Tumor Invasion Distance Based on MRI Is a Novel Prognostic Indicator for I-IIIB Cervical Cancer Patients Treated with Radiotherapy
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Evaluation and Treatment
2.3. Tumor Invasion Distance Measurement
2.4. Follow-Up and Outcomes
2.5. A New Risk Stratification System
2.5.1. The Prognostic Value of TID and a New Risk Stratification
2.5.2. The Comparison of New Risk Stratification and FIGO Staging System
2.6. Statistical Analysis
3. Results
3.1. Cohort Characteristics and Survival
3.2. The Relationship Between TID and Outcomes of CC Patients
3.3. The Relationship Between TID and Tumor Size
3.4. Identification of Prognostic Factors
3.5. Establishment of a Prognostic Model and New Risk Stratification
3.6. Comparison of New Risk Stratification and the 2018 FIGO Stage System
3.7. The Relationship Between New Risk Stratification and Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Shinagare, A.B.; Burk, K.S.; Kilcoyne, A.; Akin, E.A.; Chuang, L.; Hindman, N.M.; Huang, C.; Rauch, G.M.; Small, W., Jr.; Stein, E.B.; et al. ACR Appropriateness Criteria® Pretreatment Evaluation and Follow-Up of Invasive Cancer of the Cervix: 2023 Update. J. Am. Coll. Radiol. JACR 2024, 21, S249–S267. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.L.; Pareja, R.; Chiva, L.; Rodriguez, J.; Munsell, M.F.; Iniesta, M.D.; Manzour, N.; Frumovitz, M.; Ramirez, P.T. Accuracy of pre-operative tumor size assessment compared to final pathology and frequency of adjuvant treatment in patients with FIGO 2018 stage IB2 cervical cancer. Int. J. Gynecol. Cancer 2024, 34, 1861–1866. [Google Scholar] [CrossRef]
- Levinson, K.; Beavis, A.L.; Purdy, C.; Rositch, A.F.; Viswanathan, A.; Wolfson, A.H.; Kelly, M.G.; Tewari, K.S.; McNally, L.; Guntupalli, S.R.; et al. Corrigendum to “Beyond Sedlis-A novel histology-specific nomogram for predicting cervical cancer recurrence risk: An NRG/GOG ancillary analysis” [Gynecologic Oncology 162 (2021) 532–538]. Gynecol. Oncol. 