Dynamic Evolution of Vascular Features Based on Magnetic Resonance Imaging to Predict Pathological Response, Patterns of Recurrence and Survival Outcomes in Breast Cancer Neoadjuvant Chemotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. MRI Protocols
2.3. MRI Vascular Feature Extraction
2.4. Data Collection
2.5. Outcomes
2.6. Model Development and Validation
2.7. Statistics
3. Results
3.1. Patient Clinicopathologic Characteristics
3.2. Tumor Response to NAC
3.3. Model Development and Validation for bpCR
3.4. Model Development and Validation for RFS
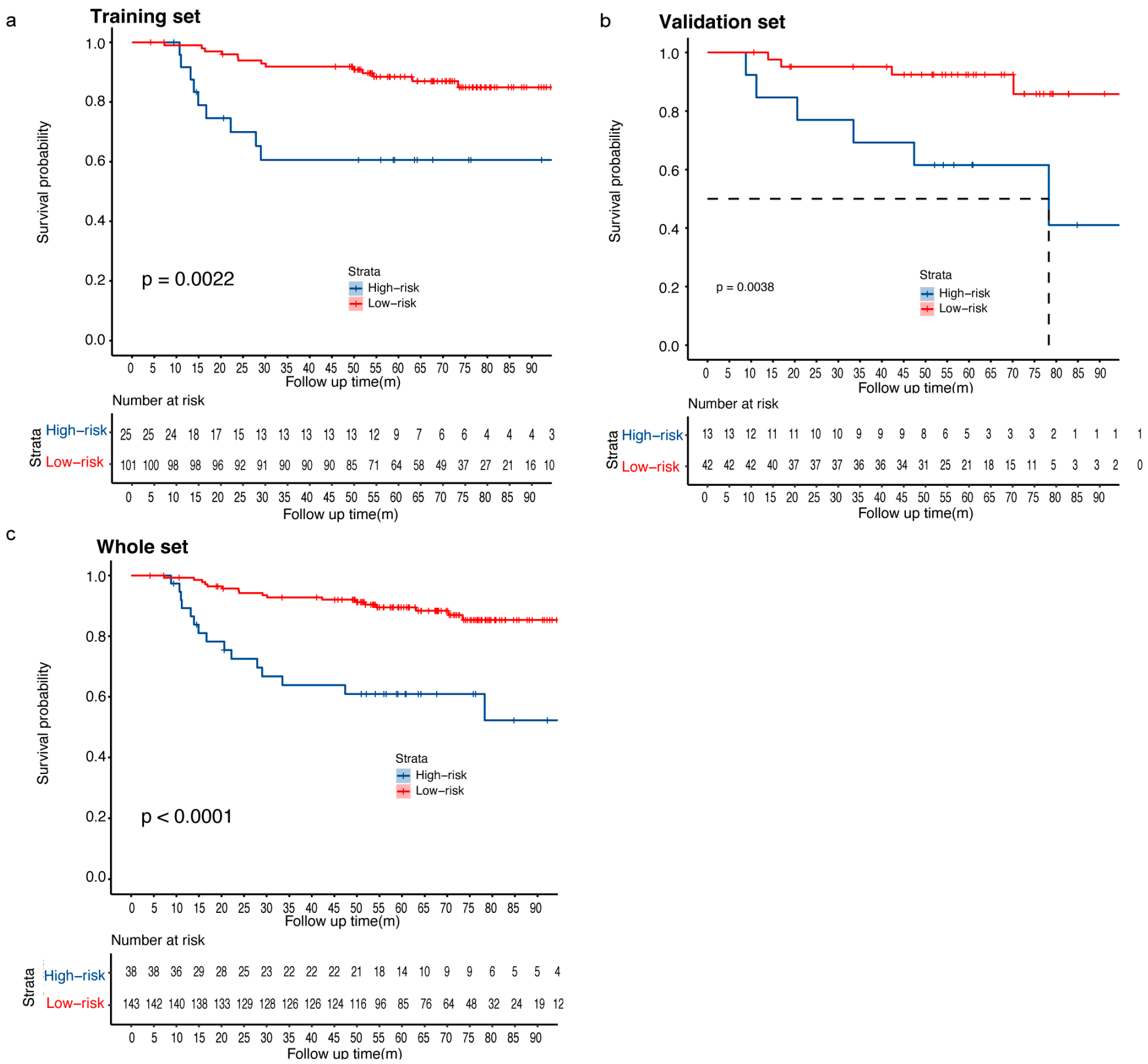
3.5. Risk Stratification of the RFS Predictive Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| bpCR | breast pathological complete response |
| DCA | decision curve analysis |
| DFS | disease-free survival |
| ER | estrogen receptor |
| HER2 | human epidermal growth factor receptor-2 |
| HR | hormone receptor |
| LABC | locally advanced breast cancer |
| LASSO | least absolute shrinkage and selection operator |
| MIP | maximum intensity projection |
| MRI | magnetic resonance imaging |
| NAC | neoadjuvant chemotherapy |
| OS | overall survival |
| PR | progesterone receptor |
| RFS | relapse-free survival |
| ROC | receiver operating characteristic |
| TNBC | triple-negative breast cancer |
| tpCR | total pathological complete response |
| TSP-1 | thrombospondin-1 |
| VEGF | vascular endothelial growth factor |
| VTL | vessel through lesion |
References
- Mauri, D.; Pavlidis, N.; Ioannidis, J.P.A. Neoadjuvant Versus Adjuvant Systemic Treatment in Breast Cancer: A Meta-Analysis. JNCI J. Natl. Cancer Inst. 2005, 97, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Tahmassebi, A.; Wengert, G.J.; Helbich, T.H.; Bago-Horvath, Z.; Alaei, S.; Bartsch, R.; Dubsky, P.; Baltzer, P.; Clauser, P.; Kapetas, P.; et al. Impact of Machine Learning With Multiparametric Magnetic Resonance Imaging of the Breast for Early Prediction of Response to Neoadjuvant Chemotherapy and Survival Outcomes in Breast Cancer Patients. Investig. Radiol. 2019, 54, 110–117. [Google Scholar] [CrossRef]
