Tumor Immune Microenvironment and Current Status of Immune Checkpoint Inhibitor Therapy in Colorectal Cancer Liver Metastasis
Simple Summary
Abstract
1. Epidemiology and Current Treatment Status of Colorectal Cancer Liver Metastasis
2. Liver Metastasis Is Associated with the Poor Efficacy of Immune Checkpoint Inhibitors
3. Liver Metastasis and the Immune Microenvironment
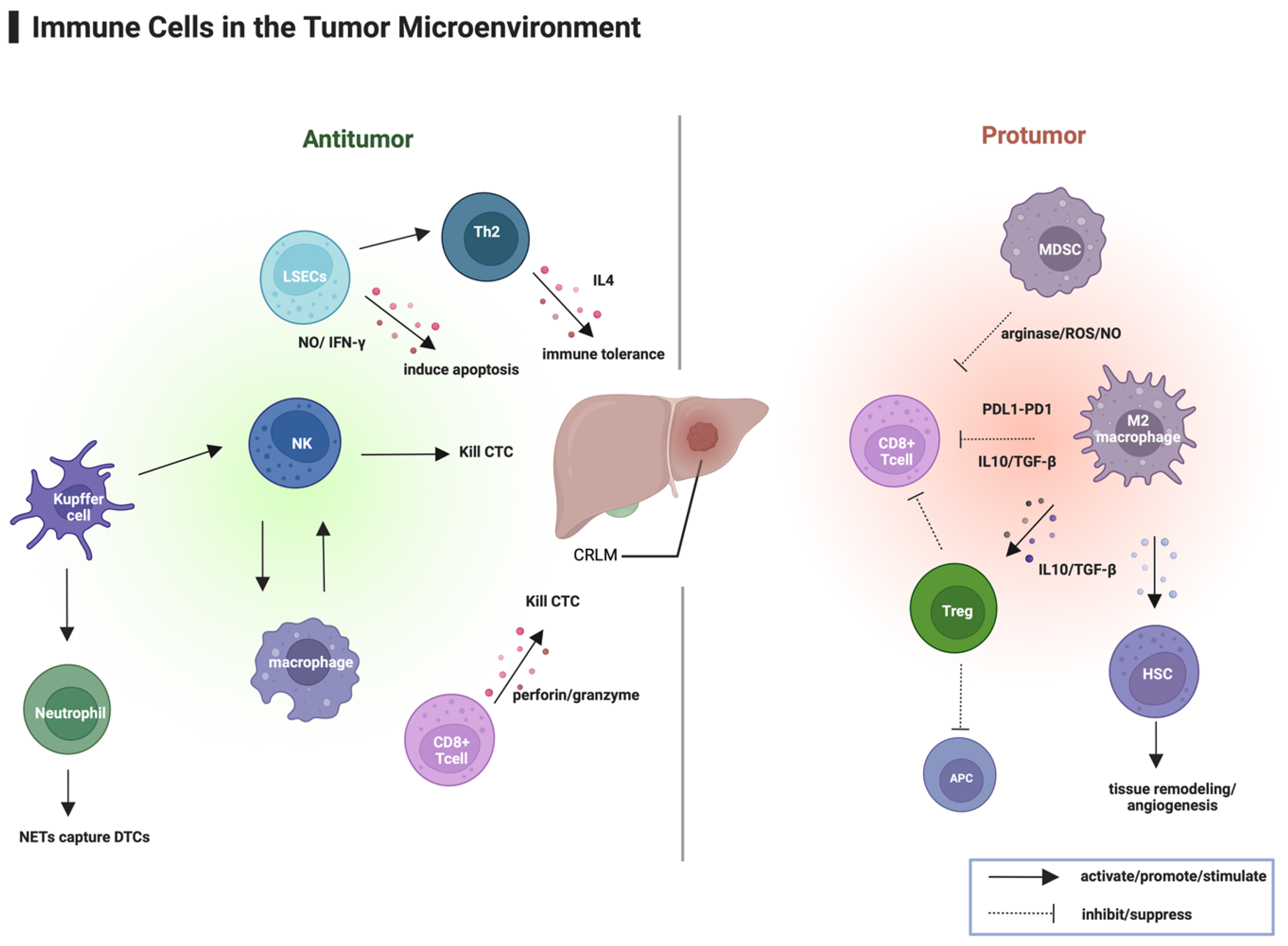
3.1. Immune Microenvironment of Liver Metastases
3.2. Systemic Immune Suppression Induced by Liver Metastasis
3.3. Influence of the Gut Microbiota on the Immune Microenvironment of Liver Metastases
3.4. Unique Immune Cell Composition and Spatial Distribution in the TME of Liver Metastases
4. ICIs for Advanced Colorectal Cancer Liver Metastasis
4.1. Preclinical Research Targeting the Immune Microenvironment of CRLM
4.2. Current Status of Immunotherapy in mCRC Liver Metastasis
4.2.1. Research on ICIs in MSI-H/dMMR mCRC
4.2.2. Research on ICIs in MSS mCRC First-Line Treatment
4.2.3. Research on ICIs in MSS mCRC Later-Line Treatment
5. Immunoscore as a Promising Biomarker for mCRC
6. Summary and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRC | Colorectal Cancer |
| CRLMs | Colorectal Cancer Liver Metastases |
| ICIs | Immune Checkpoint Inhibitors |
| dMMR | Deficient Mismatch Repair |
| MSI-H | Microsatellite Instability-High |
| pMMR | Mismatch Repair Proficient |
| MSS | Microsatellite Stable |
| mCRC | Metastatic Colorectal Cancer |
| ORR | Objective Response Rate |
| DCR | Disease Control Rate |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| mPFS | Median Progression-Free Survival |
| TME | Tumor Microenvironment |
| LSECs | Liver Sinusoidal Endothelial Cells |
| DTCs | Disseminated Tumor Cells |
| NO | Nitric Oxide |
| IFN-γ | Interferon-Gamma |
| NK cells | Natural Killer Cells |
| TGF-β | Transforming Growth Factor-Beta |
| HSCs | Hepatic Stellate Cells |
| APCs | Antigen-Presenting Cells |
| MDSCs | Myeloid-Derived Suppressor Cells |
| TCR | T-Cell Receptor |
| TANs | Tumor-Associated Neutrophils |
| NETs | Neutrophil Extracellular Traps |
| dHGP | Desmoplastic Histopathological Growth Pattern |
| TAMs | Tumor-Associated Macrophages |
| GVB | Gut Vascular Barrier |
| FMT | Fecal Microbiota Transplantation |
| TCGA | The Cancer Genome Atlas |
| scRNA-seq | Single-Cell RNA Sequencing |
| PT | Peritumoral |
| TF | Tumor Invasive Front |
| TC | Tumor Center |
| IL-10 | Interleukin-10 |
| TSCs | Tumor Slice Cultures |
| TILs | Tumor-Infiltrating Lymphocytes |
| MWA | Microwave Ablation |
| SOC | Standard-of-Care |
