accessed on 8 August 2025).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic. In Reviews of Interventions, Version 6.5 (Updated August 2024); Cochrane: London, UK, 2024; Available online: https://training.cochrane.org/handbook (accessed on 8 August 2025).
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Higashi, T.; Murase, K.; Yokoi, R.; Kuno, M.; Fukada, M.; Tajima, J.Y.; Kiyama, S.; Tanaka, Y.; Okumura, N.; Matsuhashi, N. Association of pre-operative geriatric nutritional risk index with complete adjuvant chemotherapy and prognosis post-pancreatectomy. Anticancer. Res. 2024, 44, 427–434. [Google Scholar] [CrossRef]
- Sun, K.; Chen, S.; Xu, J.; Li, G.; He, Y. The prognostic significance of the prognostic nutritional index in cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1537–1549. [Google Scholar] [CrossRef]
- Geng, Y.; Qi, Q.; Sun, M.; Chen, H.; Wang, P.; Chen, Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur. J. Surg. Oncol. 2015, 41, 1508–1514. [Google Scholar] [CrossRef]
- Maehira, H.; Mori, H.; Nitta, N.; Maekawa, T.; Nishina, Y.; Ishikawa, H.; Takebayashi, K.; Kaida, S.; Miyake, T.; Tani, M. Clinical impact of the prognostic nutritional index and skeletal muscle index for the incompletion of adjuvant chemotherapy for pancreatic cancer. Asian J. Surg. 2025, 48, 1002–1009. [Google Scholar] [CrossRef]
- Kawahara, S.; Aoyama, T.; Murakawa, M.; Kanemoto, R.; Takahashi, D.; Kamioka, Y.; Hashimoto, I.; Maezawa, Y.; Kobayashi, S.; Ueno, M.; et al. Prognostic nutritional index is an independent risk factor for continuing S-1 adjuvant chemotherapy in patients with pancreatic cancer who received neoadjuvant chemotherapy and surgical resection. BMC Cancer 2024, 24, 1469. [Google Scholar] [CrossRef]
- Hajibandeh, S.; Hajibandeh, S.; Romman, S.; Parente, A.; Laing, R.W.; Satyadas, T.; Subar, D.; Aroori, S.; Bhatt, A.; Durkin, D.; et al. Preoperative C-reactive protein-to-albumin ratio and its ability to predict outcomes of pancreatic cancer resection: A systematic review. Biomedicines 2023, 11, 1983. [Google Scholar] [CrossRef]
- Hang, J.; Xue, P.; Yang, H.; Li, S.; Chen, D.; Zhu, L.; Huang, W.; Ren, S.; Zhu, Y.; Wang, L.; et al. Pretreatment C-reactive protein to albumin ratio for predicting overall survival in advanced pancreatic cancer patients. Sci. Rep. 2017, 7, 2993. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Liu, C.; An, L.; Zhang, S.; Deng, S.; Wang, N.; Tang, H. Association between preoperative sarcopenia and prognosis of pancreatic cancer after curative-intent surgery: An updated systematic review and meta-analysis. World J. Surg. Oncol. 2024, 22, 38. [Google Scholar] [CrossRef] [PubMed]
- Raoul, P.; Cintoni, M.; Coppola, A.; Alfieri, S.; Tortora, G.; Gasbarrini, A.; Mele, M.C.; Rinninella, E. Preoperative low skeletal muscle mass index assessed using L3-CT as a prognostic marker of clinical outcomes in pancreatic cancer patients undergoing surgery: A systematic review and meta-analysis. Int. J. Surg. 2024, 110, 6126–6134. [Google Scholar] [CrossRef] [PubMed]
- Griffin, O.M.; Bashir, Y.; O’Connor, D.; Peakin, J.; McMahon, J.; Duggan, S.N.; Geoghegan, J.; Conlon, K.C. Measurement of body composition in pancreatic cancer: A systematic review, meta-analysis, and recommendations for future study design. Dig. Surg. 2022, 39, 141–152. [Google Scholar] [CrossRef]
