Trends in Prostate Cancer Incidence and Survival by Gleason Score from 2000 to 2020: A Population-Based Study in Northeastern Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
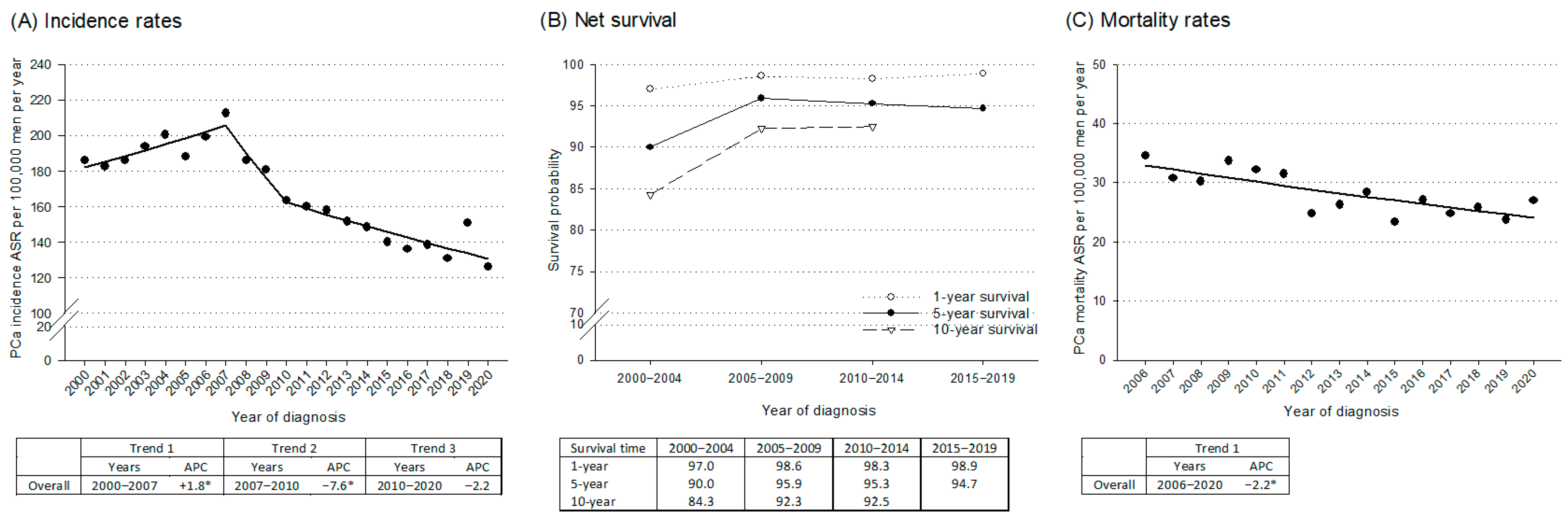
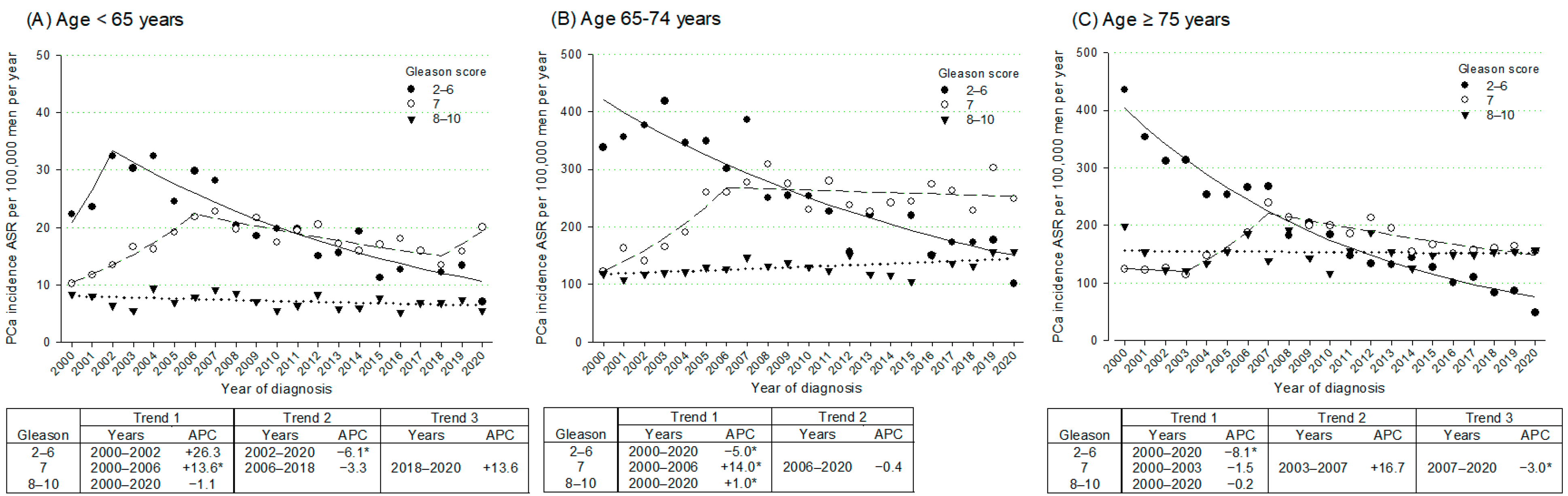
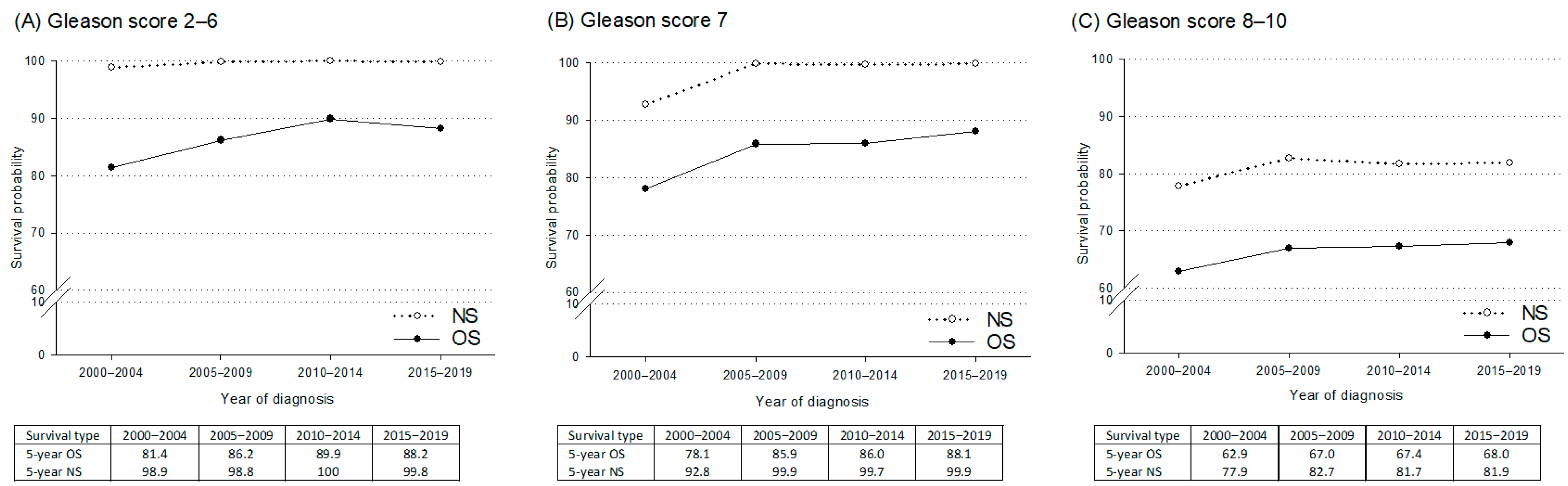
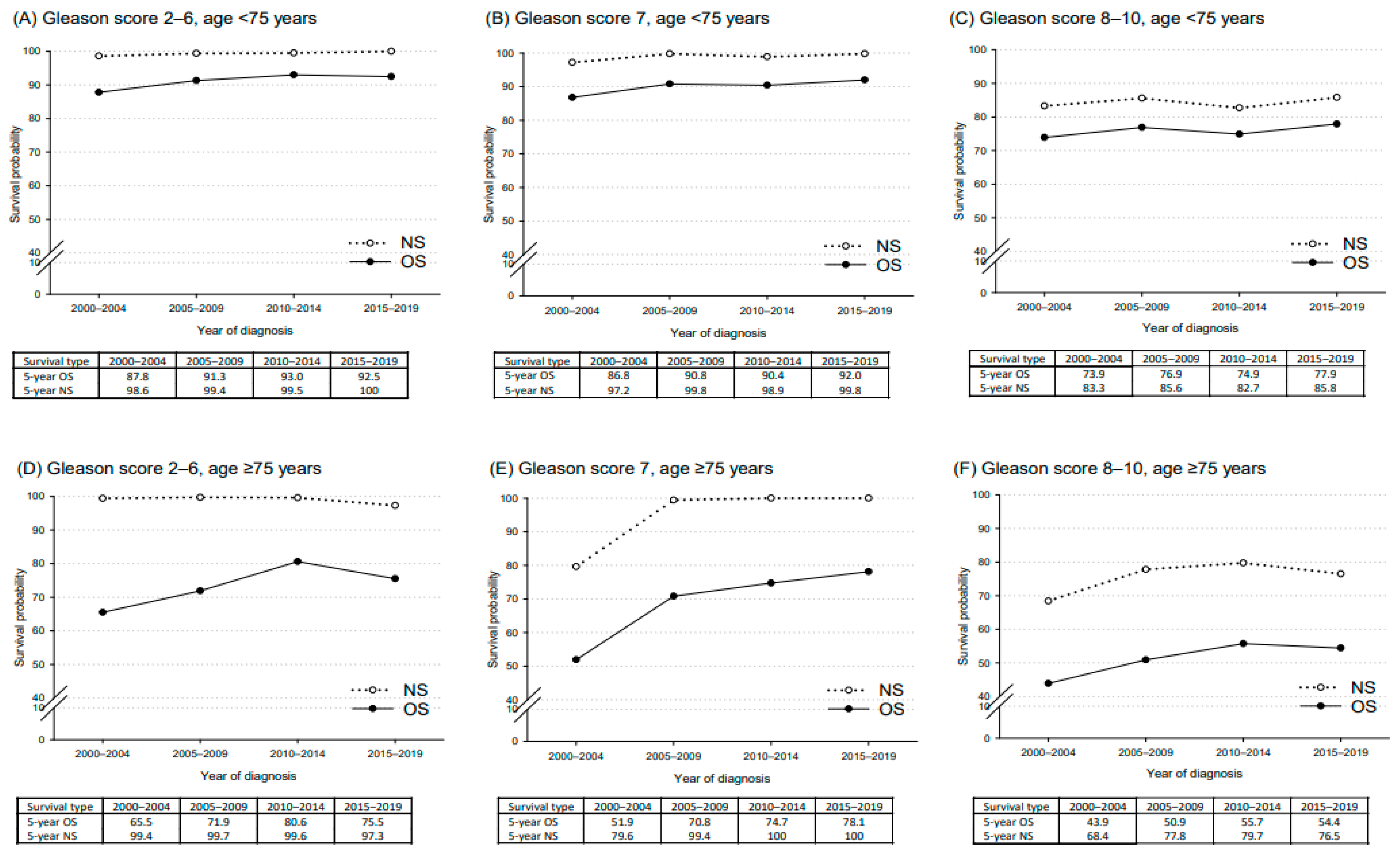
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Zhou, C.K.; Check, D.P.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Ferlay, J.; Bray, F.; Cook, M.B.; Devesa, S.S. Prostate cancer incidence in 43 populations worldwide: An analysis of time trends overall and by age group. Int. J. Cancer 2016, 138, 1388–1400. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Li, M.; Bray, F.; Kvale, R.; Serraino, D.; Lorenzoni, V.; Auvinen, A.; Dal Maso, L. Prostate cancer incidence and mortality in Europe and implications for screening activities: Population based study. BMJ 2024, 386, e077738. [Google Scholar] [CrossRef]
- Gandaglia, G.; Albers, P.; Abrahamsson, P.A.; Briganti, A.; Catto, J.W.F.; Chapple, C.R.; Montorsi, F.; Mottet, N.; Roobol, M.J.; Sønksen, J.; et al. Structured Population-based Prostate-specific Antigen Screening for Prostate Cancer: The European Association of Urology Position in 2019. Eur. Urol. 2019, 76, 142–150. [Google Scholar] [CrossRef]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2008, 149, 185–191. