Impact of Treatment Modalities on Locally Advanced Gastric Cancer—Real-World Data
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Statistical Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, W.; Tan, X.; He, W.; Zhao, L.; Liu, H.; Li, G. Long-term relative survival of patients with gastric cancer from a large-scale cohort: A period-analysis. BMC Cancer 2024, 24, 1420. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, M.; Li, J.; Yang, Z.; Feng, Q.; Zhou, M.; Zhang, Z. The patterns and timing of recurrence after curative resection for gastric cancer in China. World J. Surg. Onc. 2016, 14, 305. [Google Scholar] [CrossRef]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.-W.; Kim, Y.H.; Noh, S.-I.; Cho, J.Y.; et al. CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Coccolini, F.; Nardi, M.; Montori, G.; Ceresoli, M.; Celotti, A.; Cascinu, S.; Fugazzola, P.; Tomasoni, M.; Glehen, O.; Catena, F.; et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int. J. Surg. 2018, 51, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, F.; Papa, V.; Caprino, P.; Sgadari, A.; Bossola, M.; Doglietto, G.B. Proximal compared with distal gastric cancer: Multivariate analysis of prognostic factors. Am. Surg. 2001, 67, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; He, H.Y.; Chen, K.; Liu, H.D.; Zu, D.; Liang, C.; Bao, Q.-M.; Hu, Y.-C.; Liu, G.-X.; Zhong, Y.-K.; et al. Prognostic impact and reasons for variability by tumor location in gastric cancer. World J. Gastroenterol. 2024, 30, 4709–4724. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, M.; Barni, S.; Steccanella, F.; Sgroi, G.; Passalacqua, R.; Tomasello, G. Prognostic Role of Primary Tumor Location in Non-Metastatic Gastric Cancer: A Systematic Review and Meta-Analysis of 50 Studies. Ann. Surg. Oncol. 2017, 24, 2655–2668. [Google Scholar] [CrossRef]
