Impact of Endocrine Therapy on Osteoporosis Risk in Women with Breast Cancer Across Different Hormonal Stages: A Review
Abstract
1. Introduction
1.1. Risk Factors Synergy in Breast Cancer and Osteoporosis
1.1.1. Gender, Age, Tumor Stage and Ethnicity
1.1.2. Hormonal Status and Genetic Factors
1.1.3. Benign Breast Changes
1.1.4. Previous Treatment Using Radiation Therapy
1.1.5. Health Behaviors: Alcohol, Diet, Obesity and Smoking
2. Breast Cancer and Osteoporosis: Relationship and Impacts
2.1. Breast Cancer Classification and Its Therapeutic Implications
2.2. Impact of Cancer Treatments on Bone Health
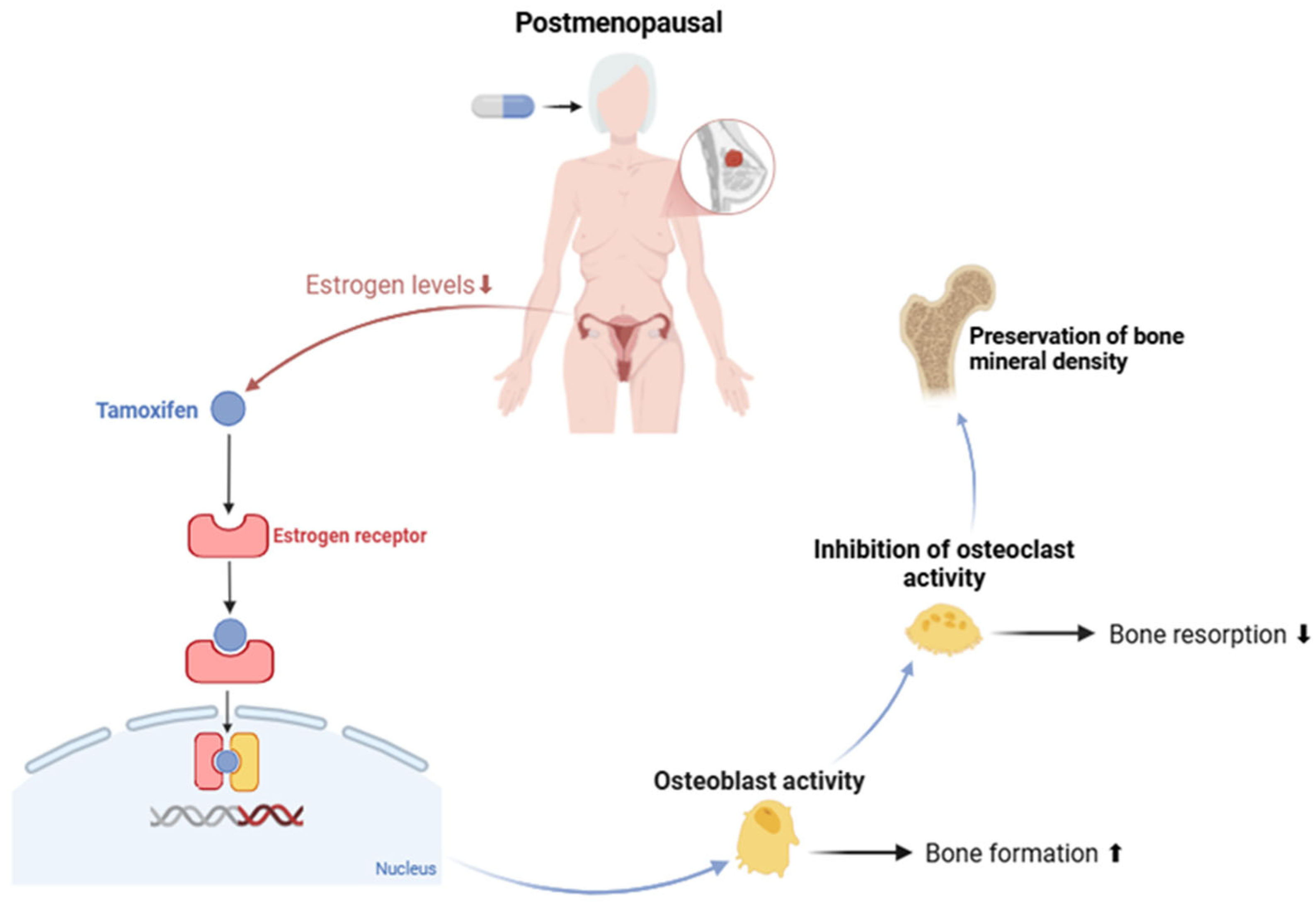
Tamoxifen
3. Therapeutic Approaches and Rehabilitation
3.1. Diagnosis and Risk Assessment
3.2. Therapeutic Approaches to Preventing Osteoporosis
3.3. Non-Pharmacological Therapy
4. Future Perspectives in Personalized Medicine
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| BMD | Bone Mineral Density |
| BC | Breast Cancer |
| WHO | World Health Organization |
| SERM | Selective Estrogen Receptor Modulator |
| FRAX | Fracture Risk Assessment Tool |
| DXA | Dual-Energy X-ray Absorptiometry |
References
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.; Alkabban, F.M.; Ferguson, T. Breast Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://pubmed.ncbi.nlm.nih.gov/29493913/ (accessed on 6 February 2025).
