The Burden and Trends of Gynecological Cancers in Asia from 1980 to 2021, with Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Measures Employed as Indicators of Disease Burden
2.3. Statistical Analysis
3. Results
3.1. Disease Burden of Gynecological Cancers in Asia
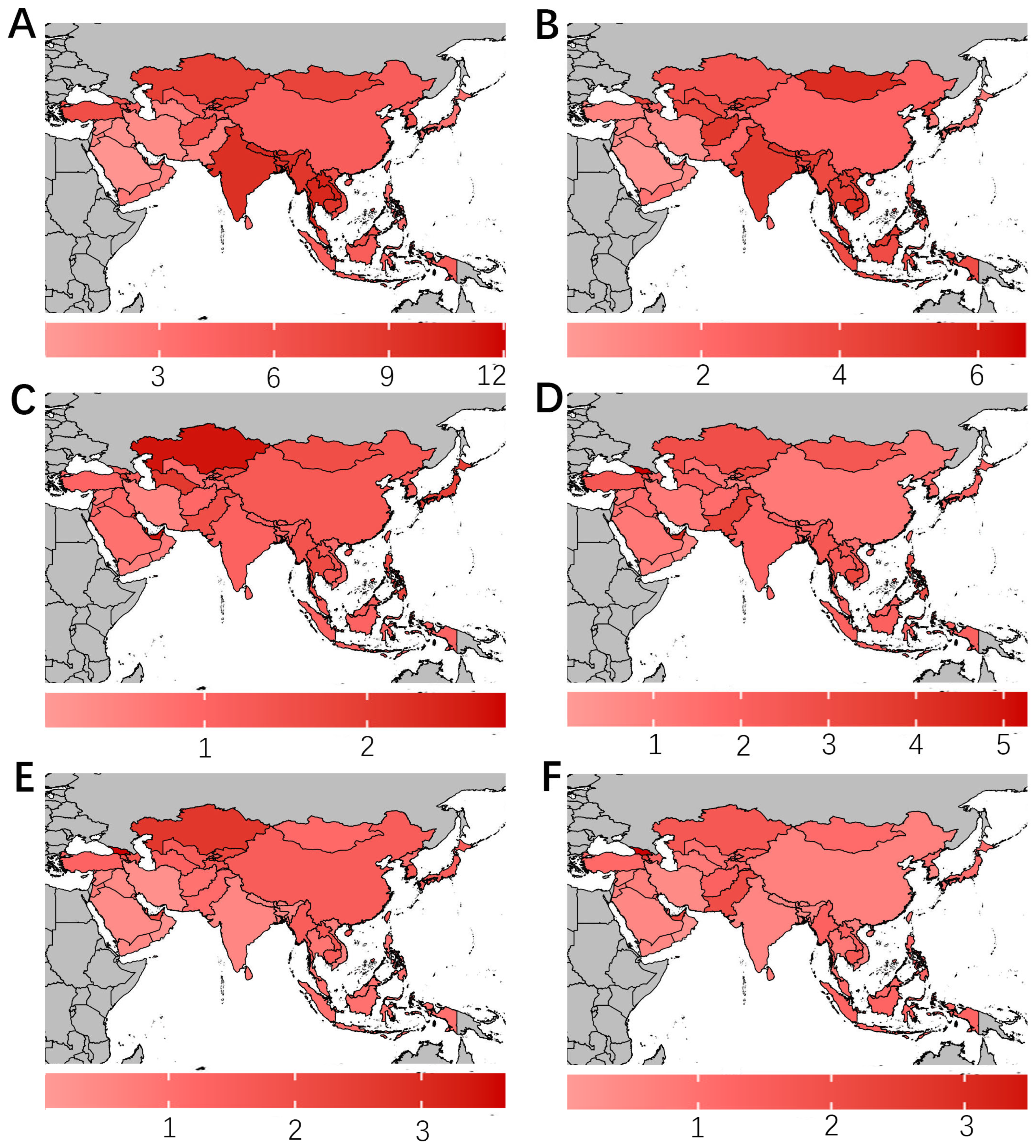
3.2. Geographical Distribution of Disease Burden
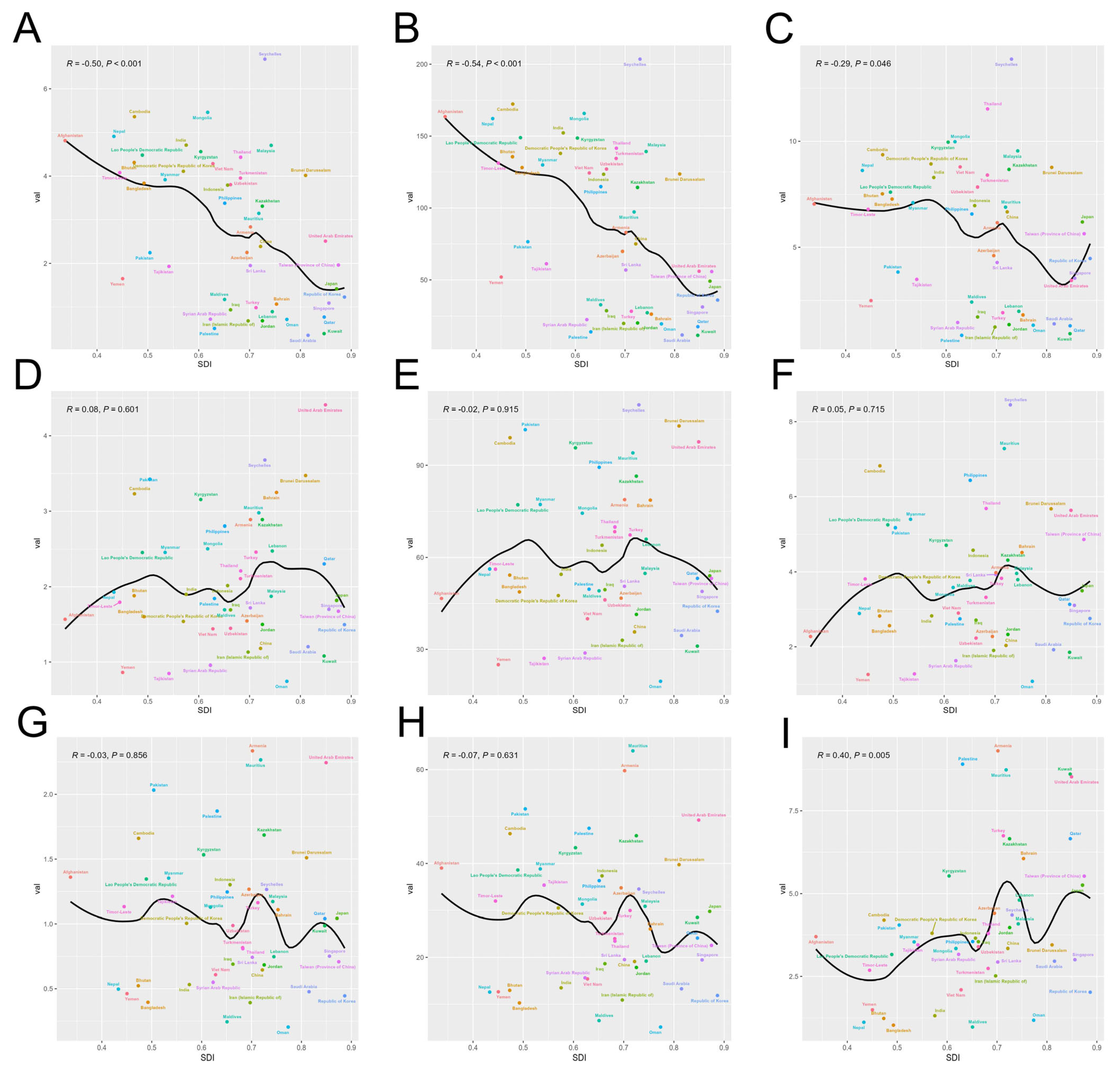
3.3. Correlation Analysis for SDI Levels and Disease Burden
3.4. Decomposition Analysis of the Factors Influencing Disease Burden
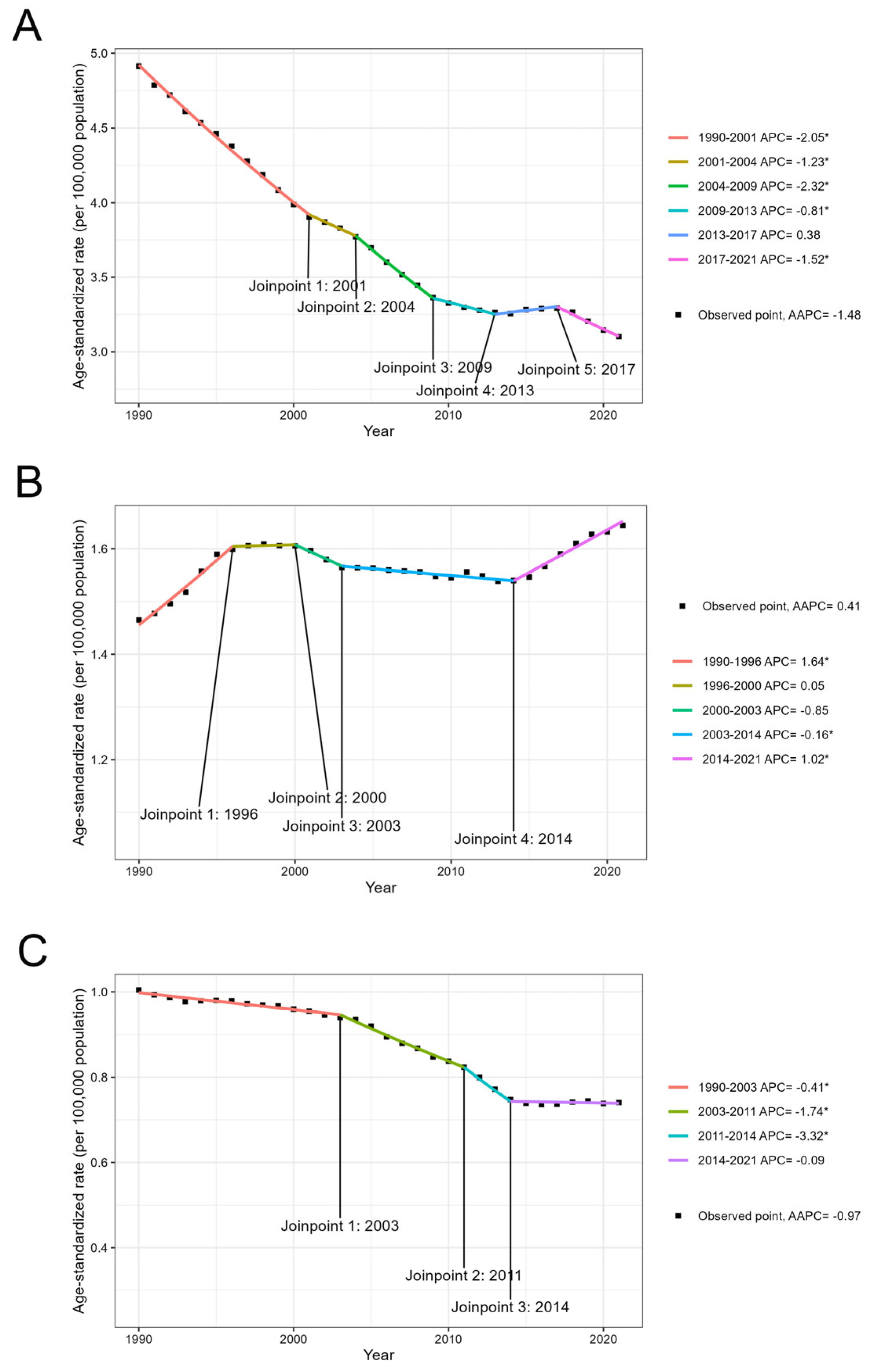
3.5. Temporal Trend of the Global Disease Burden from 1990 to 2021
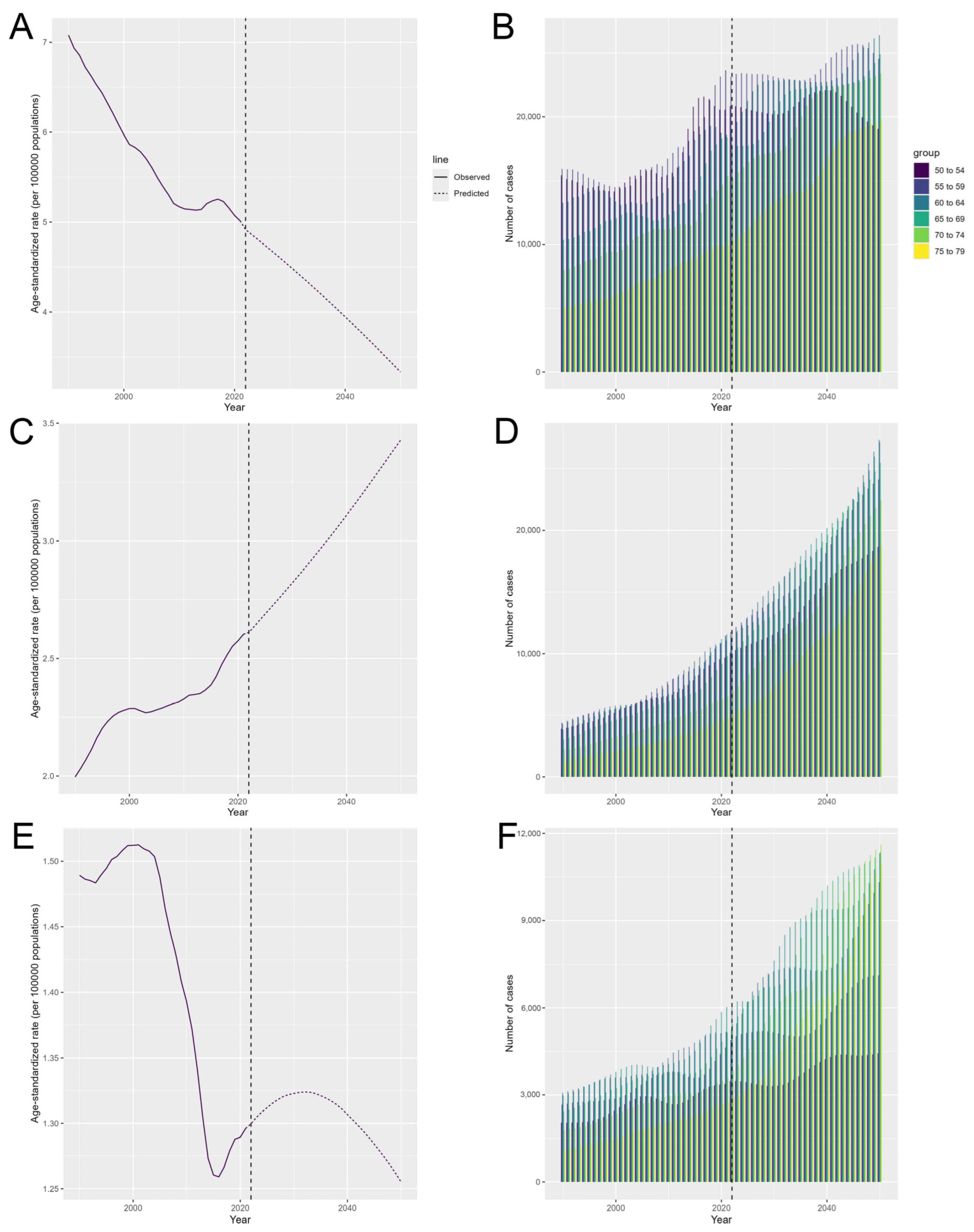
3.6. The Predicted Results from 2025 to 2050
4. Discussion
4.1. Cervical Cancer
4.2. Ovarian Cancer
4.3. Uterine Cancer
4.4. Future Trends
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DALY | Disability-adjusted life years |
| YLL | Years of life lost |
| YLD | Years lived with disability |
| ASMR | Age-standardized mortality rate |
| ASDR | Age-standardized DALY rate |
| ASIR | Age-standardized incidence rate |
| ASR | Age-standardized rate |
| EAPC | Estimated annual percent change |
| AAPC | Average annual percent change |
| SDI | Sociodemographic index |
| BAPC | Bayesian age-period-cohort model |
| GBD | Global Burden of Disease |
| APC-Web | Age-period-cohort web tool |
| CI | Confidence interval |
| UI | Uncertainty interval |
References
- Sankaranarayanan, R.; Ferlay, J. Worldwide burden of gynaecological cancer: The size of the problem. Best Pract. Res. Clin. Obs. Gynaecol. 2006, 20, 207–225. [Google Scholar] [CrossRef] [PubMed]
- The Regional Committee for the Western Pacific. Cervical Cancer. 2022. Available online: https://apps.who.int/iris/bitstream/handle/10665/365111/WPR-RC073-Res04-2022-en.pdf?sequence=1&isAllowed=y (accessed on 22 February 2025).
- Zhang, S.; Cheng, C.; Lin, Z.; Xiao, L.; Su, X.; Zheng, L.; Mu, Y.; Liao, M.; Ouyang, R.; Li, W.; et al. The global burden and associated factors of ovarian cancer in 1990–2019: Findings from the Global Burden of Disease Study 2019. BMC Public Health 2022, 22, 1455. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lin, R.; Li, H.; Wang, J.; He, H. Global and regional trends in the incidence and mortality burden of endometrial cancer, 1990–2019: Updated results from the Global Burden of Disease Study, 2019. Chin. Med. J. 2024, 137, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.; Park, S.N.; Ngan, H.Y.; Frazer, I.; Tay, E.H.; Chen, C.J.; Bhatla, N.; Pitts, M.; Shin, H.R.; Konno, R.; et al. The need for public education on HPV and cervical cancer prevention in Asia. Opinions of experts at the AOGIN conference. Vaccine 2008, 26, 5435–5440. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Ren, X.; Zhou, L.; Wang, N.; Kang, H. Disease Burden and Attributable Risk Factors of Ovarian Cancer From 1990 to 2017: Findings From the Global Burden of Disease Study 2017. Front. Public Health 2021, 9, 619581. [Google Scholar] [CrossRef]
- Yamagami, W.; Nagase, S.; Takahashi, F.; Ino, K.; Hachisuga, T.; Aoki, D.; Katabuchi, H. Clinical statistics of gynecologic cancers in Japan. J. Gynecol. Oncol. 2017, 28, e32. [Google Scholar] [CrossRef]
- Perkins, R.B.; Wentzensen, N.; Guido, R.S.; Schiffman, M. Cervical Cancer Screening: A Review. JAMA 2023, 330, 547–558. [Google Scholar] [CrossRef]
- Bray, F.; Lortet-Tieulent, J.; Znaor, A.; Brotons, M.; Poljak, M.; Arbyn, M. Patterns and trends in human papillomavirus-related diseases in Central and Eastern Europe and Central Asia. Vaccine 2013, 31 (Suppl. 7), H32–H45. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, T.T.; Liu, F.H.; Jiang, Y.T.; Sun, H.; Ma, X.X.; Zhao, Y.H.; Wu, Q.J. Global, Regional, and National Burden of Endometrial Cancer, 1990–2017: Results From the Global Burden of Disease Study, 2017. Front. Oncol. 2019, 9, 1440. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Huang, D.; Deng, K.; Wang, Q.; Li, C.; Zhu, J. Analysis of the disease burden trend of malignant tumors of the female reproductive system in China from 2006 to 2020. BMC Womens Health 2022, 22, 504. [Google Scholar] [CrossRef]
- Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef] [PubMed]
- Gupta, P.D. Standardization and Decomposition of Rates: A User’s Manual; US Department of Commerce, Economics and Statistics Administration, Bureau: Washington, DC, USA, 1993. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Goodman, A. HPV testing as a screen for cervical cancer. Bmj 2015, 350, h2372. [Google Scholar] [CrossRef]
- Vaccarella, S.; Bruni, L.; Seoud, M. Burden of human papillomavirus infections and related diseases in the extended Middle East and North Africa region. Vaccine 2013, 31 (Suppl. 6), G32–G44. [Google Scholar] [CrossRef]
