Dual PET Imaging with [68Ga]Ga-DOTA-TOC and [18F]FDG to Localize Neuroendocrine Tumors of Unknown Origin
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
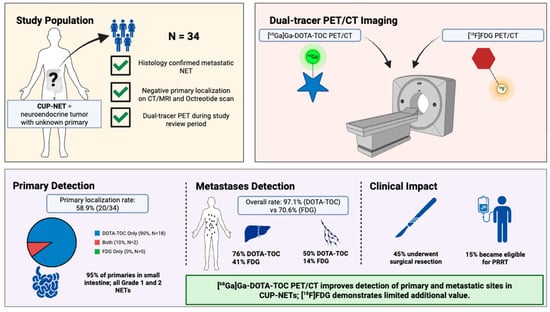
2.1. Study Population
2.2. [68Ga]Ga-DOTA-TOC and [18F]FDG Preparation
2.3. PET/CT Imaging
2.4. Visual Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Tumor Detection by PET/CT
3.3. Comparison of PET/CT Modalities for Metastatic Disease
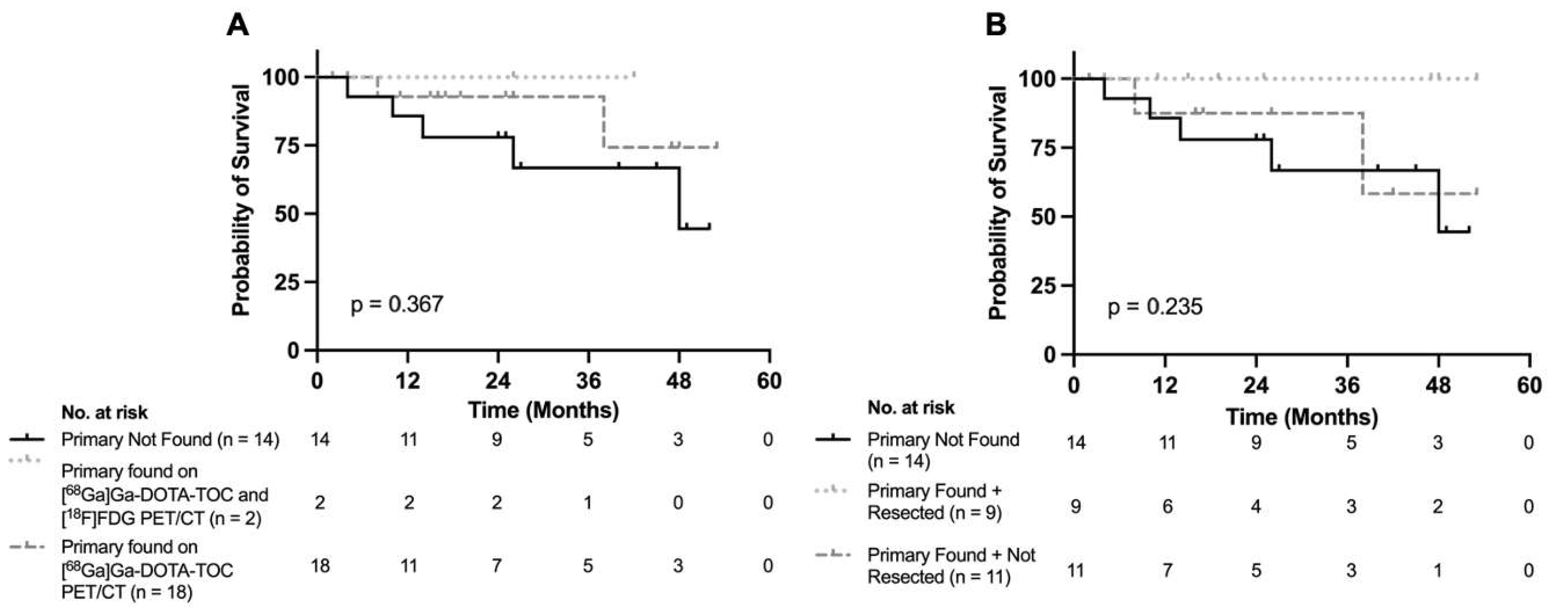
3.4. Treatment and Survival Implications Following Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NET | Neuroendocrine tumor |
| PET | Positron emission tomography |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| SUVmax | Maximum standardized uptake value |
| CUP-NET | Neuroendocrine tumor of unknown primary |
| CUP | Cancer of unknown primary |
| PRRT | Peptide receptor radionuclide therapy |
| SSTR | Somatostatin receptor |
| FDG | Fluoro-deoxy-glucose |
| LN | Lymph node |
| NEC | Neuroendocrine carcinoma |
References
- Pavlidis, N.; Pentheroudakis, G. Cancer of Unknown Primary Site. Lancet 2012, 379, 1428–1435. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O. A Real-World, Population-Based Study for the Incidence and Outcomes of Neuroendocrine Neoplasms of Unknown Primary. Neuroendocrinology 2021, 111, 876–882. [Google Scholar] [CrossRef]
- Fendrich, V.; Bartsch, D.K. Surgical Treatment of Gastrointestinal Neuroendocrine Tumors. Langenbeck’s Arch. Surg. 2011, 396, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Bertani, E.; Fazio, N.; Radice, D.; Zardini, C.; Spinoglio, G.; Chiappa, A.; Ribero, D.; Biffi, R.; Partelli, S.; Falconi, M. Assessing the Role of Primary Tumour Resection in Patients with Synchronous Unresectable Liver Metastases from Pancreatic Neuroendocrine Tumour of the Body and Tail. A Propensity Score Survival Evaluation. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 372–379. [Google Scholar] [CrossRef]
- Bertani, E.; Fazio, N.; Radice, D.; Zardini, C.; Grana, C.; Bodei, L.; Funicelli, L.; Ferrari, C.; Spada, F.; Partelli, S.; et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1–G2 Pancreatic Neuroendocrine Tumors with Unresectable Liver Metastases. Ann. Surg. Oncol. 2016, 23, 981–989. [Google Scholar] [CrossRef]
