Integrating Radiologic and Clinical Features to Predict VSX1 Expression in Clear Cell Renal Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. The Cancer Genome Atlas
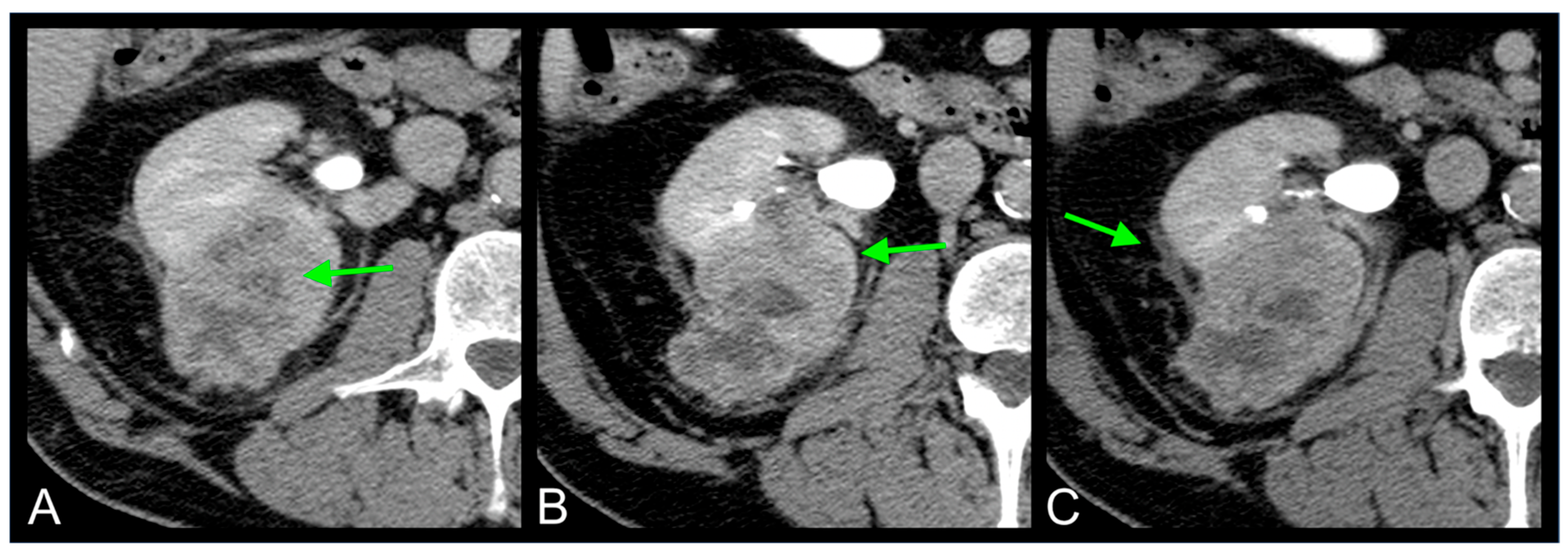
2.2. Imaging Features
2.3. Statistical Analyses
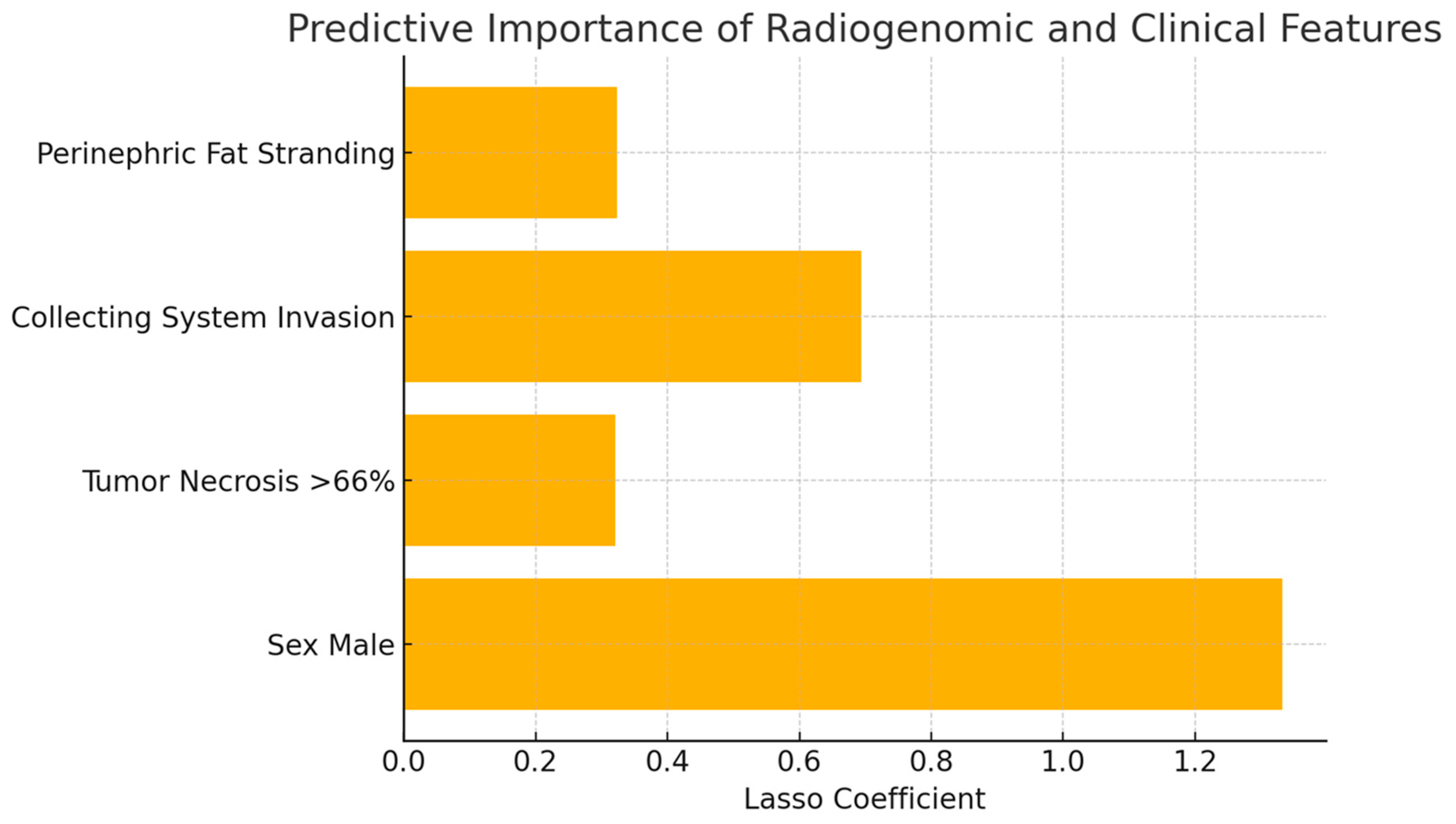
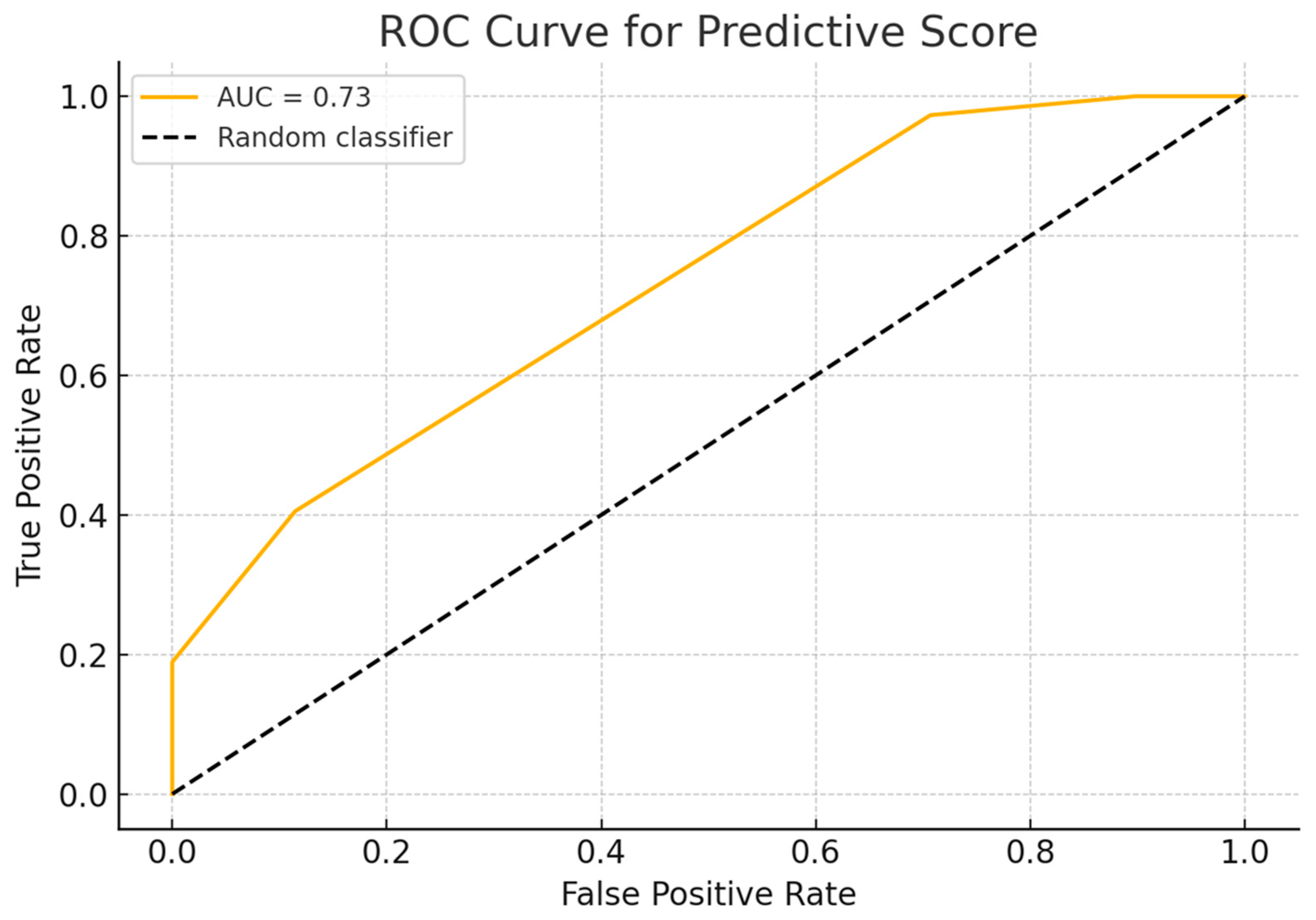
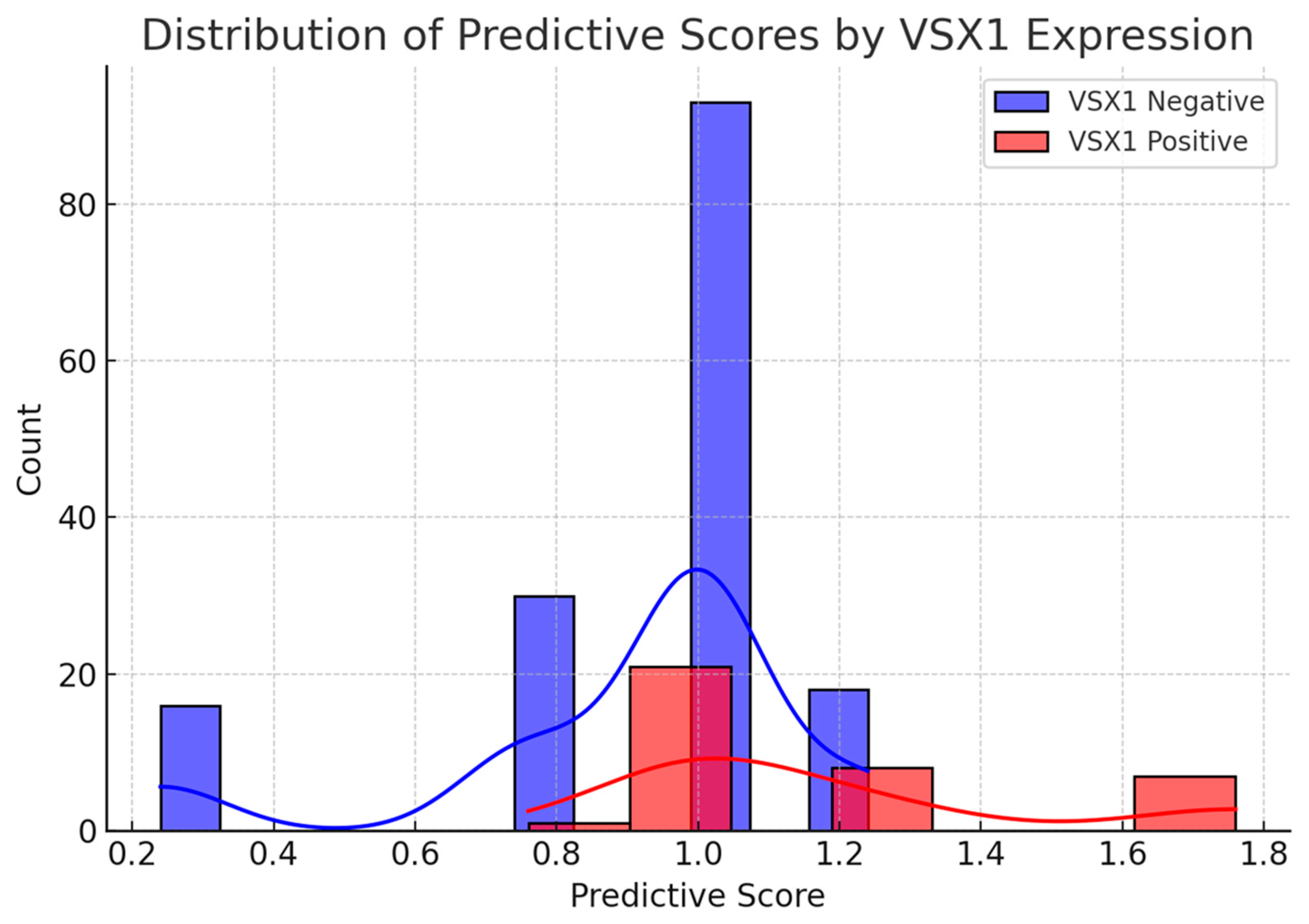
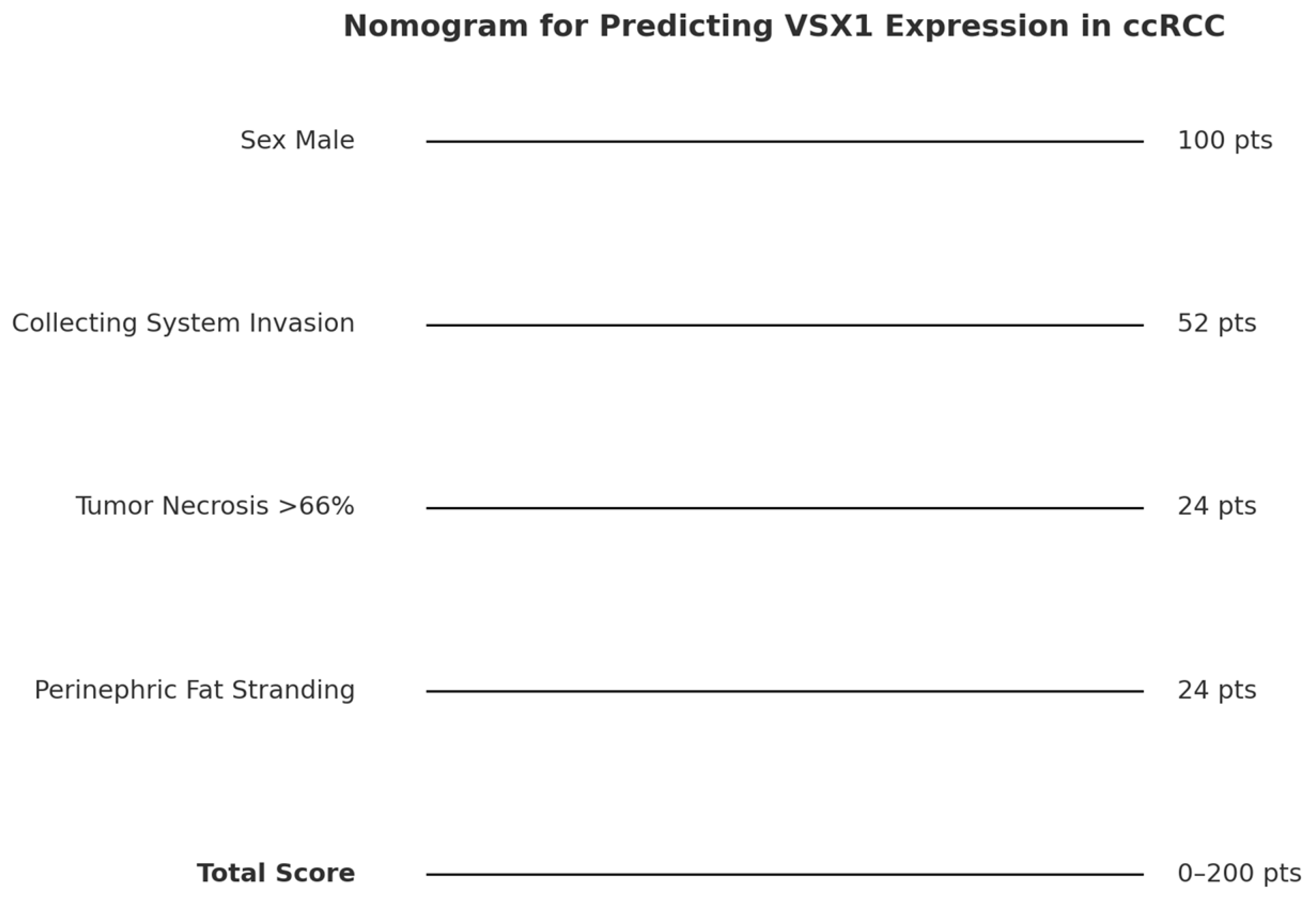
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alessandrino, F.; Krajewski, K.M.; Shinagare, A.B. Update on radiogenomics of clear cell renal cell carcinoma. Eur. Urol. Focus 2016, 2, 572–573. [Google Scholar] [CrossRef]
- Pinker, K.; Shitano, F.; Sala, E.; Do, R.K.; Young, R.J.; Wibmer, A.G.; Hricak, H.; Sutton, E.J.; Morris, E.A. Background, current role, and potential applications of radiogenomics. J. Magn. Reson. Imaging 2018, 47, 604–620. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2012, 499, 43–49. [Google Scholar]
- Akin, O.; Elnajjar, P.; Heller, M.; Jarosz, R.; Erickson, B.J.; Kirk, S.; Lee, Y.; Linehan, M.W.; Gautam, R.; Vikram, R.; et al. The Cancer Genome Atlas Kidney Renal Clear Cell Carcinoma Collection (TCGA-KIRC) (Version 3) [Data set]. Cancer Imaging Arch. 2016. [Google Scholar] [CrossRef]