| TMB | Tumor Mutational Burden |
| TKIs | Tyrosine Kinase Inhibitors |
| BSC | Best Supportive Care |
| SITC | The Society for Immunotherapy of Cancer |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Slesser, A.a.P.; Simillis, C.; Goldin, R.; Brown, G.; Mudan, S.; Tekkis, P.P. A Meta-Analysis Comparing Simultaneous versus Delayed Resections in Patients with Synchronous Colorectal Liver Metastases. Surg. Oncol. 2013, 22, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 Chemotherapy and Surgery versus Surgery Alone for Resectable Liver Metastases from Colorectal Cancer (EORTC 40983): Long-Term Results of a Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Brodt, P.; Clavien, P.-A.; Muschel, R.J.; D’Angelica, M.I.; Endo, I.; Parks, R.W.; Doyle, M.; de Santibañes, E.; Pawlik, T.M. Liver Metastases. Nat. Rev. Dis. Primers 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.-S.; Rivera, F.; et al. Bevacizumab in Combination with Oxaliplatin-Based Chemotherapy as First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.O.; Raab, H.-R.; Lordick, F.; Hartmann, J.T.; Lang, H.; Frilling, A.; Stoehlmacher, J.; Weitz, J.; et al. Tumour Response and Secondary Resectability of Colorectal Liver Metastases Following Neoadjuvant Chem-otherapy with Cetuximab: The CELIM Randomised Phase 2 Trial. Lancet Oncol. 2010, 11, 38–47. [Google Scholar] [CrossRef]
- He, W.-Z.; Hu, W.-M.; Wang, F.; Rong, Y.-M.; Yang, L.; Xie, Q.-K.; Yang, Y.-Z.; Jiang, C.; Qiu, H.-J.; Lu, J.-B.; et al. Comparison of Mismatch Repair Status Between Primary and Matched Metastatic Sites in Patients With Colo-rectal Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 1174–1183. [Google Scholar] [CrossRef]
- Jung, J.; Kang, Y.; Lee, Y.J.; Kim, E.; Ahn, B.; Lee, E.; Kim, J.Y.; Lee, J.H.; Lee, Y.; Kim, C.H.; et al. Comparison of the Mismatch Repair System between Primary and Metastatic Colorectal Cancers Using Im-munohistochemistry. J. Pathol. Transl. Med. 2017, 51, 129–136. [Google Scholar] [CrossRef]
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver Metastasis Restrains Immunotherapy Efficacy via Macrophage-Mediated T Cell Elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef]
- Johnson, B.; Haymaker, C.L.; Parra, E.R.; Soto, L.M.S.; Wang, X.; Thomas, J.V.; Dasari, A.; Morris, V.K.; Raghav, K.; Vilar, E.; et al. Phase II Study of Durvalumab (Anti-PD-L1) and Trametinib (MEKi) in Microsatellite Stable (MSS) Metastatic Colorectal Cancer (mCRC). J. Immunother. Cancer 2022, 10, e005332. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.X.; Loree, J.M.; Titmuss, E.; Jonker, D.J.; Kennecke, H.F.; Berry, S.