- Polcz, M.; Schlegel, C.; Edwards, G.C.; Wang, F.; Tan, M.; Kiernan, C.; Solórzano, C.C.; Idrees, K.; Parikh, A.; Bailey, C.E. Primary Tumor Resection Offers Survival Benefit in Patients with Metastatic Midgut Neuroendocrine Tumors. Ann. Surg. Oncol. 2020, 27, 2795–2803. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.; the Commonwealth Neuroendocrine Tumours Research Collaborative (CommNETs) Surgical Section; Hallet, J.; Law, C.; Pasieka, J.; Koea, J.; Meyer-Rochow, W. Role of Primary Tumor Resection for Metastatic Small Bowel Neuroendocrine Tumors. World J. Surg. 2021, 45, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Modica, R.; Benevento, E.; Liccardi, A.; Cannavale, G.; Minotta, R.; DI Iasi, G.; Colao, A. Recent Advances and Future Challenges in the Diagnosis of Neuroendocrine Neoplasms. Minerva Endocrinol. 2024, 49, 158–174. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; De Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Vahidfar, N.; Farzanehfar, S.; Abbasi, M.; Mirzaei, S.; Delpassand, E.S.; Abbaspour, F.; Salehi, Y.; Biersack, H.J.; Ahmadzadehfar, H. Diagnostic Value of Radiolabelled Somatostatin Analogues for Neuroendocrine Tumour Diagnosis: The Benefits and Drawbacks of [64Cu]Cu-DOTA-TOC. Cancers 2022, 14, 1914. [Google Scholar] [CrossRef]
- Fortunati, E.; Argalia, G.; Zanoni, L.; Fanti, S.; Ambrosini, V. New PET Radiotracers for the Imaging of Neuroendocrine Neoplasms. Curr. Treat. Options Oncol. 2022, 23, 703–720. [Google Scholar] [CrossRef]
- Franchina, M.; Cavalcoli, F.; Falco, O.; La Milia, M.; Elvevi, A.; Massironi, S. Biochemical Markers for Neuroendocrine Tumors: Traditional Circulating Markers and Recent Development—A Comprehensive Review. Diagnostics 2024, 14, 1289. [Google Scholar] [CrossRef]
- Lopez-Ramirez, F.; Yasrab, M.; Tixier, F.; Kawamoto, S.; Fishman, E.K.; Chu, L.C. The Role of AI in the Evaluation of Neuroendocrine Tumors: Current State of the Art. Semin. Nucl. Med. 2025, 55, 345–357. [Google Scholar] [CrossRef]
- Wang, S.C.; Parekh, J.R.; Zuraek, M.B.; Venook, A.P.; Bergsland, E.K.; Warren, R.S.; Nakakura, E.K. Identification of Unknown Primary Tumors in Patients With Neuroendocrine Liver Metastases. Arch. Surg. 2010, 145, 276. [Google Scholar] [CrossRef]
- Savelli, G.; Lucignani, G.; Seregni, E.; Marchiano, A.; Serafini, G.; Aliberti, G.; Villano, C.; Maccauro, M.; Bombardieri, E. Feasibility of Somatostatin Receptor Scintigraphy in the Detection of Occult Primary Gastro-Entero-Pancreatic (GEP) Neuroendocrine Tumours. Nucl. Med. Commun. 2004, 25, 445–449. [Google Scholar] [CrossRef]
