Allogeneic Stem Cell Transplantation: The Relevance of Conditioning Regime Intensity for Myelodysplastic Syndromes (MDS)
Abstract
1. Introduction
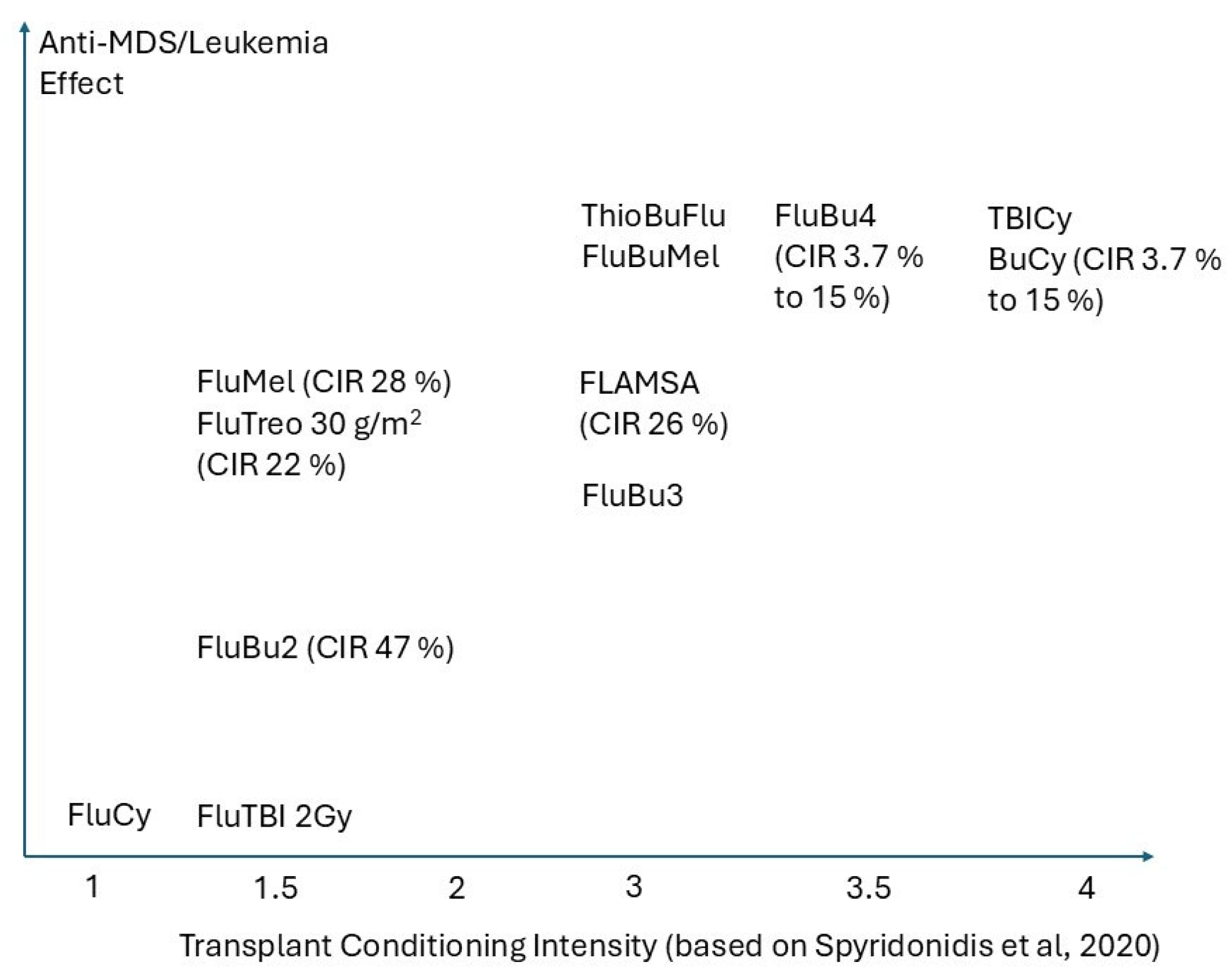
2. The Concepts of Intensity and Its Stem Cell Target, Biologic Bases, and Pragmatic Considerations
3. The Impact of Disease Burden and Cytoreduction on Patient Outcomes in Patients Who Receive Allogeneic Hematopoietic Stem Cell Transplant for MDS
4. Relationship Between Somatic Mutations and Conditioning Intensity for Allogeneic Hematopoietic Stem Cell Transplant in MDS
5. Relationship Between Minimal Residual Disease and Conditioning Intensity Prior to Allogeneic Hematopoietic Stem Cell Transplant in MDS
6. Role of the Graft-Versus-Tumor Effect in MDS
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aGvHD | acute graft-versus-host disease |
| AML | acute myeloid leukemia |
| Allo-HCT | allogeneic hematopoietic stem cell transplant |
| ASXL1 | additional sex combs-like protein 1 |
| ATG | anti thymocyte globulin |
| BM | bone marrow |
| BMT CTN | Blood and Marrow Transplant Clinical Trials Network |
| CIBMTR | Center for International Blood and Marrow Transplant Research |
| CBL | Casitas B-lineage lymphoma |
| DLI | donor lymphocyte infusion |
| DNMT3A | DNA methyltransferase 3A |
| EBMT | European Society for Blood and Marrow Transplantation |
| FIT1 | fat storage-inducing transmembrane protein 1 |
| FLAMSA | fludarabine amsacrine cytosine arabinoside |
| FLT3 | FMS-related tyrosinekinase 3 |
| Flu/Bu | fludarabine busulfan |
| Flu/Mel | fludarabine melphalan |
| cGvHD | chronic graft-versus-host disease |
| GVT | graft versus tumor effect |
| HCT | hematopoietic stem cell transplant |
| HCT-CI | hematopoietic stem cell transplant comorbidity index |
| HLA | human leukocyte antigen |
| HMA | hypomethylating agent |
| HR | hazard ratio |
| IFN | interferon |
| iKIR | inhibitory killer cell immunoglobulin-like receptor |
| IPSS | International Prognostic Scoring System |
| IPSS-M | International Prognostic Scoring System—Molecular |
| IPSS-R | International Prognostic Scoring System—Revised |
| IWG | International Working Group |
| JAK | Janus kinase |
| MAC | myeloablative conditioning |
| MAGE | melanoma antigen gene |
| MDS | myelodyplastic syndrome |
| MFC | multi-color flow cytometry |
| MID | minimal identifiable disease |
| miHA | minor histocompatibility antigen |
| MRD | measurable residual disease |
| NF1 | neurofibromatosis 1 |
| NGS-MRD | next generation sequencing measurable residual disease |
| NK | natural killer |
| NPM1 | nucleophosmin-1 |
| NRM | non-relapse mortality |
| OS | overall survival |
| PFS | progression free survival |
| RFS | relapse free survival |
| PTPN1 | protein tyrosine phosphatase 1B |
| RIC | reduced intensity conditioning |
| RCT | randomized control trial |
| RUNX | runt-related transcription factor |
| TP53 | tumor promoter 53 |
| TRM | treatment related mortality |
| VAF | variant allele frequency |
| WT1 | Wilm’s tumor 1 |
References
- DeFilipp, Z.; Ciurea, S.O.; Cutler, C.; Robin, M.; Warlick, E.D.; Nakamura, R.; Brunner, A.M.; Dholaria, B.; Walker, A.R.; Kröger, N.; et al. Hematopoietic Cell Transplantation in the Management of Myelodysplastic Syndrome: An Evidence-Based Review from the American Society for Transplantation and Cellular Therapy Committee on Practice Guidelines. Transplant. Cell. Ther. 2023, 29, 71–81. [Google Scholar] [CrossRef]
- Nakamura, R.; Saber, W.; Martens, M.J.; Ramirez, A.; Scott, B.; Oran, B.; Leifer, E.; Tamari, R.; Mishra, A.; Maziarz, R.T.; et al. Biologic Assignment Trial of Reduced-Intensity Hematopoietic Cell Transplantation Based on Donor Availability in Patients 50–75 Years of Age with Advanced Myelodysplastic Syndrome. J. Clin. Oncol. 2021, 39, 3328–3339. [Google Scholar] [CrossRef]
- van Galen, P.; Hovestadt, V.; Wadsworth, M.H., II; Hughes, T.K.; Griffin, G.K.; Battaglia, S.; Verga, J.A.; Stephansky, J.; Pastika, T.J.; Lombardi Story, J.; et al. Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell 2019, 176, 1265–1281.e24. [Google Scholar] [CrossRef]
- Zeng, A.G.X.; Bansal, S.; Jin, L.; Mitchell, A.; Chen, W.C.; Abbas, H.A.; Chan-Seng-Yue, M.; Voisin, V.; van Galen, P.; Tierens, A.; et al. A cellular hierarchy framework for understanding heterogeneity and predicting drug response in acute myeloid leukemia. Nat. Med. 2022, 28, 1212–1223. [Google Scholar] [CrossRef]
- Pei, S.; Shelton, I.T.; Gillen, A.E.; Stevens, B.M.; Gasparetto, M.; Wang, Y.; Liu, L.; Liu, J.; Brunetti, T.M.; Engel, K.; et al. A Novel Type of Monocytic Leukemia Stem Cell Revealed by the Clinical Use of Venetoclax-Based Therapy. Cancer Discov. 2023, 13, 2032–2049. [Google Scholar] [CrossRef]
- Beneyto-Calabuig, S.; Merbach, A.K.; Kniffka, J.-A.; Antes, M.; Szu-Tu, C.; Rohde, C.; Waclawiczek, A.; Stelmach, P.; Gräßle, S.; Pervan, P.; et al. Clonally resolved single-cell multi-omics identifies routes of cellular differentiation in acute myeloid leukemia. Cell Stem Cell 2023, 30, 706–721.e8. [Google Scholar] [CrossRef]