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; et al. Liver Metastases and Immune Checkpoint Inhibitor Efficacy in Patients with Refractory Metastatic Colorectal Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2346094. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, S.; Xu, H.; Yang, G.; Xu, F.; Yang, L.; Chen, D.; An, G.; Wang, Y. Organ-Specific Immune Checkpoint Inhibitor Treatment in Lung Cancer: A Systematic Review and Meta-Analysis. BMJ Open 2023, 13, e059457. [Google Scholar] [CrossRef]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-Reported Outcomes with Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Unresectable Hepatocellular Carcinoma (IMbrave150): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef]
- Zeng, X.; Ward, S.E.; Zhou, J.; Cheng, A.S.L. Liver Immune Microenvironment and Metastasis from Colorectal Cancer-Pathogenesis and Therapeutic Perspectives. Cancers 2021, 13, 2418. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, G.; Shen, C.; Zhu, L.; Yu, C.; Sartorius, K.; Ding, W.; Jiang, Y.; Lu, Y. Role of T Cells in Liver Metastasis. Cell Death Dis. 2024, 15, 1–16. [Google Scholar] [CrossRef]
- Van den Eynden, G.G.; Majeed, A.W.; Illemann, M.; Vermeulen, P.B.; Bird, N.C.; Høyer-Hansen, G.; Eefsen, R.L.; Reynolds, A.R.; Brodt, P. The Multifaceted Role of the Microenvironment in Liver Metastasis: Biology and Clinical Implications. Cancer Res. 2013, 73, 2031–2043. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, H.; Liu, Z.; Xu, J.; Gao, Y.; Zhan, X.; Zhou, S.; Zhong, W.; Wu, D.; Wang, P.; et al. Macrophage STING Signaling Promotes NK Cell to Suppress Colorectal Cancer Liver Metastasis via 4-1BBL/4-1BB Co-Stimulation. J. Immunother. Cancer 2023, 11, e006481. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, X.; He, X.; Hu, Z.; Huang, H.; Chen, J.; Chen, K.; Zhao, S.; Wei, P.; Li, D. Liver Metastasis from Colorectal Cancer: Pathogenetic Development, Immune Landscape of the Tumour Microenvironment and Therapeutic Approaches. J. Exp. Clin. Cancer Res. 2023, 42, 177. [Google Scholar] [CrossRef]
- Keirsse, J.; Van Damme, H.; Geeraerts, X.; Beschin, A.; Raes, G.; Van Ginderachter, J.A. The Role of Hepatic Macrophages in Liver Metastasis. Cell Immunol. 2018, 330, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Langhans, B.; Nischalke, H.D.; Krämer, B.; Dold, L.; Lutz, P.; Mohr, R.; Vogt, A.; Toma, M.; Eis-Hübinger, A.M.; Nattermann, J.; et al. Role of Regulatory T Cells and Checkpoint Inhibition in Hepatocellular Carcinoma. Cancer Immunol. Immunother. 2019, 68, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Sieminska, I.; Baran, J. Myeloid-Derived Suppressor Cells in Colorectal Cancer. Front. Immunol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal Liver Metastasis: Molecular Mechanism and Interventional Therapy. Signal Transduct. Target. Ther. 2022, 7, 70. [Google Scholar] [CrossRef]
