New Trends in Thyroid Malignancy: Minimally Invasive Thermal Ablation Percutaneous Techniques for T1 Papillary Thyroid Carcinomas
| PROCEDURE | Advantages | Drawbacks |
|---|---|---|
| THYROID SURGERY | Complete removal of the lobe and tumor | In-patient hospitalization (a) Risk of hypothyroidism (b) Risk of hypoparathyroidism (c) Dysphonia (d) Hematoma (e) Wound sepsis (f) Lymphocele (g) Wide neck scar (h) Horner syndrome (i) Tracheal necrosis |
| ACTIVE SURVEILLANCE | No intervention | Risk of active tumor progression and tumor spread |
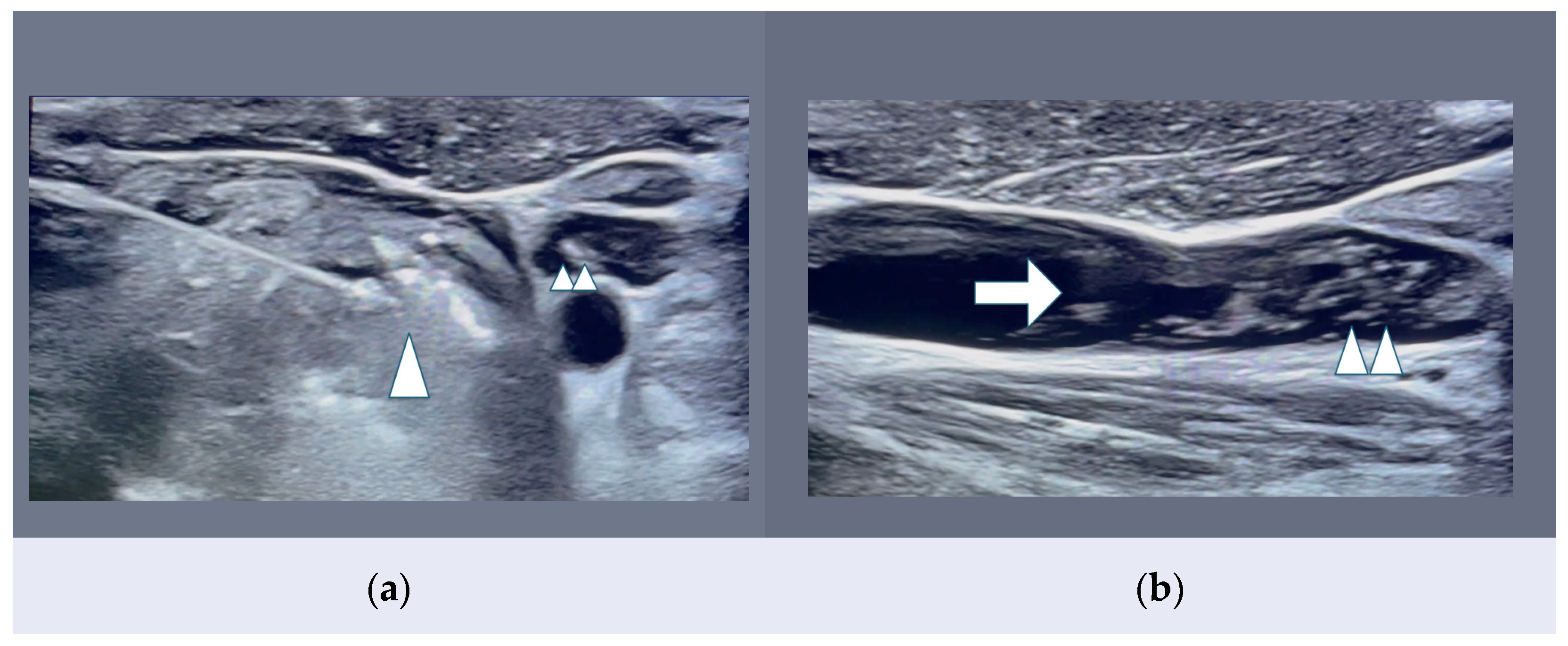
| MIT LA, RFA, MWA | -Thyroid and parathyroid gland preservation -No wide neck scar -Shorter procedure -Out-patient hospitalization -LA: small localized tumors -RFA: widely used, safe and effective in PTC <10 mm -MWA: may achieve faster ablation in larger vascularized tumors | -Treated nodule remains on site -No lymph node control -Nodular rupture (2%) -Local (skin, trachea) burns -(c,d,e,h,i) MIT < surgery |
Supplementary Materials
Conflicts of Interest
Abbreviations
References
- Pacella, C.M.; Bizzarri, G.; Guglielmi, R.; Anelli, V.; Bianchini, A.; Crescenzi, A.; Pacella, S.; Papini, E. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology 2000, 217, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Valcavi, R.; Piana, S.; Bortolan, G.S.; Lai, R.; Barbieri, V.; Negro, R. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: A feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid 2013, 23, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, J.H.; Valcavi, R.; Pacella, C.M.; Rhim, H.; Na, D.G. Thermal ablation for benign thyroid nodules: Radiofrequency and laser. Korean J. Radiol. 2011, 12, 525–540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mc Guff, P.E.; Deterling, R.A.; Gottlieb, L.S.; Fahimi, H.D.; Bushnell, D.; Robert, F. The Laser treatment of experimental malignant. tumors. Can. Med. Assoc. J. 1964, 91, 1089–1095. [Google Scholar] [PubMed] [PubMed Central]
- Mauri, G.; Orsi, F.; Carriero, S.; Della Vigna, P.; De Fiori, E.; Monzani, D.; Pravettoni, G.; Grosso, E.; Manzoni, M.F.; Ansarin, M.; et al. Image-Guided Thermal Ablation as an Alternative to Surgery for Papillary Thyroid Microcarcinoma: Preliminary Results of an Italian Experience. Front. Endocrinol. 2021, 11, 575152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruneton, J.N.; Balu-Maestro, C.; Marcy, P.Y.; Melia, P.; Mourou, M.Y. Very high frequency (13 MHz) ultrasonographic examination of the normal neck: Detection of normal lymph nodes and thyroid nodules. J. Ultrasound Med. 1994, 13, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Park, C.S.; Chung, W.Y.; Oh, K.K.; Kim, D.I.; Lee, J.T.; Yoo, H.S. New sonographic criteria for recommeding fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. Am. J. Roentgenol. 