- Bundred, J.; Kamarajah, S.K.; Roberts, K.J. Body composition assessment and sarcopenia in patients with pancreatic cancer: A systematic review and meta-analysis. HPB 2019, 21, 1603–1612. [Google Scholar] [CrossRef]
- Takagi, K.; Inoue, Y.; Oba, A.; Ono, Y.; Sato, T.; Ito, H.; Saino, Y.; Saiura, A.; Takahashi, Y. Impact of sarcopenia on S1 adjuvant chemotherapy and prognosis in pancreatic cancer patients. Biosci. Trends 2023, 17, 310–317. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Boutin, R.D.; Lenchik, L.; Kim, J.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L., 3rd; Guthrie, K.A.; Chiorean, E.G.; Ahmad, S.A.; et al. Body composition measurements and clinical outcomes in patients with resectable pancreatic adenocarcinoma: Analysis from SWOG S1505. J. Gastrointest. Surg. 2024, 28, 232–235. [Google Scholar] [CrossRef]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Satoi, S.; Toyama, H.; Matsumoto, I.; Aosasa, S.; Shirakawa, H.; Wada, K.; Fujii, T.; et al. Neoadjuvant Chemotherapy with Gemcitabine and S-1 Versus Upfront Surgery for Resectable Pancreatic Cancer: Results of the Randomized Phase II/III Prep-02/JSAP05 Trial. Ann. Surg. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, T.; Nara, S.; Ban, D.; Mizui, T.; Miyata, A.; Esaki, M. Neoadjuvant gemcitabine and S-1 in pancreatic ductal adenocarcinoma: Effects on nutritional status and pancreaticoduodenectomy outcomes. Surgery 2025, 180, 109026. [Google Scholar] [CrossRef] [PubMed]
- Sabater, L.; Ausania, F.; Bakker, O.J.; Boadas, J.; Domínguez-Muñoz, J.E.; Falconi, M.; Fernández-Cruz, L.; Frulloni, L.; González-Sánchez, V.; Lariño-Noia, J.; et al. Evidence-based guidelines for the management of exocrine pancreatic insufficiency after pancreatic surgery. Ann. Surg. 2016, 264, 949–958. [Google Scholar] [CrossRef]
- Gianotti, L.; Braga, M.; Nespoli, L.; Radaelli, G.; Beneduce, A.; Di Carlo, V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002, 122, 1763–1770. [Google Scholar] [CrossRef]
- Vella, R.; Pizzocaro, E.; Bannone, E.; Gualtieri, P.; Frank, G.; Giardino, A.; Frigerio, I.; Pastorelli, D.; Gruttadauria, S.; Mazzali, G.; et al. Nutritional intervention for the elderly during chemotherapy: A systematic review. Cancers 2024, 16, 2809. [Google Scholar] [CrossRef]
- Ueno, M.; Sugimori, K.; Taguri, M.; Ohkawa, S.; Kobayashi, S.; Miwa, H.; Kaneko, T.; Morimoto, M.; Yamanaka, T. Randomized phase II study of gemcitabine monotherapy vs. gemcitabine with an EPA-enriched oral supplement in advanced pancreatic cancer. Nutr. Cancer 2022, 74, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, A.; Krampitz, J.; Rosenberger, F.; Kind, S.; Rötzer, I. Nutritional interventions in pancreatic cancer: A systematic review. Cancers 2022, 14, 2212. [Google Scholar] [CrossRef]
- Pires, L.B.C.; Salaroli, L.B.; Podesta, O.P.G.; Haraguchi, F.K.; Lopes-Júnior, L.C. Omega-3 supplementation and nutritional status in patients with pancreatic neoplasms: A systematic review. Nutrients 2024, 16, 4036. [Google Scholar] [CrossRef]
- Trestini, I.; Cintoni, M.; Rinninella, E.; Grassi, F.; Paiella, S.; Salvia, R.; Bria, E.; Pozzo, C.; Alfieri, S.; Gasbarrini, A.; et al. Neoadjuvant treatment: A window of opportunity for nutritional prehabilitation in patients with pancreatic ductal adenocarcinoma. World J. Gastrointest. Surg. 2021, 13, 885–903. [Google Scholar] [CrossRef]