- Bitencourt, A.G.V.; Gibbs, P.; Rossi Saccarelli, C.; Daimiel, I.; Lo Gullo, R.; Fox, M.J.; Thakur, S.; Pinker, K.; Morris, E.A.; Morrow, M.; et al. MRI-based machine learning radiomics can predict HER2 expression level and pathologic response after neoadjuvant therapy in HER2 overexpressing breast cancer. EBioMedicine 2020, 61, 103042. [Google Scholar] [CrossRef]
- Ramtohul, T.; Djerroudi, L.; Lissavalid, E.; Nhy, C.; Redon, L.; Ikni, L.; Djelouah, M.; Journo, G.; Menet, E.; Cabel, L.; et al. Multiparametric MRI and Radiomics for the Prediction of HER2-Zero, -Low, and -Positive Breast Cancers. Radiology 2023, 308, e222646. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Ning, N.; Liang, H.; Wu, Y.; Wu, Q.; Wang, Z.; Tian, J.; Yang, J.; Gao, X.; et al. Association of peritumoral region features assessed on breast MRI and prognosis of breast cancer: A systematic review and meta-analysis. Eur. Radiol. 2024, 34, 6108–6120. [Google Scholar] [CrossRef]
- Schmitz, A.C.; Peters, N.H.; Veldhuis, W.B.; Gallardo, A.M.; van Diest, P.J.; Stapper, G.; van Hillegersberg, R.; Mali, W.P.; van den Bosch, M.A. Contrast-enhanced 3.0-T breast MRI for characterization of breast lesions: Increased specificity by using vascular maps. Eur. Radiol. 2008, 18, 355–364. [Google Scholar] [CrossRef]
- Sardanelli, F.; Iozzelli, A.; Fausto, A.; Carriero, A.; Kirchin, M.A. Gadobenate dimeglumine-enhanced MR imaging breast vascular maps: Association between invasive cancer and ipsilateral increased vascularity. Radiology 2005, 235, 791–797. [Google Scholar] [CrossRef]
- Carriero, A.; Di Credico, A.; Mansour, M.; Bonomo, L. Maximum intensity projection analysis in magnetic resonance of the breast. J. Exp. Clin. Cancer Res. 2002, 21, 77–81. [Google Scholar]
- Bufi, E.; Belli, P.; Di Matteo, M.; Giuliani, M.; Tumino, M.; Rinaldi, P.; Nardone, L.; Franceschini, G.; Mulé, A.; Bonomo, L. Hypervascularity Predicts Complete Pathologic Response to Chemotherapy and Late Outcomes in Breast Cancer. Clin. Breast Cancer 2016, 16, e193–e201. [Google Scholar] [CrossRef]
- Humbert, O.; Riedinger, J.-M.; Charon-Barra, C.; Berriolo-Riedinger, A.; Desmoulins, I.; Lorgis, V.; Kanoun, S.; Coutant, C.; Fumoleau, P.; Cochet, A.; et al. Identification of Biomarkers Including 18FDG-PET/CT for Early Prediction of Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Clin. Cancer Res. 2015, 21, 5460–5468. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Masuda, N.; Kodama, Y.; Ogawa, M.; Karita, M.; Yamamura, J.; Tsukuda, K.; Doihara, H.; Miyoshi, S.; Mano, M.; et al. Predictive factors for the effectiveness of neoadjuvant chemotherapy and prognosis in triple-negative breast cancer patients. Cancer Chemother. Pharmacol. 2011, 67, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Fausto, A.; Menicagli, L.; Esseridou, A. Breast vascular mapping obtained with contrast-enhanced MR imaging: Implications for cancer diagnosis, treatment, and risk stratification. Eur. Radiol. Suppl. 2007, 17, 48–51. [Google Scholar] [CrossRef]
- Nissan, N.; Massasa, E.E.M.; Bauer, E.; Halshtok-Neiman, O.; Shalmon, A.; Gotlieb, M.; Faermann, R.; Samoocha, D.; Yagil, Y.; Ziv-Baran, T.; et al. MRI can accurately diagnose breast cancer during lactation. Eur. Radiol. 2023, 33, 2935–2944. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Lorenc, P.; Sikorska, A.; Molenda, S.; Guzniczak, N.; Dams-Kozlowska, H.; Florczak, A. Physiological and tumor-associated angiogenesis: Key factors and therapy targeting VEGF/VEGFR pathway. Biomed. Pharmacother. 2024, 180, 117585. [Google Scholar] [CrossRef]
- Bahhnassy, A.; Mohanad, M.; Shaarawy, S.; Ismail, M.F.; El-Bastawisy, A.; Ashmawy, A.M.; Zekri, A.R. Transforming growth factor-β, insulin-like growth factor I/insulin-like growth factor I receptor and vascular endothelial growth factor-A: Prognostic and predictive markers in triple-negative and non-triple-negative breast cancer. Mol. Med. Rep. 2015, 12, 851–864. [Google Scholar] [CrossRef]
- Inoue, K.; Ozeki, Y.; Suganuma, T.; Sugiura, Y.; Tanaka, S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer 1997, 79, 206–213. [Google Scholar] [CrossRef]
- Han, G.; Wang, Y.; Bi, W.; Jia, J.; Wang, W.; Xu, M. Effects of vascular endothelial growth factor expression on pathological characteristics and prognosis of osteosarcoma. Clin. Exp. Med. 2016, 16, 577–584. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.; Demetri, G. Markers of angiogenesis and clinical features in patients with sarcoma. Cancer 2007, 109, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, S.I.; Arii, S.; Furutani, M.; Niwano, M.; Harada, T.; Mizumoto, M.; Mori, A.; Onodera, H.; Imamura, M. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br. J. Cancer 1998, 78, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, Y. The impact of VEGF on cancer metastasis and systemic disease. Semin. Cancer Biol. 2022, 86, 251–261. [Google Scholar] [CrossRef]