- Waclawiczek, A.; Leppä, A.-M.; Renders, S.; Trumpp, A. An arms-race against resistance: Leukemic stem cells and lineage plasticity. Mol. Oncol. 2024, 18, 475–478. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Nardi, V.; Stein, E.; Hasserjian, R.P. A practical approach on the classifications of myeloid neoplasms and acute leukemia: WHO and ICC. J. Hematol. Oncol. 2024, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Kewan, T. Clinical and Molecular Insights into the Classification Dilemma: Are MDS/AML Cases Distinct from Secondary AML? ASH. 2024. Available online: https://ash.confex.com/ash/2024/webprogram/Paper207770.html (accessed on 10 April 2025).
- Sureda, A.; Corbacioglu, S.; Greco, R.; Kröger, N.; Carreras, E. (Eds.) The EBMT Handbook: Hematopoietic Cell Transplantation and Cellular Therapies; Springer International Publishing: Cham, Switzerland, 2024; Available online: https://link.springer.com/10.1007/978-3-031-44080-9 (accessed on 10 April 2025).
- Spyridonidis, A.; Labopin, M.; Savani, B.N.; Niittyvuopio, R.; Blaise, D.; Craddock, C.; Socié, G.; Platzbecker, U.; Beelen, D.; Milpied, N.; et al. Redefining and measuring transplant conditioning intensity in current era: A study in acute myeloid leukemia patients. Bone Marrow Transplant. 2020, 55, 1114–1125. [Google Scholar] [CrossRef]
- Gagelmann, N.; Kröger, N. Dose intensity for conditioning in allogeneic hematopoietic cell transplantation: Can we recommend “when and for whom” in 2021? Haematologica 2021, 106, 1794–1804. [Google Scholar] [CrossRef]
- Scott, B.L.; Pasquini, M.C.; Logan, B.R.; Wu, J.; Devine, S.M.; Porter, D.L.; Maziarz, R.T.; Warlick, E.D.; Fernandez, H.F.; Alyea, E.P.; et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1154–1161. [Google Scholar] [CrossRef]
- Scott, B.L.; Pasquini, M.C.; Fei, M.; Fraser, R.; Wu, J.; Devine, S.M.; Porter, D.L.; Maziarz, R.T.; Warlick, E.; Fernandez, H.F.; et al. Myeloablative versus Reduced-Intensity Conditioning for Hematopoietic Cell Transplantation in Acute Myelogenous Leukemia and Myelodysplastic Syndromes-Long-Term Follow-Up of the BMT CTN 0901 Clinical Trial. Transplant. Cell. Ther. 2021, 27, 483.e1–483.e6. [Google Scholar] [CrossRef]
- Kröger, N.; Iacobelli, S.; Franke, G.-N.; Platzbecker, U.; Uddin, R.; Hübel, K.; Scheid, C.; Weber, T.; Robin, M.; Stelljes, M.; et al. Dose-Reduced Versus Standard Conditioning Followed by Allogeneic Stem-Cell Transplantation for Patients with Myelodysplastic Syndrome: A Prospective Randomized Phase III Study of the EBMT (RICMAC Trial). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2157–2164. [Google Scholar] [CrossRef]
- Niederwieser, D.; Al-Ali, H.K.; Krahl, R.; Kahl, C.; Wolf, H.-H.; Kreibich, U.; Vucinic, V.; Hegenbart, U.; Hirt, C.; Peter, N.; et al. Hematopoietic stem cell transplantation (HSCT) compared to consolidation chemotherapy (CT) to increase leukemia free survival (LFS) in acute myelogenous leukemia (AML) patients between 60 and 75 years irrespective of genetic risk: Report from the AML 2004 of the East German Study Group (OSHO). J. Clin. Oncol. 2016, 34 (Suppl. S15), e18501. [Google Scholar]
- Mitchell, E.; Spencer Chapman, M.; Williams, N.; Dawson, K.J.; Mende, N.; Calderbank, E.F.; Jung, H.; Mitchell, T.; Coorens, T.H.H.; Spencer, D.H.; et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature 2022, 606, 343–350. [Google Scholar] [CrossRef]
- Craddock, C.; Jackson, A.; Loke, J.; Siddique, S.; Hodgkinson, A.; Mason, J.; Andrew, G.; Nagra, S.; Malladi, R.; Peniket, A.; et al. Augmented Reduced-Intensity Regimen Does Not Improve Postallogeneic Transplant Outcomes in Acute Myeloid Leukemia. J. Clin. Oncol. 2021, 39, 768–778. [Google Scholar] [CrossRef]
- Sora, F.; Grazia, C.D.; Chiusolo, P.; Raiola, A.M.; Bregante, S.; Mordini, N.; Olivieri, A.; Iori, A.P.; Patriarca, F.; Grisariu, S.; et al. Allogeneic Hemopoietic Stem Cell Transplants in Patients with Acute Myeloid Leukemia (AML) Prepared with Busulfan and Fludarabine (BUFLU) or Thiotepa, Busulfan, and Fludarabine (TBF): A Retrospective Study. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2020, 26, 698–703. [Google Scholar] [CrossRef]
- Oran, B.; Ahn, K.W.; Fretham, C.; Beitinjaneh, A.; Bashey, A.; Pawarode, A.; Wirk, B.; Scott, B.L.; Savani, B.N.; Bredeson, C.; et al. Fludarabine and Melphalan Compared with Reduced Doses of Busulfan and Fludarabine Improve Transplantation Outcomes in Older Patients with Myelodysplastic Syndromes. Transplant. Cell. Ther. 2021, 27, 921.e1–921.e10. [Google Scholar] [CrossRef]
- Steckel, N.K.; Groth, C.; Mikesch, J.-H.; Trenschel, R.; Ottinger, H.; Kordelas, L.; Mueller-Tidow, C.; Schliemann, C.; Reicherts, C.; Albring, J.C.; et al. High-dose melphalan-based sequential conditioning chemotherapy followed by allogeneic haematopoietic stem cell transplantation in adult patients with relapsed or refractory acute myeloid leukaemia. Br. J. Haematol. 2018, 180, 840–853. [Google Scholar] [CrossRef]