2002, 178, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.W.; Basurrah, M.A.; Lee, J.; Hwang, S.H. Diagnostic Performance of Six Ultrasound Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Network Meta-Analysis. Am. J. Roentgenol. 2023, 220, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Cochand-Priollet, B.; Maleki, Z. Cytology and Histology of Thyroid Nodules: Exploring Novel Insights in the Molecular Era for Enhanced Patient Management. Curr. Oncol. 2023, 30, 7753–7772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawka, A.M.; Carty, S.E.; Haugen, B.R.; Hennessey, J.V.; Kopp, P.A.; Pearce, E.N.; Sosa, J.A.; Tufano, R.P.; Jonklaas, J. American Thyroid Association Guidelines and Statements: Past, Present, and Future. Thyroid 2018, 28, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Kanona, H.; Virk, J.S.; Offiah, C.; Stimpson, P. Ultrasound-guided assessment of thyroid nodules based on the 2014 British Thyroid Association guidelines for the management of thyroid cancer-how we do it. Clin. Otolaryngol. 2017, 42, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Na, D.G. Ultrasound Imaging Criteria and Protocols for Active Surveillance of Low-Risk Thyroid Cancer: A Review of International Consensus Guidelines. Endocrinol. Metab. 2025, 40, 185–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghai, S.; O’Brien, C.; Goldstein, D.P.; Sawka, A.M.; Canadian Thyroid Cancer Active Surveillance Study Group. Ultrasound in active surveillance for low-risk papillary thyroid cancer: Imaging considerations in case selection and disease surveillance. Insights Imaging 2021, 12, 130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcy, P.Y.; Russ, G.; Saba, L.; Sanglier, J.; Ghanassia, E.; Sharara, H.; Thariat, J.; Morvan, J.B.; Bizeau, A. Opinion: Leading position of ultrasound in decision algorithm for small papillary thyroid carcinoma. Insights Imaging 2022, 13, 101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, W.; Jiang, S.; Zhan, W.; Zhou, J.; Xu, S.; Zhang, L. Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: Preliminary results. Eur. Radiol. 2017, 27, 2934–2940. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Baek, S.M.; Shin, S.; Son, J.M.; Kim, H.; Baek, J.H. Radiofrequency Ablation of Low-Risk Papillary Thyroid Microcarcinoma: A Retrospective Cohort Study Including Patients with More than 10 Years of Follow-up. Thyroid 2025, 35, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Hegedüs, L.; Bandula, S.; Cazzato, R.L.; Czarniecka, A.; Dudeck, O.; Fugazzola, L.; Netea-Maier, R.; Russ, G.; Wallin, G.; et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 Clinical Practice Guideline for the Use of Minimally Invasive Treatments in Malignant Thyroid Lesions. Eur. Thyroid J. 2021, 10, 185–197. [Google Scholar] [CrossRef]
- EuroMITT International Congress on Minimally Invasive Thyroid Treatments. Available online: https://eumitt.com/mitt-2025/ (accessed on 11 July 2025).