- Höppener, D.J.; Galjart, B.; Nierop, P.M.H.; Buisman, F.E.; van der Stok, E.P.; Coebergh van den Braak, R.R.J.; van Amerongen, M.J.; Balachandran, V.P.; Jarnagin, W.R.; Kingham, T.P.; et al. Histopathological Growth Patterns and Survival After Resection of Colorectal Liver Metastasis: An External Validation Study. JNCI Cancer Spectr. 2021, 5, pkab026. [Google Scholar] [CrossRef]
- Höppener, D.J.; Nierop, P.M.H.; Hof, J.; Sideras, K.; Zhou, G.; Visser, L.; Gouw, A.S.H.; de Jong, K.P.; Sprengers, D.; Kwekkeboom, J.; et al. Enrichment of the Tumour Immune Microenvironment in Patients with Desmoplastic Colorectal Liver Metastasis. Br. J. Cancer 2020, 123, 196–206. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, S.; Ma, J.; Chen, Z.; Song, G.; Rao, D.; Cheng, Y.; Huang, S.; Liu, Y.; Jiang, S.; et al. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov. 2022, 12, 134–153. [Google Scholar] [CrossRef]
- Sampaio-Ribeiro, G.; Ruivo, A.; Silva, A.; Santos, A.L.; Oliveira, R.C.; Gama, J.; Cipriano, M.A.; Tralhão, J.G.; Paiva, A. Innate Immune Cells in the Tumor Microenvironment of Liver Metastasis from Colorectal Cancer: Contribution to a Comprehensive Therapy. Cancers 2023, 15, 3222. [Google Scholar] [CrossRef]
- Lee, J.C.; Mehdizadeh, S.; Smith, J.; Young, A.; Mufazalov, I.A.; Mowery, C.T.; Daud, A.; Bluestone, J.A. Regulatory T Cell Control of Systemic Immunity and Immunotherapy Response in Liver Metastasis. Sci. Immunol. 2020, 5, eaba0759. [Google Scholar] [CrossRef]
- Ho, W.W.; Gomes-Santos, I.L.; Aoki, S.; Datta, M.; Kawaguchi, K.; Talele, N.P.; Roberge, S.; Ren, J.; Liu, H.; Chen, I.X.; et al. Dendritic Cell Paucity in Mismatch Repair–Proficient Colorectal Cancer Liver Metastases Limits Immune Checkpoint Blockade Efficacy. Proc. Natl. Acad. Sci. USA 2021, 118, e2105323118. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Li, M.; Zhang, Y.; Sun, M.; Wang, L.; Lin, J.; Cui, Y.; Chen, Q.; Jin, C.; et al. Fusobacterium Nucleatum Reduces METTL3-Mediated m6A Modification and Contributes to Colorectal Cancer Metastasis. Nat. Commun. 2022, 13, 1248. [Google Scholar] [CrossRef]