- Vimalraj, S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef]
- Yang, S.X.; Steinberg, S.M.; Nguyen, D.; Wu, T.D.; Modrusan, Z.; Swain, S.M. Gene expression profile and angiogenic marker correlates with response to neoadjuvant bevacizumab followed by bevacizumab plus chemotherapy in breast cancer. Clin. Cancer Res. 2008, 14, 5893–5899. [Google Scholar] [CrossRef]
- Kim, R.; Kawai, A.; Wakisaka, M.; Sawada, S.; Shimoyama, M.; Yasuda, N.; Hidaka, M.; Morita, Y.; Ohtani, S.; Arihiro, K. Immune correlates of the differing pathological and therapeutic effects of neoadjuvant chemotherapy in breast cancer. Eur. J. Surg. Oncol. 2020, 46, 77–84. [Google Scholar] [CrossRef]
- Martincich, L.; Bertotto, I.; Montemurro, F.; Ponzone, R.; Carbonaro, L.A.; Regge, D.; Sardanelli, F. Variation of breast vascular maps on dynamic contrast-enhanced MRI after primary chemotherapy of locally advanced breast cancer. AJR Am. J. Roentgenol. 2011, 196, 1214–1218. [Google Scholar] [CrossRef]
- Belotti, D.; Vergani, V.; Drudis, T.; Borsotti, P.; Pitelli, M.R.; Viale, G.; Giavazzi, R.; Taraboletti, G. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin. Cancer Res. 1996, 2, 1843–1849. [Google Scholar]
- Ng, S.S.; Figg, W.D.; Sparreboom, A. Taxane-mediated antiangiogenesis in vitro: Influence of formulation vehicles and binding proteins. Cancer Res. 2004, 64, 821–824. [Google Scholar] [CrossRef]
- Grant, D.S.; Williams, T.L.; Zahaczewsky, M.; Dicker, A.P. Comparison of antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere). Int. J. Cancer 2003, 104, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Carré, M.; Pourroy, B.; Camoin, L.; Rebaï, O.; Briand, C.; Braguer, D. Antiangiogenic activity of paclitaxel is associated with its cytostatic effect, mediated by the initiation but not completion of a mitochondrial apoptotic signaling pathway. Mol. Cancer Ther. 2004, 3, 1301–1310. [Google Scholar] [CrossRef]
- Lau, D.H.; Xue, L.; Young, L.J.; Burke, P.A.; Cheung, A.T. Paclitaxel (Taxol): An inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother. Radiopharm. 1999, 14, 31–36. [Google Scholar] [CrossRef]
