Recent Advances in the Management of EGFR-Mutated Advanced Non-Small Cell Lung Cancer—A Narrative Review
Simple Summary
Abstract
1. Introduction
2. Overview of First-Line Treatment (Table 1)
| Trial (Arm) | Median Age (Range) | Liver Metastases | CNS Metastases | TP53 Mutation | Baseline ctDNA | ORR | PFS (Median) | CNS PFS (Median) | OS (Median) |
|---|---|---|---|---|---|---|---|---|---|
| FLAURA—Osimertinib [5] | 64 years (26–85) | Not reported | 19.0% | Not reported | Not reported | 80% (75–85%) | 18.9 months (95% CI 15.2–21.4) | CNS PFS (18 months) 58% (95% CI 40–72) | 38.6 months (95% CI 34.5–41.8) |
| FLAURA—Gefitinib/Erlotinib [5] | 64 years (35–93) | Not reported | 23.0% | Not reported | Not reported | 76% (70–81%) | 10.2 months (95% CI 9.6–11.1) | CNS PFS (18 months) 40% (95% CI 25–55) | 31.8 months (95% CI 26.6–36.0) |
| FLAURA2—Osimertinib + Chemo [7] | 61 years (26–83) | 15.4% | 41.6% | Not reported | Not reported | 83% (78–87%) | 25.5 months (95% CI~) (HR 0.62) | 24.9 months (patients with baseline CNS mets) | NR (95% CI 38.0- NR), interim HR 0.75, p: 0.028 |
| FLAURA2—Osimertinib (monotherapy) [7] | 62 years (30–85) | 23.7% | 39.6% | Not reported | Not reported | 76% (70–80%) | 16.7 months (95% CI~) | 13.8 months (patients with baseline CNS mets) | 36.7 months (95% CI 33.2–NR) |
| MARIPOSA—Amivantamab + Lazertinib [11] | 64 years (25–88) | 15% | 41.4% | 56% | 69.2% | 86% (83–89%) | 23.7 months (95% CI 19.1–27.7) | 25.4 months (95% CI 20.1–29.5), HR 0.79, p: 0.07 | NR (95% CI 42.9- NR) (interim HR 0.75, p < 0.005) |
| MARIPOSA—Osimertinib [11] | 63 years (28–88) | 17% | 40% | 52.5% | 71.4% | 85% (81–88%) | 16.6 months (95% CI 14.8–18.5) | 22.2 months (95%CI, 18.4–26.9) | 36.7 months (95% CI 33.4–41.0) |
3. Mechanisms of Resistance and Second-Line Treatment
4. Ongoing Trials and Subsequent Lines of Treatment
4.1. Targeting On-Target EGFR Resistance
4.2. Off-Target Inhibition
4.3. Combined On-Target and Off-Target Inhibition
4.4. Targeting Tumor Antigens—ADC’s
4.5. Currently Available Subsequent Lines of Treatment (Figure 2)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Graham, R.P.; Treece, A.L.; Lindeman, N.I.; Vasalos, P.; Shan, M.; Jennings, L.J.; Rimm, D.L. Worldwide Frequency of Commonly Detected EGFR Mutations. Arch. Pathol. Lab. Med. 2018, 142, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Kambartel, K.; Häntschel, M.; Bennetts, M.; Nickens, D.J.; Brinkmann, J.; Kayser, A.; Moran, M.; Cappuzzo, F. Worldwide Prevalence of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Meta-Analysis. Mol. Diagn. Ther. 2021, 26, 7–18. [Google Scholar] [CrossRef]
- Shi, Y.; Au, J.S.-K.; Thongprasert, S.; Srinivasan, S.; Tsai, C.-M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.-C. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients with Advanced Non–Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef]
- D’ANgelo, S.P.; Pietanza, M.C.; Johnson, M.L.; Riely, G.J.; Miller, V.A.; Sima, C.S.; Zakowski, M.F.; Rusch, V.W.; Ladanyi, M.; Kris, M.G. Incidence of EGFR Exon 19 Deletions and L858R in Tumor Specimens from Men and Cigarette Smokers with Lung Adenocarcinomas. J. Clin. Oncol. 2011, 29, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Cho, B.C.; Lu, S.; Felip, E.; Spira, A.I.; Girard, N.; Lee, J.-S.; Lee, S.-H.; Ostapenko, Y.; Danchaivijitr, P.; Liu, B.; et al. Amivantamab plus Lazertinib in Previously Untreated EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2024, 391, 1486–1498. [Google Scholar] [CrossRef]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef]
- Yang, J.-H.; Kim, Y.; Lee, S.-H.; Liu, B.; Ostapenko, Y.; Lu, S.; Alip, A.; Korbenfeld, E.; Dias, J.; Danchaivijitr, P.; et al. 4O: Amivantamab plus lazertinib vs osimertinib in first-line (1L) EGFR-mutant (EGFRm) advanced NSCLC: Final overall survival (OS) from the phase III MARIPOSA study. J. Thorac. Oncol. 2025, 20, S6–S8. [Google Scholar] [CrossRef]
- PVI_Slides_LakeTahoe24.pdf. Available online: https://c.peerview.com/live/programs/150210399-1/downloads/PVI_slides_LakeTahoe24.pdf (accessed on 22 April 2025).