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA 2018, 319, 1901–1913. [Google Scholar]
- Heidenreich, A.; Abrahamsson, P.A.; Artibani, W.; Catto, J.; Montorsi, F.; Van Poppel, H.; Wirth, M.; Mottet, N. Early detection of prostate cancer: European Association of Urology Recommendation. Eur. Urol. 2013, 64, 347–354. [Google Scholar] [CrossRef]
- Taborelli, M.; Toffolutti, F.; Bidoli, E.; Dal Maso, L.; Del Zotto, S.; Clagnan, E.; Gobbato, M.; Serraino, D.; Franceschi, S. The use of PSA testing over more than 20 years: A population-based study in North-Eastern Italy. Tumori J. 2023, 109, 406–412. [Google Scholar] [CrossRef]
- Perotti, V.; Tittarelli, A.; Contiero, P.; Dal Maso, L.; Pesce, M.T.; Zarcone, M.; Gili, A.; Mazzucco, W.; Stracci, F.; Crocetti, E.; et al. Trends in cancer incidence and mortality in Italy, 2013–2017. Cancer Epidemiol. 2025, 97, 102855. [Google Scholar] [CrossRef] [PubMed]
- Schafer, E.J.; Laversanne, M.; Sung, H.; Soerjomataram, I.; Briganti, A.; Dahut, W.; Bray, F.; Jemal, A. Recent Patterns and Trends in Global Prostate Cancer Incidence and Mortality: An Update. Eur. Urol. 2025, 87, 302–313. [Google Scholar] [CrossRef]
