Diffuse-Type Tenosynovial Giant Cell Tumor of the Knee: Clinical Course After Anterior Open Synovectomy
Abstract
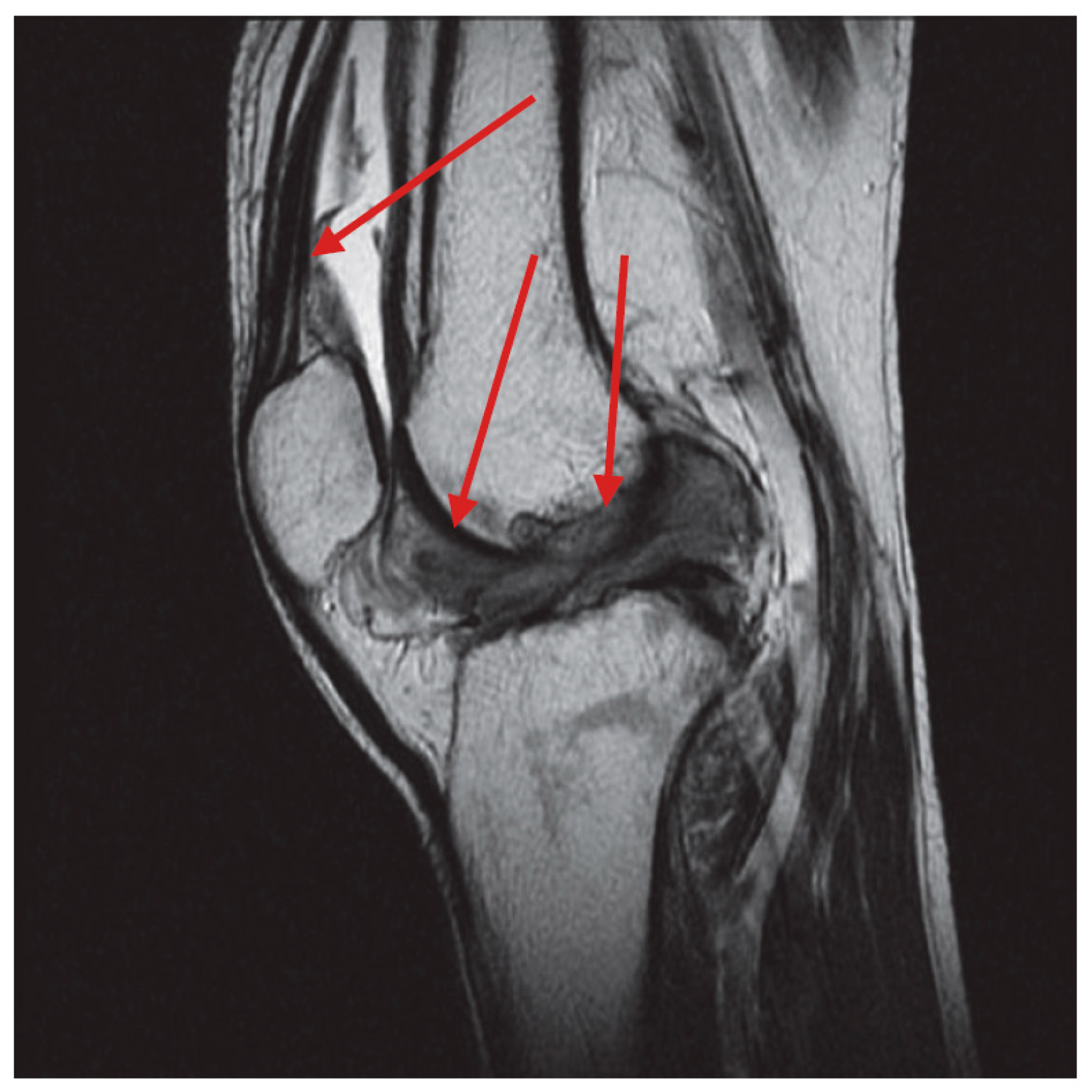
1. Introduction
2. Patients and Methods
2.1. Patient Selection
2.2. Open Anterior Synovectomy: Technique
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, N.S.; Landskroner, K.; Shah, B.; Walda, S.; Weiss, O.; Pallapotu, V. Identification of Patient Needs and Preferences in Pigmented Villonodular Synovitis (PVNS) Using a Qualitative Online Bulletin Board Study. Adv. Ther. 2020, 37, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.; Bridge, J.A.; Hogendoorn, P.C.W.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone; World Health Organization; International Agency for Research on Cancer, 4th ed.; IACR Press: Lyon, France, 2013; pp. 100–103. [Google Scholar]
- Karami, M.; Soleimani, M.; Shiari, R. Pigmented villonodular synovitis in pediatric population: Review of literature and a case report. Pediatr. Rheumatol. 2018, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- De, K.; Fj, F.; Da, F.; Aj, C. Pigmented villonodular synovitis of the knee: Diagnosis and treatment. J. Knee Surg. 2009, 22, 243–254. [Google Scholar] [CrossRef]
- Martin, R.C.; Osborne, D.L.; Edwards, M.J.; Wrightson, W.; McMasters, K.M. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: Defining the presentation, surgical therapy and recurrence. Oncol. Rep. 2000, 7, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Wang, W.-L.; Lewis, V.O. Treatment of tenosynovial giant cell tumor and pigmented villonodular synovitis. Curr. Opin. Oncol. 2011, 23, 361–366. [Google Scholar] [CrossRef]
- Peyraud, F.; Cousin, S.; Italiano, A. CSF-1R Inhibitor Development: Current Clinical Status. Curr. Oncol. Rep. 2017, 19, 70. [Google Scholar] [CrossRef]
- Brahmi, M.; Vinceneux, A.; Cassier, P.A. Current Systemic Treatment Options for Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis: Targeting the CSF1/CSF1R Axis. Curr. Treat. Options Oncol. 2016, 17, 10. [Google Scholar] [CrossRef]
- Staals, E.L.; Ferrari, S.; Donati, D.M.; Palmerini, E. Diffuse-type tenosynovial giant cell tumour: Current treatment concepts and future perspectives. Eur. J. Cancer 2016, 63, 34–40. [Google Scholar] [CrossRef]
- Chipman, D.E.; Perkins, C.A.; Lijesen, E.; Green, D.W. Pigmented villonodular synovitis/giant cell tumor in the knee. Curr. Opin. Pediatr. 2024, 36, 78–82. [Google Scholar] [CrossRef]
- Healey, J.H.; Bernthal, N.M.; van de Sande, M. Management of Tenosynovial Giant Cell Tumor: A Neoplastic and Inflammatory Disease. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e20.00028. [Google Scholar] [CrossRef]