- Felip, E.; Cho, B.C.; Gutiérrez, V.; Alip, A.; Besse, B.; Lu, S.; Girard, A.I.S.N.; Califano, R.; Gadgeel, S.M.; Yang, J.H. 2024—Amivantamab Plus Lazertinib Versus Osimertinib in.pdf. Available online: https://www.annalsofoncology.org/article/S0923-7534(24)00702-6/pdf (accessed on 22 April 2025).
- Felip, E.; Cho, B.; Gutiérrez, V.; Alip, A.; Besse, B.; Lu, S.; Spira, A.; Girard, N.; Califano, R.; Gadgeel, S.; et al. Amivantamab plus lazertinib versus osimertinib in first-line EGFR-mutant advanced non-small-cell lung cancer with biomarkers of high-risk disease: A secondary analysis from MARIPOSA. Ann. Oncol. 2024, 35, 805–816. [Google Scholar] [CrossRef]
- Study Details|Enhanced Dermatological Care to Reduce Rash and Paronychia in Epidermal Growth Factor Receptor (EGRF)-Mutated Non-Small Cell Lung Cancer (NSCLC) Treated First-Line with Amivantamab Plus Lazertinib|ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT06120140#study-overview (accessed on 22 April 2025).
- Girard, N.; Li, W.; Spira, A.; Feldman, J.; Mak, M.; Sauder, M.; Bozorgmehr, F.; Voon, P.-J.; Yang, C.; Cundom, J.; et al. 10MO: Preventing moderate to severe dermatologic adverse events in first-line EGFR-mutant advanced NSCLC treated with amivantamab plus lazertinib: Early success of the COCOON trial. J. Thorac. Oncol. 2025, 20, S14–S16. [Google Scholar] [CrossRef]
- Study Details|Premedication to Reduce Amivantamab Associated Infusion Related Reactions|ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT05663866 (accessed on 22 April 2025).
- Spira, A.I.; Paz-Ares, L.; Han, J.Y.; Shih, J.Y.; Mascau, C.; Roy, U.B.; Zugazagoitia, J.; Kim, Y.J.; Chiu, C.-H.; Kim, S.-W.; et al. Preventing Infusion-Related Reactions With Intravenous Amivantamab-Results From SKIPPirr, a Phase 2 Study: A Brief Report. J. Thorac. Oncol. 2025, 20, 695–698. [Google Scholar] [CrossRef]
- ESMO. ctDNA-Guided Treatment Influence on Survival in Advanced NSCLC. Available online: https://www.esmo.org/oncology-news/ctdna-guided-treatment-influence-on-survival-in-advanced-nsclc (accessed on 22 April 2025).
- Cancer Network. Osimertinib/Chemo Elicits Meaningful PFS in EGFR+ NSCLC. 2023. Available online: https://www.cancernetwork.com/view/osimertinib-chemo-elicits-meaningful-pfs-in-egfr-nsclc (accessed on 22 April 2025).