- Monpeyssen, H. Transitioning from Traditional Academic Decision Making to Patient-Centric Healthcare Choices: The Example of Thyroid Thermal Ablation Techniques for Papillary Thyroid Microcarcinomas. Curr. Oncol. 2023, 30, 9670–9675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grange, L.; Grange, R.; Bertholon, S.; Morisson, S.; Martin, I.; Boutet, C.; Grange, S. Virtual reality for interventional radiology patients: A preliminary study. Support. Care Cancer 2024, 32, 416. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.S.; Iannessi, A.; Buteau, S.; Jiang, X.Y.; Beaumont, H.; Grondin, B.; Baudin, G. Effects of relaxing therapies on patient’s pain during percutaneous interventional radiology procedures. Ann. Palliat. Med. 2018, 7, 455–462. [Google Scholar] [CrossRef] [PubMed]
- AFTHY: Association Francophone de Thyroïdologie. Available online: https://www.afthy.fr (accessed on 11 July 2025).
- Gao, X.; Yang, Y.; Wang, Y.; Huang, Y. Efficacy and safety of ultrasound-guided radiofrequency, microwave and laser ablation for the treatment of T1N0M0 papillary thyroid carcinoma on a large scale: A systematic review and meta-analysis. Int. J. Hyperth. 2023, 40, 2244713. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, Y.; Chu, X.; Chen, G.; Han, X.; Zhao, Y.; Liu, C.; Wang, J.; Xu, S. Ultrasound-guided microwave ablation versus surgery for low-risk solitary papillary thyroid microcarcinoma: A propensity-matched cohort study. Endocr. Connect. 2025, 14, e240366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, X.; Peng, Y.; Han, G. Comparative efficacy of different thermal ablation and conventional surgery for the treatment of Papillary Thyroid Microcarcinoma: Systematic review including traditional pooling and Bayesian network meta-analysis. Am. J. Otolaryngol. 2024, 45, 104479. [Google Scholar] [CrossRef] [PubMed]
- Austerlitz, J.; Mann, D.S.; Noel, J.E.; Orloff, L.A. Thyroid Nodule Rupture Following Radiofrequency Ablation for Benign Thyroid Nodules. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 651–657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, X.; Wang, J.; Tang, Q.; Gong, R.; Wei, T.; Li, Z. Delayed Tracheal Perforation After Microwave Ablation of Papillary Thyroid Microcarcinoma: A Case Report and Literature Review. Ear Nose Throat J. 2025, 1455613251333185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcy, P.Y., on behalf of AFTHY. New Trends in Thyroid Malignancy: Minimally Invasive Thermal Ablation Percutaneous Techniques for T1 Papillary Thyroid Carcinomas. Curr. Oncol. 2025, 32, 442. https://doi.org/10.3390/curroncol32080442
Marcy PY on behalf of AFTHY. New Trends in Thyroid Malignancy: Minimally Invasive Thermal Ablation Percutaneous Techniques for T1 Papillary Thyroid Carcinomas. Current Oncology. 2025; 32(8):442. https://doi.org/10.3390/curroncol32080442
Chicago/Turabian StyleMarcy, Pierre Yves on behalf of AFTHY. 2025. "New Trends in Thyroid Malignancy: Minimally Invasive Thermal Ablation Percutaneous Techniques for T1 Papillary Thyroid Carcinomas" Current Oncology 32, no. 8: 442. https://doi.org/10.3390/curroncol32080442
APA StyleMarcy, P. Y., on behalf of AFTHY. (2025). New Trends in Thyroid Malignancy: Minimally Invasive Thermal Ablation Percutaneous Techniques for T1 Papillary Thyroid Carcinomas. Current Oncology, 32(8), 442. https://doi.org/10.3390/curroncol32080442