- Ngo-Huang, A.T.; Parker, N.H.; Xiao, L.; Schadler, K.L.; Petzel, M.Q.B.; Prakash, L.R.; Kim, M.P.; Tzeng, C.-W.D.; Lee, J.E.; Ikoma, N.; et al. Effects of a Pragmatic Home-Based Exercise Program Concurrent with Neoadjuvant Therapy on Physical Function of Patients with Pancreatic Cancer: The PancFit Randomized Clinical Trial. Ann. Surg. 2023, 278, 22–30. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xue, K.; Zhang, Y.; Tian, B. Geriatric Nutritional Risk Index Predicts Postoperative Outcomes in Elderly Patients with Pancreatoduodenectomy: A Propensity Score-Matched Analysis. Gland. Surg. 2025, 14, 807–817. [Google Scholar] [CrossRef]
- Gündoğdu, E.; Karahan, B.N.; Şendil, A.M.; Zengin, A.; Ulaş, M.; Kılıç, M. The Prognostic Impact of Preoperative Nutritional Status on Postoperative Complications and Overall Survival in Patients with Resectable Pancreatic Cancer. Support. Care Cancer 2025, 33, 240. [Google Scholar] [CrossRef]
- Funamizu, N.; Mori, S.; Sakamoto, A.; Honjo, M.; Tamura, K.; Sakamoto, K.; Ogawa, K.; Umeda, Y.; Aoki, T.; Takada, Y.; et al. Novel Modified Frailty Index Predicts Completion of Adjuvant Chemotherapy in Resectable Pancreatic Cancer in a Dual Center Study. Sci. Rep. 2025, 15, 17000. [Google Scholar] [CrossRef]
- Harimoto, N.; Sugimachi, K.; Nishijima, T.F.; Tomino, T.; Shimagaki, T.; Mano, Y.; Onishi, E.; Sugiyama, M.; Kimura, Y.; Morita, M. Combined Effect of Frailty and Sarcopenia on Postoperative Complications in Older Adults Undergoing Curative Surgery for Hepato-Biliary-Pancreatic Cancer. Ann. Gastroenterol. Surg. 2024, 9, 587–594. [Google Scholar] [CrossRef]
- Funamizu, N.; Omura, K.; Takada, Y.; Ozaki, T.; Mishima, K.; Igarashi, K.; Wakabayashi, G. Geriatric Nutritional Risk Index Less Than 92 Is a Predictor for Late Postpancreatectomy Hemorrhage Following Pancreatoduodenectomy: A Retrospective Cohort Study. Cancers 2020, 12, 2779. [Google Scholar] [CrossRef]
- Funamizu, N.; Sogabe, K.; Shine, M.; Utsunomiya, T.; Honjo, M.; Ito, C.; Uraoka, M.; Nagaoka, T.; Tamura, K.; Sakamoto, K.; et al. Association between the Preoperative C-Reactive Protein-to-Albumin Ratio and the Risk for Postoperative Pancreatic Fistula following Distal Pancreatectomy for Pancreatic Cancer. Nutrients 2022, 14, 5277. [Google Scholar] [CrossRef]
- Mortier, V.; Wei, F.; Pellat, A.; Marchese, U.; Dohan, A.; Brezault, C.; Barat, M.; Fuks, D.; Soyer, P.; Coriat, R. Impact of Sarcopenia on Patients with Localized Pancreatic Ductal Adenocarcinoma Receiving FOLFIRINOX or Gemcitabine as Adjuvant Chemotherapy. Cancers 2022, 14, 6179. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Tamura, T.; Minagawa, N.; Hirata, K. Preoperative Body Mass Index-to-Prognostic Nutritional Index Ratio Predicts Pancreatic Fistula after Pancreaticoduodenectomy. Hepatobiliary Surg. Nutr. 2016, 5, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Cintoni, M.; Grassi, F.; Palombaro, M.; Rinninella, E.; Pulcini, G.; Di Donato, A.; Salvatore, L.; Quero, G.; Tortora, G.; Alfieri, S.; et al. Nutritional interventions during chemotherapy for pancreatic cancer: A systematic review of prospective studies. Nutrients 2023, 15, 727. [Google Scholar] [CrossRef] [PubMed]
- Poulia, K.A.; Antoniadou, D.; Sarantis, P.; Karamouzis, M.V. Pancreatic Cancer Prognosis, Malnutrition Risk, and Quality of Life: A Cross-Sectional Study. Nutrients 2022, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.; Spiro, A.; Ahern, R.; Emery, P.W. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2012, 104, 371–385. [Google Scholar] [CrossRef]