2021, 163, 616–617. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Yu, T.; Bai, H.; Liu, J.; Wang, D.; Luo, Y. Value of diffusion-weighted imaging in preoperative evaluation and prediction of postoperative supplementary therapy for patients with cervical cancer. Ann. Transl. Med. 2022, 10, 120. [Google Scholar] [CrossRef]
- Xie, N.; Lin, J.; Yu, H.; Liu, L.; Deng, S.; Liu, L.; Sun, Y. A Diagnostic Nomogram Incorporating Prognostic Nutritional Index for Predicting Vaginal Invasion in Stage IB—IIA Cervical Cancer. Cancer Control J. Moffitt Cancer Cent. 2024, 31, 10732748241278479. [Google Scholar] [CrossRef]
- Gennigens, C.; De Cuypere, M.; Hermesse, J.; Kridelka, F.; Jerusalem, G. Optimal treatment in locally advanced cervical cancer. Expert. Rev. Anticancer Ther. 2021, 21, 657–671. [Google Scholar] [CrossRef]
- Mayadev, J.S.; Ke, G.; Mahantshetty, U.; Pereira, M.D.; Tarnawski, R.; Toita, T. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int. J. Gynecol. Cancer 2022, 32, 436–445. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obs. 2018, 143 (Suppl. S2), 22–36. [Google Scholar] [CrossRef]
- Yoshida, K.; Jastaniyah, N.; Sturdza, A.; Lindegaard, J.; Segedin, B.; Mahantshetty, U.; Rai, B.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Sasaki, R.; et al. Assessment of Parametrial Response by Growth Pattern in Patients With International Federation of Gynecology and Obstetrics Stage IIB and IIIB Cervical Cancer: Analysis of Patients From a Prospective, Multicenter Trial (EMBRACE). Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, S.; Kataoka, M.; Hamamoto, Y.; Tokumasu, A.; Uwatsu, K.; Kanzaki, H.; Takata, N.; Ishikawa, H.; Ouchi, A.; Mochizuki, T. Tumor growth patterns on magnetic resonance imaging and treatment outcomes in patients with locally advanced cervical cancer treated with definitive radiotherapy. Int. J. Clin. Oncol. 2019, 24, 1119–1128. [Google Scholar] [CrossRef]