- Schreiter, N.F.; Bartels, A.-M.; Froeling, V.; Steffen, I.; Pape, U.-F.; Beck, A.; Hamm, B.; Brenner, W.; Röttgen, R. Searching for Primaries in Patients with Neuroendocrine Tumors (NET) of Unknown Primary and Clinically Suspected NET: Evaluation of Ga-68 DOTATOC PET/CT and In-111 DTPA Octreotide SPECT/CT. Radiol. Oncol. 2014, 48, 339–347. [Google Scholar] [CrossRef]
- Buchmann, I.; Henze, M.; Engelbrecht, S.; Eisenhut, M.; Runz, A.; Schäfer, M.; Schilling, T.; Haufe, S.; Herrmann, T.; Haberkorn, U. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in Patients with Neuroendocrine Tumours. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1617–1626. [Google Scholar] [CrossRef]
- Deppen, S.A.; Blume, J.; Bobbey, A.J.; Shah, C.; Graham, M.M.; Lee, P.; Delbeke, D.; Walker, R.C. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2016, 57, 872–878. [Google Scholar] [CrossRef]
- Kayani, I.; Bomanji, J.B.; Groves, A.; Conway, G.; Gacinovic, S.; Win, T.; Dickson, J.; Caplin, M.; Ell, P.J. Functional Imaging of Neuroendocrine Tumors with Combined PET/CT Using 68Ga-DOTATATE (DOTA- D Phe1,Tyr3 -octreotate) and 18F-FDG. Cancer 2008, 112, 2447–2455. [Google Scholar] [CrossRef]
- Bucau, M.; Laurent-Bellue, A.; Poté, N.; Hentic, O.; Cros, J.; Mikail, N.; Rebours, V.; Ruszniewski, P.; Lebtahi, R.; Couvelard, A. 18F-FDG Uptake in Well-Differentiated Neuroendocrine Tumors Correlates with Both Ki-67 and VHL Pathway Inactivation. Neuroendocrinology 2018, 106, 274–282. [Google Scholar] [CrossRef]
- Frilling, A.; Sotiropoulos, G.C.; Radtke, A.; Malago, M.; Bockisch, A.; Kuehl, H.; Li, J.; Broelsch, C.E. The Impact of 68Ga-DOTATOC Positron Emission Tomography/Computed Tomography on the Multimodal Management of Patients With Neuroendocrine Tumors. Ann. Surg. 2010, 252, 850–856. [Google Scholar] [CrossRef]
- Prasad, V.; Ambrosini, V.; Hommann, M.; Hoersch, D.; Fanti, S.; Baum, R.P. Detection of Unknown Primary Neuroendocrine Tumours (CUP-NET) Using 68Ga-DOTA-NOC Receptor PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 67–77. [Google Scholar] [CrossRef]
- Łapińska, G.; Bryszewska, M.; Fijołek-Warszewska, A.; Kozłowicz-Gudzińska, I.; Ochman, P.; Sackiewicz-Słaby, A. The Diagnostic Role of 68Ga-DOTATATE PET/CT in the Detection of Neuroendocrine Tumours. Nucl. Med. Rev. Cent. East. Eur. 2011, 14, 16–20. [Google Scholar] [CrossRef]
- Naswa, N.; Sharma, P.; Kumar, A.; Soundararajan, R.; Kumar, R.; Malhotra, A.; Ammini, A.C.; Bal, C. 68Ga-DOTANOC PET/CT in Patients With Carcinoma of Unknown Primary of Neuroendocrine Origin. Clin. Nucl. Med. 2012, 37, 245–251. [Google Scholar] [CrossRef]
