Efficacy and Safety of Dose-Dense Chemotherapy in Breast Cancer: Real Clinical Data and Literature Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| pCR | Pathological complete response |
| DFS | Disease-free survival |
| OS | Overall survival |
| EC | Epirubicin/cyclophosphamide |
| NAC | Neoadjuvant chemotherapy |
| TNBC | Triple-negative breast cancer |
| G-CSF | Granulocyte-colony stimulating factor |
| PD-L1 | Programmed cell death ligand 1 |
| IMP3 | Insulin-like growth factor II mRNA-binding protein 3 |
References
- Morgan, E.; Oneill, C.; Shah, R.; Langselius, O.; Su, Y.; Frick, C.; Fink, H.; Bardot, A.; Walsh, O.M.; Woods, R.R.; et al. Metastatic recurrence in women diagnosed with non-metastatic breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2024, 26, 171. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Efird, J.T.; Prasad, S.; Jindal, C.; Walker, P.R. The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer. Oncotarget 2017, 8, 112712–112719. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.; Hanna, E.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Gereenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Reynolds, K.L.; et al. Pathological complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef]
- Melichar, B.; Hornychova, H.; Kalabova, H.; Basova, G.; Mergancova, J.; Urminska, H.; Jandik, P.; Cervinka, V.; Laco, J.; Ryska, A. Increased efficacy of a dose-dense regimen of neoadjuvant chemotherapy in breast carcinoma. Med. Oncol. 2012, 29, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Citron, M.L.; Berry, D.A.; Cirrincione, C.; Hudis, C.; Winer, E.P.; Gradishar, W.J.; Davidson, N.E.; Martino, S.; Livingston, R.; Ingle, J.N.; et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J. Clin. Oncol. 2003, 21, 1431–1439. [Google Scholar] [PubMed]
- Matikas, A.; Papakonstantinou, A.; Loibi, S.; Steger, G.G.; Untch, M.; Johansson, H.; Tsiknakis, N.; Hellstrom, M.; Greil, R.; Mobus, V.J.; et al. Benefit from dose-dense adjuvant chemotherapy for breast cancer: Subgroup analyses from the randomised phase 3 PANTHER trial. Lancet Reg. Health-Eur. 2025, 49, 101162. [Google Scholar] [CrossRef]
- Filho, O.M.; Ballman, K.; Cambell, J.; Liu, M.; Ligibel, L.; Watson, M.; Chen, Z.N.; Stover, D.; Carey, L.; Partridge, A.; et al. Adjuvant Dose-Dense Chemotherapy in Hormone Receptor–Positive Breast Cancer. J. Clin. Oncol. 2025, 43, 1229–1239. [Google Scholar] [CrossRef]
- Fornier, M.N.; Seidman, A.D.; Theodoulou, M.; Moynahan, M.E.; Currie, V.; Moassr, M.; Sklarin, N.; Gilewski, T.; Andrea, G.D.; Salvaggio, R.; et al. Doxorubicin followed by sequential paclitaxel and cyclophosphamide versus concurrent paclitaxel and cyclophosphamide: 5-year results of a phase II randomized trial of adjuvant dose-dense chemotherapy for women with node-positive breast carcinoma. Clin. Cancer Res. 2001, 7, 3934–3941. [Google Scholar]
- Venturini, M.; Mastro, L.D.; Aitini, E.; Baldini, E.; Caroti, C.; Contu, A.; Testore, F.; Breme, F.; Pronzato, P.; Cavazzini, G.; et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: Results from a randomized trial. J. Natl. Cancer Inst. 2005, 97, 1724–1733. [Google Scholar] [CrossRef]
- Fornier, M.; Norton, L. Dose-dense adjuvant chemotherapy for primary breast cancer. Breast Cancer Res. 2005, 7, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Blondeaux, E.; Lambertini, M.; Michelotti, A.; Conte, B.; Benasso, M.; Dellepiane, C.; Bighin, C.; Pastorino, S.; Levaggi, A.; D’Alonzo, A.; et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: 15-year results of the Phase 3 Mammella InterGruppo (MIG)-1 study. Br. J. Cancer 2020, 122, 1611–1617. [Google Scholar] [CrossRef]