- Stelljes, M.; Middeke, J.M.; Bug, G.; Wagner-Drouet, E.-M.; Müller, L.P.; Schmid, C.; Krause, S.W.; Bethge, W.; Jost, E.; Platzbecker, U.; et al. Remission induction versus immediate allogeneic haematopoietic stem cell transplantation for patients with relapsed or poor responsive acute myeloid leukaemia (ASAP): A randomised, open-label, phase 3, non-inferiority trial. Lancet Haematol. 2024, 11, e324–e335. [Google Scholar] [CrossRef]
- Potter, V.; Gras, L.; Kroger, N.; Sockel, K.; Ganser, A.; Finke, J.; Labussiere-Wallet, H.; Peffault de Latour, R.; Koc, Y.; Salmenniemi, U.; et al. Sequential vs. reduced intensity conditioning for apteits with myelodysplastic syndromes with an excess of blasts at time of allogeneic hematopoietic cell transplantation: A retrospective study by the chronic malignancies working party of the EBMT. Bone Marrow Transplant. 2024, 59, 224–231. [Google Scholar] [CrossRef]
- Beelen, D.W.; Trenschel, R.; Stelljes, M.; Groth, C.; Masszi, T.; Reményi, P.; Wagner-Drouet, E.-M.; Hauptrock, B.; Dreger, P.; Luft, T.; et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): A randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020, 7, e28–e39. [Google Scholar]
- Shimoni, A.; Radici, V.; Nagler, A. Conditioning. In The EBMT Handbook: Hematopoietic Cell Transplantation and Cellular Therapies; Sureda, A., Corbacioglu, S., Greco, R., Kröger, N., Carreras, E., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 125–134. [Google Scholar] [CrossRef]
- Pasic, I.; Moya, T.A.; Remberger, M.; Chen, C.; Gerbitz, A.; Kim, D.D.H.; Kumar, R.; Lam, W.; Law, A.D.; Lipton, J.H.; et al. Treosulfan-Versus Busulfan-based Conditioning in Allogeneic Hematopoietic Cell Transplantation for Myelodysplastic Syndrome: A Single-center Retrospective Propensity Score-matched Cohort Study. Transplant. Cell. Ther. 2024, 30, 681.e1–681.e11. [Google Scholar] [CrossRef]
- Martino, R.; Iacobelli, S.; Brand, R.; Jansen, T.; van Biezen, A.; Finke, J.; Bacigalupo, A.; Beelen, D.; Reiffers, J.; Devergie, A.; et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood 2006, 108, 836–846. [Google Scholar] [CrossRef]
- Bellen, D.W.; Stelljes, M.; Remenyi, P.; Wagner-Drouet, E.; Dreger, P.; Bethge, W.; Ciceri, F.; Stolzel, F.; Junghanss, C.; Labussiere-Wallet, H.; et al. Treosulfan compared with reduced-intensity busulfan improves allogeneic hematopoietic cell transplantation outcomes of older acute myeloid leukemia and myelodysplastic patients: Final analysis of a prospective randomized trial. Am. J. Hematol. 2022, 97, 1023–1034. [Google Scholar] [CrossRef]
- Potter, V.T.; Iacobelli, S.; van Biezen, A.; Maertens, J.; Bourhis, J.-H.; Passweg, J.R.; Yakhoub-Agha, I.; Tabrizi, R.; Bay, J.-O.; Chevallier, P.; et al. Comparison of Intensive Chemotherapy and Hypomethylating Agents before Allogeneic Stem Cell Transplantation for Advanced Myelodysplastic Syndromes: A Study of the Myelodysplastic Syndrome Subcommittee of the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1615–1620. [Google Scholar]
- Jentzsch, M.; Doehring, C.; Linke, R.; Hille, A.; Grimm, J.; Poenisch, W.; Vucinic, V.; Franke, G.; Behre, G.; Niederwasser, D.; et al. Comparison of non-myeloablative and reduced-intensity allogeneic stem cell transplantation in older patients with myelodysplastic syndromes. Am. J. Hematol. 2019, 94, 1344–1352. [Google Scholar] [CrossRef]
- Walter, R.B.; Potter, V.; Craddock, C. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia in Adults over 70 years old. Blood 2024. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Alessandrino, E.P.; Bacigalupo, A.; van Lint, M.T.; Malcovati, L.; Pascutto, C.; Falda, M.; Bernardi, M.; Onida, F.; Guidi, S.; et al. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood 2014, 123, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Eikema, D.-J.; Stelljes, M.; Beelen, D.; de Wreede, L.; Mufti, G.; Knelange, N.S.; Niederwieser, D.; Friis, L.S.; Ehninger, G.; et al. Optimized EBMT transplant-specific risk score in myelodysplastic syndromes after allogeneic stem-cell transplantation. Haematologica 2019, 104, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Tentori, C.A.; Gregorio, C.; Robin, M.; Gagelmann, N.; Gurnari, C.; Ball, S.; Berrocal, J.; Lanino, L.; D’Amico, S.; Speafico, M.; et al. Clinical and Genomic-based decision support to define optimal timing of allogeneic hematopoietic stem-cell transplantation in patients with myelodysplastic syndrome. J. Clin. Oncol. 2024, 42, 2873–2886. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Zhou, W.; Wang, R.; Li, Y.; Yu, L. Pre-transplant therapy for patients with myelodysplastic syndromes: A systematic review and meta-analysis. Leuk. Res. 2021, 110, 106645. [Google Scholar] [CrossRef]
- Park, S.-S.; Jeon, Y.-W.; Min, G.J.; Park, S.; Yahng, S.-A.; Yoon, J.-H.; Shin, S.-H.; Lee, S.-E.; Cho, B.-S.; Eom, K.-S.; et al. Graft-versus-Host Disease-Free, Relapse-Free Survival after Allogeneic Stem Cell Transplantation for Myelodysplastic Syndrome. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2019, 25, 63–72. [Google Scholar] [CrossRef]