- Obeagu, E.I.; Obeagu, G.U. Breast Cancer: A Review of Risk Factors and Diagnosis. Medicine 2024, 103, E36905. [Google Scholar] [CrossRef] [PubMed]
- Star, J.; Bandi, P.; Siegel, R.L.; Han, X.; Minihan, A.; Smith, R.A.; Jemal, A. Cancer Screening in the United States During the Second Year of the COVID-19 Pandemic. J. Clin. Oncol. 2023, 41, 4352–4359. [Google Scholar] [CrossRef]
- Wilkinson, L.; Gathani, T. Understanding Breast Cancer as a Global Health Concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Cancer Today. Available online: https://gco.iarc.fr/today/en/dataviz/bars?mode=cancer&key=total&sort_by=value0&populations=900&types=0&values_position=in (accessed on 4 February 2025).
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Song, S.; Guo, Y.; Yang, Y.; Fu, D. Advances in Pathogenesis and Therapeutic Strategies for Osteoporosis. Pharmacol. Ther. 2022, 237, 108168. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I. Management of Bone Loss Due to Endocrine Therapy During Cancer Treatment. Osteoporos. Int. 2023, 34, 671–680. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Parimalakrishnan, S.; Anand, D.C.P.; Kaviya, E.; Vijayakumar, A.R. Osteoporosis: Exploring Causes, Pathophysiology and Advances in Treatment: A Comprehensive Review. J. Young Pharm. 2024, 16, 613–619. [Google Scholar] [CrossRef]
- Poloni, P.F.; De Luca Vespoli, H.; de Sousa Almeida-Filho, B.; Bueloni-Dias, F.; Nahas-Neto, J.; Nahas, E.A.P. Low Bone Mineral Density Is Associated with Breast Cancer in Postmenopausal Women: A Case-Control Study. Climacteric 2017, 20, 491–497. [Google Scholar] [CrossRef]
- Sunderland, M.C.; Kent Osborne, C. Tamoxifen in Premenopausal Patients with Metastatic Breast Cancer: A Review. J. Clin. Oncol. 1991, 9, 1283–1297. [Google Scholar] [CrossRef]
- Breast Cancer Risk Factors|Breast Cancer|CDC. Available online: https://www.cdc.gov/breast-cancer/risk-factors/index.html (accessed on 4 February 2025).
- Seiler, A.; Chen, M.A.; Brown, R.L.; Fagundes, C.P. Obesity, Dietary Factors, Nutrition, and Breast Cancer Risk. Curr. Breast Cancer Rep. 2018, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Tański, W.; Kosiorowska, J.; Szymaḿska-Chabowska, A. Osteoporosis—Risk Factors, Pharmaceutical and Non-Pharmaceutical Treatment. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global Breast Cancer Incidence and Mortality Trends by Region, Age-Groups, and Fertility Patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef]
- Salari, N.; Darvishi, N.; Bartina, Y.; Larti, M.; Kiaei, A.; Hemmati, M.; Shohaimi, S.; Mohammadi, M. Global Prevalence of Osteoporosis among the World Older Adults: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Miner. Res. 2014, 29, 2520. [Google Scholar] [CrossRef]
- Jorgetti, V.; Dos Reis, L.M.; Ott, S.M. Ethnic Differences in Bone and Mineral Metabolism in Healthy People and Patients with CKD. Kidney Int. 2014, 85, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Risk Factors: Hormones—NCI. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones (accessed on 25 February 2025).
- Satpathi, S.; Gaurkar, S.S.; Potdukhe, A.; Wanjari, M.B. Unveiling the Role of Hormonal Imbalance in Breast Cancer Development: A Comprehensive Review. Cureus 2023, 15, e41737. [Google Scholar] [CrossRef]
- Sioka, C.; Fotopoulos, A.; Georgiou, A.; Xourgia, X.; Papadopoulos, A.; Kalef-Ezra, J.A. Age at Menarche, Age at Menopause and Duration of Fertility as Risk Factors for Osteoporosis. Climacteric 2010, 13, 63–71. [Google Scholar] [CrossRef]
- Qiu, C.; Chen, H.; Wen, J.; Zhu, P.; Lin, F.; Huang, B.; Wu, P.; Lin, Q.; Lin, Y.; Rao, H.; et al. Associations between Age at Menarche and Menopause with Cardiovascular Disease, Diabetes, and Osteoporosis in Chinese Women. J. Clin. Endocrinol. Metab. 2013, 98, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Fistarol, M.; Rezende, C.R.; Figueiredo Campos, A.L.; Kakehasi, A.M.; Geber, S. Time Since Menopause, but Not Age, Is Associated with Increased Risk of Osteoporosis. Climacteric 2019, 22, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Risk Factors You Can’t Change|American Cancer Society. Available online: https://www.cancer.org/cancer/types/breast-cancer/risk-and-prevention/breast-cancer-risk-factors-you-cannot-change.html (accessed on 6 February 2025).