- Giordano, G.; Cincione, R.I.; Losavio, F.; Senia, T.; Aquilini Mummolo, A.; Pacilli, M.; Lizzi, V.; Bruno, G.; Piscazzi, A.; Conteduca, V.; et al. Pancreatic Enzyme Replacement and Nutritional Support with nab-Paclitaxel-Based First-Line Chemotherapy Regimens in Metastatic Pancreatic Cancer. Oncologist 2023, 28, e793–e800. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-Associated Cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type-Specific Intracellular Bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.-Y.; Pan, R.-L.; Zhang, X.-T.; Si, X.-Y.; Chen, M.-J.; Wang, M.-Z.; Zhang, L. Tocilizumab for Advanced Non-Small-Cell Lung Cancer With Concomitant Cachexia: An Observational Study. J. Cachexia Sarcopenia Muscle 2024, 15, 2815–2825. [Google Scholar] [CrossRef]
- Chen, P.; Wang, D.; Zhan, Z.; Chen, L.; Chen, Y. Tocilizumab in Combination with Corticosteroids: Potential for Managing Cancer Cachexia with Systemic Hyperinflammation. Front. Immunol. 2024, 15, 1477310. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Vining, D.J.; Arora, S.P.; de Achaval, S.; Larson, J.; Kauh, J.; Cartwright, C.; Avritscher, R.; Alibhai, I.; Tweardy, D.J.; et al. Phase I trial of TTI-101, a first-in-class oral inhibitor of STAT3, in patients with advanced solid tumors. Clin. Cancer Res. 2025, 31, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Latenstein, A.E.J.; Dijksterhuis, W.P.M.; Mackay, T.M.; Beijer, S.; van Eijck, C.H.J.; de Hingh, I.H.J.T.; Molenaar, I.Q.; van Oijen, M.G.H.; van Santvoort, H.C.; de van der Schueren, M.A.E.; et al. Cachexia, dietetic consultation, and survival in patients with pancreatic and periampullary cancer: A multicenter cohort study. Cancer Med. 2020, 9, 9385–9395. [Google Scholar] [CrossRef] [PubMed]
- Petzel, M.Q.B.; Hoffman, L. Nutrition Implications for Long-Term Survivors of Pancreatic Cancer Surgery. Nutr. Clin. Pract. 2017, 32, 588–598. [Google Scholar] [CrossRef]
- Klassen, P.N.; Mazurak, V.C.; Baracos, V.; Martin, L.; Ghosh, S.; Kasnik, K.; Sawyer, M.B. Dose Optimization of Pancreatic Enzyme Replacement Therapy Is Essential to Mitigate Muscle Loss in Patients with Advanced Pancreatic Cancer and Exocrine Pancreatic Insufficiency. Clin. Nutr. 2024, 43, 1900–1906. [Google Scholar] [CrossRef]
- Di Martino, M.; de la Hoz Rodriguez, Á.; Saibanti, A.; Salvador Camarmo, G.; Pagano, N.; Martín-Pérez, E.; Donadon, M. Pancreatic Exocrine Insufficiency after Pancreatic Resection: A Systematic Review. BMC Surg. 2025, 25, 53. [Google Scholar] [CrossRef]
- Rovesti, G.; Valoriani, F.; Rimini, M.; Bardasi, C.; Ballarin, R.; Di Benedetto, F.; Menozzi, R.; Dominici, M.; Spallanzani, A. Clinical implications of malnutrition in the management of patients with pancreatic cancer: Introducing the concept of the nutritional oncology board. Nutrients 2021, 13, 3522. [Google Scholar] [CrossRef]
- Flint, T.R.; Janowitz, T.; Connell, C.M.; Roberts, E.W.; Denton, A.E.; Coll, A.P.; Jodrell, D.I.; Fearon, D.T. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-Tumor Immunity. Cell Metab. 2016, 24, 672–684. [Google Scholar] [CrossRef]
- Dasgupta, A.; Gibbard, D.F.; Schmitt, R.E.; Arneson-Wissink, P.; Ducharme, J.; Bruinsma, M.W.; Hawse, J.R.; Jatoi, A.; Doles, J. A TGF-β/KLF10 Signaling Axis Regulates Atrophy-Associated Genes to Induce Muscle Wasting in Pancreatic Cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2215095120. [Google Scholar] [CrossRef] [PubMed]