- Mastro, L.D.; Placido, S.D.; Bruzzi, P.; Laurentiis, M.D.; Boni, C.; Cavazzini, G.; Durando, A.; Turletti, A.; Nistico, C.; Valle, E.; et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: An open-label, 2 × 2 factorial, randomized phase 3 trial. Lancet 2015, 385, 1863–1872. [Google Scholar] [CrossRef]
- Mastro, L.D.; Poggio, F.; Blondeaux, E.; Placido, S.D.; Giuliano, M.; Forestieri, V.; Laurentiis, M.D.; Gravina, A.; Bisagni, G.; Rimanti, A.; et al. Fluorouracil and dose-dense adjuvant chemotherapy in patients with early-stage breast cancer (GIM2): End-of-study results from a randomized, phase3 trial. Lancet Oncol. 2022, 23, 1571–1582. [Google Scholar] [CrossRef]
- Baldini, E.; Gardin, G.; Giannessi, P.G.; Evangelista, G.; Roncella, M.; Prochilo, T.; Collecchi, P.; Rosso, R.; Lionetto, R.; Bruzzi, P.; et al. Accelerated versus standard cyclophosphamide, epirubicin and 5-fluorouracil or cyclophosphamide, methotrexate and 5-fluorouracil: A randomized phase III trial in locally advanced breast cancer. Ann. Oncol. 2003, 14, 227–232. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; He, Y.; Li, J.; Wang, T.; Ouyang, T.; Fan, Z. Observation Effectiveness of Dose-Dense Neoadjuvant Anthracycline Sequential Weekly Paclitaxel for Triple-Negative Breast Cancer Patients. Clin. Breast Cancer 2023, 23, 423–430. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, S.; Xu, F.; Zeng, X. Survival benefit of pure dose-dense chemotherapy in breast cancer: A meta-analysis of randomized controlled trials. World J. Surg. Oncol. 2018, 16, 144. [Google Scholar] [CrossRef]
- Ohashi, R.; Sangen, M.; Namimatsu, S.; Yanagihara, K.; Yamashita, K.; Sakatani, T.; Takei, H.; Naito, A. Prognostic value of IMP3 expression as a determinant of chemosensitivity in triple negative breast cancer. Pathol. Res. Pract. 2017, 213, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Nagata, K.; Sano, M.; Yanagihara, K.; Ohashi, R.; Takei, H. Impact of IMP3 Expression on Chemotherapy Response and Prognosis in Triple-Negative Breast Cancer: A Retrospective Cohort Study. J. Nippon. Med. Sch. 2025, 92, 44–51. [Google Scholar] [CrossRef]
- Japanese Breast Cancer Society. Breast Cancer Practice Guidelines 2022 (Treatment Edition); Kanehara & Co., Ltd.: Tokyo, Japan, 2022. (In Japanese) [Google Scholar]
- Cameron, D.; Morden, J.P.; Canney, P.; Velikova, G.; Coleman, R.; Bartlett, J.; Agrawal, R.; Banerji, J.; Bertelli, G.; Boomfield, D.; et al. Accelerated versus standard epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil or capecitabine as adjuvant therapy for breast cancer in the randomised UK TACT2 trial (CRUK/05/19): A multicentre, phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2017, 18, 929–945. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Georgy, J.T.; Andrews, A.G.; John, A.O.; Joel, A.; Chacko, R.T.; Premkumar, P.S.; Singh, A. Anemia requiring transfusion in breast cancer patients on dose-dense chemotherapy: Prevalence, risk factors, cost and effect on disease outcome. Support. Care Cancer 2022, 30, 5519–5526. [Google Scholar] [CrossRef]
- Yagi, M.; Yoneto, T.; Yanagihara, K.; Nagata, K.; Matsuki, S.; Takei, H. Organizing Pneumonia Associated with Pneumocystis jirovecii in a Patient Receiving Dose-Dense Chemotherapy for Breast Cancer: A Case Report. J. Nippon. Med. Sch. 2024, 91, 567–573. [Google Scholar] [CrossRef]
- Truong, J.; Ashurst, J.V. Pneumocystis jirovecii Pneumonia. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2025; Available online: https://pubmed.ncbi.nlm.nih.gov/29493992/ (accessed on 21 January 2023).