- Pao, W.; Miller, V.A.; Politi, K.A.; Riely, G.J.; Somwar, R.; Zakowski, M.F.; Kris, M.G.; Varmus, H. Acquired Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib Is Associated with a Second Mutation in the EGFR Kinase Domain. PLoS Med. 2005, 2, e73. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Cheng, Y.; Zhou, C.; Ohe, Y.; Imamura, F.; Cho, B.C.; Lin, M.-C.; Majem, M.; Shah, R.; Rukazenkov, Y.; et al. Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann. Oncol. 2018, 29, viii740. Available online: https://www.annalsofoncology.org/article/S0923-7534(19)50454-9/fulltext (accessed on 13 December 2024). [CrossRef]
- Chmielecki, J.; Mok, T.; Wu, Y.-L.; Han, J.-Y.; Ahn, M.-J.; Ramalingam, S.S.; John, T.; Okamoto, I.; Yang, J.C.-H.; Shepherd, F.A.; et al. Analysis of acquired resistance mechanisms to osimertinib in patients with EGFR-mutated advanced non-small cell lung cancer from the AURA3 trial. Nat. Commun. 2023, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Hu, Y.; Mileham, K.F.; Husain, H.; Costa, D.B.; Tracy, P.; Feeney, N.; Sholl, L.M.; Dahlberg, S.E.; Redig, A.J.; et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients with EGFRT790M–Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018, 4, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, N.; Ou, Q.; Xiang, Y.; Jiang, T.; Wu, X.; Bao, H.; Tong, X.; Wang, X.; Shao, Y.W.; et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 3097–3107. [Google Scholar] [CrossRef]
- Yang, J.C.; Robichaux, J.; Planchard, D.; Kobayashi, K.; Lee, C.K.; Sugawara, S.; Yang, T.-Y.; Kim, T.M.; Kim, S.-W.; Yanagitani, N.; et al. MA12.03 FLAURA2: Resistance, and Impact of Baseline TP53 Alterations in Patients Treated with 1L Osimertinib ± Platinum-Pemetrexed. J. Thorac. Oncol. 2024, 19, S101–S102. [Google Scholar] [CrossRef]
- Besse, B.; Lee, S.-H.; Lu, S.; Stroyakovskiy, D.; Yazici, O.; Cid, J.R.; Hayashi, H.; Nguyen, D.; Yang, J.-H.; Gottfried, M.; et al. LBA55 Mechanisms of acquired resistance to first-line amivantamab plus lazertinib versus osimertinib in patients with EGFR-mutant advanced non-small cell lung cancer: An early analysis from the phase III MARIPOSA study. Ann. Oncol. 2024, 35, S1245–S1246. [Google Scholar] [CrossRef]
- Shum, E.; Elamin, Y.Y.; Piotrowska, Z.; Spigel, D.R.; Reckamp, K.L.; Rotow, J.K.; Tan, D.S.-W.; Lim, S.M.; Kim, T.M.; Lin, C.-C.; et al. A phase 1/2 study of BLU-945 in patients with common activating EGFR-mutant non–small cell lung cancer (NSCLC): SYMPHONY trial in progress. J. Clin. Oncol. 2022, 40, TPS9156. [Google Scholar] [CrossRef]
- Black Diamond Therapeutics, Inc. A Phase 1/2 Study to Assess BDTX-1535, an Oral EGFR Inhibitor, in Patients with Glioblastoma or Non-Small Cell Lung Cancer. clinicaltrials.gov; 2025 March. Report No.: NCT05256290. Available online: https://clinicaltrials.gov/study/NCT05256290 (accessed on 22 April 2025).
- AstraZeneca. A Biomarker-Directed Phase 2 Platform Study in Patients with Advanced Non-Small Lung Cancer Whose Disease Has Progressed on First-Line Osimertinib Therapy; clinicaltrials.gov; 2025 January. Report No.: NCT03944772. Available online: https://clinicaltrials.gov/study/NCT03944772 (accessed on 22 April 2025).