- Buda, A.; Fanfani, F. The patterns of growth of cervical cancer: A challenge to personalized radical surgery. Int. J. Gynecol. Cancer 2023, 33, 1162–1163. [Google Scholar] [CrossRef] [PubMed]
- Trimbos, J.B.; Lambeek, A.F.; Peters, A.A.; Wolterbeek, R.; Gaarenstroom, K.N.; Fleuren, G.J.; Kenter, G.G. Prognostic difference of surgical treatment of exophytic versus barrel-shaped bulky cervical cancer. Gynecol. Oncol. 2004, 95, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, Y.; Yang, J.J.; Zhao, J.; Xiang, Y.; Xia, C.; Cao, Y.; Chen, B.; Guan, H.; Qi, Y.F.; et al. MRI-based radiomics analysis improves preoperative diagnostic performance for the depth of stromal invasion in patients with early stage cervical cancer. Insights Into Imaging 2022, 13, 17. [Google Scholar] [CrossRef]
- Bizzarri, N.; Pedone Anchora, L.; Zannoni, G.F.; Carbone, V.; Bruno, M.; Fedele, C.; Gallotta, V.; Chiantera, V.; Avesani, G.; Gui, B.; et al. Validation of tumour-free distance as novel prognostic marker in early-stage cervical cancer: A retrospective, single-centre, cohort study. Br. J. Cancer 2021, 125, 561–568. [Google Scholar] [CrossRef]
- Fiorentino, A.; Laudicella, R.; Ciurlia, E.; Annunziata, S.; Lancellotta, V.; Mapelli, P.; Tuscano, C.; Caobelli, F.; Evangelista, L.; Marino, L.; et al. Positron emission tomography with computed tomography imaging (PET/CT) for the radiotherapy planning definition of the biological target volume: PART 2. Crit. Rev. Oncol./Hematol. 2019, 139, 117–124. [Google Scholar] [CrossRef]
- Salvo, G.; Odetto, D.; Pareja, R.; Frumovitz, M.; Ramirez, P.T. Revised 2018 International Federation of Gynecology and Obstetrics (FIGO) cervical cancer staging: A review of gaps and questions that remain. Int. J. Gynecol. Cancer 2020, 30, 873–878. [Google Scholar] [CrossRef]
- Pálsdóttir, K.; Fridsten, S.; Blomqvist, L.; Alagic, Z.; Fischerova, D.; Gaurilcikas, A.; Hasselrot, K.; Jäderling, F.; Testa, A.C.; Sundin, A.; et al. Interobserver agreement of transvaginal ultrasound and magnetic resonance imaging in local staging of cervical cancer. Ultrasound Obstet. Gynecol. 2021, 58, 773–779. [Google Scholar] [CrossRef]