- Tan, D.S.-W.; Montoya, J.; Ng, Q.-S.; Chan, K.-S.; Lynette, O.; Sakktee Krisna, S.; Takano, A.; Lim, W.-T.; Tan, E.-H.; Lim, K.-H. Molecular Profiling for Druggable Genetic Abnormalities in Carcinoma of Unknown Primary. J. Clin. Oncol. 2013, 31, e237–e239. [Google Scholar] [CrossRef]
- Alonso, O.; Rodríguez-Taroco, M.; Savio, E.; Bentancourt, C.; Gambini, J.P.; Engler, H. 68Ga-DOTATATE PET/CT in the Evaluation of Patients with Neuroendocrine Metastatic Carcinoma of Unknown Origin. Ann. Nucl. Med. 2014, 28, 638–645. [Google Scholar] [CrossRef]
- Nakamoto, Y.; Sano, K.; Ishimori, T.; Ueda, M.; Temma, T.; Saji, H.; Togashi, K. Additional Information Gained by Positron Emission Tomography with 68Ga-DOTATOC for Suspected Unknown Primary or Recurrent Neuroendocrine Tumors. Ann. Nucl. Med. 2015, 29, 512–518. [Google Scholar] [CrossRef]
- Pruthi, A.; Pankaj, P.; Verma, R.; Jain, A.; Belho, E.S.; Mahajan, H. Ga-68 DOTANOC PET/CT Imaging in Detection of Primary Site in Patients with Metastatic Neuroendocrine Tumours of Unknown Origin and Its Impact on Clinical Decision Making: Experience from a Tertiary Care Centre in India. J. Gastrointest. Oncol. 2016, 7, 449–461. [Google Scholar] [CrossRef]
- Sadowski, S.M.; Neychev, V.; Millo, C.; Shih, J.; Nilubol, N.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Kebebew, E. Prospective Study of 68 Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J. Clin. Oncol. 2016, 34, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, P.M.; Rominger, A.; Wenter, V.; Spitzweg, C.; Auernhammer, C.; Angele, M.K.; Rist, C.; Cyran, C.C. The Added Value of 68Ga-DOTA-TATE-PET to Contrast-Enhanced CT for Primary Site Detection in CUP of Neuroendocrine Origin. Eur. Radiol. 2017, 27, 1676–1684. [Google Scholar] [CrossRef]
- Menda, Y.; O’Dorisio, T.M.; Howe, J.R.; Schultz, M.; Dillon, J.S.; Dick, D.; Watkins, G.L.; Ginader, T.; Bushnell, D.L.; Sunderland, J.J.; et al. Localization of Unknown Primary Site with 68Ga-DOTATOC PET/CT in Patients with Metastatic Neuroendocrine Tumor. J. Nucl. Med. 2017, 58, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Sampathirao, N.; Basu, S. MIB-1 Index–Stratified Assessment of Dual-Tracer PET/CT with 68Ga-DOTATATE and 18F-FDG and Multimodality Anatomic Imaging in Metastatic Neuroendocrine Tumors of Unknown Primary in a PRRT Workup Setting. J. Nucl. Med. Technol. 2017, 45, 34–41. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chang, Y.-C.; Hwang, T.-L.; Chen, J.-S.; Chou, W.-C.; Hsieh, C.-H.; Yeh, T.-S.; Hsu, J.-T.; Yeh, C.-N.; Tseng, J.-H.; et al. 68Ga-DOTATOC and 18F-FDG PET/CT for Identifying the Primary Lesions of Suspected and Metastatic Neuroendocrine Tumors: A Prospective Study in Taiwan. J. Formos. Med. Assoc. 2018, 117, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef]