- Zhao, X.L.; Hu, S.Y.; Hu, J.W.; Wang, H.H.; Wen, T.M.; Feng, Y.S.; Qiao, Y.L.; Zhao, F.H.; Zhang, Y. Tackling barriers to scale up human papillomavirus vaccination in China: Progress and the way forward. Infect. Dis. Poverty 2023, 12, 86. [Google Scholar] [CrossRef]
- Du, M.; Yan, W.; Jing, W.; Qin, C.; Liu, Q.; Liu, M.; Liu, J. Increasing incidence rates of sexually transmitted infections from 2010 to 2019: An analysis of temporal trends by geographical regions and age groups from the 2019 Global Burden of Disease Study. BMC Infect. Dis. 2022, 22, 574. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Pellecchia, G.; Arcieri, M.; Bogani, G.; Taliento, C.; Greco, P.; Driul, L.; Chiantera, V.; Ercoli, A.; Fanfani, F.; et al. Management for Cervical Cancer Patients: A Comparison of the Guidelines from the International Scientific Societies (ESGO-NCCN-ASCO-AIOM-FIGO-BGCS-SEOM-ESMO-JSGO). Cancers 2024, 16, 2541. [Google Scholar] [CrossRef]
- Katanoda, K.; Matsuda, T.; Matsuda, A.; Shibata, A.; Nishino, Y.; Fujita, M.; Soda, M.; Ioka, A.; Sobue, T.; Nishimoto, H. An updated report of the trends in cancer incidence and mortality in Japan. Jpn. J. Clin. Oncol. 2013, 43, 492–507. [Google Scholar] [CrossRef]
- World Health Organization. New WHO Recommendations on Screening and Treatment to Prevent Cervical Cancer Among Women Living with HIV: Policy Brief; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Elit, L.; Haruyama, R.; Gatti, A.; Howard, S.C.; Lam, C.G.; Fidarova, E.; Angioli, R.; Cao, X.; Trapani, D.; Ilbawi, A. Examining policy cohesion for cervical cancer worldwide: Analysis of WHO country reports. ESMO Open 2020, 5, e000878. [Google Scholar] [CrossRef]
- Tabuchi, T.; Hoshino, T.; Nakayama, T.; Ito, Y.; Ioka, A.; Miyashiro, I.; Tsukuma, H. Does removal of out-of-pocket costs for cervical and breast cancer screening work? A quasi-experimental study to evaluate the impact on attendance, attendance inequality and average cost per uptake of a Japanese government intervention. Int. J. Cancer 2013, 133, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Song, S.; Cho, H.N.; Park, B.; Jun, J.K.; Choi, E.; Kim, Y.; Choi, K.S. Trends in Participation Rates for the National Cancer Screening Program in Korea, 2002–2012. Cancer Res. Treat. 2017, 49, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.U.; Muhammad, N.; Naeemi, H.; Shehzad, U.; Hamann, U. Chasing the origin of 23 recurrent BRCA1 mutations in Pakistani breast and ovarian cancer patients. Int. J. Cancer 2022, 151, 402–411. [Google Scholar] [CrossRef]