- Semina, E.V.; Mintz-Hittner, H.A.; Murray, J.C. Isolation and characterization of a novel human paired-like homeodomain-containing transcription factor gene, VSX1, expressed in ocular tissues. Genomics 2000, 63, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Aldave, A.J.; Han, J.; Frausto, R.F. Genetics of the corneal endothelial dystrophies: An evidence-based review. Clin. Genet. 2013, 84, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Burdon, K.P.; Vincent, A.L. Insights into keratoconus from a genetic perspective. Clin. Exp. Optom. 2013, 96, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Héon, E.; Greenberg, A.; Kopp, K.K.; Rootman, D.; Vincent, A.L.; Billingsley, G.; Priston, M.; Dorval, K.M.; Chow, L.C.; McInnes, R.R.; et al. VSX1: A gene for posterior polymorphous dystrophy and keratoconus. Hum. Mol. Genet. 2002, 11, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Yang, L.; Pan, J.; Chen, Y.; Lu, Y.; Dong, X.; Li, D.; Gan, W. High VSX1 expression promotes the aggressiveness of clear cell renal cell carcinoma by transcriptionally regulating FKBP10. J. Transl. Med. 2022, 20, 554. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Imaging Archive Wiki. CIP TCGA Radiology Initiative—The Cancer Imaging Archive (TCIA) Public Access. Available online: https://wiki.cancerimagingarchive.net/display/Public/CIP+TCGA+Radiology+Initiative (accessed on 1 November 2019).