- Barnes, G.; Pathak, A.; Schwartzberg, L. G-CSF utilization rate and prescribing patterns in United States: Associations between physician and patient factors and GCSF use. Cancer Med. 2014, 3, 1477–1484. [Google Scholar] [CrossRef]
- Goodman, L.M.; Moeller, M.B.; Azzouqa, A.G.; Guthrie, A.E.; Dalby, C.A.; Earl, M.A.; Cheng, C.; Pannell, N.A.; Shapiro, M.; Velcheti, V.; et al. Reduction of inappropriate prophylactic pegylated granulocyte colonystimulating factor use for patients with non-small-cell. lung cancer who receive chemotherapy: An ASCO quality training program project of the Cleveland Clinic Taussig Cancer Institute. Am. Soc. Clin. Oncol. 2016, 12, e101–e107. [Google Scholar] [CrossRef]
- Lyman, G.H.; Abella, E.; Pettengell, R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit. Rev. Oncol. Hematol. 2014, 90, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.M.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef]
- Waters, G.E.; Corrigan, P.; Gatesman, M.; Smith, T.J. Comparison of pegfilgrastim prescribing practice to national guidelines at a university hospital outpatient oncology clinic. J. Oncol. Pract. 2012, 9, 203–206. [Google Scholar] [CrossRef][Green Version]
- Mahtani, R.; Crawford, J.; Flannery, S.M.; Lawrence, T.; Schenfeld, J.; Gawade, P.L. Prophylactic pegfilgrastim to prevent febrile neutropenia among patients receiving biweekly (Q2W) chemotherapy regimens: A systematic review of efficacy, effectiveness and safety. BMC Cancer 2021, 21, 621. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, H.; Ishiguro, C.; Ando, T.; Kubota, Y.; Kinoshita, N.; Oniyama, Y.; Iguchi, T.; Uyama, Y. Nested Case-Control Study Utilizing MID-NET® on Thrombocytopenia Associated with Pegfilgrastim in Patients Treated with Antineoplastic Agents. Clin. Pharmacol. Ther. 2021, 110, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.C.; Atag, E.; Semiz, H.S.; Unal, O.U.; Uzun, M.; Aksoy, S.O.; Durak, M.G.; Karaoglu, A. Achieving treatment goals in older breast cancer patients receiving neoadjuvant chemotherapy. Sci. Rep. 2015, 15, 9866. [Google Scholar] [CrossRef]