- Yahng, S.-A.; Kim, M.; Kim, T.-M.; Jeon, Y.-W.; Yoon, J.-H.; Shin, S.-H.; Lee, S.-E.; Eom, K.-S.; Lee, S.; Min, C.-K.; et al. Better transplant outcome with pre-transplant marrow response after hypomethylating treatment in higher-risk MDS with excess blasts. Oncotarget 2017, 8, 12342–12354. [Google Scholar] [CrossRef]
- Welch, J.S.; Petti, A.A.; Miller, C.A.; Fronick, C.C.; O’Laughlin, M.; Fulton, R.S.; Wilson, R.K.; Baty, J.D.; Duncavage, E.J.; Tandon, B.; et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N. Engl. J. Med. 2016, 375, 2023–2036. [Google Scholar] [CrossRef]
- Symeonidis, A.; van Biezen, A.; de Wreede, L.; Piciocchi, A.; Finke, J.; Beelen, D.; Bornhäuser, M.; Cornelissen, J.; Volin, L.; Mufti, G.; et al. Achievement of complete remission predicts outcome of allogeneic haematopoietic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Br. J. Haematol. 2015, 171, 239–246. [Google Scholar]
- Warlick, E.D.; Cioc, A.; Defor, T.; Dolan, M.; Weisdorf, D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: Importance of pretransplant disease burden. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2009, 15, 30–38. [Google Scholar] [CrossRef]
- de Witte, T.; Suciu, S.; Verhoef, G.; Labar, B.; Archimbaud, E.; Aul, C.; Selleslag, D.; Ferrant, A.; Wijermans, P.; Mandelli, F.; et al. Intensive chemotherapy followed by allogeneic or autologous stem cell transplantation for patients with myelodysplastic syndromes (MDSs) and acute myeloid leukemia following MDS. Blood 2001, 98, 2326–2331. [Google Scholar] [CrossRef]
- Strati, P.; Garcia-Manero, G.; Zhao, C.; Kadia, T.; Borthakur, G.; Konopleva, M.; Daver, N.; DiNardo, C.D.; Short, N.J.; Yilmaz, M.; et al. Intensive chemotherapy is more effective than hypomethylating agents for the treatment of younger patients with myelodysplastic syndrome and elevated bone marrow blasts. Am. J. Hematol. 2019, 94, E188–E190. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.; Pérez, W.S.; Rozman, C.; Carreras, E.; Klein, J.P.; Rizzo, J.D.; Davies, S.M.; Lazarus, H.M.; Bredeson, C.N.; Marks, D.I.; et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood 2002, 100, 1997–2004. [Google Scholar] [PubMed]
- Schroeder, T.; Wegener, N.; Lauseker, M.; Rautenberg, C.; Nachtkamp, K.; Schuler, E.; Kondakci, M.; Haas, R.; Germing, U.; Kobbe, G. Comparison between Upfront Transplantation and different Pretransplant Cytoreductive Treatment Approaches in Patients with High-Risk Myelodysplastic Syndrome and Secondary Acute Myelogenous Leukemia. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2019, 25, 1550–1559. [Google Scholar] [CrossRef]
- Schroeder, J.C.; Mix, L.; Faustmann, P.; Weller, J.F.; Fehn, A.; Phely, L.; Riedel, A.; Vogel, W.; Faul, C.; Lengerke, C.; et al. Superior outcome of upfront allogeneic hematopoietic cell transplantation versus hypomethylating agent induction in myelodysplastic syndrome. Bone Marrow Transplant. 2024, 59, 1332–1334. [Google Scholar] [CrossRef]
- Tobiasson, M.; Pandzic, T.; Illman, J.; Nillson, L.; Westrom, S.; Ejerblad, E.; Olesen, G.; Bjorklund, A.; Kittang, A.; Werlenius, O.; et al. Patient-specific measurable disease markers predict outcome in patients with myelodysplastic syndrome and related disorders after hematopoietic stem-cell transplantation. J. Clin. Oncol. 2024, 42, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Konuma, T.; Shimomura, Y.; Ozawa, Y.; Ueda, Y.; Uchida, N.; Onizuka, M.; Akiyama, M.; Mori, T.; Nakamae, H.; Ohno, Y.; et al. Induction chemotherapy followed by allogeneic HCT versus upfront allogeneic HCT for advanced myelodysplastic syndrome: A propensity score matched analysis. Hematol. Oncol. 2019, 37, 85–95. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, F.; Xuan, L.; Zhang, Y.; Fan, Z.; Xu, N.; Zhao, K.; Xu, J.; Liu, H.; Shi, P.; et al. Upfront transplantation may have better outcomes than pretransplant cytoreductive therapy for treating patients with MDS-EB-1 or MDS-EB-2. Int. J. Cancer 2021, 149, 1109–1120. [Google Scholar] [CrossRef]
- Onida, F.; Brand, R.; van Biezen, A.; Schaap, M.; von dem Borne, P.A.; Maertens, J.; Beelen, D.W.; Carreras, E.; Alessandrino, E.P.; Volin, L.; et al. Impact of the International Prognostic Scoring System cytogenetic risk groups on the outcome of patients with primary myelodysplastic syndromes undergoing allogeneic stem cell transplantation from human leukocyte antigen-identical siblings: A retrospective analysis of the European Society for Blood and Marrow Transplantation-Chronic Malignancies Working Party. Haematologica 2014, 99, 1582–1590. [Google Scholar]
- Rolles, B. Impact of Response to Hypomethylating Agent-Based Therapy on Survival Outcomes in the Context of Baseline Clinical-Molecular Risk and Transplant Status in Patients with Myelodysplastic Syndromes/Neoplasms (MDS): An Analysis from the International Consortium for MDS (icMDS) Validate Database. ASH. 2024. Available online: https://ash.confex.com/ash/2024/webprogram/Paper208034.html (accessed on 7 February 2025).