- Bertocchi, A.; Carloni, S.; Ravenda, P.S.; Bertalot, G.; Spadoni, I.; Lo Cascio, A.; Gandini, S.; Lizier, M.; Braga, D.; Asnicar, F.; et al. Gut Vascular Barrier Impairment Leads to Intestinal Bacteria Dissemination and Colorectal Cancer Metastasis to Liver. Cancer Cell 2021, 39, 708–724.e11. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fan, L.; Lin, Y.; Shen, W.; Qi, Y.; Zhang, Y.; Chen, Z.; Wang, L.; Long, Y.; Hou, T.; et al. Fusobacterium Nucleatum Promotes Colorectal Cancer Metastasis through miR-1322/CCL20 Axis and M2 Polarization. Gut Microbes 2021, 13, 1980347. [Google Scholar] [CrossRef]
- Mignini, I.; Piccirilli, G.; Galasso, L.; Termite, F.; Esposto, G.; Ainora, M.E.; Gasbarrini, A.; Zocco, M.A. From the Colon to the Liver: How Gut Microbiota May Influence Colorectal Cancer Metastatic Potential. J. Clin. Med. 2024, 13, 420. [Google Scholar] [CrossRef]
- Jiang, S.-S.; Xie, Y.-L.; Xiao, X.-Y.; Kang, Z.-R.; Lin, X.-L.; Zhang, L.; Li, C.-S.; Qian, Y.; Xu, P.-P.; Leng, X.-X.; et al. Fusobacterium Nucleatum-Derived Succinic Acid Induces Tumor Resistance to Immunotherapy in Colorectal Cancer. Cell Host Microbe 2023, 31, 781–797.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lei, J.; Ke, S.; Chen, Y.; Xiao, J.; Tang, Z.; Wang, L.; Ren, Y.; Alnaggar, M.; Qiu, H.; et al. Fecal Microbiota Transplantation plus Tislelizumab and Fruquintinib in Refractory Microsatellite Stable Metastatic Colorectal Cancer: An Open-Label, Single-Arm, Phase II Trial (RENMIN-215). EClinicalMedicine 2023, 66, 102315. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xing, B.; Luo, N.; Gao, R.; Yu, K.; Hu, X.; Bu, Z.; Peng, J.; Ren, X.; et al. Immune Phenotypic Linkage between Colorectal Cancer and Liver Metastasis. Cancer Cell 2022, 40, 424–437.e5. [Google Scholar] [CrossRef]
- Sathe, A.; Mason, K.; Grimes, S.M.; Zhou, Z.; Lau, B.T.; Bai, X.; Su, A.; Tan, X.; Lee, H.; Suarez, C.J.; et al. Colorectal Cancer Metastases in the Liver Establish Immunosuppressive Spatial Networking between Tu-mor-Associated SPP1+ Macrophages and Fibroblasts. Clin. Cancer Res. 2023, 29, 244–260. [Google Scholar] [CrossRef]
- He, Y.; Han, Y.; Fan, A.; Li, D.; Wang, B.; Ji, K.; Wang, X.; Zhao, X.; Lu, Y. Multi-Perspective Comparison of the Immune Microenvironment of Primary Colorectal Cancer and Liver Metastases. J. Transl. Med. 2022, 20, 454. [Google Scholar] [CrossRef]
- Zhou, S.-N.; Pan, W.-T.; Pan, M.-X.; Luo, Q.-Y.; Zhang, L.; Lin, J.-Z.; Zhao, Y.-J.; Yan, X.-L.; Yuan, L.-P.; Zhang, Y.-X.; et al. Comparison of Immune Microenvironment Between Colon and Liver Metastatic Tissue in Colon Cancer Patients with Liver Metastasis. Dig. Dis. Sci. 2021, 66, 474–482. [Google Scholar] [CrossRef]