- Greco, F.; Panunzio, A.; Tafuri, A.; Bernetti, C.; Pagliarulo, V.; Beomonte Zobel, B.; Scardapane, A.; Mallio, C.A. CT-Based Radiogenomics of P4HA3 Expression in Clear Cell Renal Cell Carcinoma. Acad. Radiol. 2024, 31, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Panunzio, A.; Bernetti, C.; Tafuri, A.; Beomonte Zobel, B.; Mallio, C.A. Exploring the ADAM12 Expression in Clear Cell Renal Cell Carcinoma: A Radiogenomic Analysis on CT Imaging. Acad. Radiol. 2024, 31, 3672–3677. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Panunzio, A.; Bernetti, C.; Tafuri, A.; Beomonte Zobel, B.; Mallio, C.A. The Radiogenomic Landscape of Clear Cell Renal Cell Carcinoma: Insights into Lipid Metabolism through Evaluation of ADFP Expression. Diagnostics 2024, 14, 1667. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.; Muhealdeen, D.N.; Fakhralddin, S.S.; Bapir, R.; Tahir, S.H.; Rashid, R.J.; Omer, C.S.; Abdullah, H.O.; Abdalla, B.A.; Mohammed, S.H.; et al. Prognostic factors in renal cell carcinoma: A single center study. Mol. Clin. Oncol. 