- Socinski, M.A.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 1909–1924. [Google Scholar] [CrossRef]
- Fang, W.; Zhao, Y.; Yang, Y.; Zhou, N.; Chen, L.; Huang, Y.; Chen, J.; Zhuang, L.; Du, Y.; Zhuang, W.; et al. Phase II results of ivonescimab (AK112/ SMT112), a novel PD-1/VEGF bispecific, in combination with chemotherapy for first line treatment of advanced or metastatic non-small cell lung cancer (NSCLC) without actionable genomic alterations (AGA) in EGFR/ALK. J. Clin. Oncol. 2023, 41, 9087. [Google Scholar] [CrossRef]
- HARMONi-A Study Investigators; Fang, W.; Zhao, Y.; Luo, Y.; Yang, R.; Huang, Y.; He, Z.; Zhao, H.; Li, M.; Li, K.; et al. Ivonescimab Plus Chemotherapy in Non–Small Cell Lung Cancer with EGFR Variant. JAMA 2024, 332, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.-H.; Melosky, B.; Shih, J.-Y.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; Felip, E.; et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study. Ann. Oncol. 2023, 35, 77–90. [Google Scholar] [CrossRef]
- Fang, W.; Li, X.; Wang, Q.; Meng, X.; Zheng, W.; Sun, L.; Yao, W.; Zhuang, W.; Fan, Y.; Zhuo, M.; et al. Sacituzumab tirumotecan versus docetaxel for previously treated EGFR-mutated advanced non-small cell lung cancer: Multicentre, open label, randomised controlled trial. BMJ 2025, 389, e085680. [Google Scholar] [CrossRef]
- Leighl, N.B.; Akamatsu, H.; Lim, S.M.; Cheng, Y.; Minchom, A.R.; Marmarelis, M.E.; Sanborn, R.E.; Yang, J.C.-H.; Liu, B.; John, T.; et al. Subcutaneous Versus Intravenous Amivantamab, Both in Combination with Lazertinib, in Refractory Epidermal Growth Factor Receptor–Mutated Non–Small Cell Lung Cancer: Primary Results from the Phase III PALOMA-3 Study. J. Clin. Oncol. 2024, 42, 3593–3605. [Google Scholar] [CrossRef]