- Cibula, D.; Köhler, C.; Jarkovský, J.; Kocián, R.; Dundr, P.; Klát, J.; Zapardiel, I.; Landoni, F.; Frühauf, F.; Fischbach, R.; et al. Magnetic resonance imaging and ultrasound examination in preoperative pelvic staging of early-stage cervical cancer: Post-hoc analysis of SENTIX study. Ultrasound Obstet. Gynecol. 2025, 65, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, J.L.; García, E.; Machuca, M.; Quintana, R.; Escrig, J.; Chacón, E.; Mínguez, J.A.; Chiva, L. Magnetic resonance imaging and ultrasound for assessing parametrial infiltration in cervical cancer. A systematic review and meta-analysis. Med. Ultrason. 2020, 22, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kocián, R.; Siegler, K.; Klát, J.; Benešová, K.; Paderno, M.; Van Lonkhuijzen, L.; Chaloupková, B.; Pilka, R.; Michal, M.; Kašcák, P.; et al. #451 Magnetic resonance imaging or expert ultrasound in preoperative local staging of patients with early-stage cervical cancer: Final results of the SENTIX prospective, single-arm, international trial (CEEGOG CX-01; ENGOT-CX2). Int. J. Gynecol. Cancer 2023, 33, A2–A3. [Google Scholar] [CrossRef]
- Fischerova, D.; Frühauf, F.; Burgetova, A.; Haldorsen, I.S.; Gatti, E.; Cibula, D. The Role of Imaging in Cervical Cancer Staging: ESGO/ESTRO/ESP Guidelines (Update 2023). Cancers 2024, 16, 775. [Google Scholar] [CrossRef]
- Lura, N.; Wagner-Larsen, K.S.; Forsse, D.; Trovik, J.; Halle, M.K.; Bertelsen, B.I.; Salvesen, Ø.; Woie, K.; Krakstad, C.; Haldorsen, I.S. What MRI-based tumor size measurement is best for predicting long-term survival in uterine cervical cancer? Insights Into Imaging 2022, 13, 105. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Cao, D.; Shen, K. MRI-based three-dimensional reconstruction for staging cervical cancer and predicting high-risk patients. Ann. Transl. Med. 2021, 9, 1398. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Magnetic resonance imaging for detection of parametrial invasion in cervical cancer: An updated systematic review and meta-analysis of the literature between 2012 and 2016. Eur. Radiol. 2018, 28, 530–541. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer (Version 4.2025). [EB/OL]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 3 June 2025).
- Fang, C.; Zhang, P.; Yu, A.; Yang, Y.; Zhang, J. Different prognosis of stage IIIB cervical cancer patients with lower third of vaginal invasion and those without. Gynecol. Oncol. 2021, 162, 50–55. [Google Scholar] [CrossRef]
- Kotha, N.V.; Williamson, C.W.; Marra, K.V.; McHale, M.; Mell, L.K.; Mayadev, J.S. Incomplete cisplatin regimens in chemoradiation and its effect on outcomes for locally advanced cervical cancer. Int. J. Gynecol. Cancer 2022, 32, 1540–1548. [Google Scholar] [CrossRef]
- Balcacer, P.; Shergill, A.; Litkouhi, B. MRI of cervical cancer with a surgical perspective: Staging, prognostic implications and pitfalls. Abdom. Radiol. 2019, 44, 2557–2571. [Google Scholar] [CrossRef]
- Gurram, L.; Patil, R.; Chopra, S.; Maheshwari, A.; Shylasree, T.S.; Gupta, S.; Ghosh, J.; Gulia, S.; Ghadi, Y.; Mahantshetty, U. Evaluation of outcomes in patients of cervical Cancer with lower one third vaginal involvement: A single institutional experience. Gynecol. Oncol. 2020, 159, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lin, J.; Deng, S.; Yu, H.; Xie, N.; Sun, Y. A novel nomogram and risk stratification for early metastasis in cervical cancer after radical radiotherapy. Cancer Med. 2023, 12, 21798–21806. [Google Scholar] [CrossRef] [PubMed]
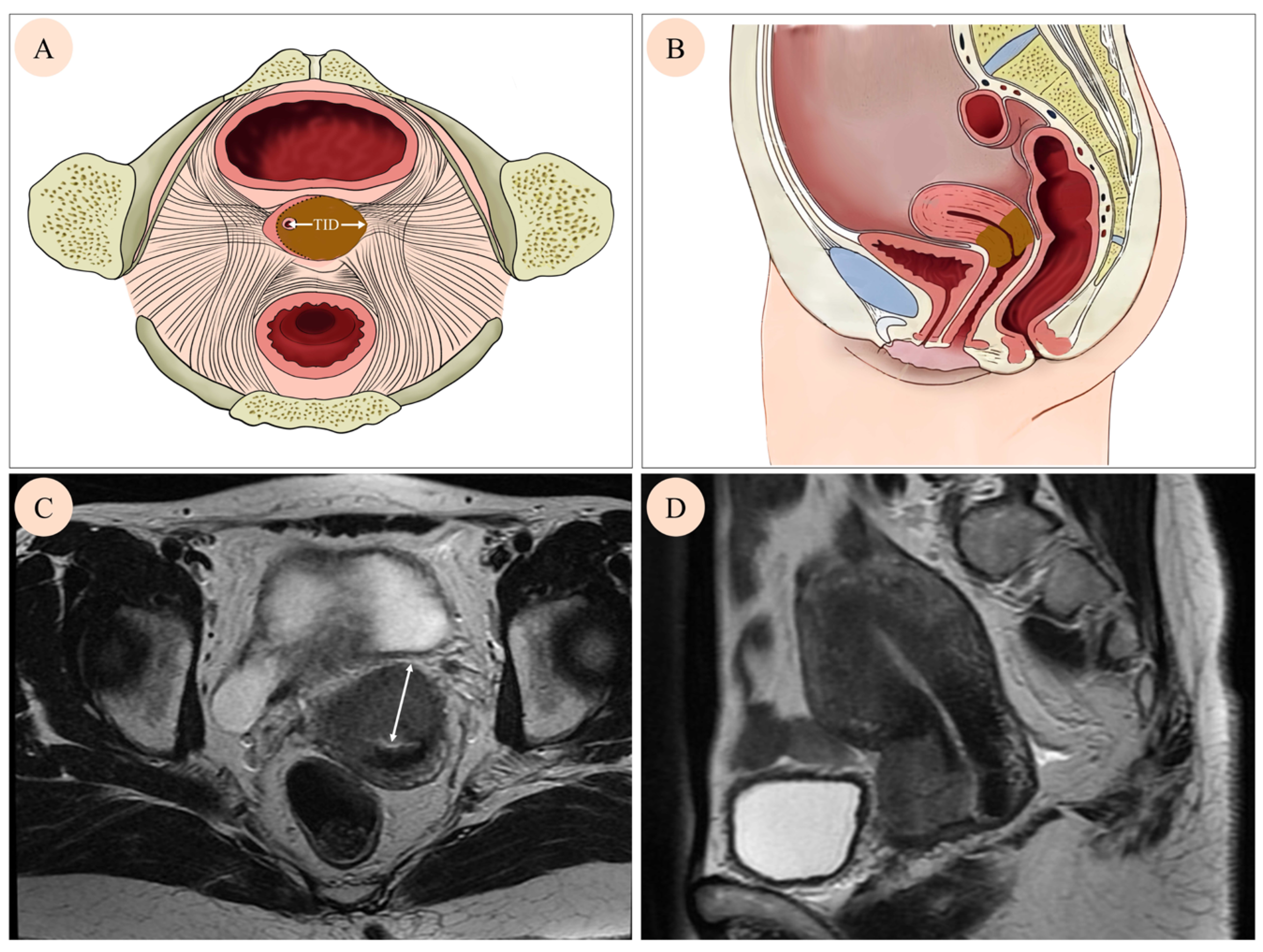

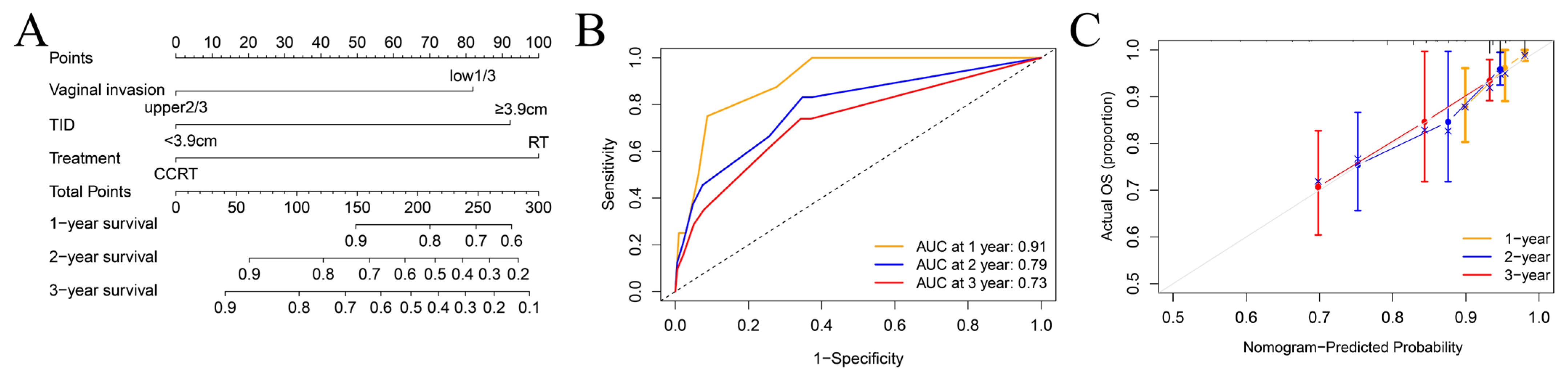
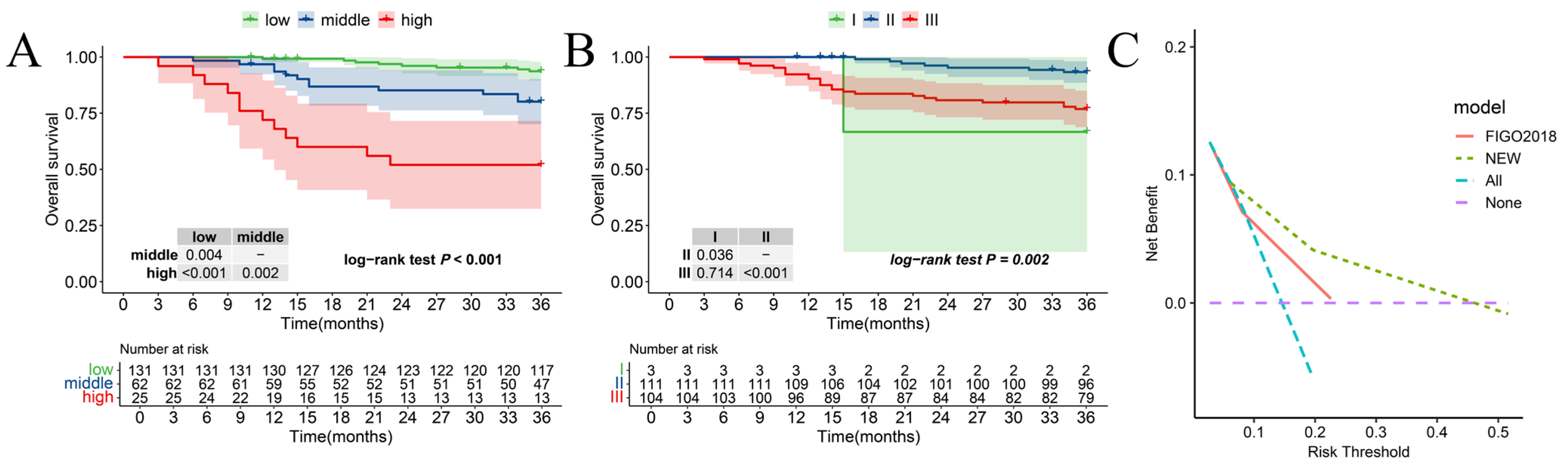
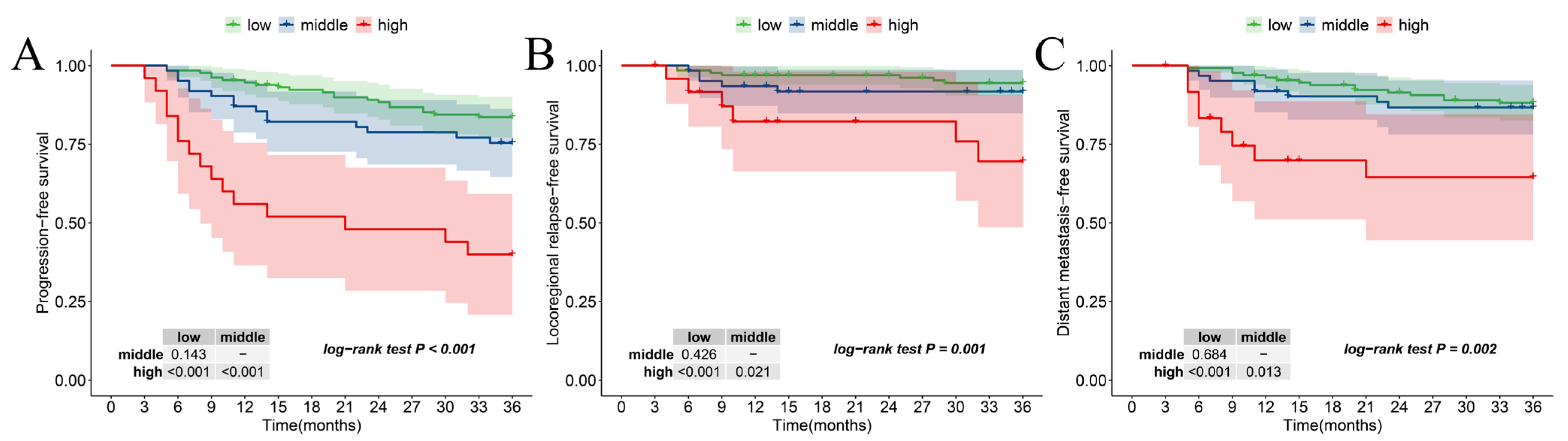
| Characteristic | Level | Number of Patients (%) |
|---|---|---|
| Total number | 218 | |
| Age | 25–84 (median = 59) | |