- Vaynrub, A.; Healey, J.H.; Tap, W.; Vaynrub, M. Pexidartinib in the Management of Advanced Tenosynovial Giant Cell Tumor: Focus on Patient Selection and Special Considerations. OncoTargets Ther. 2022, 15, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Wagner, A.J.; Tap, W.D.; Palmerini, E.; Wainberg, Z.A.; Desai, J.; Healey, J.H.; Van De Sande, M.A.J.; Bernthal, N.M.; Staals, E.L.; et al. Long-term outcomes of pexidartinib in tenosynovial giant cell tumors. Cancer 2021, 127, 884–893. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Healey, J.H.; Palmerini, E.; Bauer, S.; Schreuder, H.; Leithner, A.; Martin-Broto, J.; Gouin, F.; Lopez-Bastida, J.; Gelderblom, H.; et al. A prospective real-world study of the diffuse-type tenosynovial giant cell tumor patient journey: A 2-year observational analysis. J. Surg. Oncol. 2022, 126, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Keyhani, S.; Soleymanha, M.; Vosoughi, F.; Nikibakhsh, A.; Zadgari, E.; Mousavi, M.; LaPrade, R.F. Recurrence of arthroscopic treatment of pigmented villonodular synovitis of the knee: A systematic review and meta-analysis. J. Exp. Orthop. 2025, 12, e70169. [Google Scholar] [CrossRef]
- Lingamfelter, M.; Novaczyk, Z.B.; Cheng, E.Y. Extensile Anterior and Posterior Knee Exposure for Complete Synovectomy of Diffuse Tenosynovial Giant Cell Tumor (Pigmented Villonodular Synovitis). JBJS Essent. Surg. Tech. 2022, 12. [Google Scholar] [CrossRef]
- Chandra, A.A.; Agarwal, S.; Donahue, A.; Handorf, E.; Abraham, J.A. Arthroscopic Versus Open Management of Diffuse-Type Tenosynovial Giant Cell Tumor of the Knee: A Meta-analysis of Retrospective Cohort Studies. JAAOS Glob. Res. Rev. 2021, 4, e21.00035. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, M.B.; Portela, J.; Soares do Brito, J. Open versus arthroscopic surgery for diffuse tenosynovial giant-cell tumours of the knee: A systematic review. EFORT Open Rev. 2020, 5, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Panciera, A.; Colangelo, A.; Di Martino, A.; Ferri, R.; Bulzacki Bogucki, B.D.; Cecchin, D.; Brunello, M.; Benvenuti, L.; Digennaro, V. Total knee arthroplasty in pigmented villonodular synovitis osteoarthritis: A systematic review of literature. Musculoskelet. Surg. 2024, 108, 145–152. [Google Scholar] [CrossRef]
- Verspoor, F.G.M.; Hannink, G.; Scholte, A.; Van Der Geest, I.C.M.; Schreuder, H.W.B. Arthroplasty for tenosynovial giant cell tumors. Acta Orthop. 2016, 87, 497–503. [Google Scholar] [CrossRef]
- Cosseddu, F.; Ipponi, E.; Ruinato, A.D.; Shytaj, S.; Capanna, R.; Andreani, L. Surgical management of villonodular-pigmented synovitis of knee: Decisional algorithm. Orthop. Rev. 2022, 14, 39644. [Google Scholar] [CrossRef]