- Oxnard, G.R.; Yang, J.C.-H.; Yu, H.; Kim, S.-W.; Saka, H.; Horn, L.; Goto, K.; Ohe, Y.; Mann, H.; Thress, K.S.; et al. TATTON: A multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann. Oncol. 2020, 31, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Guarneri, V.; Voon, P.J.; Lim, B.K.; Yang, J.-J.; Wislez, M.; Huang, C.; Liam, C.K.; Mazieres, J.; Tho, L.M.; et al. Tepotinib plus osimertinib in patients with EGFR-mutated non-small-cell lung cancer with MET amplification following progression on first-line osimertinib (INSIGHT 2): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2024, 25, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.P.; de Marinis, F.; Bonanno, L.; Sacher, A.G.; Chu, Q.S.; Baik, C.S.; Bironzo, P.; Bazhenova, L.; Tiseo, M.; Proto, C.; et al. Efficacy and CNS results from a randomized subset of the phase 2 SAVANNAH study comparing savolitinib (savo) + osimertinib (osi) combination with savo + placebo (PBO). J. Clin. Oncol. 2025, 43, 8513. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Tanaka, K.; Paz-Ares, L.; Cornelissen, R.; Girard, N.; Pons-Tostivint, E.; Baz, D.V.; Sugawara, S.; Cobo, M.; Pérol, M.; et al. Datopotamab Deruxtecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III TROPION-Lung01 Study. J. Clin. Oncol. 2025, 43, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Sands, J.; Ahn, M.-J.; Lisberg, A.; Cho, B.C.; Blumenschein, G.; Shum, E.; Tostivint, E.P.; Goto, Y.; Yoh, K.; Heist, R.; et al. Datopotamab Deruxtecan in Advanced or Metastatic Non–Small Cell Lung Cancer With Actionable Genomic Alterations: Results From the Phase II TROPION-Lung05 Study. J. Clin. Oncol. 2025, 43, 1254–1265. [Google Scholar] [CrossRef]