| Age (years) | <73 | 192 (88.1) |
| ≥73 | 26 (11.9) | |
| Stage | I | 3(1.4) |
| II | 111(50.9) | |
| III | 104(47.7) | |
| Pathology | NSCC | 14 (6.4) |
| SCC | 204 (93.6) | |
| Invasion distance (median [IQR]) | 2.71 [2.11, 3.55] | |
| Invasion distance (cm) | <3.9 | 181 (83.0) |
| ≥3.9 | 37 (17.0) | |
| Uterus invasion | no | 113 (51.8) |
| yes | 105 (48.2) | |
| Vaginal involvement | upper2/3 | 193 (88.5) |
| low1/3 | 25 (11.5) | |
| Hydronephrosis | no | 213 (97.7) |
| yes | 5 (2.3) | |
| Treatment | CCRT | 159 (72.9) |
| RT | 59 (27.1) | |
| Items | OS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univarite | Multivariate | Univarite | Multivariate | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | ||||||||
| ≥73 vs. <73 | 3.82 (1.67–8.28) | 0.001 | 1.38 (0.58–3.29) | 0.466 | 2.5 (1.28–4.88) | 0.007 | 1.1 (0.51–2.36) | 0.800 |
| Histology | ||||||||
| SCC VS NSCC | 2.23 (0.3–16.33) | 0.430 | 1.16 (0.36–3.74) | 0.798 | ||||
| Treatment | ||||||||
| CCRT vs. RT | 0.28 (0.14–0.56) | <0.001 | 0.32 (0.15–0.7) | 0.004 | 0.39 (0.23–0.68) | 0.001 | 0.41 (0.22–0.77) | 0.005 |
| Vaginal invasion | ||||||||
| low1/3 vs. upper 2/3 | 4.64 (2.2–9.82) | <0.001 | 2.58 (1.16–5.76) | 0.021 | 3.12 (1.63–5.96) | 0.001 | 2.02 (1.01–4.04) | 0.048 |
| Uterus involvement | ||||||||
| Yes vs. No | 0.72 (0.36–1.46) | 0.367 | 0.88 (0.51–1.53) | 0.648 | ||||
| TID | ||||||||
| ≥3.9 vs. <3.9 | 3.42 (1.67–7) | 0.001 | 3.00 (1.41–6.38) | 0.004 | 2.49 (1.36–4.55) | 0.003 | 2.37 (1.25–4.49) | 0.008 |
| Hydronephrosis | ||||||||
| Yes vs. No | 1.26 (0.17–9.25) | 0.819 | 0.73 (0.1–5.32) | 0.76 | ||||
| Items | DMFS | LRFS | ||||||
| Univarite | Multivariate | Univarite | Multivariate | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | ||||||||
| ≥73 vs. <73 | 0.94 (0.29–3.11) | 0.923 | 2.49 (0.82–7.57) | 0.109 | ||||
| Histology | ||||||||
| SCC VS NSCC | 0.66 (0.2–2.19) | 0.502 | 27051606.71 (0–Inf) | 0.997 | ||||
| Treatment | ||||||||