- Scheid, C.; Eikema, D.; van Gelder, M.; Salmenniemi, U.; Maertens, J.; Passweg, J.; Blaise, D.; Byrne, J.; Kroeger, N.; Sockel, K.; et al. Does IPSS-R downstaging before transplantation improve the prognosis of patients with myelodysplastic syndrome? Blood 2024, 144, 445–456. [Google Scholar] [CrossRef]
- Scott, B.L. Change is not always good. Blood 2024, 144, 355–357. [Google Scholar] [CrossRef]
- Garcia-Manero, G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 1307–1325. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Haase, D.; Santini, V.; Sanz, G.F.; Platzbecker, U.; Mey, U. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 142–156. [Google Scholar] [CrossRef]
- de Witte, T.; Bowen, D.; Robin, M.; Malcovati, L.; Niederwieser, D.; Yakoub-Agha, I.; Mufti, G.J.; Fenaux, P.; Sanz, G.; Martino, R.; et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: Recommendations from an international expert panel. Blood 2017, 129, 1753–1762. [Google Scholar] [CrossRef]
- Damaj, G.; Duhamel, A.; Robin, M.; Beguin, Y.; Michallet, M.; Mohty, M.; Vigouroux, S.; Bories, P.; Garnier, A.; El Cheikh, J.; et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: A study by the Société Française de Greffe de Moelle et de Thérapie-Cellulaire and the Groupe-Francophone des Myélodysplasies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 4533–4540. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Kanda, Y.; Fukuhara, S.; Sakamaki, H.; Okamoto, S.; Kodera, Y.; Tanosaki, R.; Takahashi, S.; Matsushima, T.; Atsuta, Y.; et al. Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia 2005, 19, 396–401. [Google Scholar] [CrossRef]
- Kröger, N.; Sockel, K.; Wolschke, C.; Bethge, W.; Schlenk, R.F.; Wolf, D.; Stadler, M.; Kobbe, G.; Wulf, G.; Bug, G.; et al. Comparison Between 5-Azacytidine Treatment and Allogeneic Stem-Cell Transplantation in Elderly Patients with Advanced MDS According to Donor Availability (VidazaAllo Study). J. Clin. Oncol. 2021, 39, 3318–3327. [Google Scholar] [CrossRef]
- Lin, R.J.; Artz, A.S. Allogeneic hematopoietic cell transplantation for older patients. Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Bejar, R.; Stevenson, K.E.; Caughey, B.; Lindsley, R.C.; Mar, B.G.; Stojanov, P.; Getz, G.; Steensma, D.P.; Ritz, J.; Soiffer, R.; et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2691–2698. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Gallì, A.; Bacigalupo, A.; Zibellini, S.; Bernardi, M.; Rizzo, E.; Allione, B.; van Lint, M.T.; Pioltelli, P.; Marenco, P.; et al. Clinical Effects of Driver Somatic Mutations on the Outcomes of Patients with Myelodysplastic Syndromes Treated with Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2016, 34, 3627–3637. [Google Scholar] [CrossRef]
- Yoshizato, T.; Nannya, Y.; Atsuta, Y.; Shiozawa, Y.; Iijima-Yamashita, Y.; Yoshida, K.; Shiraishi, Y.; Suzuki, H.; Nagata, Y.; Sato, Y.; et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: Impact on outcome of stem cell transplantation. Blood 2017, 129, 2347–2358. [Google Scholar] [CrossRef]
- Hunter, A.M.; Komrokji, R.S.; Yun, S.; Al Ali, N.; Chan, O.; Song, J.; Hussaini, M.; Talati, C.; Sweet, K.L.; Lancet, J.E.; et al. Baseline and serial molecular profiling predicts outcomes with hypomethylating agents in myelodysplastic syndromes. Blood Adv. 2021, 5, 1017–1028. [Google Scholar] [CrossRef]
- Versluis, J.; Saber, W.; Tsai, H.K.; Gibson, C.J.; Dillon, L.W.; Mishra, A.; McGuirk, J.; Maziarz, R.T.; Westervelt, P.; Hegde, P.; et al. Allogeneic Hematopoietic Cell Transplantation Improves Outcome in Myelodysplastic Syndrome Across High-Risk Genetic Subgroups: Genetic Analysis of the Blood and Marrow Transplant Clinical Trials Network 1102 Study. J. Clin. Oncol. 2023, 41, 4497–4510. [Google Scholar] [CrossRef] [PubMed]
- Bally, C.; Adès, L.; Renneville, A.; Sebert, M.; Eclache, V.; Preudhomme, C.; Mozziconacci, M.-J.; de The, H.; Lehmann-Che, J.; Fenaux, P. Prognostic value of TP53 gene mutations in myelodysplastic syndromes and acute myeloid leukemia treated with azacitidine. Leuk. Res. 2014, 38, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Kadia, T.M.; Jain, P.; Ravandi, F.; Garcia-Manero, G.; Andreef, M.; Takahashi, K.; Borthakur, G.; Jabbour, E.; Konopleva, M.; Daver, N.G.; et al. TP53 mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer 2016, 122, 3484–3491. [Google Scholar] [CrossRef]
- Diepstraten, S.; Yuan, Y.; La Marca, J.; Young, S.; Chang, C.; Whelan, L.; Ross, A.; Fischer, K.; Pomillo, G.; Morris, R.; et al. Putting the STING back into BH3-mimetic drugs for T53-mutant blood cancers. Cancer Cell 2024, 42, 850–868. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Scruggs, D.; Ayoub, E.; Patsilevas, V.; Mak, P.; Carter, B.; Boettcher, S.; Maiti, A.; Zhou, Q.; Yang, Z.; et al. AML-147 C_MYC targeting by degradation: Novel dual Myc/GSTP1 degrader GT19715 exerts profound cell kill in vitro and in vivo in acute myeloid leukemia and lymphomas. Clin. Lymphoma Myeloma Leuk. 2022, 22, S218. [Google Scholar] [CrossRef]
- Lindsley, R.C.; Saber, W.; Mar, B.G.; Redd, R.; Wang, T.; Haagenson, M.D.; Grauman, P.V.; Hu, Z.-H.; Spellman, S.R.; Lee, S.J.; et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. N. Engl. J. Med. 2017, 376, 536–547. [Google Scholar] [CrossRef]
- Chan, O.; Hunter, A.; Talati, C.; Sallman, D.A.; Asghari, H.; Song, J.; Hussaini, M.; Bejanyan, N.; Elmariah, H.; Kuykendall, A.T.; et al. Impact of TP53 gene Mutation Clearance and Conditioning Intensity on Outcome in MDS or AML Patients Prior to Allogeneic Stem Cell Transplantation. Blood 2019, 134, 149. [Google Scholar] [CrossRef]
- Scott, B.L. Long-Term Follow up of BMT CTN 0901, a Randomized Phase III Trial Comparing Myeloablative (MAC) to Reduced Intensity Conditioning (RIC) Prior to Hematopoietic Cell Transplantation (HCT) for Acute Myeloid Leukemia (AML) or Myelodysplasia (MDS) (MAvRIC Trial). Biol. Blood Marrow Transplant. 2020, 26, S11. [Google Scholar]