- Vacca, A.; Ribatti, D.; Iurlaro, M.; Merchionne, F.; Nico, B.; Ria, R.; Dammacco, F. Docetaxel Versus Paclitaxel for Antiangiogenesis. J. Hematotherapy Stem Cell Res. 2002, 11, 103–118. [Google Scholar] [CrossRef]
- Jiang, H.; Tao, W.; Zhang, M.; Pan, S.; Kanwar, J.R.; Sun, X. Low-dose metronomic paclitaxel chemotherapy suppresses breast tumors and metastases in mice. Cancer Investig. 2010, 28, 74–84. [Google Scholar] [CrossRef]
- Gasparini, G.; Biganzoli, E.; Bonoldi, E.; Morabito, A.; Fanelli, M.; Boracchi, P. Angiogenesis sustains tumor dormancy in patients with breast cancer treated with adjuvant chemotherapy. Breast Cancer Res. Treat. 2001, 65, 71–75. [Google Scholar] [CrossRef]
- Gasparini, G.; Barbareschi, M.; Boracchi, P.; Verderio, P.; Caffo, O.; Meli, S.; Dalla Palma, P.; Marubini, E.; Bevilacqua, P. Tumor angiogenesis predicts clinical outcome of node-positive breast cancer patients treated with adjuvant hormone therapy or chemotherapy. Cancer J. Sci. Am. 1995, 1, 131–141. [Google Scholar]
- Gasparini, G.; Toi, M.; Miceli, R.; Vermeulen, P.B.; Dittadi, R.; Biganzoli, E.; Morabito, A.; Fanelli, M.; Gatti, C.; Suzuki, H.; et al. Clinical relevance of vascular endothelial growth factor and thymidine phosphorylase in patients with node-positive breast cancer treated with either adjuvant chemotherapy or hormone therapy. Cancer J. Sci. Am. 1999, 5, 101–111. [Google Scholar]
- Tufail, M.; Jiang, C.H.; Li, N. Tumor dormancy and relapse: Understanding the molecular mechanisms of cancer recurrence. Mil. Med. Res. 2025, 12, 7. [Google Scholar] [CrossRef]
- Tamamouna, V.; Pavlou, E.; Neophytou, C.M.; Papageorgis, P.; Costeas, P. Regulation of Metastatic Tumor Dormancy and Emerging Opportunities for Therapeutic Intervention. Int. J. Mol. Sci. 2022, 23, 13931. [Google Scholar] [CrossRef]
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef] [PubMed]
- Kazerounian, S.; Lawler, J. Integration of pro- and anti-angiogenic signals by endothelial cells. J. Cell Commun. Signal. 2018, 12, 171–179. [Google Scholar] [CrossRef] [PubMed]


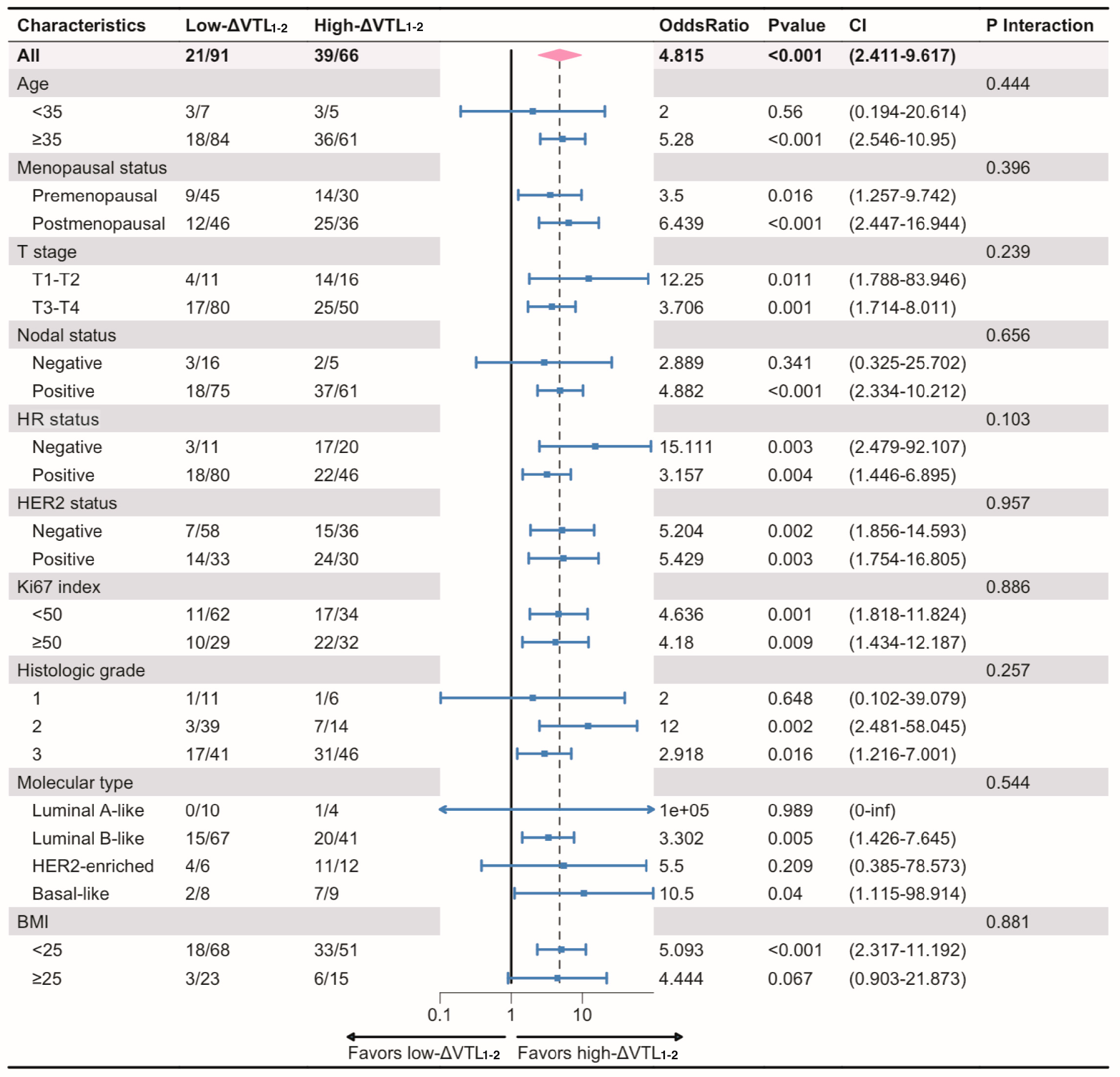
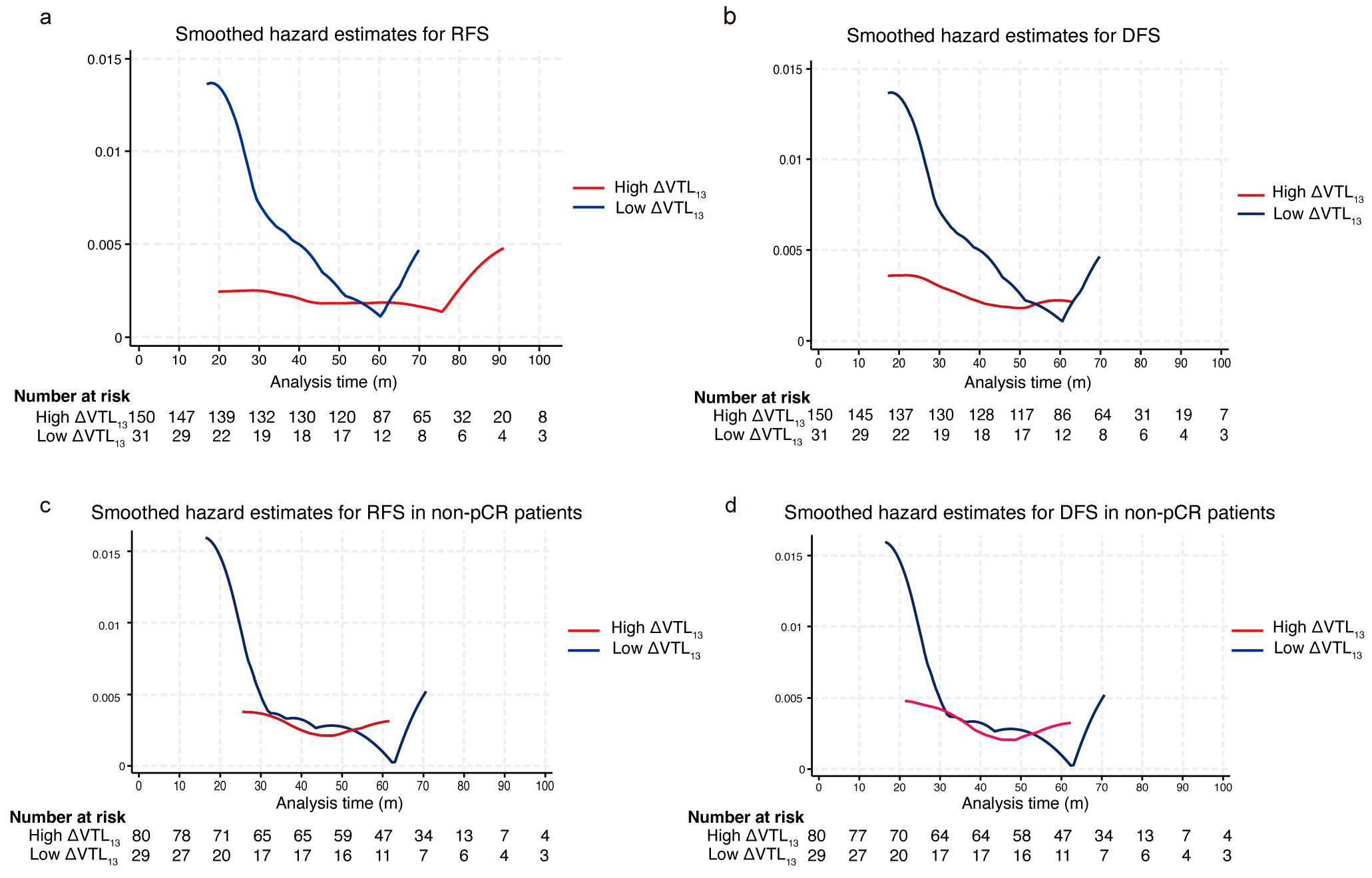