- Xue, J.; Yang, H.; Huang, S.; Zhou, T.; Zhang, X.; Zu, G. Comparison of the overall survival of proximal and distal gastric cancer after gastrectomy: A systematic review and meta-analysis. World J. Surg. Oncol. 2021, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Kakish, H.H.; Ahmed, F.A.; Pei, E.; Dong, W.; Elshami, M.; Ocuin, L.M.; Rothermel, L.D.; Ammori, J.B.; Hoehn, R.S. Understanding Factors Leading to Surgical Attrition for “Resectable” Gastric Cancer. Ann. Surg. Oncol. 2023, 30, 4207–4216. [Google Scholar] [CrossRef]
- Kronenfeld, J.P.; Collier, A.L.; Turgeon, M.K.; Ju, M.; Alterio, R.; Wang, A.; Fernandez, M.; Porembka, M.R.; Richter, H.; Lee, A.Y.; et al. Attrition During Neoadjuvant Chemotherapy for Gastric Adenocarcinoma is Associated with Decreased Survival: A United States Safety-Net Collaborative Analysis. J. Surg. Oncol. 2021, 124, 1317–1328. [Google Scholar] [CrossRef]
- Choi, A.H.; Kim, J.; Chao, J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J. Gastroenterol. J. Surg. Oncol. 2021, 21, 7343–7348. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Isobe, T.; Hashimoto, K.; Kizaki, J.; Miyagi, M.; Aoyagi, K.; Koufuji, K.; Shirouzu, K. Characteristics and prognosis of gastric cancer in young patients. Oncol. Rep. 2013, 30, 43–49. [Google Scholar] [CrossRef]
- Rona, K.A.; Schwameis, K.; Zehetner, J.; Samakar, K.; Green, K.; Samaan, J.; Sandhu, K.; Bildzukewicz, N.; Katkhouda, N.; Lipham, J.C. Gastric cancer in the young: An advanced disease with poor prognostic features. J. Surg. Oncol. 2017, 115, 371–375. [Google Scholar] [CrossRef]
- Li, J. Gastric Cancer in Young Adults: A Different Clinical Entity from Carcinogenesis to Prognosis. Gastroenterol. Res. Pract. 2020, 2020, 9512707. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Sato, Y.; Nakagawa, M.; Aburatani, T.; Matsuyama, T.; Nakajima, Y.; Kinugasa, Y. Perioperative chemotherapy for locally advanced gastric cancer in Japan: Current and future perspectives. Surg. Today 2020, 50, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Hong, J.; Du, N.; Wang, Y.; Chen, J.; He, Y.; Chen, P. Long-Term Outcomes of Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer/Esophagogastric Junction Cancer: A Systematic Review and Meta-Analysis. Anticancer Agents Med. Chem. 2022, 22, 143–151. [Google Scholar]