- AIOM; AIRTUM; Fondazione AIOM; Osservatorio Nazionale Screening (ONS); PASSI; PASSI d’Argento; SIAPeC-IAP. I Numeri Del Cancro in Italia 2024. 2024. Available online: https://www.aiom.it/wp-content/uploads/2024/12/2024_NDC-def.pdf (accessed on 25 July 2025).
- Vicentini, M.; Sacchettini, C.; Trama, A.; Nicolai, N.; Gatta, G.; Botta, L.; Valdagni, R.; Giorgi Rossi, P.; Prostate Cancer High-Resolution Study Working Group; Pannozzo, F.; et al. Changes in Mortality and Incidence of Prostate Cancer by Risk Class in Different Periods in Italy: The Possible Effects of PSA Spread. Tumori J. 2017, 103, 292–298. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Meisner, A.L.; Arap, W.; Barry, M.; Shah, S.K.; Zeliadt, S.B.; Wiggins, C.L. Trends in United States Prostate Cancer Incidence Rates by Age and Stage, 1995–2012. Cancer Epidemiol. Biomark. Prev. 2016, 25, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Negoita, S.; Feuer, E.J.; Mariotto, A.; Cronin, K.A.; Petkov, V.I.; Hussey, S.K.; Benard, V.; Henley, S.J.; Anderson, R.N.; Fedewa, S.; et al. Annual Report to the Nation on the Status of Cancer, part II: Recent changes in prostate cancer trends and disease characteristics. Cancer 2018, 124, 2801–2814. [Google Scholar] [CrossRef]