2023, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Mattila, K.E.; Vainio, P.; Jaakkola, P.M. Prognostic Factors for Localized Clear Cell Renal Cell Carcinoma and Their Application in Adjuvant Therapy. Cancers 2022, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Abou Elkassem, A.M.; Lo, S.S.; Gunn, A.J.; Shuch, B.M.; Dewitt-Foy, M.E.; Abouassaly, R.; Vaidya, S.S.; Clark, J.I.; Louie, A.V.; Siva, S.; et al. Role of Imaging in Renal Cell Carcinoma: A Multidisciplinary Perspective. Radiographics 2021, 41, 1387–1407. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Loya, A.; Hameed, M.; Akhtar, N.; Mushtaq, S.; Hassan, U. Prognostic Significance of Percentage Necrosis in Clear Cell Renal Cell Carcinoma. Am. J. Clin. Pathol. 2022, 157, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.B.; Clark, P.E.; Morgan, T.M.; Stratton, K.L.; Herrell, S.D.; Davis, R.; Cookson, M.S.; Smith, J.A., Jr.; Chang, S.S. Urinary collecting system invasion is a predictor for overall and disease-specific survival in locally invasive renal cell carcinoma. Urology 2011, 78, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Brookman-Amissah, S.; May, M.; Albrecht, K.; Herrmann, T.; Roigas, J.; Gilfrich, C.P.; Pflanz, S.; Gunia, S. Urinary collecting system invasion reflects adverse