- Ye, J.; Guo, W.; Wang, C.; Egelston, C.A.; D’Apuzzo, M.; Shankar, G.; Fakih, M.G.; Lee, P.P. Peritumoral Immune-Suppressive Mechanisms Impede Intratumoral Lymphocyte Infiltration into Colorectal Cancer Liver versus Lung Metastases. Cancer Res. Commun. 2023, 3, 2082–2095. [Google Scholar] [CrossRef]
- Deng, J.-Y.; Gou, Q.; Yang, L.; Chen, Z.-H.; Yang, M.-Y.; Yang, X.-R.; Yan, H.-H.; Wei, X.-W.; Liu, J.-Q.; Su, J.; et al. Immune Suppressive Microenvironment in Liver Metastases Contributes to Organ-Specific Response of Immunotherapy in Advanced Non-Small Cell Lung Cancer. J. Immunother. Cancer 2023, 11, e007218. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Jiang, X.; Guha, P.; Lausted, C.; Carter, J.A.; Hsu, C.; Labadie, K.P.; Kohli, K.; Kenerson, H.L.; Daniel, S.K.; et al. Blockade of Interleukin 10 Potentiates Antitumour Immune Function in Human Colorectal Cancer Liver Metastases. Gut 2023, 72, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Chen, Y.; Huang, H.; Liu, Y.; Chen, J.; Zhu, D.; Zheng, X.; Chen, L.; Jiang, J. LAG3 Blockade Coordinates with Microwave Ablation to Promote CD8+ T Cell-Mediated Anti-Tumor Immunity. J. Transl. Med. 2022, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, P.; Gallage, S.; Heikenwälder, M.F. Unique Tumour Microenvironment: When Ferroptosis Activation Boosts ICI of Liver Cancer. Gut 2023, 72, 1639–1641. [Google Scholar] [CrossRef]
- Conche, C.; Finkelmeier, F.; Pešić, M.; Nicolas, A.M.; Böttger, T.W.; Kennel, K.B.; Denk, D.; Ceteci, F.; Mohs, K.; Engel, E.; et al. Combining Ferroptosis Induction with MDSC Blockade Renders Primary Tumours and Metastases in Liver Sensitive to Immune Checkpoint Blockade. Gut 2023, 72, 1774–1782. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Parikh, A.; Spigel, D.R.; Cohn, A.L.; Yoshino, T.; Kochenderfer, M.; Elez, E.; Shao, S.H.; Deming, D.; Holdridge, R.; et al. Modified FOLFOX6 plus Bevacizumab with and without Nivolumab for First-Line Treatment of Metastatic Colorectal Cancer: Phase 2 Results from the CheckMate 9X8 Randomized Clinical Trial. J. Immunother. Cancer 2024, 12, e008409. [Google Scholar] [CrossRef]
- Upfront FOLFOXIRI Plus Bevacizumab with or Without Atezolizumab in the Treatment of Patients with Metastatic Colorectal Cancer (AtezoTRIBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35636444/ (accessed on 27 August 2024).
- Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients with Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study|Cancer Biomarkers|JAMA Oncology|JAMA Network. Available online: https://jamanetwork.com/journals/jamaoncology/fullarticle/2765332 (accessed on 13 February 2025).