- Bhaskaran, S.P.; Huang, T.; Rajendran, B.K.; Guo, M.; Luo, J.; Qin, Z.; Zhao, B.; Chian, J.; Li, S.; Wang, S.M. Ethnic-specific BRCA1/2 variation within Asia population: Evidence from over 78 000 cancer and 40 000 non-cancer cases of Indian, Chinese, Korean and Japanese populations. J. Med. Genet. 2021, 58, 752–759. [Google Scholar] [CrossRef]
- Ford, D.; Easton, D.F.; Peto, J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am. J. Hum. Genet. 1995, 57, 1457–1462. [Google Scholar]
- Liede, A.; Malik, I.A.; Aziz, Z.; Rios Pd Pde, L.; Kwan, E.; Narod, S.A. Contribution of BRCA1 and BRCA2 mutations to breast and ovarian cancer in Pakistan. Am. J. Hum. Genet. 2002, 71, 595–606. [Google Scholar] [CrossRef]
- Najafi-Sharjabad, F.; Zainiyah Syed Yahya, S.; Abdul Rahman, H.; Hanafiah Juni, M.; Abdul Manaf, R. Barriers of modern contraceptive practices among Asian women: A mini literature review. Glob. J. Health Sci. 2013, 5, 181–192. [Google Scholar] [CrossRef]
- Tabassum, S.; Masood, A.I.; Khakwani, M. Pattern of female gynecological malignancies in south Punjab Region of Pakistan: An overview of 5 years. Prof. Med. J. 2021, 28, 90–95. [Google Scholar] [CrossRef]
- Akhmedullin, R.; Aimyshev, T.; Zhakhina, G.; Yerdessov, S.; Beyembetova, A.; Ablayeva, A.; Biniyazova, A.; Seyil, T.; Abdukhakimova, D.; Segizbayeva, A.; et al. In-depth analysis and trends of cancer mortality in Kazakhstan: A joinpoint analysis of nationwide healthcare data 2014–2022. BMC Cancer 2024, 24, 1340. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, Z.; Wang, H.; Peng, J.; Hong, L. Burden of ovarian cancer in China from 1990 to 2030: A systematic analysis and comparison with the global level. Front. Public Health 2023, 11, 1136596. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Li, L.; Murugasen, S.; Hong, L.-S.; Yang, L.; Guo, Y.; Bian, Z.; Collins, R.; Chen, J.; He, H.; et al. Temporal trends of main reproductive characteristics in ten urban and rural regions of China: The China Kadoorie Biobank study of 300 000 women. Int. J. Epidemiol. 2014, 43, 1252–1262. [Google Scholar] [CrossRef]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef]