- Byrne, M.T.; Kurian, T.J.; Patel, D.A.; Tamari, R.; Hong, S.; Abdelhakim, H.; Klein, V.; Rojas, P.; Madhavan, R.; Kent, A.; et al. Non-Relapse Mortality in TP53-Mutated MDS/AML—A Multi-Center Collaborative Study. Blood 2021, 138, 2922. [Google Scholar] [CrossRef]
- Levis, M.J.; Hamadani, M.; Logan, B.; Jones, R.J.; Singh, A.K.; Litzow, M.; Wingard, J.R.; Papadopoulos, E.B.; Perl, A.E.; Soiffer, R.J.; et al. Gilteritinib as Post-Transplant Maintenance for AML with Internal Tandem Duplication Mutation of FLT3. J. Clin. Oncol. 2024, 42, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; DeZern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.; Garcia-Manero, G.; Daver, N.; Aboud, C.; Stein, E.; Larkin, K.; Halpern, A.; McCurdy, S.; Al Malki, M.; Murthy, G.; et al. Magrolimab + azacytidine vs. placebo + azacytidine in patients with untreated high-risk myelodysplastic syndrome: Phase 3 ENHANCE study final analysis. EHA Libr. 2024, S181. [Google Scholar]
- Duncavage, E.J.; Jacoby, M.A.; Chang, G.S.; Miller, C.A.; Edwin, N.; Shao, J.; Elliott, K.; Robinson, J.; Abel, H.; Fulton, R.S.; et al. Mutation Clearance after Transplantation for Myelodysplastic Syndrome. N. Engl. J. Med. 2018, 379, 1028–1041. [Google Scholar] [CrossRef]
- Yun, S.; Geyer, S.M.; Komrokji, R.S.; Al Ali, N.H.; Song, J.; Hussaini, M.; Sweet, K.L.; Lancet, J.E.; List, A.F.; Padron, E.; et al. Prognostic Significance of Serial Molecular Annotation in Myelodysplastic Syndromes (MDS) and Secondary Acute Myeloid Leukemia (sAML). Leukemia 2021, 35, 1145–1155. [Google Scholar] [CrossRef]
- Hou, C.; Zhou, L.; Yang, M.; Jiang, S.; Shen, H.; Zhu, M.; Chen, J.; Miao, M.; Xu, Y.; Wu, D. The Prognostic Value of Early Detection of Minimal Residual Disease as Defined by Flow Cytometry and Gene Mutation Clearance for Myelodysplastic Syndrome Patients After Myeloablative Allogeneic Hematopoietic Stem-Cell Transplantation. Front. Oncol. 2021, 11, 700234. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Wei, Z.-L.; Xu, Y.-J.; Shi, J.-M.; Yi, H.; Lai, Y.-R.; Jiang, E.-L.; Wang, S.-B.; Wu, T.; Gao, L.; et al. Poor pretransplantation minimal residual disease clearance as an independent prognostic risk factor for survival in myelodysplastic syndrome with excess blasts: A multicenter, retrospective cohort study. Cancer 2023, 129, 2013–2022. [Google Scholar] [CrossRef]
- Festuccia, M.; Deeg, H.J.; Gooley, T.A.; Baker, K.; Wood, B.L.; Fang, M.; Sandmaier, B.M.; Scott, B.L. Minimal Identifiable Disease and the Role of Conditioning Intensity in Hematopoietic Cell Transplantation for Myelodysplastic Syndrome and Acute Myelogenous Leukemia Evolving from Myelodysplastic Syndrome. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1227–1233. [Google Scholar] [CrossRef]
- Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; Devine, S.M.; et al. Impact of Conditioning Intensity and Genomics on Relapse After Allogeneic Transplantation for Patients with Myelodysplastic Syndrome. JCO Precis. Oncol. 2021, 5, 265–274. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia with Genomic Evidence of Residual Disease. J. Clin. Oncol. 2020, 38, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Dillon, L.; Gui, G.; Page, K.; Ravindra, N.; Wong, Z.; Andrew, G.; Mukherjee, D.; Zeger, S.; El Chare, F.; Spellman, S.; et al. DNA sequencing to detect residual disease in adults with acute myeloid leukemia prior to hematopoietic cell transplant. JAMA 2023, 32, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Platzbecker, U.; Bewersdorf, J.P.; Stahl, M.; Adès, L.; Borate, U.; Bowen, D.; Buckstein, R.; Brunner, A.; Carraway, H.E.; et al. Consensus proposal for revised International Working Group 2023 response criteria for higher-risk myelodysplastic syndromes. Blood 2023, 141, 2047–2061. [Google Scholar]
- Radford, M.; Garcia-Horton, A.; Badami, R.; Jin, E.; Usmani, N.; Grafodatskaya, D.; McCready, E.; Khalaf, D.; Walker, I.; Leber, B.; et al. Early Mixed Donor Chimerism is a Strong Negative Prognostic Indicator in Allogeneic Stem Cell Transplant for AML and MDS. Transplant. Cell. Ther. 2025, 31, 77.e1–77.e20. [Google Scholar] [CrossRef]
- Lee, H.C.; Saliba, R.M.; Rondon, G.; Chen, J.; Charafeddine, Y.; Medeiros, L.J.; Alatrash, G.; Andersson, B.S.; Popat, U.; Kebriaei, P.; et al. Mixed T Lymphocyte Chimerism after Allogeneic Hematopoietic Transplantation Is Predictive for Relapse of Acute Myeloid Leukemia and Myelodysplastic Syndromes. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 1948–1954. [Google Scholar] [CrossRef]
- Baron, F.; Baker, J.E.; Storb, R.; Gooley, T.A.; Sandmaier, B.M.; Maris, M.B.; Maloney, D.G.; Heimfeld, S.; Oparin, D.; Zellmer, E.; et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 2004, 104, 2254–2262. [Google Scholar] [CrossRef]
- Felix, N.J.; Allen, P.M. Specificity of T-cell alloreactivity. Nat. Rev. Immunol. 2007, 7, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.P.; Horowitz, M.M.; Ash, R.C.; Champlin, R.E.; Goldman, J.M.; Rimm, A.A.; Ringdén, O.; Stone, J.A.; Bortin, M.M. Identical-twin bone marrow transplants for leukemia. Ann. Intern. Med. 1994, 120, 646–652. [Google Scholar] [CrossRef]
- Heining, C.; Spyridonidis, A.; Bernhardt, E.; Schulte-Mönting, J.; Behringer, D.; Grüllich, C.; Jakob, A.; Bertz, H.; Finke, J. Lymphocyte reconstitution following allogeneic hematopoietic stem cell transplantation: A retrospective study including 148 patients. Bone Marrow Transplant. 2007, 39, 613–622. [Google Scholar] [CrossRef]
- Dekker, L.; de Koning, C.; Lindemans, C.; Nierkens, S. Reconstitution of T Cell Subsets Following Allogeneic Hematopoietic Cell Transplantation. Cancers 2020, 12, 1974. [Google Scholar] [CrossRef]