| CCRT vs. RT | 0.7 (0.33–1.49) | 0.355 | 0.33 (0.13–0.83) | 0.018 | 0.29 (0.12–0.75) | 0.01 | ||
| Vaginal invasion | ||||||||
| Low 1/3 vs. upper 2/3 | 1.9 (0.73–4.96) | 0.188 | 2.68 (0.88–8.15) | 0.083 | ||||
| Uterus involvement | ||||||||
| Yes vs. No | 0.57 (0.28–1.2) | 0.140 | 2.19 (0.82–5.83) | 0.118 | ||||
| TID | ||||||||
| ≥3.9 vs. <3.9 | 2.45 (1.13–5.31) | 0.024 | 2.45 (1.12–5.32) | 0.024 | 3.5 (1.36–9.04) | 0.010 | 3.94 (1.52–10.24) | 0.005 |
| Hydronephrosis | ||||||||
| Yes vs. No | 0 (0–Inf) | 0.996 | 0 (0–Inf) | 0.997 | ||||
| System | χ2 Test or Linear Trend | AIC | C Statistic |
|---|---|---|---|
| New risk stratification | 28.03 | 314 | 0.74 |
| 2018 FIGO stage system | 9.35 | 331 | 0.646 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Lin, J.; Li, A.; Xie, N.; Zheng, J.; Xiao, Y.; Lin, X.; Wu, S.; Yu, H.; Sun, Y. Tumor Invasion Distance Based on MRI Is a Novel Prognostic Indicator for I-IIIB Cervical Cancer Patients Treated with Radiotherapy. Curr. Oncol. 2025, 32, 355. https://doi.org/10.3390/curroncol32060355
Liu L, Lin J, Li A, Xie N, Zheng J, Xiao Y, Lin X, Wu S, Yu H, Sun Y. Tumor Invasion Distance Based on MRI Is a Novel Prognostic Indicator for I-IIIB Cervical Cancer Patients Treated with Radiotherapy. Current Oncology. 2025; 32(6):355. https://doi.org/10.3390/curroncol32060355
Chicago/Turabian StyleLiu, Linying, Jie Lin, Anyang Li, Ning Xie, Jianfeng Zheng, Youping Xiao, Xuefen Lin, Shizhong Wu, Haijuan Yu, and Yang Sun. 2025. "Tumor Invasion Distance Based on MRI Is a Novel Prognostic Indicator for I-IIIB Cervical Cancer Patients Treated with Radiotherapy" Current Oncology 32, no. 6: 355. https://doi.org/10.3390/curroncol32060355
APA StyleLiu, L., Lin, J., Li, A., Xie, N., Zheng, J., Xiao, Y., Lin, X., Wu, S., Yu, H., & Sun, Y. (2025). Tumor Invasion Distance Based on MRI Is a Novel Prognostic Indicator for I-IIIB Cervical Cancer Patients Treated with Radiotherapy. Current Oncology, 32(6), 355. https://doi.org/10.3390/curroncol32060355