- Liang, Y.; Ding, X.; Wang, X.; Wang, B.; Deng, J.; Zhang, L.; Liang, H. Prognostic value of surgical margin status in gastric cancer patients. ANZ J. Surg. 2015, 85, 678–684. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, C.; Cai, Z.; Shen, C.; Yin, Y.; Yin, X.; Zhao, Z.; Mu, M.; Yin, Y.; Zhang, B. Impact of Surgical Margin Status on Survival in Gastric Cancer: A Systematic Review and Meta-Analysis. Cancer Control 2021, 28, 10732748211043665. [Google Scholar] [CrossRef]
- Uzun, O.; Gulmez, S.; Senger, A.S.; Percem, A.; Polat, E.; Duman, M. Prognostic Factors in Operated T3-T4 Gastric Cancer. J. Coll. Physicians Surg. Pak. 2020, 30, 1047–1052. [Google Scholar] [CrossRef]
- Deng, J.; You, Q.; Gao, Y.; Yu, Q.; Zhao, P.; Zheng, Y.; Fang, W.; Xu, N.; Teng, L.; Singh, P.K. Prognostic value of perineural invasion in gastric cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e88907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, H.; Luo, D.; Xiong, Z.; Li, X.; Yin, S.; Jin, L.; Chen, S.; Peng, J.; Lian, L. Lymphovascular or perineural invasion is associated with lymph node metastasis and survival outcomes in patients with gastric cancer. Cancer Med. 2023, 12, 9401–9408. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Kuo, C.J.; Hsu, J.T.; Chen, T.H.; Lin, C.J.; Tseng, J.H.; Yeh, T.-S.; Hwang, T.-L.; Jan, Y.-Y. Clinicopathologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am. J. Surg. 2013, 205, 623–630. [Google Scholar] [CrossRef]


| Characteristic | ACT Group (n = 56) | NACT Group (n = 47) | Test Statistic | p-Value |
|---|---|---|---|---|
| Age | 61.86 ± 12.7 | 60.55 ± 11.17 | 0.548 | 0.585 t |
| Gender | ||||
| Female | 17 (30.4) | 9 (19.1) | 1.701 | 0.192 x |
| Male | 39 (69.6) | 38 (80.9) | ||
| Comorbidities | ||||
| None | 31 (55.4) | 27 (57.4) | 0.045 | 0.831 x |
| Present | 25 (44.6) | 20 (42.6) | ||
| Comorbidities | ||||
| Diabetes mellitus (DM) | 10 (40) | 9 (45) | 4.425 | 0.817 x |