- Handgretinger, R.; Lang, P.; André, M.C. Exploitation of natural killer cells for the treatment of acute leukemia. Blood 2016, 127, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- McLarnon, A.; Piper, K.P.; Goodyear, O.C.; Arrazi, J.M.; Mahendra, P.; Cook, M.; Clark, F.; Pratt, G.; Craddock, C.; Moss, P.A.H. CD8(+) T-cell immunity against cancer-testis antigens develops following allogeneic stem cell transplantation and reveals a potential mechanism for the graft-versus-leukemia effect. Haematologica 2010, 95, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, J.J.; Scheinberg, P.; Chattopadhyay, P.K.; Gostick, E.; Ladell, K.; Roederer, M.; Hensel, N.F.; Douek, D.C.; Barrett, A.J.; Price, D.A. High avidity myeloid leukemia-associated antigen-specific CD8+ T cells preferentially reside in the bone marrow. Blood 2009, 113, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Ehx, G.; Larouche, J.-D.; Durette, C.; Laverdure, J.-P.; Hesnard, L.; Vincent, K.; Hardy, M.-P.; Thériault, C.; Rulleau, C.; Lanoix, J.; et al. Atypical acute myeloid leukemia-specific transcripts generate shared and immunogenic MHC class-I-associated epitopes. Immunity 2021, 54, 737–752.e10. [Google Scholar] [CrossRef]
- Langenhorst, J.B.; Dorlo, T.P.C.; van Maarseveen, E.M.; Nierkens, S.; Kuball, J.; Boelens, J.J.; van Kesteren, C.; Huitema, A.D.R. Population Pharmacokinetics of Fludarabine in Children and Adults during Conditioning Prior to Allogeneic Hematopoietic Cell Transplantation. Clin. Pharmacokinet. 2019, 58, 627–637. [Google Scholar] [CrossRef]
- Mackall, C.L.; Fleisher, T.A.; Brown, M.R.; Andrich, M.P.; Chen, C.C.; Feuerstein, I.M.; Horowitz, M.E.; Magrath, I.T.; Shad, A.T.; Steinberg, S.M. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N. Engl. J. Med. 1995, 332, 143–149. [Google Scholar] [CrossRef]
- Sfikakis, P.P.; Gourgoulis, G.M.; Moulopoulos, L.A.; Kouvatseas, G.; Theofilopoulos, A.N.; Dimopoulos, M.A. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. Eur. J. Clin. Investig. 2005, 35, 380–387. [Google Scholar] [CrossRef]
- Cheson, B.D. Infectious and immunosuppressive complications of purine analog therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1995, 13, 2431–2448. [Google Scholar] [CrossRef]
- Priebe, T.; Platsoucas, C.D.; Seki, H.; Fox, F.E.; Nelson, J.A. Purine nucleoside modulation of functions of human lymphocytes. Cell. Immunol. 1990, 129, 321–328. [Google Scholar] [CrossRef]
- Morecki, S.; Gelfand, Y.; Nagler, A.; Or, R.; Naparstek, E.; Varadi, G.; Engelhard, D.; Akerstein, A.; Slavin, S. Immune reconstitution following allogeneic stem cell transplantation in recipients conditioned by low intensity vs. myeloablative regimen. Bone Marrow Transplant. 2001, 28, 243–249. [Google Scholar] [CrossRef]
- Petersen, S.L.; Ryder, L.P.; Björk, P.; Madsen, H.O.; Heilmann, C.; Jacobsen, N.; Sengeløv, H.; Vindeløv, L.L. A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplant. 2003, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg, A.; Fischer, M.; Kalhs, P.; Mitterbauer, M.; Rabitsch, W.; Greinix, H.T.; Leitner, G. Immune recovery after conventional and non-myeloablative allogeneic stem cell transplantation. Leuk. Lymphoma 2005, 46, 1755–1760. [Google Scholar] [CrossRef]
- Willemsen, L.; Jol-van der Zijde, C.M.; Admiraal, R.; Putter, H.; Jansen-Hoogendijk, A.M.; Ostaijen-Ten Dam, M.M.; Wijnen, J.T.; van Kesteren, C.; Waaijer, J.L.M.; Lankester, A.C.; et al. Impact of serotherapy on immune reconstitution and survival outcomes after stem cell transplantations in children: Thymoglobulin versus alemtuzumab. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Dhadda, M.; Hoegh-Petersen, M.; Liu, Y.; Hagel, L.M.; Podgorny, P.; Ugarte-Torres, A.; Khan, F.M.; Luider, J.; Auer-Grzesiak, I.; et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy 2012, 14, 1258–1275. [Google Scholar] [CrossRef]
- Forcade, E.; Chevret, S.; Finke, J.; Ehninger, G.; Ayuk, F.; Beelen, D.; Koster, L.; Ganser, A.; Volin, L.; Sengeloev, H.; et al. Impact of in vivo T-cell depletion in patients with myelodysplastic syndromes undergoing allogeneic hematopoietic stem cell transplant: A registry study from the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant. 2022, 57, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Laporte, J.; Jackson, K.; Zhang, X.; Bashey, A.; Morris, L.E.; Bachier Rodriguez, L.; Solh, M.M.; Solomon, S.R.; Holland, H.K. Survival Impact of Early Versus Late Discontinuation of Immunosuppression after Allogeneic Stem Cell Transplantation. Blood 2024, 144 (Suppl. S1), 7333. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Nagler, A.; Bornhäuser, M.; Finke, J.; Fassas, A.; Volin, L.; Gürman, G.; Maertens, J.; Bordigoni, P.; et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: A retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4938–4945. [Google Scholar]
- Schroeder, T.; Rachlis, E.; Bug, G.; Stelljes, M.; Klein, S.; Steckel, N.K.; Wolf, D.; Ringhoffer, M.; Czibere, A.; Nachtkamp, K.; et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions--a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 653–660. [Google Scholar] [CrossRef]
- Poiré, X.; Graux, C.; Ory, A.; Herman, J.; Baron, F.; Schoemans, H.; Lewalle, P.; De Becker, A.; Deeren, D.; Berneman, Z.; et al. Sequential administration of low dose 5-azacytidine (AZA) and donor lymphocyte infusion (DLI) for patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) in relapse after allogeneic stem cell transplantation (SCT): A prospective study from the Belgian Hematology Society (BHS). Bone Marrow Transplant. 2022, 57, 116–118. [Google Scholar]
- Alessandrì, L.; Arigoni, M.; Calogero, R. Differential Expression Analysis in Single-Cell Transcriptomics. In Single Cell Methods: Sequencing and Proteomics; Proserpio, V., Ed.; Springer: New York, NY, USA, 2019; pp. 425–432. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Schleuning, M.; Stadler, M.; Finke, J.; Hurst, E.; Baron, F.; Ringden, O.; et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia—A matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br. J. Haematol. 2019, 184, 782–787. [Google Scholar] [CrossRef]
- Jedlickova, Z.; Schmid, C.; Koenecke, C.; Hertenstein, B.; Baurmann, H.; Schwerdtfeger, R.; Tischer, J.; Kolb, H.-J.; Schleuning, M. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 663–667. [Google Scholar] [CrossRef]