- DiSilvestro, J.B.; Haddad, J.; Robison, K.; Beffa, L.; Laprise, J.; Scalia-Wilbur, J.; Raker, C.; Clark, M.A.; Lokich, E.; Hofstatter, E.; et al. Ovarian Cancer Risk-Reduction and Screening in BRCA1/2 Mutation Carriers. J. Womens Health 2024, 33, 624–628. [Google Scholar] [CrossRef]
- Gu, B.; Shang, X.; Yan, M.; Li, X.; Wang, W.; Wang, Q.; Zhang, C. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol. Oncol. 2021, 161, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Akhmedkhanov, A.; Zeleniuch-Jacquotte, A.; Toniolo, P. Role of exogenous and endogenous hormones in endometrial cancer: Review of the evidence and research perspectives. Ann. N. Y. Acad. Sci. 2001, 943, 296–315. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, E.Y.; Jung, K.W.; Shin, A.; Chan, K.K.; Aoki, D.; Kim, J.W.; Low, J.J.; Won, Y.J. Trends in gynecologic cancer mortality in East Asian regions. J. Gynecol. Oncol. 2014, 25, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Gulinazi, Y.; Du, Y.; Ning, C.C.; Cheng, Y.L.; Shan, W.W.; Luo, X.Z.; Zhang, H.W.; Zhu, Q.; Ma, F.H. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: A randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 848–857. [Google Scholar] [CrossRef]
- Sasano, T.; Mabuchi, S.; Komura, N.; Maeda, M.; Kamiura, S.; Morishima, T.; Miyashiro, I. Evaluation of survival outcomes between minimally invasive and open surgery in the treatment of early-stage endometrial cancer: A population-based study in Osaka Japan. Jpn. J. Clin. Oncol. 2023, 53, 791–797. [Google Scholar] [CrossRef]
- Restaino, S.; Paglietti, C.; Arcieri, M.; Biasioli, A.; Della Martina, M.; Mariuzzi, L.; Andreetta, C.; Titone, F.; Bogani, G.; Raimondo, D.; et al. Management of Patients Diagnosed with Endometrial Cancer: Comparison of Guidelines. Cancers 2023, 15, 1091. [Google Scholar] [CrossRef]
- Enomoto, T.; Okamoto, A.; Kim, J.H.; Lai, C.H.; Wu, X.; Kim, Y.M. East Asian Gynecologic Oncology Trial Group (EAGOT): Founding history and future perspective. J. Gynecol. Oncol. 2023, 34, e86. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, M.; Long, Z.; Ning, H.; Li, J.; Cao, Y.; Liao, Y.; Liu, G.; Wang, F.; Pan, A. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: Systematic analysis of the Global Burden of Disease Study 2019. BMJ 2022, 379, e072385. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Respiratory Tract Cancers Collaborators. Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir. Med. 2021, 9, 1030–1049. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Cancer mortality in Serbia, 1991–2015: An age-period-cohort and joinpoint regression analysis. Cancer Commun. 2018, 38, 10. [Google Scholar] [CrossRef]