| Hypertension (HT) | 12 (48) | 9 (45) | ||
| Coronary artery disease (CAD) | 3 (12) | 5 (25) | ||
| COPD/asthma | 3 (12) | 2 (10) | ||
| Stroke/Parkinson’s disease | 1 (4) | - | ||
| Malignancy | 1 (4) | 1 (5) | ||
| Dementia | 1 (4) | - | ||
| Hepatitis B (HBV) | - | 1 (5) | ||
| ECOG PS | ||||
| 0 | 17 (30.4) | 12 (25.5) | 2.382 | 0.304 x |
| 1 | 31 (55.4) | 32 (68.1) | ||
| 2 | 8 (14.3) | 3 (6.4) | ||
| Histology | ||||
| Adenocarcinoma | 42 (75) | 37 (78.7) | 0.198 | 0.656 x |
| Signet ring cell | 14 (25) | 10 (21.3) | ||
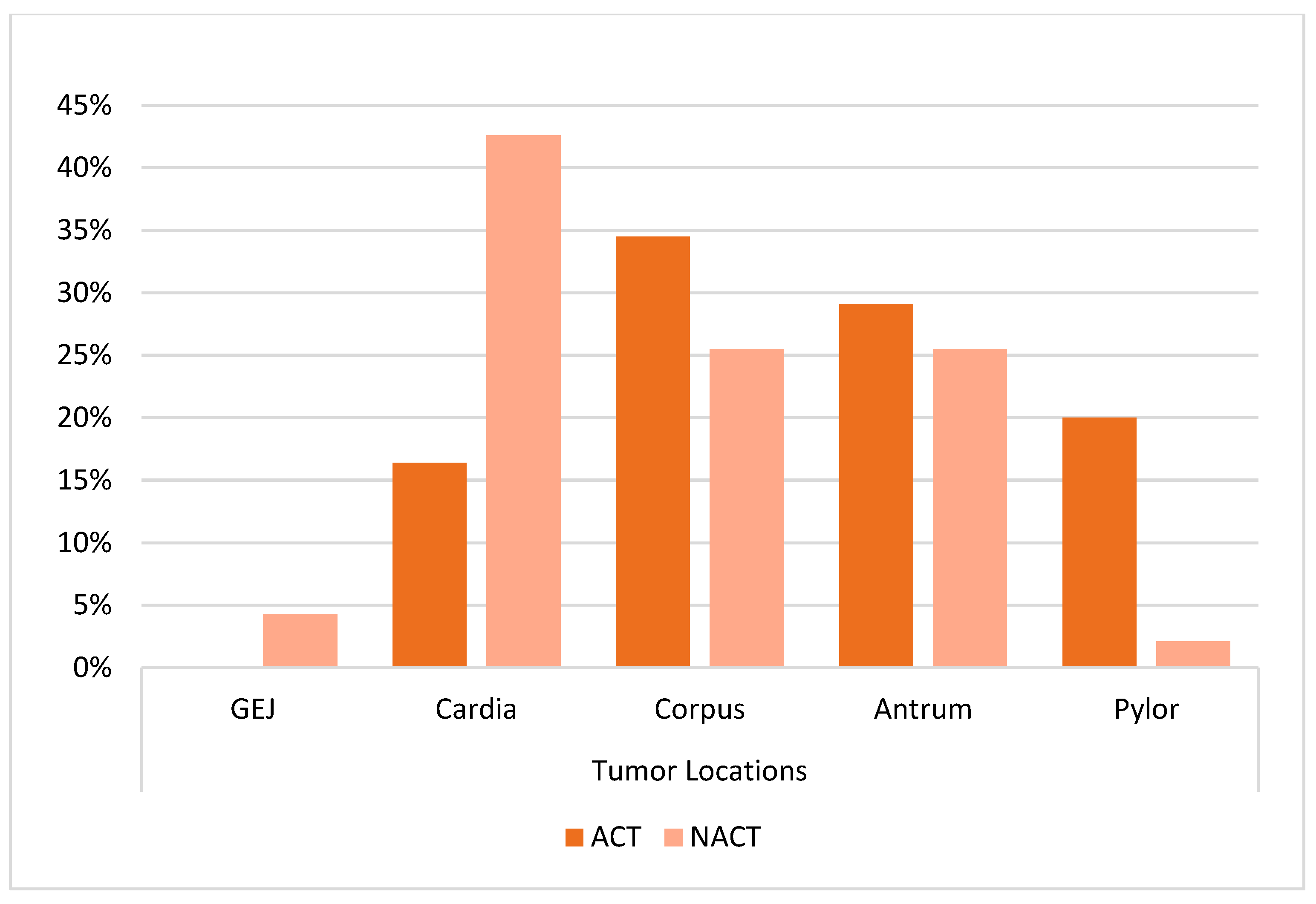
| Tumor location | ||||
| GEJ | - | 2 (4.3) | 16.03 | 0.001 f |
| Cardia | 9 (16.4) a | 20 (42.6) b | ||
| Corpus | 19 (34.5) a | 12 (25.5) a | ||
| Antrum | 16 (29.1) a | 12 (25.5) a | ||
| Pyloric | 11 (20) a | 1 (2.1) b | ||
| Grade | ||||
| 1 | 4 (7.7) | 1 (2.4) | 1.332 | 0.511 f |
| 2 | 24 (46.2) | 18 (43.9) | ||
| 3 | 24 (46.2) | 22 (53.7) | ||
| Pathological stage | ||||
| 0 | - | 2 (5.7) | 7.988 | 0.030 f |
| 1 | 4 (7.1) a | 7 (20) a | ||
| 2 | 22 (39.3) a | 15 (42.9) a | ||
| 3 | 30 (53.6) a | 11 (31.4) b | ||
| Clinical stage | ||||
| 1 | - | 1 (2.2) | - | |
| 2 | - | 11 (23.9) | ||
| 3 | - | 32 (69.6) | ||
| 4 | - | 2 (4.3) | ||
| cT | ||||
| 2 | - | 12 (26.1) | - | |
| 3 | - | 25 (54.3) | ||
| 4 | - | 9 (19.6) | ||
| cN | ||||
| 0 | - | 1 (2.2) | - | |
| 1 | - | 18 (40) | ||
| 2 | - | 16 (35.6) | ||
| 3 | - | 10 (22.2) | ||
| pT/ypT | ||||
| 0 | - | 2 (5.7) | 14.570 | 0.002 f |
| 1 | 2 (3.6) a | 7 (20) b | ||
| 2 | 3 (5.4) a | 5 (14.3) a | ||
| 3 | 30 (53.6) a | 16 (45.7) a | ||
| 4 | 21 (37.5) a | 5 (14.3) b | ||
| pN/ypN | ||||
| 0 | 15 (26.8) | 14 (40) | 4.280 | 0.233 x |
| 1 | 13 (23.2) | 5 (14.3) | ||
| 2 | 11 (19.6) | 10 (28.6) | ||
| 3 | 17 (30.4) | 6 (17.1) | ||
| Cerbb2 | ||||
| Negative | 35 (68.6) | 34 (79.1) | 1.449 | 0.558 f |
| Positive | 5 (9.8) | 2 (4.7) | ||
| Not Evaluated | 11 (21.6) | 7 (16.3) | ||
| Pdl-1 | ||||
| Negative | 2 (5) | 7 (17.9) | ||
| Positive | 1 (2.5) | 1 (2.6) | ||