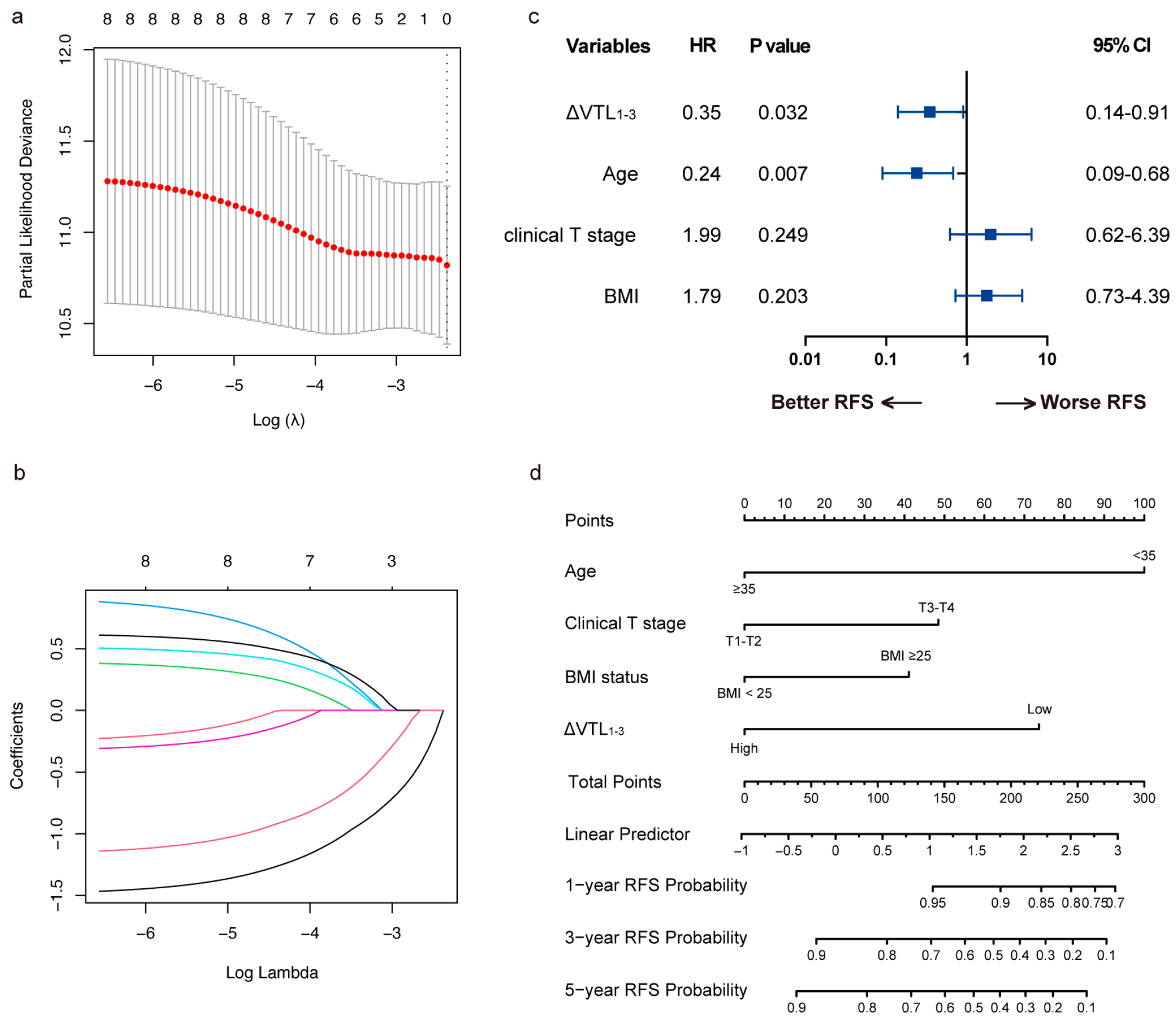
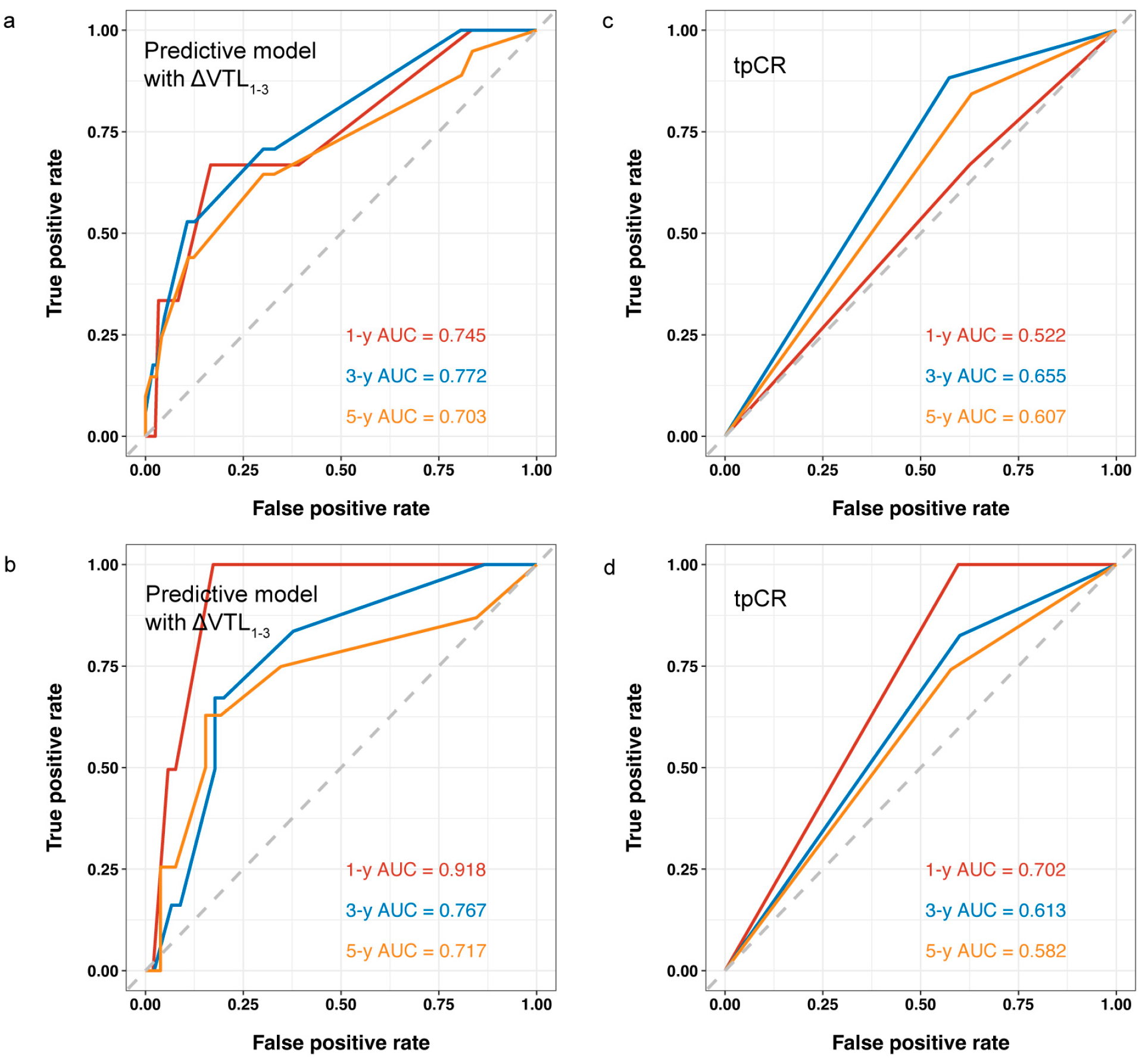
| Variable | Whole Set (n = 182) | Training Set (n = 127) | Validation Set (n = 55) | Statistic | p-Value |
|---|---|---|---|---|---|
| Age, n (%) | χ2 = 0.196 | 0.658 | |||
| <35 | 14 (7.69) | 11 (8.66) | 3 (5.45) | ||
| ≥35 | 168 (92.31) | 116 (91.34) | 52 (94.55) | ||
| Menopausal status, n (%) | χ2 = 0.052 | 0.819 | |||
| Premenopausal | 87 (47.8) | 60 (47.24) | 27 (49.09) | ||
| Postmenopausal | 95 (52.2) | 67 (52.76) | 28 (50.91) | ||
| Clinical T stage, n (%) | χ2 = 0.580 | 0.446 | |||
| T1–T2 | 36 (19.78) | 27 (21.26) | 9 (16.36) | ||
| T3–T4 | 146 (80.22) | 100 (78.74) | 46 (83.64) | ||
| Nodal status, n (%) | χ2 = 0.278 | 0.598 | |||
| Negative | 26 (14.29) | 17 (13.39) | 9 (16.36) | ||
| Positive | 156 (85.71) | 110 (86.61) | 46 (83.64) | ||
| HER2 status, n (%) | χ2 = 0.829 | 0.363 | |||
| Negative | 110 (60.44) | 74 (58.27) | 36 (65.45) | ||
| Positive | 72 (39.56) | 53 (41.73) | 19 (34.55) | ||
| Ki-67 index, n (%) | χ2 = 0.751 | 0.386 | |||
| <50% | 108 (59.34) | 78 (61.42) | 30 (54.55) | ||
| ≥50% | 74 (40.66) | 49 (38.58) | 25 (45.45) | ||
| Histologic grade, n (%) | χ2 = 2.481 | 0.289 | |||
| G1 | 20 (10.99) | 13 (10.24) | 7 (12.73) | ||
| G2 | 60 (32.97) | 38 (29.92) | 22 (40.00) | ||
| G3 | 102 (56.04) | 76 (59.84) | 26 (47.27) | ||
| Molecular subtype, n (%) | χ2 = 1.762 | 0.623 | |||
| Luminal A-like | 19 (10.44) | 14 (11.02) | 5 (9.09) | ||
| Luminal B-like | 122 (67.03) | 82 (64.57) | 40 (72.73) | ||
| HER2-enriched | 21 (11.54) | 17 (13.39) | 4 (7.27) | ||
| Basal-like | 20 (10.99) | 14 (11.02) | 6 (10.91) | ||
| BMI, n (%) | χ2 = 0.023 | 0.881 | |||
| <25 | 137 (75.27) | 96 (75.59) | 41 (74.55) | ||
| ≥25 | 45 (24.73) | 31 (24.41) | 14 (25.45) | ||
| bpCR, n (%) | χ2 = 0.006 | 0.936 | |||
| Non-pCR | 110 (60.44) | 77 (60.63) | 33 (60.00) | ||
| pCR | 72 (39.56) | 50 (39.37) | 22 (40.00) | ||
| ΔVTL1-2, %, Mean ± SD | 34.50 ± 38.36 | 32.59 ± 39.08 | 38.83 ± 36.70 | t = −0.938 | 0.349 |
| ΔVTL1-3, %, Mean ± SD | 53.72 ± 39.59 | 54.34 ± 40.89 | 52.28 ± 36.73 | t = 0.322 | 0.748 |
| ΔVTL1-2, n (%) | χ2 = 0.004 | 0.950 | |||
| Low | 91 (57.96) | 63 (57.80) | 28 (58.33) | ||
| High | 66 (42.04) | 46 (42.20) | 20 (41.67) | ||