- Vaes, R.D.W.; van Bijnen, A.A.; Olde Damink, S.W.M.; Rensen, S.S. Pancreatic Tumor Organoid-Derived Factors from Cachectic Patients Disrupt Contractile Smooth Muscle Cells. Cancers 2024, 16, 542. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Knees, J.W.; Falkenthal, J.; Geisel, D.; Neumann, C.C.M.; Hilfenhaus, G.; Stephan, L.U.; Schöning, W.; Malinka, T.; Pratschke, J.; Stintzing, S.; et al. Cachexia-Affected Survival Based on Inflammatory Parameters Compared to Complex Conventional Nutritional Assessments in Patients with Pancreatic Cancer and Other Gastrointestinal Tumors—The CONKO 020 Investigation. Cancers 2024, 16, 1194. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- von Haehling, S.; Coats, A.J.S.; Anker, S.D. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: Update 2023. J. Cachexia Sarcopenia Muscle 2023, 14, 2981–2983. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Therapeutic Strategies Against Cancer Cachexia. Eur. J. Transl. Myol. 2019, 29, 7960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Shi, G.; Shi, J.; Li, Z.; Xiao, Y.; Qiu, Y.; He, L.; Xie, F.; Yu, D.; Cao, H.; et al. Research progress on the mechanism and treatment of cachexia based on tumor microenvironment. Nutrition 2025, 133, 112697. [Google Scholar] [CrossRef]
- Funamizu, N.; Honjo, M.; Tamura, K.; Sakamoto, K.; Ogawa, K.; Takada, Y. microRNAs Associated with Gemcitabine Resistance via EMT, TME, and Drug Metabolism in Pancreatic Cancer. Cancers 2023, 15, 1230. [Google Scholar] [CrossRef]
- Funamizu, N.; Hu, C.; Lacy, C.R.; Schetter, A.N.; Zhang, G.; He, P.; Gaedcke, J.; Ghadimi, M.B.; Ried, T.; Yfantis, H.G.; et al. Macrophage migration inhibitory factor induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int. J. Cancer 2013, 132, 785–794. [Google Scholar]
- Funamizu, N.; Lacy, C.R.; Parpart, S.T.; Takai, A.; Hiyoshi, Y.; Yanaga, K. MicroRNA-301b promotes cell invasiveness through targeting TP63 in pancreatic carcinoma cells. Int. J. Oncol. 2014, 44, 725–734. [Google Scholar] [CrossRef]
- Funamizu, N.; Lacy, C.R.; Kamada, M.; Yanaga, K.; Manome, Y. MicroRNA-200b and -301 are associated with gemcitabine response as biomarkers in pancreatic carcinoma cells. Int. J. Oncol. 2019, 54, 991–1000. [Google Scholar] [CrossRef]
- Yu, J.; Ohuchida, K.; Mizumoto, K.; Sato, N.; Kayashima, T.; Fujita, H.; Nakata, K.; Tanaka, M. MicroRNA, hsa-miR-200c, Is an Independent Prognostic Factor in Pancreatic Cancer and Its Upregulation Inhibits Pancreatic Cancer Invasion but Increases Cell Proliferation. Mol. Cancer 2010, 9, 169. [Google Scholar] [CrossRef]
- LaConti, J.J.; Shivapurkar, N.; Preet, A.; Deslattes Mays, A.; Peran, I.; Kim, S.E.; Marshall, J.L.; Riegel, A.T.; Wellstein, A. Tissue and serum microRNAs in the Kras(G12D) transgenic animal model and in patients with pancreatic cancer. PLoS ONE 2011, 6, e20687. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol. 2017, 15, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Deng, D.; Wang, B.; Donati, V.; Frampton, A.E.; Giovannetti, E. Metabolites Derived from Gut Microbiota Mitigate Chemoresistance in Pancreatic Cancer. Expert. Rev. Gastroenterol. Hepatol. 2024, 18, 597–604. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential Role of Intratumor Bacteria in Mediating Tumor Resistance to Gemcitabine Chemotherapy. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).