- Santhanam, P.; Chandramahanti, S.; Kroiss, A.; Yu, R.; Ruszniewski, P.; Kumar, R.; Taïeb, D. Nuclear Imaging of Neuroendocrine Tumors with Unknown Primary: Why, When and How? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1144–1155. [Google Scholar] [CrossRef]
- Breeman, W.A.P.; De Jong, M.; De Blois, E.; Bernard, B.F.; Konijnenberg, M.; Krenning, E.P. Radiolabelling DOTA-Peptides with 68Ga. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 478–485. [Google Scholar] [CrossRef]
- De Dosso, S.; Treglia, G.; Pascale, M.; Tamburello, A.; Santhanam, P.; Kroiss, A.S.; Pereira Mestre, R.; Saletti, P.; Giovanella, L. Detection Rate of Unknown Primary Tumour by Using Somatostatin Receptor PET/CT in Patients with Metastatic Neuroendocrine Tumours: A Meta-Analysis. Endocrine 2019, 64, 456–468. [Google Scholar] [CrossRef]
- Navin, P.J.; Ehman, E.C.; Liu, J.B.; Halfdanarson, T.R.; Gupta, A.; Laghi, A.; Yoo, D.C.; Carucci, L.R.; Schima, W.; Sheedy, S.P. Imaging of Small-Bowel Neuroendocrine Neoplasms: AJR Expert Panel Narrative Review. Am. J. Roentgenol. 2023, 221, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Massimino, K.P.; Han, E.; Pommier, S.J.; Pommier, R.F. Laparoscopic Surgical Exploration Is an Effective Strategy for Locating Occult Primary Neuroendocrine Tumors. Am. J. Surg. 2012, 203, 628–631. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Chauhan, A.; Rau, J.; Diebold, A.E.; Opoku-Boateng, A.; Ramcharan, T.; Boudreaux, J.P.; Woltering, E.A. Neuroendocrine Tumors (NETs) of Unknown Primary: Is Early Surgical Exploration and Aggressive Debulking Justifiable? Chin. Clin. Oncol. 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed]
| Primary Site Localization Status | All (n = 34) | Yes (n =20) | No (n = 14) | p |
|---|---|---|---|---|
| Sex, n (%) | 0.127 | |||
| Female | 19 (55.9) | 9 (45.0) | 10 (71.4) | |
| Median Age at Diagnosis (Range) | 68 (28–86) | 66.5 (35–85) | 69 (28–86) | 0.752 |
| Median Days from Diagnosis to First PET Scan (Range) | 275 (17–3206) | 181 (17–2894) | 1575 (78–3206) | 0.003 |
| Median Days between DOTA-TOC and Octreotide Scans (Range) | 150 (23–1654) | 103.5 (23–610) | 168.5 (43–1654) | 0.087 |
| Median Days between DOTA-TOC and FDG scans (Range) | 4 (1–48) | 4 (1–48) | 5 (1–11) | 0.704 |