- Aurégan, J.-C.; Klouche, S.; Bohu, Y.; Lefèvre, N.; Herman, S.; Hardy, P. Treatment of Pigmented Villonodular Synovitis of the Knee. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Spierenburg, G.; Suevos Ballesteros, C.; Stoel, B.C.; Navas Cañete, A.; Gelderblom, H.; Van De Sande, M.A.J.; Van Langevelde, K. MRI of diffuse-type tenosynovial giant cell tumour in the knee: A guide for diagnosis and treatment response assessment. Insights Imaging 2023, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.-T.; Wu, J.-S. The Visual Analogue Scale for Rating, Ranking and Paired-Comparison (VAS-RRP): A new technique for psychological measurement. Behav. Res. 2018, 50, 1694–1715. [Google Scholar] [CrossRef]
- Norkin, C.; White, D.J. Measurement of Joint Motion, 5e: A Guide to Goniometry, 5th ed.; F.A. Davis: Philadelphia, PA, USA, 2016; p. 4699. ISBN 978-0-8036-4566-0. [Google Scholar]
- Servodio Iammarrone, C.; Cadossi, M.; Sambri, A.; Grosso, E.; Corrado, B.; Servodio Iammarrone, F. Is there a role of pulsed electromagnetic fields in management of patellofemoral pain syndrome? Randomized controlled study at one year follow-up. Bioelectromagnetics 2016, 37, 81–88. [Google Scholar] [CrossRef]
- Mastboom, M.J.L.; Palmerini, E.; Verspoor, F.G.M.; Rueten-Budde, A.J.; Stacchiotti, S.; Staals, E.L.; Schaap, G.R.; Jutte, P.C.; Aston, W.; Gelderblom, H.; et al. Surgical outcomes of patients with diffuse-type tenosynovial giant-cell tumours: An international, retrospective, cohort study. Lancet Oncol. 2019, 20, 877–886. [Google Scholar] [CrossRef]
- Lashgari, R.J.; Chen, B.W.; Ventimiglia, D.J.; Henry, L.E.; Kolevar, M.P.; Leong, N.L.; Meredith, S.J.; Packer, J.D.; Henn, R.F. Patient-Reported Outcomes after Surgery for Pigmented Villonodular Synovitis in the Knee: A Cohort Study. J. Knee Surg. 2025, 38, 180–187. [Google Scholar] [CrossRef]
- Gelhorn, H.L.; Tong, S.; McQuarrie, K.; Vernon, C.; Hanlon, J.; Maclaine, G.; Lenderking, W.; Ye, X.; Speck, R.M.; Lackman, R.D.; et al. Patient-reported Symptoms of Tenosynovial Giant Cell Tumors. Clin. Ther. 2016, 38, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Zvijac, J.E.; Lau, A.C.; Hechtman, K.S.; Uribe, J.W.; Tjin-A-Tsoi, E.W. Arthroscopic treatment of pigmented villonodular synovitis of the knee. Arthroscopy 1999, 15, 613–617. [Google Scholar] [CrossRef]
- Kubat, O.; Mahnik, A.; Smoljanović, T.; Bojanić, I. Arthroscopic treatment of localized and diffuse pigmented villonodular synovitis of the knee. Coll. Antropol. 2010, 34, 1467–1472. [Google Scholar]
- Mastboom, M.J.; Planje, R.; van de Sande, M.A. The Patient Perspective on the Impact of Tenosynovial Giant Cell Tumors on Daily Living: Crowdsourcing Study on Physical Function and Quality of Life. Interact. J. Med. Res. 2018, 7, e4. [Google Scholar] [CrossRef]
- The Diffuse-Type Tenosynovial Giant Cell Tumor (dt-TGCT) Patient Journey: A Prospective Multicenter Study—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33926503/ (accessed on 26 February 2025).