- Zauderer, M.; Patil, S.; Hurria, A. Feasibility and toxicity of dose-dense adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res. Treat. 2009, 117, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar]
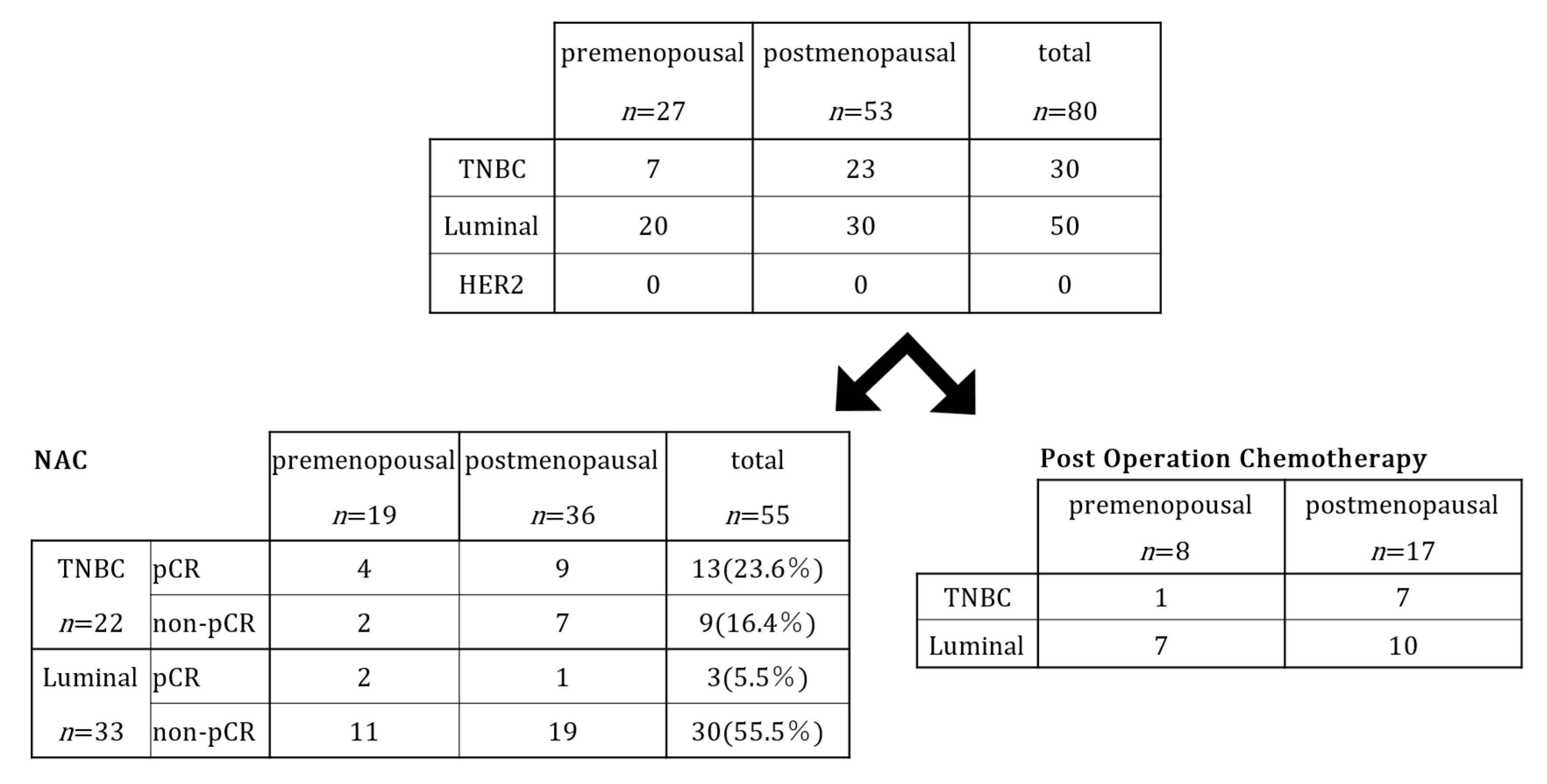
| Premenopause 27 (33.8%) | Postmenopause 53 (66.3%) | Total 80 (100%) | |
|---|---|---|---|
| Median age (range) | 44.1 (30–50) | 63.0 (46–78) | 56.7 (30–78) |
| Observation period (months) | 35 (9–52) | 40 (3–61) | 38 (3–64) |
| Menopausal status | |||
| Premenopausal | 25 | 0 | 25 (31.2%) |
| Postmenopausal | 0 | 51 | 51 (63.8%) |
| Unknown *1 | 2 | 2 | 4 (5.0%) |
| Histopathological type | |||
| Invasive ductal carcinoma | 25 | 50 | 75 (93.8%) |
| Invasive lobular carcinoma | 2 | 2 | 4 (5.0%) |
| Other | 0 | 1 | 1 (1.3%) |
| Clinical stage (before chemotherapy) | |||
| I | 4 | 11 | 15 (18.8%) |
| II | 16 | 30 | 46 (57.5%) |
| III | 7 | 12 | 19 (23.8%) |
| Tumor stage (before chemotherapy) | |||
| T1 | 6 | 18 | 24 (30.0%) |
| T2 | 15 | 21 | 36 (45.0%) |
| T3 | 3 | 4 | 7 (8.8%) |
| T4 | 3 | 10 | 13 (16.3%) |
| Tumor grade (before chemotherapy) | |||
| 1 | 12 | 16 | 28 (35.0%) |
| 2 | 10 | 24 | 34 (42.5%) |
| 3 | 5 | 13 | 18 (22.5%) |
| Hormone receptor | |||
| ER and/or PgR: positive | 20 | 30 | 50 (62.5%) |
| ER and PgR: negative | 7 | 23 | 30 (37.5%) |
| HER2 | |||
| positive | 0 | 0 | 0 |
| negative | 27 | 53 | 80 (100%) |
| Ki67 | |||
| ≤20 | 15 | 20 | 35 (43.8%) |
| >20 | 12 | 33 | 45 (56.3%) |
| Subtype | |||
| Luminal | 20 | 30 | 50 (62.5%) |
| Triple-negative | 7 | 23 | 30 (37.5%) |
| HER2 | 0 | 0 | 0 |