- Rosenberg, P.S.; Check, D.P.; Anderson, W.F. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2296–2302. [Google Scholar] [CrossRef]

| Location | 1980/1990 | 2021 | EAPC from 1980 to 2021 b | ||
|---|---|---|---|---|---|
| Counts a | ASR a | Counts a | ASR a | ||
| Deaths | |||||
| Cervical cancer | |||||
| Asia | 105,469.25 (91,934.2–124,165.45) | 6.10 (5.36–7.16) | 160,723.84 (143,073.14–179,396.56) | 3.10 (2.75–3.47) | −1.68 |
| (−1.77–−1.59) | |||||
| Central Asia | 2614.76 (2465.78–2765.99) | 6.35 (5.96–6.73) | 3093.38 (2734.71–3497.22) | 3.47 (3.09–3.90) | −1.43 |
| (−1.54–−1.31) | |||||
| East Asia | 35,015.35 (27,246.63–43,843.21) | 5.00 (3.93–6.20) | 52,032.08 (39,399.83–66,509.9) | 2.41 (1.83–3.07) | −1.58 |
| (−1.74–−1.41) | |||||
| High-income Asia–Pacific | 5652.76 (5283.89–6114.84) | 3.74 (3.48–4.03) | 5074.08 (4303.28–5602.42) | 1.32 (1.18–1.43) | −2.37 |
| (−2.53–−2.22) | |||||
| South Asia | 46,998.03 (37,972.51–58,456.86) | 8.79 (7.04–10.98) | 70,314.60 (61,026.36–79,858.14) | 4.42 (3.83–5.00) | −1.92 |
| (−2.09–−1.75) | |||||
| Southeast Asia | 13,923.40 (11,203.51–17,143.76) | 6.25 (5.09–7.70) | 27,513.07 (23,668.86–31,860.21) | 3.96 (3.42–4.56) | −1.09 |
| (−1.17–−1.00) | |||||
| Ovarian cancer | |||||
| Asia | 21,138.66 (16,819.56–28,334.13) | 1.31 (1.05–1.74) | 84,278.89 (73,874.6–97,734.25) | 1.64 (1.44–1.91) | 0.43 |
| (0.33–0.53) | |||||
| Central Asia | 644.84 (560.24–736.9) | 1.57 (1.36–1.80) | 1949.03 (1705.34–2219.78) | 2.27 (2.00–2.58) | 1.15 |
| (1.06–1.25) | |||||
| East Asia | 8746.25 (6158.32–13,134.29) | 1.28 (0.93–1.87) | 26,337.19 (19,516.44–34,082.62) | 1.20 (0.89–1.55) | −0.46 |
| (−0.62–−0.30) | |||||
| High-income Asia–Pacific | 2803.27 (2678.11–2974.64) | 1.81 (1.72–1.92) | 7200.86 (6064.30–7968.63) | 1.72 (1.51–1.85) | −0.25 |
| (−0.38–−0.11) | |||||
| South Asia | 5207.88 (3507.82–7227.2) | 1.11 (0.72–1.54) | 30,585.13 (26,424.78–36,402.65) | 2.00 (1.73–2.39) | 1.49 |
| (1.43–1.55) | |||||
| Southeast Asia | 2564.91 (1851.37–3818) | 1.22 (0.89–1.76) | 14,523.22 (11,286.29–18,865.47) | 2.11 (1.64–2.73) | 1.33 |
| (1.23–1.42) | |||||
| Uterine cancer | |||||