- Jensen, O.M.; Storm, H.H. Cancer registration: Principles and methods. In Reporting of Results; IARC Scientific Publications: Lyon, France, 1991; Volume 95, pp. 108–125. [Google Scholar]
- Tyczynski, J.E.; Démaret, E.; Parkin, D.M. (Eds.) Standards and Guidelines for Cancer Registration in Europe. Volume I: The ENCR Recommendations; IARC Technical Publication No. 40; International Agency for Research on Cancer: Lyon, France, 2003. [Google Scholar]
- Forman, D.; Bray, F.; Brewster, D.H.; Gombe Mbalawa, C.; Kohler, B.; Piñeros, M.; Steliarova-Foucher, E.; Swaminathan, R.; Ferlay, J. (Eds.) Cancer Incidence in Five Continents, Vol. X.; IARC Scientific Publication No. 164; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. (Eds.) Cancer Incidence in Five Continents, Vol. XI.; IARC Scientific Publication No. 166; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Bray, F.; Colombet, M.; Aitken, J.F.; Bardot, A.; Eser, S.; Galceran, J.; Hagenimana, M.; Matsuda, T.; Mery, L.; Piñeros, M.; et al. (Eds.) Cancer Incidence in Five Continents, Vol. XII.; IARC Scientific Publication No. 169; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Surveillance Research Program, National Cancer Institute. SEER*Stat Software, Version 8.4.4. Available online: https://seer.cancer.gov/seerstat (accessed on 25 July 2025).