| Axillary lymph node status (at the diagnosis) *2 | |||
| Positive | 16 | 37 | 53 (66.3%) |
| Negative | 11 | 16 | 27 (33.8%) |
| No. of positive nodes (post-surgery) | |||
| 0 | 7 | 33 | 40 (50.0%) |
| 1–3 | 13 | 13 | 26 (32.5%) |
| 4–9 | 5 | 6 | 11 (13.8%) |
| ≥10 | 2 | 1 | 3 (3.8%) |
| Chemotherapy | |||
| Neoadjuvant | 19 | 36 | 55 (68.8%) |
| Post-operative | 8 | 17 | 25 (31.3%) |
| Pathological therapeutic effect after NAC (n = 55) | |||
| pCR | 5 | 11 | 16 |
| Non-pCR | 14 | 25 | 39 |
| Post-chemotherapy | |||
| Endocrine | 20 | 30 | 50 (62.5%) |
| CDK4/6 inhibitor | 5 | 9 | 14 (17.5%) |
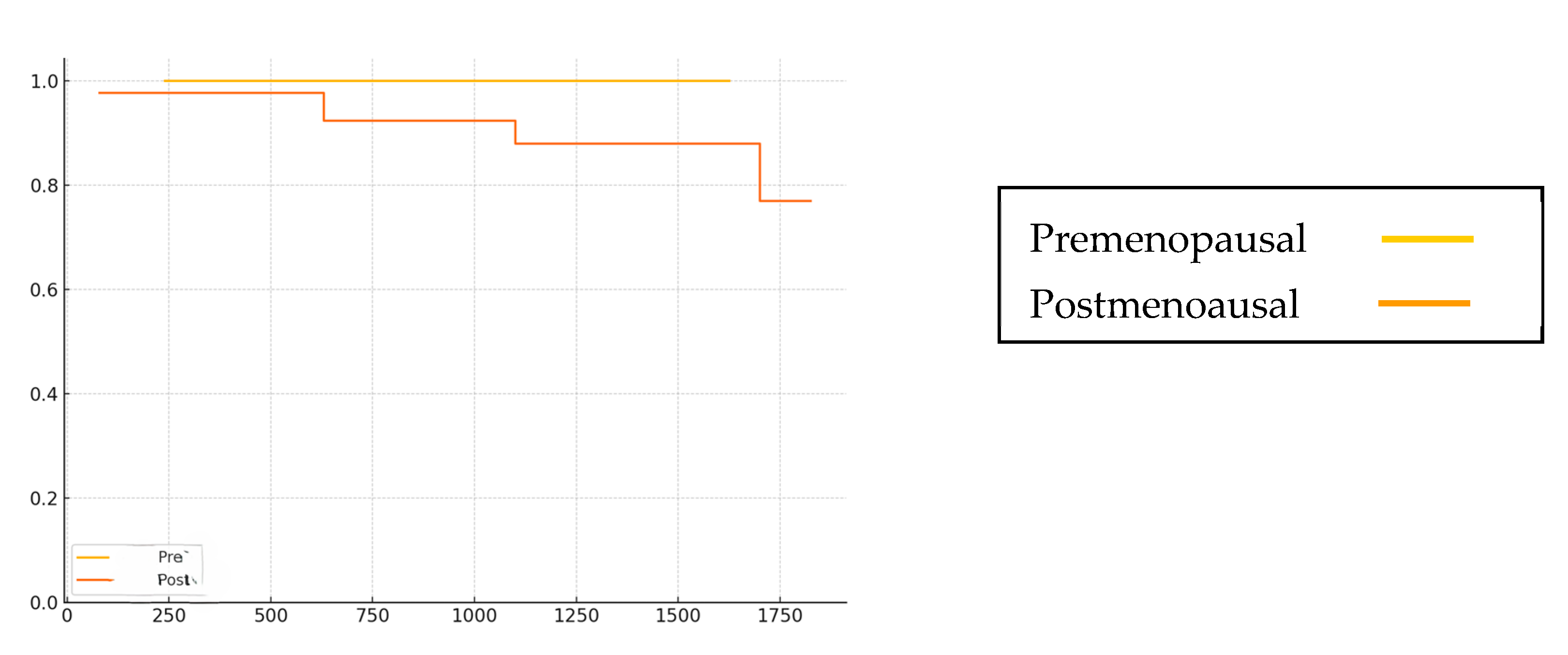
| Event | |||
| Distant recurrence | 0 | 5 | 5 (6.3%) |
| Death | 0 | 3 | 3 (3.8%) |
| Triple-Negative Type (n = 22) | Luminal Type (n = 33) | |
|---|---|---|
| Clinical chemotherapy effect, no (%) | ||
| cCR | 11 (50.0) | 5 (15.1) |
| cPR | 11 (50.0) | 27 (81.8) |
| cSD | 0 (0) | 1 (3.0) |
| cPD | 0 (0) | 0 (0) |
| Pathological chemotherapy effect, no (%) * | ||
| pCR | 13 (59.1) * | 3 (9.1) * |
| non-pCR | 9 (40.9) | 30 (90.9) |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Leukopenia (18.8%) | 6 (7.5) | 0 (0) | 5 (6.3) | 4 (5.0) |
| Neutropenia (18.8%) | 5 (6.2) | 1 (1.3) | 5 (6.3) | 4 (5.0) |
| Febrile neutropenia (8.8%) | 0 (0) | 0 (0) | 7 (8.8) | 0 (0) |
| Anemia (57.6%) | 33 (41.3) | 10 (12.5) | 3 (3.8) | 0 (0) |
| Thrombocytopenia (22.6%) | 16 (20.0) | 1 (1.3) | 0 (0) | 1 (1.3) |
| aspartate aminotransferase (58.8%) | 40 (50.0) | 6 (7.5) | 1(1.3) | 0 (0) |
| Anorexia (27.6%) | 20 (25.0) | 1 (1.3) | 1 (1.3) | 0 (0) |
| Nausea (37.6%) | 24 (30.0) | 5 (6.3) | 1 (1.3) | 0 (0) |
| Constipation (25.1%) | 15 (18.8) | 5 (6.3) | 0 (0) | 0 (0) |
| Diarrhea (3.8%) | 3 (3.8) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue (43.8%) | 29 (36.3) | 4 (5.0) | 2 (2.5) | 0 (0) |