- Sultan, N.; Memon, S.A.; Mooghal, M.; Wali, S.; Khan, W.; Tahseen, H.; Khan, M.; Monis, D. Ethnic Predisposition, Risk Factors and Breast Cancer Presentation; a 10-Year Data. Single Centered Prospective Cohort Study from Karachi. Ann. Med. Surg. 2022, 82, 104612. [Google Scholar] [CrossRef]
- Chung, S.Y.; Oh, J.; Chang, J.S.; Shin, J.; Kim, K.H.; Chun, K.H.; Keum, K.C.; Suh, C.O.; Kang, S.M.; Kim, Y.B. Risk of Cardiac Disease in Patients With Breast Cancer: Impact of Patient-Specific Factors and Individual Heart Dose From Three-Dimensional Radiation Therapy Planning. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 473–481. [Google Scholar] [CrossRef]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A Comprehensive Overview on Osteoporosis and Its Risk Factors. Ther. Clin. Risk Manag. 2018, 14, 2029. [Google Scholar] [CrossRef]
- Román, M.; Louro, J.; Posso, M.; Alcántara, R.; Peñalva, L.; Sala, M.; del Riego, J.; Prieto, M.; Vidal, C.; Sánchez, M.; et al. Breast Density, Benign Breast Disease, and Risk of Breast Cancer over Time. Eur. Radiol. 2021, 31, 4839–4847. [Google Scholar] [CrossRef]
- Lynge, E.; Vejborg, I.; Lillholm, M.; Nielsen, M.; Napolitano, G.; von Euler-Chelpin, M. Breast Density and Risk of Breast Cancer. Int. J. Cancer 2022, 152, 1150. [Google Scholar] [CrossRef] [PubMed]
- Radiation for Breast Cancer|Breast Cancer Treatment|American Cancer Society. Available online: https://www.cancer.org/cancer/types/breast-cancer/treatment/radiation-for-breast-cancer.html. (accessed on 11 February 2025).
- Carlson, L.E.; Watt, G.P.; Tonorezos, E.S.; Chow, E.J.; Yu, A.F.; Woods, M.; Lynch, C.F.; John, E.M.; Mellemkjær, L.; Brooks, J.D.; et al. Coronary Artery Disease in Young Women After Radiation Therapy for Breast Cancer: The WECARE Study. JACC CardioOncol. 2021, 3, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Berk, L. The Effects of High-Dose Radiation Therapy on Bone: A Scoping Review. Radiat. Oncol. J. 2024, 42, 95. [Google Scholar] [CrossRef]
- Costa, S.; Reagan, M.R. Therapeutic Irradiation: Consequences for Bone and Bone Marrow Adipose Tissue. Front. Endocrinol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Freudenheim, J.L. Alcohol’s Effects on Breast Cancer in Women. Alcohol. Res. 2020, 40, 11. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Alcohol and Cancer Risk; Office of the Assistant Secretary for Health: Washington, DC, USA, 2023. Available online: https://www.hhs.gov/sites/default/files/oash-alcohol-cancer-risk.pdf (accessed on 24 February 2025).
- Starek-Świechowicz, B.; Budziszewska, B.; Starek, A. Alcohol and Breast Cancer. Pharmacol. Rep. 2022, 75, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Giampieri, F.; Chisari, E.; Micek, A.; Paladino, N.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; La Vignera, S.; Musumeci, G.; et al. Alcohol Consumption, Bone Mineral Density, and Risk of Osteoporotic Fractures: A Dose–Response Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1515. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.W. Alcohol and Other Factors Affecting Osteoporosis Risk in Women. Alcohol. Res. Health 2002, 26, 292. [Google Scholar]
- Dehesh, T.; Fadaghi, S.; Seyedi, M.; Abolhadi, E.; Ilaghi, M.; Shams, P.; Ajam, F.; Mosleh-Shirazi, M.A.; Dehesh, P. The Relation between Obesity and Breast Cancer Risk in Women by Considering Menstruation Status and Geographical Variations: A Systematic Review and Meta-Analysis. BMC Womens Health 2023, 23, 392. [Google Scholar] [CrossRef]
- Sellami, M.; Bragazzi, N.L. Nutrigenomics and Breast Cancer: State-of-Art, Future Perspectives and Insights for Prevention. Nutrients 2020, 12, 512. [Google Scholar] [CrossRef]