| ΔVTL1-3, n (%) | χ2 = 0.318 | 0.573 | |||
| Low | 32 (17.58) | 21 (16.54) | 11 (20.00) | ||
| High | 150 (82.42) | 106 (83.46) | 44 (80.00) | ||
| RFS rate, % (95% CI) | t = 0.167 | 0.875 | |||
| 1-y | 97.2 (94.8–99.6) | 97.6 (94.9–100.0) | 96.3 (91.5–100.0) | ||
| 3-y | 86.8 (82.0–92.0) | 86.0 (80.0–92.4) | 88.7 (80.6–97.7) | ||
| 5-y | 83.6 (78.3–89.4) | 83.2 (76.7–90.2) | 84.6 (75.3–95.0) | ||
| OS rate, % (95% CI) | t = 0.472 | 0.661 | |||
| 1-y | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | ||
| 3-y | 94.0 (90.6–97.5) | 93.7 (89.6–98.0) | 94.5 (88.7–100.0) | ||
| 5-y | 91.7 (87.8–95.8) | 90.6 (85.6–95.8) | 94.5 (88.7–100.0) |
| Characteristics | ΔVTL1-2 | ΔVTL1-3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Low, n = 91 | High, n = 66 | p-Value | Adjusted p-Value | Low, n = 32 | High, n = 150 | p-Value | Adjusted p-Value | |
| Age(yr) | 1 | 1 | 0.275 | 0.393 | ||||
| <35 | 7 (7.69%) | 5 (7.58%) | 4 (12.5%) | 10 (6.67%) | ||||
| ≥35 | 84 (92.3%) | 61 (92.4%) | 28 (87.5%) | 140 (93.3%) | ||||
| Menopausal status | 0.739 | 0.924 | 0.101 | 0.337 | ||||
| Premenopausal | 45 (49.5%) | 30 (45.5%) | 20 (62.5%) | 67 (44.7%) | ||||
| Postmenopausal | 46 (50.5%) | 36 (54.5%) | 12 (37.5%) | 83 (55.3%) | ||||
| BMI | 0.858 | 0.953 | 1 | 1 | ||||
| <25 | 68 (74.7%) | 51 (77.3%) | 24 (75.0%) | 113 (75.3%) | ||||
| ≥25 | 23 (25.3%) | 15 (22.7%) | 8 (25.0%) | 37 (24.7%) | ||||
| HR status | 0.009 | 0.045 | 0.167 | 0.347 | ||||
| Negative | 11 (12.1%) | 20 (30.3%) | 3 (9.38%) | 33 (22.0%) | ||||
| Positive | 80 (87.9%) | 46 (69.7%) | 29 (90.6%) | 117 (78.0%) | ||||
| HER2 status | 0.32 | 0.457 | 0.208 | 0.347 | ||||
| Negative | 58 (63.7%) | 36 (54.5%) | 23 (71.9%) | 87 (58.0%) | ||||
| Positive | 33 (36.3%) | 30 (45.5%) | 9 (28.1%) | 63 (42.0%) | ||||
| Ki-67 status | 0.052 | 0.150 | 0.549 | 0.686 | ||||
| <50% | 62 (68.1%) | 34 (51.5%) | 21 (65.6%) | 87 (58.0%) | ||||
| ≥50% | 29 (31.9%) | 32 (48.5%) | 11 (34.4%) | 63 (42.0%) | ||||
| Clinical T Stage | 0.075 | 0.150 | 1 | 1 | ||||
| T1–2 | 11 (12.1%) | 16 (24.2%) | 6 (18.8%) | 30 (20.0%) | ||||
| T3–4 | 80 (87.9%) | 50 (75.8%) | 26 (81.2%) | 120 (80.0%) | ||||
| Nodal status | 0.114 | 0.190 | 0.175 | 0.347 | ||||
| Negative | 16 (17.6%) | 5 (7.58%) | 7 (21.9%) | 19 (12.7%) | ||||