- Mok, T.; Jänne, P.A.; Nishio, M.; Novello, S.; Reck, M.; Steuer, C.; Wu, Y.-L.; Fougeray, R.; Fan, P.-D.; Meng, J.; et al. HERTHENA-Lung02: Phase III study of patritumab deruxtecan in advanced EGFR-mutated NSCLC after a third-generation EGFR TKI. Futur. Oncol. 2023, 20, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Cantarini, M.; Frewer, P.; Hawkins, G.; Peters, J.; Howarth, P.; Ahmed, G.F.; Sahota, T.; Hartmaier, R.; Li-Sucholeiki, X.; et al. SAVANNAH: A Phase II trial of osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-driven (MET+), locally advanced or metastatic non-small cell lung cancer (NSCLC), following disease progression on osimertinib. J. Clin. Oncol. 2019, 37 (Suppl. 15), TPS9119. [Google Scholar] [CrossRef]
- Tsai, C.J.; Yang, J.T.; Shaverdian, N.; Patel, J.; Shepherd, A.F.; Guttmann, D.; Yeh, R.; Gelblum, D.Y.; Namakydoust, A.; Preeshagul, I.; et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): An open-label, randomised, controlled, phase 2 study. Lancet 2023, 403, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.; Badra, E.V.; Adilovic, S.; Ahmadsei, M.; Christ, S.M.; Tanadini-Lang, S.; Mayinger, M.; Guckenberger, M.; Andratschke, N. Stereotactic body radiotherapy for oligoprogression with or without switch of systemic therapy. Clin. Transl. Radiat. Oncol. 2024, 45, 100748. [Google Scholar] [CrossRef]
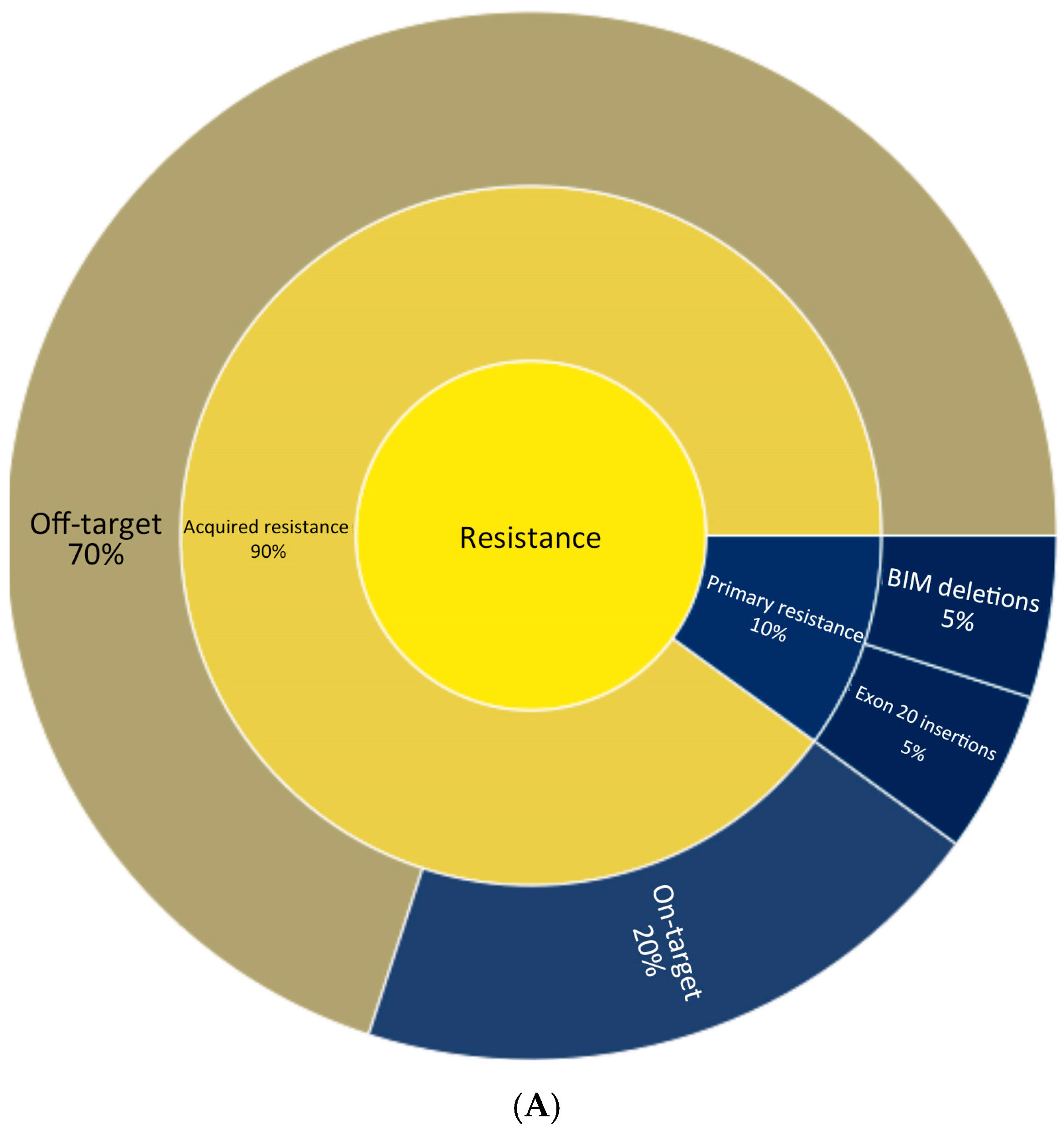
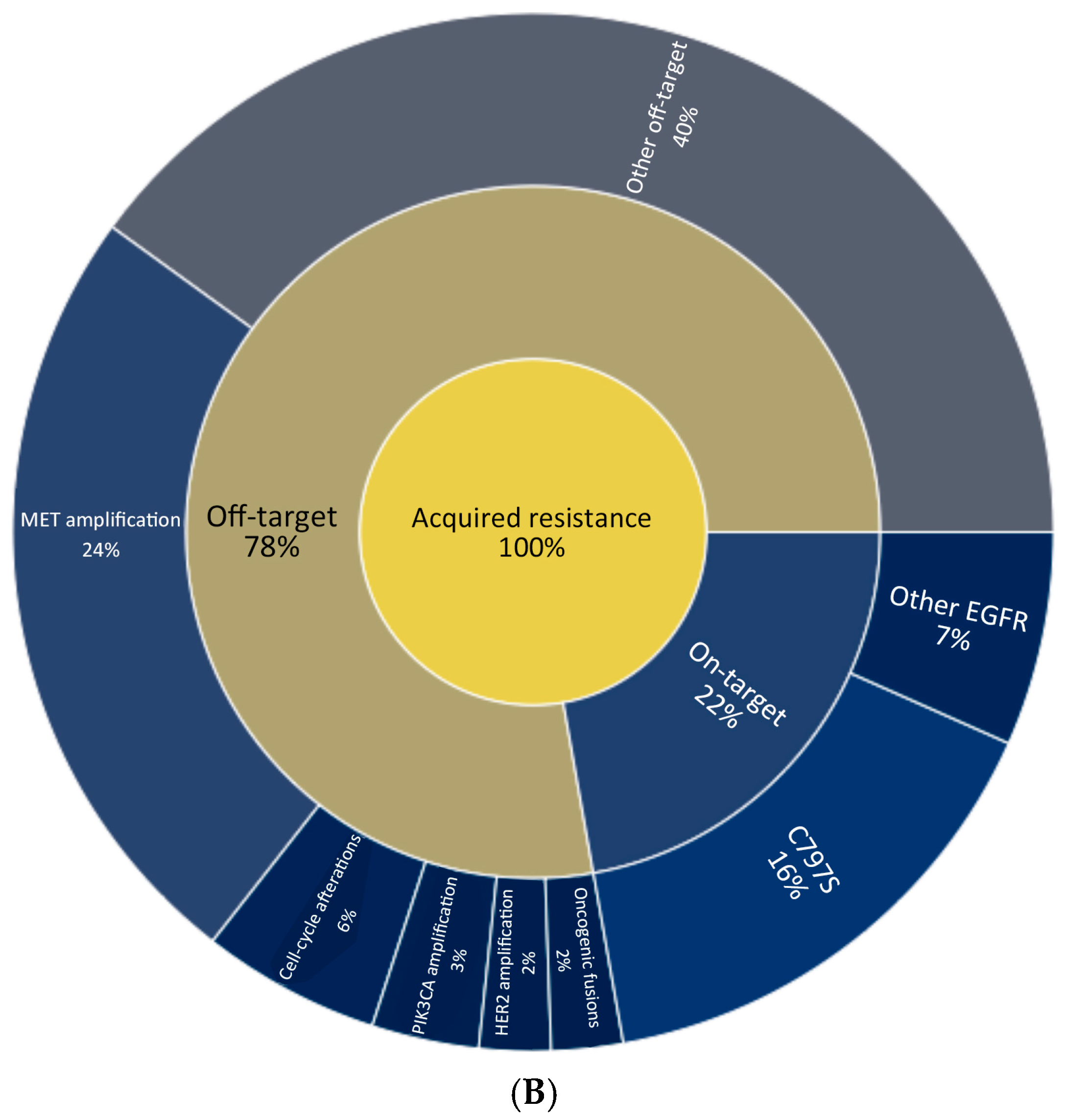
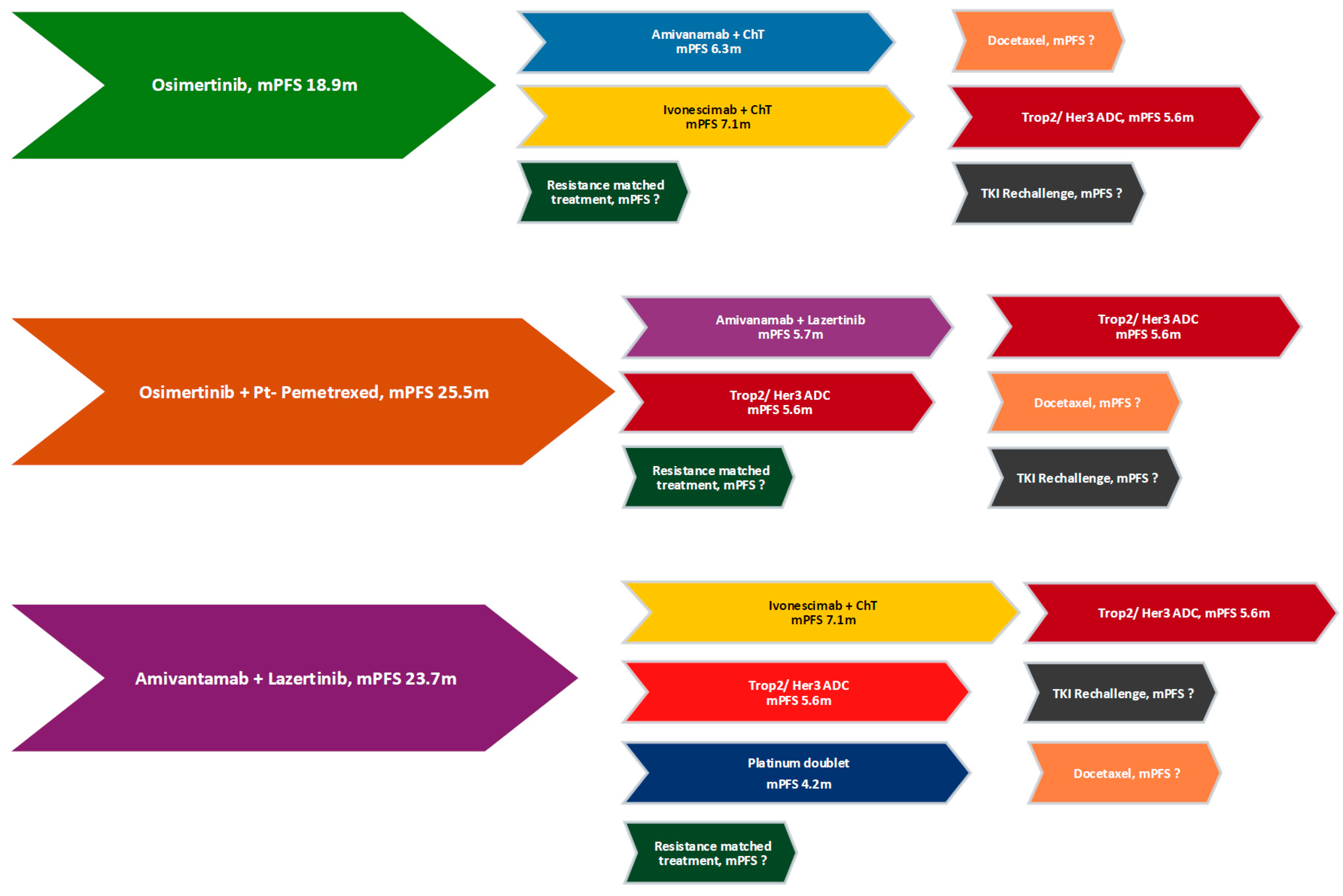
| Trial (Phase, NCT) | Phase | NCT | Population (Inclusion) | Intervention(s) | Status | Region(s) | Enrollment |
|---|---|---|---|---|---|---|---|
| ORCHARD [25] | Phase II | NCT03944772 | EGFR—mutant advanced NSCLC with disease progression on first-line Osimertinib | Biomarker-directed multiple arms (e.g., continuing Osimertinib plus Savolitinib, or adding EGFR/HER3-directed ADCs like patritumab-deruxtecan or datopotamab-deruxtecan, cetuximab + gefitinib, etc.) | Active (recruitment complete, treatment ongoing) | Global (multi-continent) | 247 |