| Not Evaluated | 37 (92.5) | 31 (79.5) | ||
| LVI | ||||
| Negative | 10 (19.2) | 14 (41.2) | 4.921 | 0.027 x |
| Positive | 42 (80.8) | 20 (58.8) | ||
| PNI | ||||
| Negative | 13 (24.5) | 18 (52.9) | 7.290 | 0.007 x |
| Positive | 40 (75.5) | 16 (47.1) | ||
| ACT Group (n =56) | NACT Group (n =47) | Test Statistic | p-Value | |
|---|---|---|---|---|
| Surgery | ||||
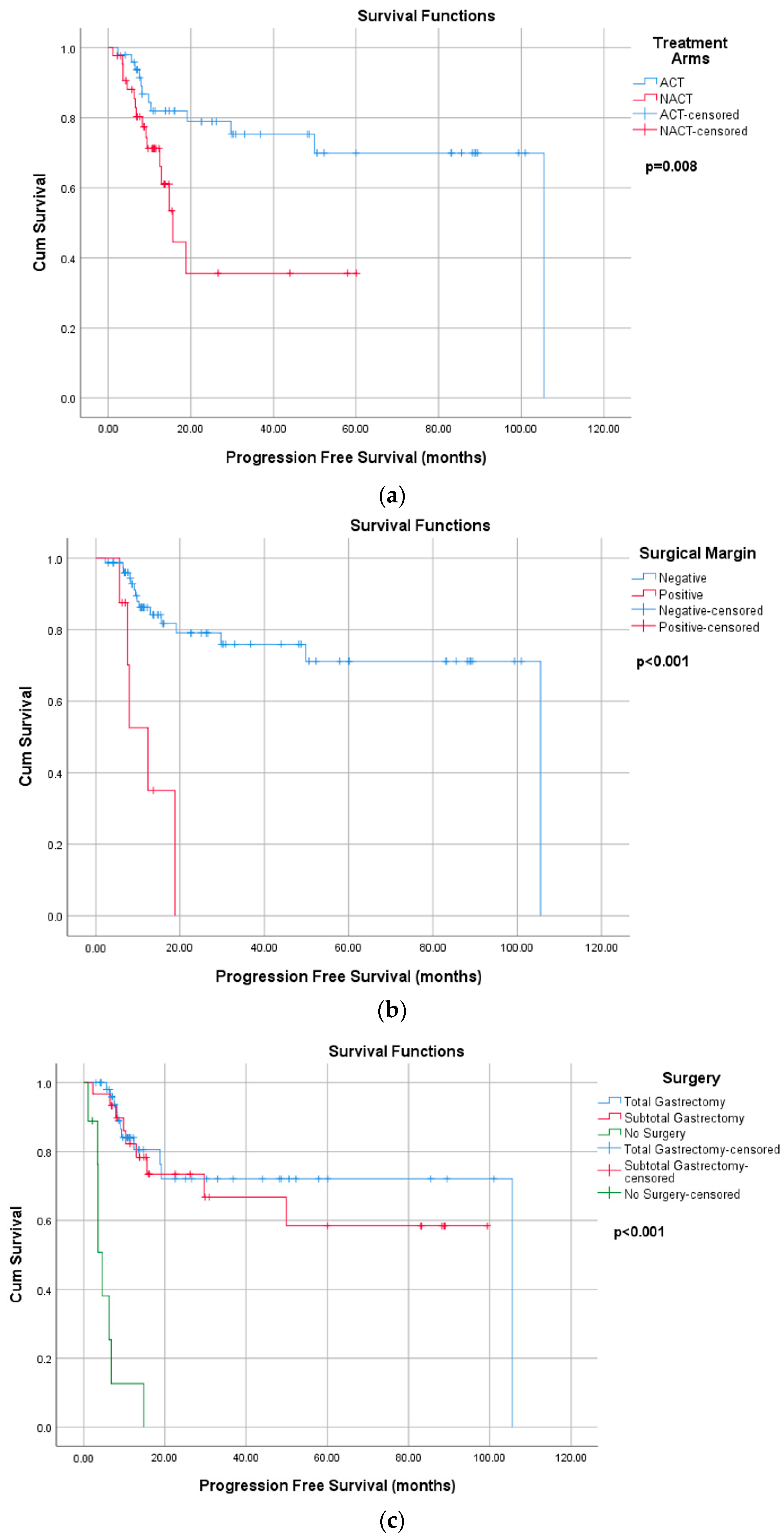
| Total gastrectomy | 26 (46.4) a | 32 (69.6) b | 32.44 | <0.001 f |
| Subtotal gastrectomy | 30 (53.6) a | 4 (8.7) b | ||
| No Surgery | - | 10 (21.7) | ||
| Surgical margin | ||||
| Negative | 49 (87.5) | 33 (91.7) | - | 0.735 f |
| Positive | 7 (12.5) | 3 (8.3) | ||
| D2 dissection | ||||
| Yes | 45 (80.4) | 27 (75) | 0.370 | 0.543 x |
| No | 11 (19.6) | 9 (25) | ||
| Number of lymph node dissection | 23 (0–70) | 20 (3–62) | -0.765 | 0.445 m |
| ACT Group (n = 56) | NACT Group (n = 47) | |
|---|---|---|
| Neoadjuvant treatment | ||
| Flot | - | 39 (83) |
| Folfox | - | 3 (6.4) |
| EOX | - | 1 (2.1) |
| Xelox | - | 1 (2.1) |
| CF | - | 1 (2.1) |
| mDCF | - | 2 (4.3) |
| Number of neoadjuvant cycles | - | 4.38 ± 1.39 |
| Tumor regression score | ||
| 0 | - | 3 (13.6) |
| 1 | - | 5 (22.7) |
| 2 | - | 7 (31.8) |
| 3 | - | 7 (31.8) |
| Radiological response | ||
| CR | - | 2 (4.7) |
| PR | - | 28 (65.1) |
| SD | - | 8 (18.6) |
| PD | - | 5 (11.6) |
| Adjuvant treatment | ||
| No treatment | - | 11 (30.6) |
| Xelox | 23 (41.8) | 1 (2.8) |
| Folfox | 21 (38.2) | 2 (5.6) |
| Fufa/Capecitabin | 3 (5.5) | - |
| mDFC | 5 (9.1) | - |
| CRT | 8 (14.5) | 3 (8.3) |
| Flot | - | 21 (58.3) |
| Number of adjuvant cycles | 6.89 ±3.31 | 3.22 ± 1.45 |
| Adjuvant radiotherapy | ||
| No | 32 (58.2) | 37 (86) |
| Yes | 23 (41.8) | 6 (14) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age | 0.985 (0.953–1.019) | 0.377 | ||
| Treatment arms (Ref: adjuvant) | 2.836 (1.269–6.335) | 0.011 | 1.564 (0.478–5.120) | 0.459 |
| Number of lymph node dissection | 1.013 (0.984–1.044) | 0.377 | ||
| Surgery margin (Ref: negative) | 7.347 (2.516–21.458) | <0.001 | 8.555 (2.581–28.359) | <0.001 |