| Arthralgia (57.6%) | 40 (50.0) | 5 (6.3) | 1 (1.3) | 0 (0) |
| Myalgia (70.1%) | 50 (62.5) | 5 (6.3) | 1 (1.3) | 0 (0) |
| Peripheral neuropathy (55.0%) | 34 (42.5) | 10 (12.5) | 0 (0) | 0 (0) |
| Edema (16.3%) | 10 (12.5) | 3 (3.8) | 0 (0) | 0 (0) |
| Eczema (8.8%) | 7 (8.8) | 0 (0) | 0 (0) | 0 (0) |
| Stomatitis (10.1%) | 7 (8.8) | 1 (1.3) | 0 (0) | 0 (0) |
| Lung infection (7.5%) | 0 (0) | 2 (2.5) | 4 (5.0) | 0 (0) |
| Fever (12.5%) | 8 (10.0) | 2 (2.5) | 0 (0) | 0 (0) |
| Dysgeusia (17.5%) | 12 (15.0) | 2 (2.5) | 0 (0) | 0 (0) |
| Facial nerve disorder (1.3%) | 0 (0) | 1 (1.3) | 0 (0) | 0 (0) |
| Headache (3.8%) | 3 (3.8) | 0 (0) | 0 (0) | 0 (0) |
| Alopecia (100%) | 0 (0) | 80 (100.0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagihara, K.; Yoshida, M.; Yamakawa, T.; Kato, S.; Tamura, M.; Nagata, K. Efficacy and Safety of Dose-Dense Chemotherapy in Breast Cancer: Real Clinical Data and Literature Review. Curr. Oncol. 2025, 32, 441. https://doi.org/10.3390/curroncol32080441
Yanagihara K, Yoshida M, Yamakawa T, Kato S, Tamura M, Nagata K. Efficacy and Safety of Dose-Dense Chemotherapy in Breast Cancer: Real Clinical Data and Literature Review. Current Oncology. 2025; 32(8):441. https://doi.org/10.3390/curroncol32080441
Chicago/Turabian StyleYanagihara, Keiko, Masato Yoshida, Tamami Yamakawa, Sena Kato, Miki Tamura, and Koji Nagata. 2025. "Efficacy and Safety of Dose-Dense Chemotherapy in Breast Cancer: Real Clinical Data and Literature Review" Current Oncology 32, no. 8: 441. https://doi.org/10.3390/curroncol32080441
APA StyleYanagihara, K., Yoshida, M., Yamakawa, T., Kato, S., Tamura, M., & Nagata, K. (2025). Efficacy and Safety of Dose-Dense Chemotherapy in Breast Cancer: Real Clinical Data and Literature Review. Current Oncology, 32(8), 441. https://doi.org/10.3390/curroncol32080441