| BLU-945 SYMPHONY [26] | Phase I/II | NCT04862780 | Metastatic EGFR—mutant NSCLC with acquired resistance (e.g., EGFR T790M and/or C797S mutations) after ≥1 prior EGFR TKI | BLU-945 (oral selective EGFR inhibitor)—dose-escalation and expansion cohorts, with and without Osimertinib | Recruiting (ongoing dose-escalation/expansion) | Global (US, Asia, etc.) | (ongoing; not yet reported) |
| HARMONi-A [27] | Phase III | AK112-301 | EGFR—mutant advanced NSCLC progressed on EGFR TKIs (including third-generation Osimertinib) | Arm A: Ivonescimab (anti–PD1/VEGF) Arm B: pemetrexed + carboplatin vs. placebo Arm C: pemetrexed + carboplatin | Completed | China (55 sites) | 322 |
| SAVANNAH [28] | Phase II | NCT03778229 | EGFR—mutant NSCLC with high MET overexpression and/or amplification, progressed on first-line Osimertinib | Osimertinib + Savolitinib(MET kinase inhibitor) | Recruiting | Global (multicenter) | ~360 (enrolled) |
| MARIPOSA-2 [29] | Phase III | NCT04988295 | EGFR—mutant (exon 19del/L858R) advanced NSCLC after progression on Osimertinib | Arm 1: Amivantamab (bispecific EGFR-MET antibody) + platinum chemotherapy (carboplatin + pemetrexed) + Lazertinib; Arm 2: Amivantamab + platinum chemo; Arm 3: Platinum chemo alone | Completed | Global (North America, Europe, Asia, etc.) | 657 |
| HERTHENA-Lung02 [30] | Phase III | NCT05338970 | EGFR—mutant NSCLC with progression on ≥1 EGFR TKI (including third-gen) | Patritumab-deruxtecan (HER3-directed ADC) vs. platinum doublet (cisplatin or carboplatin + pemetrexed) | Recruiting | Global (Asia, Europe, North America, Oceania) | ~560 |