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus Chemotherapy for Microsatellite Instability-High or Mismatch Repair-Deficient Metastatic Colorectal Cancer (KEYNOTE-177): Final Analysis of a Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Cutsem, E.V.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2021, 40, 161–170. [Google Scholar] [CrossRef]
- Manca, P.; Corti, F.; Intini, R.; Mazzoli, G.; Miceli, R.; Germani, M.M.; Bergamo, F.; Ambrosini, M.; Cristarella, E.; Cerantola, R.; et al. Tumour Mutational Burden as a Biomarker in Patients with Mismatch Repair Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer Treated with Immune Checkpoint Inhibitors. Eur. J. Cancer 2023, 187, 15–24. [Google Scholar] [CrossRef]
- Wang, F.; Peng, J.; Liang, X.; Cheng, Y.; Deng, Y.; Chen, K.; Zhang, M.; Zhang, J.; Wang, W.; Cao, B.; et al. First-Line Serplulimab plus HLX04 and XELOX versus Placebo plus Bevacizumab and XELOX in Metastatic Colorectal Cancer: A Phase 2/3 Study. J. Clin. Oncol. 2024, 42, 124. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.-X.; Peng, J.; Liang, X.; Cheng, Y.; Deng, Y.; Chen, K.; Zhang, M.; Zhang, J.; Wang, W.; et al. First-Line Serplulimab plus HLX04 and XELOX versus Placebo plus Bevacizumab and XELOX in Metastatic Colorectal Cancer: A Phase 2/3 Study. J. Clin. Oncol. 2024, 42, 3569. [Google Scholar] [CrossRef]
- First-Line Durvalumab and Tremelimumab with Chemotherapy in RAS-Mutated Metastatic Colorectal Cancer: A Phase 1b/2 Trial-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/37563240/ (accessed on 27 August 2024).
- Kawazoe, A.; Xu, R.-H.; García-Alfonso, P.; Passhak, M.; Teng, H.-W.; Shergill, A.; Gumus, M.; Qvortrup, C.; Stintzing, S.; Towns, K.; et al. Lenvatinib Plus Pembrolizumab Versus Standard of Care for Previously Treated Metastatic Colorectal Cancer: Final Analysis of the Randomized, Open-Label, Phase III LEAP-017 Study. J. Clin. Oncol. 2024, 42, 2918–2927. [Google Scholar] [CrossRef]
- Fakih, M.; Sandhu, J.; Lim, D.; Li, X.; Li, S.; Wang, C. Regorafenib, Ipilimumab, and Nivolumab for Patients With Microsatellite Stable Colorectal Cancer and Dis-ease Progression With Prior Chemotherapy: A Phase 1 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 627–634. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Zang, A.; Cheng, Y.; Zhang, Y.; Wang, X.; Chen, Z.; Qu, S.; He, J.; Chen, C.; et al. First-in-Human Phase I/Ib Study of QL1706 (PSB205), a Bifunctional PD1/CTLA4 Dual Blocker, in Patients with Advanced Solid Tumors. J. Hematol. Oncol. 2023, 16, 50. [Google Scholar] [CrossRef]
- Wang, F.; Jin, Y.; Wang, M.; Luo, H.-Y.; Fang, W.-J.; Wang, Y.-N.; Chen, Y.-X.; Huang, R.-J.; Guan, W.-L.; Li, J.-B.; et al. Combined Anti-PD-1, HDAC Inhibitor and Anti-VEGF for MSS/pMMR Colorectal Cancer: A Randomized Phase 2 Trial. Nat. Med. 2024, 30, 1035–1043. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International Validation of the Consensus Immunoscore for the Classification of Colon Cancer: A Prognostic and Accuracy Study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Salvatore, L.; Lonardi, S.; Tamberi, S.; Marmorino, F.; Moretto, R.; Prisciandaro, M.; Tamburini, E.; et al. Upfront Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Bevacizumab with or Without Atezoli-zumab for Patients with Metastatic Colorectal Cancer: Updated and Overall Survival Results of the ATEZOTRIBE Study. J. Clin. Oncol. 2024, 42, 2637–2644. [Google Scholar] [CrossRef]