| Surgery type (Ref: no surgery) | ||||
| Total gastrectomy | 0.050 (0.018–0.135) | <0.001 | 0.205 (0.052–0.808) | 0.023 |
| Subtotal gastrectomy | 0.064 (0.023–0.182) | <0.001 | - | - |
| Tumor location (Ref: cardia) | 0.617 (0.307–1.239) | 0.174 | ||
| Corpus | 1.365 (0.536–3.478) | 0.514 | ||
| Antrum | 0.755 (0.253–2.253) | 0.615 | ||
| Pyloric | 0.422 (0.087–2.039) | 0.283 | ||
| GEJ | - | 0.984 | ||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.043 (1.017–1.068) | 0.001 | 1.069 (1.034–1.105) | <0.001 |
| Treatment arms (Ref: adjuvant) | 1.761 (0.999–3.104) | 0.051 | ||
| Number of lymph node dissection | 0.999 (0.977–1.021) | 0.92 | ||
| Surgery margin (Ref: negative) | 6.956 (3.226–15.000) | <0.001 | 9.316 (3.769–23.024) | <0.001 |
| Surgery type (Ref: no surgery) | 0.393 (0.135–1.149) | 0.088 | ||
| Total gastrectomy | 0.278 (0.124–0.623) | 0.002 | ||
| Subtotal gastrectomy | 0.178 (0.072–0.439) | <0.001 | ||
| Tumor location (Ref: cardia) | 0.679 (0.41–1.124) | 0.132 | ||
| Corpus | 0.772 (0.403–1.478) | 0.435 | ||
| Antrum | 0.612 (0.304–1.233) | 0.170 | ||
| Pyloric | 0.229 (0.068–0.772) | 0.017 | ||
| GEJ | - | 0.974 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uguztemur, E.; Oztürk, B. Impact of Treatment Modalities on Locally Advanced Gastric Cancer—Real-World Data. Curr. Oncol. 2025, 32, 463. https://doi.org/10.3390/curroncol32080463
Uguztemur E, Oztürk B. Impact of Treatment Modalities on Locally Advanced Gastric Cancer—Real-World Data. Current Oncology. 2025; 32(8):463. https://doi.org/10.3390/curroncol32080463
Chicago/Turabian StyleUguztemur, Esma, and Banu Oztürk. 2025. "Impact of Treatment Modalities on Locally Advanced Gastric Cancer—Real-World Data" Current Oncology 32, no. 8: 463. https://doi.org/10.3390/curroncol32080463
APA StyleUguztemur, E., & Oztürk, B. (2025). Impact of Treatment Modalities on Locally Advanced Gastric Cancer—Real-World Data. Current Oncology, 32(8), 463. https://doi.org/10.3390/curroncol32080463