| Asia | 17,792.51 (12,812.07–22,268.12) | 1.17 (0.87–1.43) | 37,508.92 (31,519.05–45,758.59) | 0.74 (0.62–0.91) | −1.09 |
| (−1.17–−1.02) | |||||
| Central Asia | 876.77 (817.83–937.34) | 2.24 (2.08–2.40) | 1243.44 (1105.49–1403.90) | 1.53 (1.37–1.71) | −1.14 |
| (−1.32–−0.96) | |||||
| East Asia | 10,011.71 (6213.31–13,815.56) | 1.51 (0.98–2.03) | 14,233.24 (10,580.48–19,159.53) | 0.65 (0.48–0.88) | −2.07 |
| (−2.29–−1.85) | |||||
| High-income Asia–Pacific | 2251.53 (2047.66–2450.62) | 1.58 (1.44–1.71) | 3936.83 (3194.38–4379.49) | 0.88 (0.75–0.96) | −1.17 |
| (−1.43–−0.92) | |||||
| South Asia | 2447.90 (1812.16–3015.68) | 0.58 (0.43–0.71) | 9236.53 (7688.44–12,339.17) | 0.64 (0.53–0.85) | 0.18 |
| (0.10–0.26) | |||||
| Southeast Asia | 1949.66 (1395.71–2557.88) | 1.00 (0.73–1.29) | 7378.74 (5306.59–9002.82) | 1.09 (0.79–1.32) | 0.21 |
| (0.12–0.30) | |||||
| DALY | |||||
| Cervical cancer | |||||
| Asia | 4,049,060.64 (3,572,517.1–4,543,446.09) | 163.87 (144.52–183.89) | 5,318,837.66 (4,733,229.92–5,916,376.88) | 100.99 (89.92–112.21) | −1.55 |
| (−1.68–−1.42) | |||||
| Central Asia | 89,741.98 (86,415.34–94,113.3) | 174.67 (167.79–183.27) | 108,285.23 (94,717.7–12,2647.14) | 113.66 (99.9–128.44) | −1.13 |
| (−1.28–−0.98) | |||||
| East Asia | 1,178,714.01 (958,919.39–1,452,779.21) | 114.35 (93.56–140.57) | 1,616,240.23 (1,195,414.47–2,080,056.88) | 75.79 (56.14–97.92) | −1.06 |
| (−1.2–−0.92) | |||||
| High-income Asia–Pacific | 144,581.72 (135,082.87–155,151.29) | 70.72 (66.01–75.87) | 132,808.21 (119,523.11–144,704) | 44.07 (40.95–47.42) | −1.43 |
| (−1.52–−1.34) | |||||
| South Asia | 1,967,172.06 (1,640,297.67–2,286,747.31) | 261.61 (216.39–304.23) | 2,432,061.02 (2,097,849.72–2,773,134.49) | 143.24 (123.88–162.82) | −2.04 |
| (−2.33–−1.75) | |||||
| Southeast Asia | 613,110.37 (526,601.48–699,789.93) | 188.24 (162.71–214.67) | 931,500.99 (800,256.95–1,086,328.05) | 125.03 (107.37–145.35) | −1.49 |
| (−1.60–−1.39) | |||||
| Ovarian cancer | |||||
| Asia | 1,036,435.48 (844,239.2–1,272,438.2) | 43.73 (35.98–53.57) | 2,568,854.39 (2,233,137.32–2,992,340.5) | 48.94 (42.61–57.02) | 0.14 |
| (0.05–0.22) | |||||
| Central Asia | 25,758.05 (23,408.19–28,171.07) | 50.44 (45.77–55.24) | 61,881.11 (53,807.39–70,556.41) | 67.03 (58.39–76.28) | 1 |
| (0.85–1.14) | |||||
| East Asia | 416,565.35 (301,040.22–542,960.91) | 40.88 (30.43–52.84) | 787,095.89 (580,908.59–1,024,657.54) | 36.19 (26.75–47.23) | −0.86 |