- Nakahara, H.; Matsuda, S.; Harimaya, K.; Sakamoto, A.; Matsumoto, Y.; Okazaki, K.; Tashiro, Y.; Iwamoto, Y. Clinical results of open synovectomy for treatment of diffuse pigmented villonodular synovitis of the knee: Case series and review of literature. Knee 2012, 19, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Q.; Jiang, X.; Tang, X.; Li, J. Effectiveness of arthroscopy and/or arthrotomy therapy for diffuse pigmented villonodular synovitis of the knee. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2012, 26, 518–521. [Google Scholar] [PubMed]
- Yao, L.; Li, Y.; Li, T.; Fu, W.; Chen, G.; Li, Q.; Tang, X.; Li, J.; Xiong, Y. What Are the Recurrence Rates, Complications, and Functional Outcomes After Multiportal Arthroscopic Synovectomy for Patients With Knee Diffuse-type Tenosynovial Giant-cell Tumors? Clin. Orthop. Relat. Res. 2024, 482, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, V.; Andersen, S.L.; Qazi, I.; Sankar, N.; Pedersen, A.B.; Sikorski, R.; Acquavella, J.F. Tenosynovial Giant Cell Tumor: Incidence, Prevalence, Patient Characteristics, and Recurrence. A Registry-based Cohort Study in Denmark. J. Rheumatol. 2017, 44, 1476–1483. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruschi, A.; Staals, E.; Sambri, A.; Cevolani, L.; Gambarotti, M.; Righi, A.; Fiore, M.; Villari, E.; Pasini, S.; Pirini, M.G.; et al. Diffuse-Type Tenosynovial Giant Cell Tumor of the Knee: Clinical Course After Anterior Open Synovectomy. Curr. Oncol. 2025, 32, 342. https://doi.org/10.3390/curroncol32060342
Bruschi A, Staals E, Sambri A, Cevolani L, Gambarotti M, Righi A, Fiore M, Villari E, Pasini S, Pirini MG, et al. Diffuse-Type Tenosynovial Giant Cell Tumor of the Knee: Clinical Course After Anterior Open Synovectomy. Current Oncology. 2025; 32(6):342. https://doi.org/10.3390/curroncol32060342
Chicago/Turabian StyleBruschi, Alessandro, Eric Staals, Andrea Sambri, Luca Cevolani, Marco Gambarotti, Alberto Righi, Michele Fiore, Eleonora Villari, Stefano Pasini, Maria Giulia Pirini, and et al. 2025. "Diffuse-Type Tenosynovial Giant Cell Tumor of the Knee: Clinical Course After Anterior Open Synovectomy" Current Oncology 32, no. 6: 342. https://doi.org/10.3390/curroncol32060342
APA StyleBruschi, A., Staals, E., Sambri, A., Cevolani, L., Gambarotti, M., Righi, A., Fiore, M., Villari, E., Pasini, S., Pirini, M. G., De Paolis, M., & Donati, D. M. (2025). Diffuse-Type Tenosynovial Giant Cell Tumor of the Knee: Clinical Course After Anterior Open Synovectomy. Current Oncology, 32(6), 342. https://doi.org/10.3390/curroncol32060342