- Voulgaridou, G.; Papadopoulou, S.K.; Detopoulou, P.; Tsoumana, D.; Giaginis, C.; Kondyli, F.S.; Lymperaki, E.; Pritsa, A. Vitamin D and Calcium in Osteoporosis, and the Role of Bone Turnover Markers: A Narrative Review of Recent Data from RCTs. Diseases 2023, 11, 29. [Google Scholar] [CrossRef]
- Siddique, N.; O’Donoghue, M.; Casey, M.C.; Walsh, J.B. Malnutrition in the Elderly and Its Effects on Bone Health—A Review. Clin. Nutr. ESPEN 2017, 21, 31–39. [Google Scholar] [CrossRef]
- Chen, R.; Armamento-Villareal, R. Obesity and Skeletal Fragility. J. Clin. Endocrinol. Metab. 2023, 109, e466. [Google Scholar] [CrossRef]
- Scanlon, E.F.; Suh, O.; Murthy, S.M.; Mettlin, C.; Reid, S.E.; Cummings, K.M. Influence of smoking on the development of lung metastases from breast cancer. Cancer 1995, 11, 2693–2699. [Google Scholar] [CrossRef]
- Gaudet, M.M.; Carter, B.D.; Brinton, L.A.; Falk, R.T.; Gram, I.T.; Luo, J.; Milne, R.L.; Nyante, S.J.; Weiderpass, E.; Freeman, L.E.B.; et al. Pooled analysis of active cigarette smoking and invasive breast cancer risk in 14 cohort studies. Int. J. Epidemiol. 2016, 46, 881. [Google Scholar] [CrossRef]
- Passarelli, M.N.; Newcomb, P.A.; Hampton, J.M.; Trentham-Dietz, A.; Titus, L.J.; Egan, K.M.; Baron, J.A.; Willett, W.C. Cigarette Smoking Before and After Breast Cancer Diagnosis: Mortality From Breast Cancer and Smoking-Related Diseases. J. Clin. Oncol. 2016, 34, 1315. [Google Scholar] [CrossRef]
- Kiyota, Y.; Muramatsu, H.; Sato, Y.; Kobayashi, T.; Miyamoto, K.; Iwamoto, T.; Matsumoto, M.; Nakamura, M.; Tateno, H.; Sato, K.; et al. Smoking Cessation Increases Levels of Osteocalcin and Uncarboxylated Osteocalcin in Human Sera. Sci. Rep. 2020, 10, 16845. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Kypriotakis, G.; Karam-Hage, M.; Kim, S.; Jizzini, M.; Seoudy, K.S.; Robinson, J.D.; Barcenas, C.H.; Cinciripini, P.M.; Tripathy, D.; et al. The Impact of Treatment for Smoking on Breast Cancer Patients’ Survival. Cancers 2022, 14, 1464. [Google Scholar] [CrossRef] [PubMed]
- Li, C.I.; Uribe, D.J.; Daling, J.R. Clinical Characteristics of Different Histologic Types of Breast Cancer. Br. J. Cancer 2005, 93, 1046–1052. [Google Scholar] [CrossRef]
- Galappaththi, S.P.L.; Smith, K.R.; Alsatari, E.S.; Hunter, R.; Dyess, D.L.; Turbat-Herrera, E.A.; Dasgupta, S. The Genomic and Biologic Landscapes of Breast Cancer and Racial Differences. Int. J. Mol. Sci. 2024, 25, 13165. [Google Scholar] [CrossRef]
- Darida, M.; Rubovszky, G.; Kiss, Z.; Székely, B.; Madaras, B.; Horváth, Z.; Kocsis, J.; Sipőcz, I.; Várnai, M.; Balogh, É.; et al. Improvement in Breast Cancer Survival across Molecular Subtypes in Hungary between 2011 and 2020: A Nationwide, Retrospective Study. Front. Oncol. 2025, 15, 1465511. [Google Scholar] [CrossRef]
- Janni, W.; Untch, M.; Harbeck, N.; Gligorov, J.; Jacot, W.; Chia, S.; Boileau, J.F.; Gupta, S.; Mishra, N.; Akdere, M.; et al. Systematic Literature Review and Trial-Level Meta-Analysis of Aromatase Inhibitors vs Tamoxifen in Patients with HR+/HER2− Early Breast Cancer. Breast Off. J. Eur. Soc. Mastology 2025, 81, 104429. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, L.; Niu, N.; Hou, P.; Chen, G.; Wang, H.; Zhang, Z.; Jiang, X.; Xu, Q.; Zhao, Y.; et al. Molecular Classification of Hormone Receptor-Positive /HER2-Positive Breast Cancer Reveals Potential Neoadjuvant Therapeutic Strategies. Signal Transduct. Target. Ther. 2025, 10, 97. [Google Scholar] [CrossRef]
- Kay, C.; Martinez-Perez, C.; Meehan, J.; Gray, M.; Webber, V.; Dixon, J.M.; Turnbull, A.K. Current Trends in the Treatment of HR+/HER2+ Breast Cancer. Future Oncol. 2021, 17, 1665–1681. [Google Scholar] [CrossRef]