long-term outcome and is associated with simultaneous metastatic spread at the time of surgery and with multilocular dissemination during postsurgical follow-up in renal cell cancer. World J. Urol. 2010, 28, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Shinagare, A.B.; Vikram, R.; Jaffe, C.; Akin, O.; Kirby, J.; Huang, E.; Freymann, J.; Sainani, N.I.; Sadow, C.A.; Bathala, T.K.; et al. Radiogenomics of Clear Cell Renal Cell Carcinoma: Preliminary Findings of The Cancer Genome Atlas–Renal Cell Carcinoma (TCGA–RCC) Imaging Research Group. Abdom. Imaging 2015, 40, 1684–1692. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | N | Overall, N = 194 1 | VSX1 = 0, N = 157 (81%) 1 | VSX1 = 1, N = 37 (19%) 1 | p-Value 2 |
|---|---|---|---|---|---|
| Age | 194 | 59 (52, 69) | 58 (51, 67) | 63 (54, 74) | 0.077 |
| Primary tumor size | 194 | 53 (38, 81) | 54 (37, 80) | 51 (39, 81) | >0.9 |
| Sex | 194 | 0.022 | |||
| Female | 68 (35%) | 61 (39%) | 7 (19%) | ||
| Male | 126 (65%) | 96 (61%) | 30 (81%) | ||
| Race | 194 | >0.9 | |||
| White | 177 (91%) | 143 (91%) | 34 (92%) | ||
| Not White | 17 (8.8%) | 14 (8.9%) | 3 (8.1%) | ||
| Laterality | 194 | 0.8 | |||
| Left | 92 (47%) | 75 (48%) | 17 (46%) | ||
| Right | 102 (53%) | 82 (52%) | 20 (54%) | ||