- Surveillance Research Program, National Cancer Institute. Joinpoint Regression Software, Version 5.4.0—April 2025. Available online: https://surveillance.cancer.gov/joinpoint (accessed on 25 July 2025).
- Irimata, K.E.; Bastian, B.A.; Clarke, T.C.; Curtin, S.C.; Rui, P. Guidance for Selecting Model Options in the National Cancer Institute Joinpoint Regression Software, Vital Health Stat 2; National Center for Health Statistics: Hyattsville, MD, USA, 2022. [Google Scholar]
- Pohar Perme, M.; Stare, J.; Estève, J. On estimation in relative survival. Biometrics 2012, 68, 113–120. [Google Scholar] [CrossRef]
- Toffolutti, F.; Guzzinati, S.; De Paoli, A.; Francisci, S.; De Angelis, R.; Crocetti, E.; Botta, L.; Rossi, S.; Mallone, S.; Zorzi, M.; et al. Complete prevalence and indicators of cancer cure: Enhanced methods and validation in Italian population-based cancer registries. Front. Oncol. 2023, 13, 1168325. [Google Scholar] [CrossRef] [PubMed]
- Van Blarigan, E.L.; McKinley, M.A.; Washington, S.L., 3rd; Cooperberg, M.R.; Kenfield, S.A.; Cheng, I.; Gomez, S.L. Trends in Prostate Cancer Incidence and Mortality Rates. JAMA Netw. Open 2025, 8, e2456825. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Herget, K.A.; Patel, D.P.; Hanson, H.A.; Sweeney, C.; Lowrance, W.T. Recent decline in prostate cancer incidence in the United States, by age, stage, and Gleason score. Cancer Med. 2016, 5, 136–141. [Google Scholar] [CrossRef]
- Chandran, A.; van Harten, M.; Singh, D.; Vilaseca, J.; Patasius, A.; Tupikowski, K.; Amorín, Á.G.; Galvin, D.; López, H.; Salazar, J.P.; et al. Risk-stratified Approach to Implementing Population-based Prostate Cancer Screening in Five Pilot Sites in the European Union: A Protocol for the PRAISE-U Project. Eur. Urol. Open Sci. 2024, 70, 8–17. [Google Scholar] [CrossRef]
- Wright, J.L.; Salinas, C.A.; Lin, D.W.; Kolb, S.; Koopmeiners, J.; Feng, Z.; Stanford, J.L. Prostate cancer specific mortality and Gleason 7 disease differences in prostate cancer outcomes between cases with Gleason 4 + 3 and Gleason 3 + 4 tumors in a population-based cohort. J. Urol. 2009, 182, 2702–2707. [Google Scholar] [CrossRef]
- Smith, S.; Wolanski, P. Metastatic prostate cancer incidence in Australia after amendment to prostate-specific antigen screening guidelines. ANZ J. Surg. 2018, 88, E589–E593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, G.; Lan, F.; Wang, H.; Shen, D.; Xu, K.; Xu, T.; Hu, H. Exploration on Gleason score variation trend of patients with prostate carcinoma from 1996 to 2019: A retrospective single center study. Gland. Surg. 2021, 10, 607–617. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, C.; Hu, Z.; Yang, C.; Zhang, R.; Ding, Y.; Wang, Z.; Tao, S.; Qin, Y. Differences in survival of prostate cancer Gleason 8–10 disease and the establishment of a new Gleason survival grading system. Cancer Med. 2021, 10, 87–97. [Google Scholar] [CrossRef]
- Egevad, L.; Micoli, C.; Samaratunga, H.; Delahunt, B.; Garmo, H.; Stattin, P.; Eklund, M. Prognosis of Gleason Score 9–10 Prostatic Adenocarcinoma in Needle Biopsies: A Nationwide Population-based Study. Eur. Urol. Oncol. 2024, 7, 213–221. [Google Scholar] [CrossRef]