- Zagami, P.; Carey, L.A. Triple Negative Breast Cancer: Pitfalls and Progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Fessele, K.L. Bone Health Considerations in Breast Cancer. Semin. Oncol. Nurs. 2022, 38, 151273. [Google Scholar] [CrossRef] [PubMed]
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in Adjuvant Chemotherapy for Breast Cancer: An Overview. BMC Med. 2015, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Thareja, S. Aromatase Inhibitors for the Treatment of Breast Cancer: An Overview (2019–2023). Bioorg. Chem. 2024, 151, 107607. [Google Scholar] [CrossRef]
- PDQ Adult Treatment Editorial Board. Breast Cancer Treatment (PDQ®): Patient Version; National Cancer Institute: Bethesda, MD, USA, 2002. [Google Scholar]
- Mohamed, A.; Krajewski, K.; Cakar, B.; Ma, C.X. Targeted Therapy for Breast Cancer. Am. J. Pathol. 2013, 183, 1096–1112. [Google Scholar] [CrossRef]
- Sarkar, S.; Schechter, C.; Kurian, A.W.; Caswell-Jin, J.L.; Jayasekera, J.; Mandelblatt, J.S. Impact of Endocrine Therapy Regimens for Early-Stage ER+/HER2-Breast Cancer on Contralateral Breast Cancer Risk. NPJ Breast Cancer 2025, 11, 30. [Google Scholar] [CrossRef]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar]
- Mezger, J. Effects of Tamoxifen on Bone Mineral Density in Postmenopausal Women with Breast Cancer: Comment. Aktuel Endokrinol. Stoffwechs. 1992, 13, 189. [Google Scholar] [CrossRef]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J. Clin. Oncol. 2016, 34, 1689–1701. [Google Scholar] [CrossRef]
- Bichoo, R.A.; Mishra, A.; Lal, P.; Gyan, C.; Agarwal, G.; Agarwal, A.; Mishra, S.K. Quality of Life (QoL) in Postmenopausal Breast Cancer Patients Receiving Adjuvant Hormonal Therapy. Indian J. Surg. 2021, 83, 461–467. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, B.H.; Min, S.Y.; Chae, S. Effects of Anticancer Therapy on Osteoporosis in Breast Cancer Patients: A Nationwide Study Using Data from the National Health Insurance Service-National Health Information Database. J. Clin. Med. 2025, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Nisha, Y.; Dubashi, B.; Bobby, Z.; Sahoo, J.P.; Kayal, S. Effect of Cytotoxic Chemotherapy on Bone Health among Breast Cancer Patients. Does It Require Intervention? Support. Care Cancer 2021, 29, 6957–6972. [Google Scholar] [CrossRef] [PubMed]
- Body, J.J.; Bergmann, P.; Boonen, S.; Boutsen, Y.; Devogelaer, J.P.; Goemaere, S.; Reginster, J.Y.; Rozenberg, S.; Kaufman, J.M. Management of Cancer Treatment-Induced Bone Loss in Early Breast and Prostate Cancer—A Consensus Paper of the Belgian Bone Club. Osteoporos. Int. 2007, 18, 1439–1450. [Google Scholar] [CrossRef][Green Version]
- Taxel, P.; Choksi, P.; Van Poznak, C. The Management of Osteoporosis in Breast Cancer Survivors. Maturitas 2012, 73, 275–279. [Google Scholar] [CrossRef][Green Version]
- Rees, M.; Angioli, R.; Coleman, R.L.; Glasspool, R.; Plotti, F.; Simoncini, T.; Terranova, C. European Menopause and Andropause Society (EMAS) and International Gynecologic Cancer Society (IGCS) Position Statement on Managing the Menopause after Gynecological Cancer: Focus on Menopausal Symptoms and Osteoporosis. Maturitas 2020, 134, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.C.; Jacobs, T.F. Tamoxifen; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kanji, C.R.; Nyabadza, G.; Nhachi, C.; Masimirembwa, C. Pharmacokinetics of Tamoxifen and Its Major Metabolites and the Effect of the African Ancestry Specific CYP2D6*17 Variant on the Formation of the Active Metabolite, Endoxifen. J. Pers. Med. 2023, 13, 272. [Google Scholar] [CrossRef]
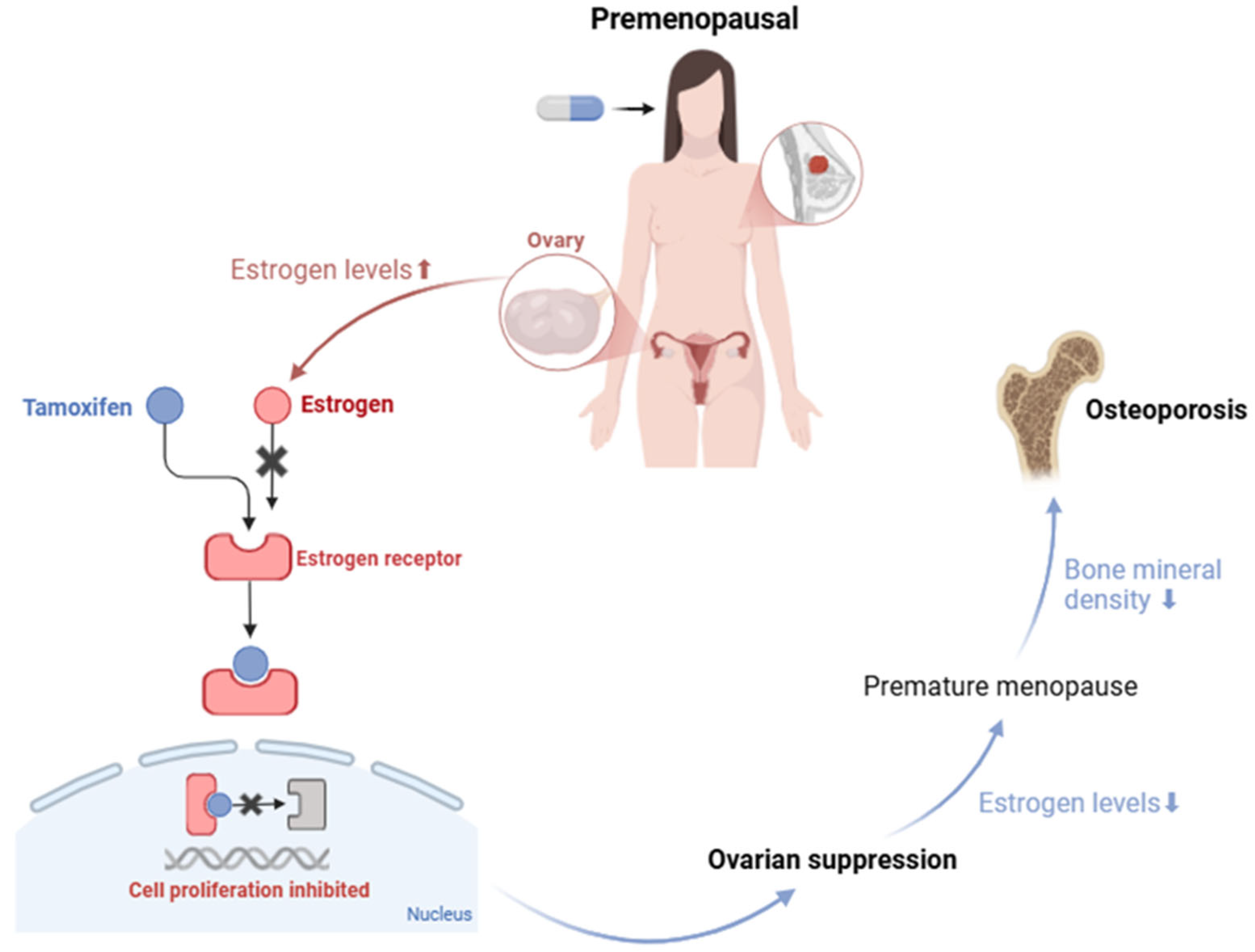