| Tumor grade (Fuhrman) | 194 | >0.9 | |||
| Grade 1–2 | 80 (41%) | 65 (41%) | 15 (41%) | ||
| Grade 3–4 | 114 (59%) | 92 (59%) | 22 (59%) | ||
| Tumor stage (American Joint Committee on Cancer) | 194 | >0.9 | |||
| 1 | 103 (53%) | 84 (54%) | 19 (51%) | ||
| 2 | 18 (9.3%) | 14 (8.9%) | 4 (11%) | ||
| 3 | 46 (24%) | 38 (24%) | 8 (22%) | ||
| 4 | 27 (14%) | 21 (13%) | 6 (16%) | ||
| History of other neoplasms | 194 | 0.7 | |||
| 0 | 157 (81%) | 128 (82%) | 29 (78%) | ||
| 1 | 37 (19%) | 29 (18%) | 8 (22%) | ||
| Collateral vascular supply | 194 | 0.7 | |||
| 0 | 84 (43%) | 67 (43%) | 17 (46%) | ||
| 1 | 110 (57%) | 90 (57%) | 20 (54%) | ||
| Tumor margins | 194 | 0.7 | |||
| 0 | 126 (65%) | 103 (66%) | 23 (62%) | ||
| 1 | 68 (35%) | 54 (34%) | 14 (38%) | ||
| Tumor composition | 194 | 0.7 | |||
| 0 | 14 (7.2%) | 11 (7.0%) | 3 (8.1%) | ||
| 1 | 180 (93%) | 146 (93%) | 34 (92%) | ||
| Tumor necrosis | 194 | 0.6 | |||
| 1 | 11 (5.7%) | 9 (5.7%) | 2 (5.4%) | ||
| 2 | 114 (59%) | 92 (59%) | 22 (59%) | ||
| 3 | 48 (25%) | 41 (26%) | 7 (19%) | ||
| 4 | 21 (11%) | 15 (9.6%) | 6 (16%) | ||
| Tumor growth pattern | 194 | 0.7 | |||
| 0 | 13 (6.7%) | 11 (7.0%) | 2 (5.4%) | ||
| 1 | 58 (30%) | 49 (31%) | 9 (24%) | ||