| Key Trial/Clinical Trial Information | Phase | Design (N) | Treatment Line Number | Subject | Intervention | Main Results |
|---|---|---|---|---|---|---|
| KEYNOTE-177/NCT02563002 | 3 | 307 | First-line | MSI-H/dMMR mCRC | Pembrolizumab (n = 153) versus chemotherapy (n = 154) | mOS: not reached (95% CI: 49.2-not reached) vs. 36.7 m (95% CI: 27.6-not reached) |
| checkmate-142/NCT02060188 | 2 | 45 | First-line | MSI-H/dMMR mCRC | Nivolumab plus low-dose ipilimumab | ORR: 69% (95% CI: 53 to 82) DCR: 84% (95% CI: 70.5 to 93.5) |
| CheckMate 9X8/NCT03414983 | 2 | 195 | First-line | mCRC | Nivolumab plus SOC versus SOC (mFOLFOX6 + bevacizumab) | PFS did not meet the prespecified threshold for statistical significance. |
| AtezoTRIBE/NCT03721653 | 2 | 218 | First-line | mCRC | Atezolizumab combined with SOC versus SOC (FOLFOXIRI + bevacizumab) | mPFS: 13.1 m (80% CI:12.5–13.8) vs. 11.5 m (10.0–12.6) p = 0.012 |
| ASTRUM-015/NCT04547166 | 2/3 | 114 | First-line | mCRC | Serplulimab plus HLX04 and XELOX group versus placebo plus bevacizumab and XELOX | mPFS: (17.2 vs. 10.7 m; stratified HR 0.60, 95% CI 0.31–1.14) mOS was not reached in either group (stratified HR 0.77, 95% CI: 0.41–1.45) |
| MEDITREME/NCT03202758 | 1b/2 | 57 | First-line | RAS-mutated mCRC | Durvalumab + tremelimumab + mFOLFOX6 versus mFOLFOX6 | 3-month PFS of 90.7% (95% CI: 79.2–96%) ORR: 64.5% mPFS: 8.2 m (95% CI: 5.9–8.6) |
| LEAP-017/NCT04776148 | 3 | 480 | Laterline | MSS/pMMR mCRC | Lenvatinib plus pembrolizumab versus SOC | mOS: 9.8 m versus 9.3 m (HR, 0.83 (95% CI, 0.68 to 1.02) p = 0.0379 |
| REGONIVO/NCT03406871 | Ib | 50 | Laterline | Advanced Gastric or Colorectal Cancer | Regorafenib plus nivolumab | ORR in gastric and colorectal cancer 44% vs. 36%; mPFS in gastric and colorectal cancer 5.6 m vs. 7.9 m |
| RIN/NCT04362839 | 1 | 39 | Laterline | MSS/pMMR mCRC the RP2D cohort | Regorafenib, ipilimumab, and nivolumab | ORR: 27.6%; PFS: 4 m (IQR, 2–9 m) OS: 20 m (IQR, 7 m to NE) |
| QL1706/NCT05576272, NCT05179317 | Ib | 27 | Laterline | Advanced Colorectal Cancer | QL1706 | ORR: 7.4% (95% CI: 0.9 to 24.3) DCR: 25.9% (95% CI: 11.1 to 46.3) |
| CAPability-01/NCT04724239 | 2 | 48 | Laterline | MSS/pMMR mCRC | Sintilimab, chidamide with or without bevacizumab (the triplet arm vs. the doublet arm) | 18wPFS rate (64.0% vs. 21.7%, p = 0.003) ORR (44.0% vs. 13.0%, p = 0.027) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, D.; Zhou, A. Tumor Immune Microenvironment and Current Status of Immune Checkpoint Inhibitor Therapy in Colorectal Cancer Liver Metastasis. Curr. Oncol. 2025, 32, 493. https://doi.org/10.3390/curroncol32090493
Cao D, Zhou A. Tumor Immune Microenvironment and Current Status of Immune Checkpoint Inhibitor Therapy in Colorectal Cancer Liver Metastasis. Current Oncology. 2025; 32(9):493. https://doi.org/10.3390/curroncol32090493
Chicago/Turabian StyleCao, Dandan, and Aiping Zhou. 2025. "Tumor Immune Microenvironment and Current Status of Immune Checkpoint Inhibitor Therapy in Colorectal Cancer Liver Metastasis" Current Oncology 32, no. 9: 493. https://doi.org/10.3390/curroncol32090493
APA StyleCao, D., & Zhou, A. (2025). Tumor Immune Microenvironment and Current Status of Immune Checkpoint Inhibitor Therapy in Colorectal Cancer Liver Metastasis. Current Oncology, 32(9), 493. https://doi.org/10.3390/curroncol32090493