- Lee, S.J.; Cha, C.D.; Hong, H.; Choi, Y.Y.; Chung, M.S. Adverse Effects of Tamoxifen Treatment on Bone Mineral Density in Premenopausal Patients with Breast Cancer: A Systematic Review and Meta-Analysis. Breast Cancer 2024, 31, 717–725. [Google Scholar] [CrossRef]
- Abe, O.; Abe, R.; Enomoto, K.; Kikuchi, K.; Koyama, H.; Masuda, H.; Nomura, Y.; Ohashi, Y.; Sakai, K.; Sugimachi, K.; et al. Relevance of Breast Cancer Hormone Receptors and Other Factors to the Efficacy of Adjuvant Tamoxifen: Patient-Level Meta-Analysis of Randomised Trials. Lancet 2011, 378, 771. [Google Scholar] [CrossRef]
- Kadakia, K.C.; Lynn Henry, N. Adjuvant Endocrine Therapy in Premenopausal Women with Breast Cancer. Clin. Adv. Hematol. Oncol. 2015, 13, 663. [Google Scholar]
- Bryce, C.J.; Arriagada, R.; Saibara, T.; Ogawa, Y.; Onishi, S.; Benson, J.R. Tamoxifen in Early Breast Cancer (Multiple Letters) (3). Lancet 1998, 352, 403–405. [Google Scholar] [CrossRef]
- Farrar, M.C.; Jacobs, T.F. Tamoxifen. Encycl. Toxicol. (Fourth Ed.) 2023, 8, V8-923–V8-927. [Google Scholar] [CrossRef]
- Van Poznak, C.; Sauter, N.P. Clinical Management of Osteoporosis in Women with a History of Breast Carcinoma. Cancer 2005, 104, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.J.; Hickish, T.; Kanis, J.A.; Tidy, A.; Ashley, S. Effect of Tamoxifen on Bone Mineral Density Measured by Dual-Energy x-Ray Absorptiometry in Healthy Premenopausal and Postmenopausal Women. J. Clin. Oncol. 1996, 14, 78–84. [Google Scholar] [CrossRef]
- Kristensen, B.; Ejlertsen, B.; Dalgaard, P.; Larsen, L.; Holmegaard, S.N.; Transbøl, I.; Mouridsen, H.T. Tamoxifen and Bone Metabolism in Postmenopausal Low-Risk Breast Cancer Patients: A Randomized Study. J. Clin. Oncol. 1994, 12, 992–997. [Google Scholar] [CrossRef]
- Love, R.R.; Barden, H.S.; Mazess, R.B.; Epstein, S.; Chappell, R.J. Effect of Tamoxifen on Lumbar Spine Bone Mineral Density in Postmenopausal Women After 5 Years. Arch. Intern. Med. 1994, 154, 2585–2588. [Google Scholar] [CrossRef] [PubMed]
- Stokes, G.; Herath, M.; Samad, N.; Trinh, A.; Milat, F. Bone Health—Across a Woman’s Lifespan. Clin. Endocrinol. 2025, 102, 389–402. [Google Scholar] [CrossRef]
- Waqas, K.; Lima Ferreira, J.; Tsourdi, E.; Body, J.J.; Hadji, P.; Zillikens, M.C. Updated Guidance on the Management of Cancer Treatment-Induced Bone Loss (CTIBL) in Pre- and Postmenopausal Women with Early-Stage Breast Cancer. J. Bone Oncol. 2021, 28, 100355. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.A.; Douglas, S.; Brown, J.E.; Anderson, R.A. Bone Mineral Density Loss during Adjuvant Chemotherapy in Pre-Menopausal Women with Early Breast Cancer: Is It Dependent on Oestrogen Deficiency? Breast Cancer Res. Treat. 2010, 123, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L. Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments. Cancers 2020, 12, 3094. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Shapiro, C.L. Osteopenia and Osteoporosis in Women with Breast Cancer. Semin. Oncol. 2003, 30, 763–775. [Google Scholar] [CrossRef]
- Vehmanen, L.; Elomaa, I.; Blomqvist, C.; Saarto, T. Tamoxifen Treatment After Adjuvant Chemotherapy Has Opposite Effects on Bone Mineral Density in Premenopausal Patients Depending on Menstrual Status. J. Clin. Oncol. 2006, 24, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Kyvernitakis, I.; Kostev, K.; Hadji, P. The Tamoxifen Paradox—Influence of Adjuvant Tamoxifen on Fracture Risk in Pre- and Postmenopausal Women with Breast Cancer. Osteoporos. Int. 2018, 29, 2557–2564. [Google Scholar] [CrossRef]