| 2 | 123 (63%) | 97 (62%) | 26 (70%) | ||
| Calcifications | 194 | 0.7 | |||
| 0 | 156 (80%) | 127 (81%) | 29 (78%) | ||
| 1 | 38 (20%) | 30 (19%) | 8 (22%) | ||
| Thrombosis or infiltration of renal artery | 194 | 0.6 | |||
| 0 | 190 (98%) | 154 (98%) | 36 (97%) | ||
| 1 | 4 (2.1%) | 3 (1.9%) | 1 (2.7%) | ||
| Thrombosis or infiltration of renal vein | 194 | >0.9 | |||
| 0 | 179 (92%) | 145 (92%) | 34 (92%) | ||
| 1 | 15 (7.7%) | 12 (7.6%) | 3 (8.1%) | ||
| Collecting system invasion | 194 | 0.3 | |||
| 0 | 135 (70%) | 112 (71%) | 23 (62%) | ||
| 1 | 59 (30%) | 45 (29%) | 14 (38%) | ||
| Intralesional hemorrhage | 194 | 0.6 | |||
| 0 | 190 (98%) | 154 (98%) | 36 (97%) | ||
| 1 | 4 (2.1%) | 3 (1.9%) | 1 (2.7%) | ||
| Hydronephrosis | 194 | 0.6 | |||
| 0 | 188 (97%) | 151 (96%) | 37 (100%) | ||
| 1 | 6 (3.1%) | 6 (3.8%) | 0 (0%) | ||
| Signs of infiltrations | 194 | >0.9 | |||
| 0 | 189 (97%) | 153 (97%) | 36 (97%) | ||
| 1 | 5 (2.6%) | 4 (2.5%) | 1 (2.7%) | ||
| Perinephric fat stranding | 194 | 0.3 | |||
| 0 | 93 (48%) | 78 (50%) | 15 (41%) | ||
| 1 | 101 (52%) | 79 (50%) | 22 (59%) | ||
| Gerota’s fascia thickening | 194 | 0.5 | |||
| 0 | 120 (62%) | 99 (63%) | 21 (57%) | ||