| Octreotide Positive Scan Rate for Metastases, n (%) | 31 (91.2) | 19 (95.0) | 12 (85.7) | 0.556 |
| Metastatic Site of Biopsy, n (%) | ||||
| Liver | 26 (76.5) | 15 (75.0) | 11 (78.6) | |
| Lymph Node | 7 (20.6) | 5 (25.0) | 3 (21.4) | |
| Breast | 1 (2.9) | 0 (0.0) | 1 (7.1) | |
| Tumor Grade, n (%) | ||||
| Grade 1 | 14 (41.2) | 12 (60.0) | 2 (14.3) | |
| Grade 2 | 18 (52.9) | 8 (40.0) | 10 (71.4) | |
| Grade 3 | 2 (5.9) | 0 (0.0) | 2 (14.3) | |
| Tumor Differentiation, n (%) | ||||
| Well Differentiated | 34 (100) | 20 (100) | 14 (100) | |
| Poorly Differentiated | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No. | Primary Site | Ki-67 (%) | DOTA-TOC Assessment | FDG Assessment | Localizing Modality | Metastasis | Surgical Resection |
|---|---|---|---|---|---|---|---|
| 1 | Small Intestine | 5 | + | − | DOTA-TOC | Liver | No |
| 2 | Small Intestine | NR | + | + | DOTA-TOC | Liver, LN, | Yes |
| 3 | Small Intestine | 5 | + | + | DOTA-TOC | Liver | Yes |
| 4 | Small Intestine | 3 | + | − | DOTA-TOC | Liver | Yes |
| 5 | Small Intestine | <2 | + | + | Both | Liver, LN, Bone | No |
| 6 | Small Intestine | 1–2 | + | − | DOTA-TOC | LN | No |
| 7 | Small Intestine | 1–2 | + | + | DOTA-TOC | Liver, LN | No |
| 8 | Small Intestine | 7 | + | + | Both | Liver, LN, Bone | No |
| 9 | Small Intestine | 2 | + | − | DOTA-TOC | Liver, LN | Yes |
| 10 | Small Intestine | 2 | + | − | DOTA-TOC | Liver, LN | Yes |
| 11 | Small Intestine | <1 | + | + | DOTA-TOC | Liver, LN | No |
| 12 | Small Intestine | <3 | + | − | DOTA-TOC | Liver, LN, Bone | No |
| 13 | Small Intestine | <1 | + | − | DOTA-TOC | Liver | Yes |
| 14 | Small Intestine | 5 | + | + | DOTA-TOC | Liver | Yes |
| 15 | Small Intestine | <1 | + | + | DOTA-TOC | LN | Yes |
| 16 | Small Intestine | <1 | + | + | DOTA-TOC | LN | Yes |
| 17 | Small Intestine | <3 | + | + | DOTA-TOC | Liver | No |
| 18 | Stomach | 12 | + | + | DOTA-TOC | Liver, Spleen | No |
| 19 | Small Intestine | 10 | + | − | DOTA-TOC | Liver | No |
| 20 | Small Intestine | 4 | + | + | DOTA-TOC | LN, Bone, Lung | No |
| Imaging Characteristic | Value |
|---|---|
| Primary Site, n (%) | |
| Small Intestine | 19 (95.0) |
| Ileum | 7 (36.8) |
| Jejunum and ileum | 2 (10.5) |
| Not specified | 9 (47.4) |
| Stomach | 1 (5.0) |
| DOTA-TOC SUVmax, mean ± SD | 15.7 ± 9.0 |
| DOTA-TOC Lesion Size, mean ± SD (mm) | 14.8 ± 3.4 |
| Primary Site Localization Status | All Patients with CUP-NET (n = 34) | p | CUP-NETs with a Primary Identified by PET/CT (n = 20) | p | No Primary Identified by PET/CT (n = 14) | p | |||