- Kim, D.; Oh, J.; Lee, H.S.; Jeon, S.; Park, W.C.; Yoon, C.I. Association between Tamoxifen and Incidence of Osteoporosis in Korean Patients with Ductal Carcinoma in Situ. Front. Oncol. 2023, 13, 1236188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Alqudaihi, H.M.; Kang, M.S.; Kim, J.; Lee, J.W.; Ko, B.S.; Son, B.H.; Ahn, S.H.; Lee, J.E.; Han, S.W.; et al. Effect of Tamoxifen on the Risk of Osteoporosis and Osteoporotic Fracture in Younger Breast Cancer Survivors: A Nationwide Study. Front. Oncol. 2020, 10, 366. [Google Scholar] [CrossRef]
- Altundag, K. Not All Patients with Premenopausal Breast Cancer Will Experience the Negative Effects of Tamoxifen Treatment on Their Bone Mineral Density. Breast Cancer 2024, 31, 997. [Google Scholar] [CrossRef]
- Body, J.J. Prevention and Treatment of Side-Effects of Systemic Treatment: Bone Loss. Ann. Oncol. 2010, 21, vii180–vii185. [Google Scholar] [CrossRef]
- Paolucci, T.; Saraceni, V.M.; Piccinini, G. Management of Chronic Pain in Osteoporosis: Challenges and Solutions. J. Pain. Res. 2016, 9, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland; Flamen, P.; Kurth, A.; et al. Bone Health in Cancer: ESMO Clinical Practice Guidelines†. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Pepe, J.; Body, J.J.; Hadji, P.; McCloskey, E.; Meier, C.; Obermayer-Pietsch, B.; Palermo, A.; Tsourdi, E.; Zillikens, M.C.; Langdahl, B.; et al. Osteoporosis in Premenopausal Women: A Clinical Narrative Review by the ECTS and the IOF. J. Clin. Endocrinol. Metab. 2020, 105, 2487–2506. [Google Scholar] [CrossRef]
- Leib, E. Diagnosis of Osteoporosis in Men, Premenopausal Women, and Children. J. Clin. Densitom. 2004, 7, 17–26. [Google Scholar] [CrossRef]
- Cohen, A.; Shane, E. Evaluation and Management of the Premenopausal Woman with Low BMD. Curr. Osteoporos. Rep. 2013, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Gnant, M.; Body, J.J.; Bundred, N.J.; Brufsky, A.; Coleman, R.E.; Guise, T.A.; Lipton, A.; Aapro, M.S. Cancer Treatment-Induced Bone Loss in Premenopausal Women: A Need for Therapeutic Intervention? Cancer Treat. Rev. 2012, 38, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Siris, E.S.; Wehren, L.E.; Miller, P.D.; Abbott, T.A.; Berger, M.L.; Santora, A.C.; Sherwood, L.M. Osteoporosis and Fracture Risk in Women of Different Ethnic Groups. J. Bone Miner. Res. 2005, 20, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Lui, L.Y.; Stone, K.L.; Hillier, T.A.; Zmuda, J.M.; Hochberg, M.; Beck, T.J.; Ensrud, K.E. Longitudinal Study of Changes in Hip Bone Mineral Density in Caucasian and African-American Women. J. Am. Geriatr. Soc. 2005, 53, 183–189. [Google Scholar] [CrossRef]
- Lüftner, D.; Niepel, D.; Steger, G.G. Therapeutic Approaches for Protecting Bone Health in Patients with Breast Cancer. Breast 2018, 37, 28–35. [Google Scholar] [CrossRef]
- Cohen, A. PREMENOPAUSAL OSTEOPOROSIS. Endocrinol. Metab. Clin. N. Am. 2016, 46, 117. [Google Scholar] [CrossRef]
- Conradie, M.; de Villiers, T. Premenopausal Osteoporosis. Climacteric 2022, 25, 73–80. [Google Scholar] [CrossRef]
- Stevenson, J. Prevention and Treatment of Osteoporosis in Women. Post. Reprod. Health 2023, 29, 11–14. [Google Scholar] [CrossRef]
- Okada, Y.; Nawata, M.; Nakayamada, S.; Saito, K.; Tanaka, Y.; Okada, Y.; Nawata, M.; Nakayamada, S.; Saito, K.; Tanaka, Y. Alendronate Protects Premenopausal Women from Bone Loss and Fracture Associated with High-Dose Glucocorticoid Therapy. J. Rheumatol. 2008, 35, 2249–2254. [Google Scholar] [CrossRef]
- Shane, E.; Shiau, S.; Recker, R.R.; Lappe, J.M.; Agarwal, S.; Kamanda-Kosseh, M.; Bucovsky, M.; Stubby, J.; Cohen, A. Denosumab After Teriparatide in Premenopausal Women With Idiopathic Osteoporosis. J. Clin. Endocrinol. Metab. 2022, 107, E1528–E1540. [Google Scholar] [CrossRef]