| 1 | 74 (38%) | 58 (37%) | 16 (43%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, F.; Panunzio, A.; Sergi, D.; Cataldo, M.; Bernetti, C.; Tafuri, A.; Beomonte Zobel, B.; Mallio, C.A. Integrating Radiologic and Clinical Features to Predict VSX1 Expression in Clear Cell Renal Cell Carcinoma. Curr. Oncol. 2025, 32, 348. https://doi.org/10.3390/curroncol32060348
Greco F, Panunzio A, Sergi D, Cataldo M, Bernetti C, Tafuri A, Beomonte Zobel B, Mallio CA. Integrating Radiologic and Clinical Features to Predict VSX1 Expression in Clear Cell Renal Cell Carcinoma. Current Oncology. 2025; 32(6):348. https://doi.org/10.3390/curroncol32060348
Chicago/Turabian StyleGreco, Federico, Andrea Panunzio, Daniele Sergi, Marco Cataldo, Caterina Bernetti, Alessandro Tafuri, Bruno Beomonte Zobel, and Carlo Augusto Mallio. 2025. "Integrating Radiologic and Clinical Features to Predict VSX1 Expression in Clear Cell Renal Cell Carcinoma" Current Oncology 32, no. 6: 348. https://doi.org/10.3390/curroncol32060348
APA StyleGreco, F., Panunzio, A., Sergi, D., Cataldo, M., Bernetti, C., Tafuri, A., Beomonte Zobel, B., & Mallio, C. A. (2025). Integrating Radiologic and Clinical Features to Predict VSX1 Expression in Clear Cell Renal Cell Carcinoma. Current Oncology, 32(6), 348. https://doi.org/10.3390/curroncol32060348