|---|---|---|---|---|---|---|---|---|---|
| [68Ga]Ga-DOTA-TOC | [18F]FDG | [68Ga]Ga-DOTA-TOC | [18F]FDG | [68Ga]Ga-DOTA-TOC | [18F]FDG | ||||
| Overall Scan Positivity, n (%) [95% CI] | 33 (97.1) [0.84–1.00] | 24 (70.6) [0.54–0.83] | 0.006 | 20 (100) [0.82–1.0] | 12 (60.0) [0.36–0.81] | 0.003 | 13 (92.9) [0.66–1.00] | 12 (85.7) [0.59–0.97] | 1.0 |
| Total Lesions, n (%) | 0.001 | 0.001 | 0.407 | ||||||
| 0 | 1 (2.9) | 10 (29.4) | 0 (0.0) | 8 (40.0) | 1 (7.1) | 2 (14.3) | |||
| 1–5 | 11 (32.4) | 15 (44.1) | 7 (35.0) | 8 (40.0) | 4 (28.6) | 7 (50.0) | |||
| >5 | 22 (64.7) | 9 (26.5) | 13 (65.0) | 4 (20.0) | 9 (64.3) | 5 (35.7) | |||
| Sites of Metastasis, n (%) | |||||||||
| Liver | 26 (76.4) | 14 (41.2) | 0.003 | 16 (80.0) | 7 (35.0) | 0.010 | 11 (78.6) | 7 (50.0) | 0.237 |
| LN | 17 (50.0) | 5 (14.7) | 0.001 | 11 (55.0) | 2 (10.0) | 0.006 | 6 (42.9) | 3 (21.4) | 0.420 |
| Bone | 9 (26.5) | 4 (11.8) | 0.123 | 3 (15.0) | 1 (5.0) | 0.605 | 6 (42.9) | 3 (21.4) | 0.420 |
| Lung | 2 (5.9) | 2 (5.9) | 1.0 | 0 (0.0) | 0 (0.0) | 1.0 | 2 (14.3) | 2 (14.3) | 1.0 |
| Median Krenning Score (Range) | 4 (0–4) | NA | 4 (3–4) | NA | 3 (0–4) | NA | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaidi, A.; Ravi, P.; Bloise, I.; Harsini, S.; Stuart, H.C.; Kennecke, H.F.; Alberts, I.; Bénard, F.; Wilson, D.; Martineau, P.; et al. Dual PET Imaging with [68Ga]Ga-DOTA-TOC and [18F]FDG to Localize Neuroendocrine Tumors of Unknown Origin. Curr. Oncol. 2025, 32, 497. https://doi.org/10.3390/curroncol32090497
Zaidi A, Ravi P, Bloise I, Harsini S, Stuart HC, Kennecke HF, Alberts I, Bénard F, Wilson D, Martineau P, et al. Dual PET Imaging with [68Ga]Ga-DOTA-TOC and [18F]FDG to Localize Neuroendocrine Tumors of Unknown Origin. Current Oncology. 2025; 32(9):497. https://doi.org/10.3390/curroncol32090497
Chicago/Turabian StyleZaidi, Ali, Pavithraa Ravi, Ingrid Bloise, Sara Harsini, Heather C. Stuart, Hagen F. Kennecke, Ian Alberts, François Bénard, Don Wilson, Patrick Martineau, and et al. 2025. "Dual PET Imaging with [68Ga]Ga-DOTA-TOC and [18F]FDG to Localize Neuroendocrine Tumors of Unknown Origin" Current Oncology 32, no. 9: 497. https://doi.org/10.3390/curroncol32090497
APA StyleZaidi, A., Ravi, P., Bloise, I., Harsini, S., Stuart, H. C., Kennecke, H. F., Alberts, I., Bénard, F., Wilson, D., Martineau, P., & Loree, J. M. (2025). Dual PET Imaging with [68Ga]Ga-DOTA-TOC and [18F]FDG to Localize Neuroendocrine Tumors of Unknown Origin. Current Oncology, 32(9), 497. https://doi.org/10.3390/curroncol32090497