- Konuma, T.; Ishiyama, K.; Igarashi, A.; Uchida, N.; Ozawa, Y.; Fukuda, T.; Ueda, Y.; Matsuoka, K.-I.; Mori, T.; Katayama, Y.; et al. Effects of Acute and Chronic Graft-versus-myelodysplastic Syndrome on Long-term Outcomes Following Allogeneic Hematopoietic Cell Transplantation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 6483–6493. [Google Scholar] [CrossRef] [PubMed]
- Cruijsen, M.; Hobo, W.; van der Velden, W.J.F.M.; Bremmers, M.E.J.; Woestenenk, R.; Bär, B.; Falkenburg, J.H.F.; Kester, M.; Schaap, N.P.M.; Jansen, J.; et al. Addition of 10-Day Decitabine to Fludarabine/Total Body Irradiation Conditioning is Feasible and Induces Tumor-Associated Antigen-Specific T Cell Responses. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lu, X.; Tang, L.V.; Wang, H.-F.; Yan, H.; You, Y.; Zhong, Z.-D.; Shi, W.; Xia, L.-H. Comparative analysis of Decitabine intensified BUCY2 and BUCY2 conditioning regimen for high-risk MDS patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022, 57, 1063–1071. [Google Scholar] [CrossRef]
- Choi, J.; Cooper, M.L.; Staser, K.; Ashami, K.; Vij, K.R.; Wang, B.; Marsala, L.; Niswonger, J.; Ritchey, J.; Alahmari, B.; et al. Baricitinib-induced blockade of interferon gamma receptor and interleukin-6 receptor for the prevention and treatment of graft-versus-host disease. Leukemia 2018, 32, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
| Trial | Condition | Regimen | Type of Study | Patients | Outcome |
|---|---|---|---|---|---|
| Multicenter retrospective study (Martino, Blood 2006) [29] | MDS | RIC vs. standard myeloablative conditioning | Registry Analysis | Total 836 patients | OS at 3 years 45% for MAC vs. 41% for RIC, p = 0.8 CIR at 3 years 27% for MAC vs. 45% for RIC, p < 0.01 |
| BMT CTN 0901 (Scott et al., JCO 2017) [15] | AML and MDS | RIC regimens: fludarabine with busulfan (≤8 mg/kg orally or 6.4 mg/kg intravenously; Flu/Bu2) or melphalan (≤150 mg/m2, Flu/Mel). MAC regimens: busulfan (16 mg/kg orally or 12.8 mg/kg intravenously) with cyclophosphamide (120 mg/kg) or fludarabine (120 to 180 mg/m2; Flu/Bu4) or cyclophosphamide (120 mg/kg) and total-body irradiation (12 to 14.2 Gy) | RCT | Total 272, 54 with MDS (terminated early because of benefit of MAC in total group) | OS for MDS at 18 months: 81.5% for MAC vs. 85.2% for RIC, p = 0.175 CIR for MDS at 18 months: 3.7% for MAC vs. 37.0% for RIC (p value not calculated by confidence intervals do not overlap) |
| RICMAC Trial (Kröger et al., JCO 2017) [17] | MDS | MAC: busulfan (16 mg/kg orally or 12.8 mg/kg intravenously) and cyclophosphamide (120 mg/kg). RIC: busulfan (8 mg/kg orally or 6.4 mg/kg intravenously) and fludarabine (150 mg/m2) | RCT | Total 129 with MDS (terminated early because of slow accrual) | OS at 2 years: 63.2% for MAC vs. 76.3% for RIC, p = 0.08 CIR at 2 years: 14.8% for MAC and 17% for RIC, p = 0.64 |
| CIBMTR Analysis (Oran et al., TCT 2021) [22] | MDS | fludarabine with either total melphalan dose ≤ 150 mg/m2, or busulfan ≤ 7.2 mg/kg intravenously (IV) | Registry Analysis | Total 1045 patients | OS at 2 years 61% for FluBu vs. 63% for FluMel, p = 0.4 CIR at 2 years 47% for FluBu vs. 28% for FluMel, p < 0.0001 |
| MC-FludT.14/L (Beelen et al., Lancet Haematology, [26] Beelen et al., AJH 2022 for final analysis) [30] | AML and MDS | intravenous (IV) fludarabine with either treosulfan (30 g/m2 IV) or busulfan (6.4 mg/kg IV) | RCT | Total 570 patients, MDS 199 patients | OS at 3 years 56.3% in FluBu and 66.8% % in FluTreo, p = 0.0037 CIR at 3 years 26% in FluBu and 25.9% in FluTreo, non-significant |
| University Leipzig analysis (Jentzsch et al., 2019) [31] | MDS and MDS/MPN | NMA vs. RIC | Single-center retrospective | Total 151 patients | No difference in OS p = 0.21, CIR, p = 0.38 |
| EBMT analysis (Shimoni et al., BJH 2021) [27] | MDS | MAC vs. RIC vs. Treo | Registry Analysis | Total 1722 patients | OS at 5 years 50% in FluTreo and 43% in MAC and 43% in RIC; p = 0.03; 5 year CIR 25% FluTreo, 25% for MAC, 38% for RIC, p < 0.001 |
| Figaro (Craddock et al., JCO 2020) [20] | AML/MDS | FLAMSA-Bu vs. Control (FluBu or FluMel) | RCT | Total 244 patients, MDS 78 patients | OS at 2 years 58.8% in control group and 60.9% in FLAMSA-Bu, p = 0.81 CIR at 2 years 29.5% in control group and 26.7% in FLAMSA-Bu, p= 0.81 |
| EBMT analysis MAC vs. RIC vs. sequential (Potter et al., BMT 2024) [31] | MDS | MAC vs. RIC vs. Sequential Conditioning | Registry Analysis | Total 303 patients | OS at 3 years 62% for MAC, 46% for RIC, 52% for sequential conditioning p = 0.13 CIR at 3 years 18% for MAC, 25% for RIC, 22 for sequential conditioning, p = 0.14 |
| PMCC Analysis (Pasic et al., 2024) [28] | MDS | Treosulfan vs. Busulfan | Single-center Retrospective Propensity Score-matched Cohort Study | Total 138 patients | OS at 2 years 66.9% for FT and 44.5% for FBT200 (p = 0.013) CIR at 2 years 15.6% for FT and 27.6% for FBT200 p = 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berg, T.; Salter, B.; Radford, M.; Chen, H.T.T.; Leber, B. Allogeneic Stem Cell Transplantation: The Relevance of Conditioning Regime Intensity for Myelodysplastic Syndromes (MDS). Curr. Oncol. 2025, 32, 319. https://doi.org/10.3390/curroncol32060319
Berg T, Salter B, Radford M, Chen HTT, Leber B. Allogeneic Stem Cell Transplantation: The Relevance of Conditioning Regime Intensity for Myelodysplastic Syndromes (MDS). Current Oncology. 2025; 32(6):319. https://doi.org/10.3390/curroncol32060319
Chicago/Turabian StyleBerg, Tobias, Brittany Salter, Michael Radford, He Tian Tony Chen, and Brian Leber. 2025. "Allogeneic Stem Cell Transplantation: The Relevance of Conditioning Regime Intensity for Myelodysplastic Syndromes (MDS)" Current Oncology 32, no. 6: 319. https://doi.org/10.3390/curroncol32060319
APA StyleBerg, T., Salter, B., Radford, M., Chen, H. T. T., & Leber, B. (2025). Allogeneic Stem Cell Transplantation: The Relevance of Conditioning Regime Intensity for Myelodysplastic Syndromes (MDS). Current Oncology, 32(6), 319. https://doi.org/10.3390/curroncol32060319