- Orrason, A.W.; Garmo, H.; Styrke, J.; Dickman, P.W.; Stattin, P. Comparison of Relative Survival and Cause-Specific Survival in Men With Prostate Cancer According to Age and Risk Category: A Nationwide, Population-Based Study. Am. J. Epidemiol. 2021, 190, 2053–2063. [Google Scholar] [CrossRef]
- Bellier, A.; Colonna, M.; Delafosse, P.; Seigneurin, A. Incidence of prostate cancer and net survival by grade in a geriatric population: A population-based study in a French administrative entity from 1991 to 2013. Cancer Epidemiol. 2018, 56, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Salinas, C.A.; Tsodikov, A.; Ishak-Howard, M.; Cooney, K.A. Prostate cancer in young men: An important clinical entity. Nat. Rev. Urol. 2014, 11, 317–323. [Google Scholar] [CrossRef]
- Bleyer, A.; Spreafico, F.; Barr, R. Prostate cancer in young men: An emerging young adult and older adolescent challenge. Cancer 2020, 126, 46–57. [Google Scholar] [CrossRef] [PubMed]

| Total PCa Cases | PCa Cases Missing Gleason Score | PCa Cases with Gleason Score | Gleason Score | |||
|---|---|---|---|---|---|---|
| 2–6 | 7 | 8–10 | ||||
| No. | No. (row%) | No. | No. (row%) | No. (row%) | No. (row%) | |
| All | 21,571 | 2339 (10.8) | 19,232 | 7821 (40.7) | 7115 (37.0) | 4296 (22.3) |
| Age at diagnosis | ||||||
| <65 | 5253 | 394 (7.5) | 4859 | 2194 (45.2) | 1888 (38.8) | 777 (16.0) |
| 65–74 | 9553 | 828 (8.7) | 8725 | 3558 (40.8) | 3341 (38.3) | 1826 (20.9) |
| ≥75 | 6765 | 1117 (16.5) | 5648 | 2069 (36.6) | 1886 (33.4) | 1693 (30.0) |
| Period of diagnosis | ||||||
| 2000–2004 | 5127 (~1025/year) | 692 (13.5) | 4435 | 2522 (56.9) | 1074 (24.2) | 839 (18.9) |
| 2005–2009 | 5760 (~1152/year) | 600 (10.4) | 5160 | 2200 (42.6) | 1926 (37.3) | 1034 (20.1) |
| 2010–2014 | 5014 (~1003/year) | 510 (10.2) | 4504 | 1643 (36.5) | 1845 (41.0) | 1016 (22.5) |
| 2015–2020 | 5670 (~945/year) | 537 (9.5) | 5133 | 1456 (28.4) | 2270 (44.2) | 1407 (27.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taborelli, M.; Serraino, D.; Toffolutti, F.; Bidoli, E.; De Vidi, S.; Fratino, L.; Dal Maso, L.; the FVG Cancer Registry Working Group. Trends in Prostate Cancer Incidence and Survival by Gleason Score from 2000 to 2020: A Population-Based Study in Northeastern Italy. Curr. Oncol. 2025, 32, 426. https://doi.org/10.3390/curroncol32080426
Taborelli M, Serraino D, Toffolutti F, Bidoli E, De Vidi S, Fratino L, Dal Maso L, the FVG Cancer Registry Working Group. Trends in Prostate Cancer Incidence and Survival by Gleason Score from 2000 to 2020: A Population-Based Study in Northeastern Italy. Current Oncology. 2025; 32(8):426. https://doi.org/10.3390/curroncol32080426
Chicago/Turabian StyleTaborelli, Martina, Diego Serraino, Federica Toffolutti, Ettore Bidoli, Sara De Vidi, Lucia Fratino, Luigino Dal Maso, and the FVG Cancer Registry Working Group. 2025. "Trends in Prostate Cancer Incidence and Survival by Gleason Score from 2000 to 2020: A Population-Based Study in Northeastern Italy" Current Oncology 32, no. 8: 426. https://doi.org/10.3390/curroncol32080426
APA StyleTaborelli, M., Serraino, D., Toffolutti, F., Bidoli, E., De Vidi, S., Fratino, L., Dal Maso, L., & the FVG Cancer Registry Working Group. (2025). Trends in Prostate Cancer Incidence and Survival by Gleason Score from 2000 to 2020: A Population-Based Study in Northeastern Italy. Current Oncology, 32(8), 426. https://doi.org/10.3390/curroncol32080426