- Delmas, P.D.; Balena, R.; Confravreux, E.; Hardouin, C.; Hardy, P.; Bremond, A. Bisphosphonate Risedronate Prevents Bone Loss in Women with Artificial Menopause Due to Chemotherapy of Breast Cancer: A Double-Blind, Placebo-Controlled Study. J. Clin. Oncol. 1997, 15, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Fuleihan, G.E.H.; Salamoun, M.; Mourad, Y.A.; Chehal, A.; Salem, Z.; Mahfoud, Z.; Shamseddine, A. Pamidronate in the Prevention of Chemotherapy-Induced Bone Loss in Premenopausal Women with Breast Cancer: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2005, 90, 3209–3214. [Google Scholar] [CrossRef]
- Levine, J.P. Pharmacologic and Nonpharmacologic Management of Osteoporosis. Clin. Cornerstone 2006, 8, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Kohrt, W.M. Exercise and Bone Mineral Density in Premenopausal Women: A Meta-Analysis of Randomized Controlled Trials. Int. J. Endocrinol. 2013, 2013, 741639. [Google Scholar] [CrossRef]
- Fornusek, C.P.; Kilbreath, S.L. Exercise for Improving Bone Health in Women Treated for Stages I–III Breast Cancer: A Systematic Review and Meta-Analyses. J. Cancer Surviv. 2017, 11, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Dhiman, V.K.; Pandey, M.; Dhiman, V.K.; Sharma, A.; Pandey, H.; Verma, S.K.; Pandey, R. Personalized Medicine: An Alternative for Cancer Treatment. Cancer Treat. Res. Commun. 2024, 42, 100860. [Google Scholar] [CrossRef]
- Andrade, P.; Santamarina, A.B.; de Freitas, J.A.; Marum, A.B.R.F.; Pessoa, A.F.M. Personalized Nutrition and Precision Medicine in Perimenopausal Women: A Minireview of Genetic Polymorphisms COMT, FUT2, and MTHFR. Clinics 2025, 80, 100549. [Google Scholar] [CrossRef]
- Samson Enitan, S.; Ngozi Adejumo, E.; Osaigbovoh Imaralu, J.; Ademola Adelakun, A.; Anike Ladipo, O.; Bosede Enitan, C. Personalized Medicine Approach to Osteoporosis Management in Women: Integrating Genetics, Pharmacogenomics, and Precision Treatments. Clin. Res. Commun. 2023, 6, 18. [Google Scholar] [CrossRef]
| Category | Z-Score | Meaning |
|---|---|---|
| Normal | z-score ≥ −2.0 | Bone density is within the expected range for age, sex, and ethnic group. |
| Low BMD | z-score < −2.0 | Bone density is lower than expected for age, sex, and ethnic group. |
| Pharmacological Class | Drug | References |
|---|---|---|
| Bisphosphonate (Antiresorptive agent) | Alendronate | [105,106,108,110,111] |
| Risedronate | ||
| Pamidronate | ||
| Anabolic agent (Parathyroid hormone analog) | Teriparatide | [106] |
| Monoclonal antibody against RANKL (Antiresorptive) | Denosumab | [109] |
| Hormonal therapy (Antiresorptive/Anabolic effect) | Estrogens | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, B.; Vale, N. Impact of Endocrine Therapy on Osteoporosis Risk in Women with Breast Cancer Across Different Hormonal Stages: A Review. Curr. Oncol. 2025, 32, 305. https://doi.org/10.3390/curroncol32060305
Gomes B, Vale N. Impact of Endocrine Therapy on Osteoporosis Risk in Women with Breast Cancer Across Different Hormonal Stages: A Review. Current Oncology. 2025; 32(6):305. https://doi.org/10.3390/curroncol32060305
Chicago/Turabian StyleGomes, Beatriz, and Nuno Vale. 2025. "Impact of Endocrine Therapy on Osteoporosis Risk in Women with Breast Cancer Across Different Hormonal Stages: A Review" Current Oncology 32, no. 6: 305. https://doi.org/10.3390/curroncol32060305
APA StyleGomes, B., & Vale, N. (2025). Impact of Endocrine Therapy on Osteoporosis Risk in Women with Breast Cancer Across Different Hormonal Stages: A Review. Current Oncology, 32(6), 305. https://doi.org/10.3390/curroncol32060305