| Positive | 75 (82.4%) | 61 (92.4%) | 25 (78.1%) | 131 (87.3%) | ||||
| Molecular type | 0.069 | 0.150 | 0.056 | 0.280 | ||||
| Luminal A-like | 10 (11.0%) | 4 (6.06%) | 7 (21.9%) | 12 (8.00%) | ||||
| Luminal B-like | 67 (73.6%) | 41 (62.1%) | 22 (68.8%) | 100 (66.7%) | ||||
| HER2-enriched | 6 (6.59%) | 12 (18.2%) | 1 (3.12%) | 20 (13.3%) | ||||
| Basel-like | 8 (8.79%) | 9 (13.6%) | 2 (6.25%) | 18 (12.0%) | ||||
| Histologic grade | 0.007 | 0.045 | 0.001 | 0.010 | ||||
| G1 | 11 (12.1%) | 6 (9.09%) | 6 (18.8%) | 14 (9.33%) | ||||
| G2 | 39 (42.9%) | 14 (21.2%) | 17 (53.1%) | 43 (28.7%) | ||||
| G3 | 41 (45.1%) | 46 (69.7%) | 9 (28.1%) | 93 (62.0%) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Zhu, M.; Xie, H.; Geng, X.; Wang, Y.; Wu, Z.; Lin, Y.; Xu, S.; Ye, Y.; Yin, W.; et al. Dynamic Evolution of Vascular Features Based on Magnetic Resonance Imaging to Predict Pathological Response, Patterns of Recurrence and Survival Outcomes in Breast Cancer Neoadjuvant Chemotherapy. Curr. Oncol. 2025, 32, 350. https://doi.org/10.3390/curroncol32060350
Wu Q, Zhu M, Xie H, Geng X, Wang Y, Wu Z, Lin Y, Xu S, Ye Y, Yin W, et al. Dynamic Evolution of Vascular Features Based on Magnetic Resonance Imaging to Predict Pathological Response, Patterns of Recurrence and Survival Outcomes in Breast Cancer Neoadjuvant Chemotherapy. Current Oncology. 2025; 32(6):350. https://doi.org/10.3390/curroncol32060350
Chicago/Turabian StyleWu, Qiong, Mingxi Zhu, Huaying Xie, Xiaochuan Geng, Yan Wang, Ziping Wu, Yanping Lin, Shuguang Xu, Yumei Ye, Wenjin Yin, and et al. 2025. "Dynamic Evolution of Vascular Features Based on Magnetic Resonance Imaging to Predict Pathological Response, Patterns of Recurrence and Survival Outcomes in Breast Cancer Neoadjuvant Chemotherapy" Current Oncology 32, no. 6: 350. https://doi.org/10.3390/curroncol32060350
APA StyleWu, Q., Zhu, M., Xie, H., Geng, X., Wang, Y., Wu, Z., Lin, Y., Xu, S., Ye, Y., Yin, W., Zhuang, Z., Lu, J., & Zhou, L. (2025). Dynamic Evolution of Vascular Features Based on Magnetic Resonance Imaging to Predict Pathological Response, Patterns of Recurrence and Survival Outcomes in Breast Cancer Neoadjuvant Chemotherapy. Current Oncology, 32(6), 350. https://doi.org/10.3390/curroncol32060350