| INSIGHT 2 [31] | Phase II | NCT03940703 | EGFR—mutant NSCLC with MET amplification after progression on first-line Osimertinib | Tepotinib (MET inhibitor) 500 mg + Osimertinib80 mg daily | Completed | Multi-national (17 countries) | 128 |
| OptiTROP—Lung03 [32] | Phase II | NCT05631262 | EGFR—mutant NSCLC after progression on EGFR TKI and platinum-based chemotherapy | Arm A: Sac-TMT Arm B: Docetaxel | Completed | China (48 sites) | 137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam Roy, P.; Reingold, D.; Pathak, N.; Verma, S.; Gupta, A.; Meti, N.; Molto, C.; Malik, P.S.; Linford, G.; Mittal, A. Recent Advances in the Management of EGFR-Mutated Advanced Non-Small Cell Lung Cancer—A Narrative Review. Curr. Oncol. 2025, 32, 448. https://doi.org/10.3390/curroncol32080448
Gautam Roy P, Reingold D, Pathak N, Verma S, Gupta A, Meti N, Molto C, Malik PS, Linford G, Mittal A. Recent Advances in the Management of EGFR-Mutated Advanced Non-Small Cell Lung Cancer—A Narrative Review. Current Oncology. 2025; 32(8):448. https://doi.org/10.3390/curroncol32080448
Chicago/Turabian StyleGautam Roy, Prabhat, Davida Reingold, Neha Pathak, Saurav Verma, Aarushi Gupta, Nicholas Meti, Consolacion Molto, Prabhat Singh Malik, Geordie Linford, and Abhenil Mittal. 2025. "Recent Advances in the Management of EGFR-Mutated Advanced Non-Small Cell Lung Cancer—A Narrative Review" Current Oncology 32, no. 8: 448. https://doi.org/10.3390/curroncol32080448
APA StyleGautam Roy, P., Reingold, D., Pathak, N., Verma, S., Gupta, A., Meti, N., Molto, C., Malik, P. S., Linford, G., & Mittal, A. (2025). Recent Advances in the Management of EGFR-Mutated Advanced Non-Small Cell Lung Cancer—A Narrative Review. Current Oncology, 32(8), 448. https://doi.org/10.3390/curroncol32080448