| (−1.02–−0.70) | |||||
| High-income Asia–Pacific | 128,562.40 (123,646.42–132,898.81) | 62.41 (59.95–64.54) | 168,116.95 (148,704.1–180,427.51) | 50.30 (45.19–53.24) | −0.74 |
| (−0.81–−0.67) | |||||
| South Asia | 264,386.13 (200,200.75–344,157.49) | 37.70 (28.43–48.66) | 960,207.82 (822,850.63–1,142,385.68) | 58.40 (50.28–69.55) | 1.3 |
| (1.22–1.38) | |||||
| Southeast Asia | 150,719.00 (119,298.73–213,939.65) | 47.58 (38.24–65.85) | 484,774.21 (368,984.4–633,791.17) | 65.65 (50.15–85.54) | 0.97 |
| (0.89–1.05) | |||||
| Uterine cancer | |||||
| Asia | 626,813.16 (462,084.19–746,542.48) | 27.70 (20.87–32.64) | 1,076,127.45 (889,738.88–1,303,328.41) | 20.49 (16.95–24.90) | −1.17 |
| (−1.29–−1.04) | |||||
| Central Asia | 29,872.56 (28,015.68–31,657.86) | 60.86 (56.99–64.51) | 36,940.92 (32,386.42–42,248.7) | 41.59 (36.74–47.29) | −1.23 |
| (−1.49–−0.97) | |||||
| East Asia | 353,039.19 (232,773.4–446,225.4) | 35.73 (24.38–44.70) | 425,141.95 (320,034.77–571,293.09) | 19.36 (14.58–26.02) | −2.33 |
| (−2.65–−2.02) | |||||
| High-income Asia–Pacific | 53,335.23 (47,425.64–58,592.43) | 26.02 (23.17–28.54) | 88,362.34 (77,557.35–96,560.38) | 24.29 (21.84–26.27) | 0.08 |
| (−0.10–0.26) | |||||
| South Asia | 94,296.04 (75,543.98–112,766.55) | 14.96 (11.94–17.89) | 256,842.42 (214,336.05–344,464.65) | 16.42 (13.72–21.96) | 0.12 |
| (−0.02–0.26) | |||||
| Southeast Asia | 89,095.68 (61,687.2–108,414.93) | 30.49 (21.6–37.07) | 226,681.94 (156,600.79–279,317.23) | 31.22 (21.81–38.21) | −0.06 |
| (−0.15–0.03) | |||||
| Incidence | |||||
| Cervical cancer | |||||
| Asia | 196,998.33 (175,157.37–218,833.15) | 8.06 (7.17–8.95) | 357,665.5 (314,247.51–403,188.49) | 6.84 (6.02–7.71) | −0.43 |
| (−0.55–−0.31) | |||||
| Central Asia | 5168.85 (4951.55–5420.21) | 9.97 (9.54–10.46) | 7178.83 (6265.57–8081.45) | 7.50 (6.56–8.41) | −0.54 |
| (−0.71–−0.38) | |||||
| East Asia | 61,909.14 (50,407.48–76,000.79) | 6.03 (4.95–7.37) | 137,863.79 (101,144.48–177,754.73) | 6.69 (4.93–8.67) | 0.76 |
| (0.6–0.93) | |||||
| High-income Asia–Pacific | 12,720.39 (12,005.79–13,515.63) | 6.30 (5.94–6.68) | 15,577.84 (14,044.14–16,881.51) | 5.52 (5.12–5.97) | −0.18 |
| (−0.29–−0.07) | |||||
| South Asia | 83,506.85 (69,576.79–96,968.91) | 11.24 (9.26–13.04) | 132,481.99 (114,540.70–151,294.70) | 7.79 (6.75–8.87) | −1.28 |
| (−1.59–−0.97) | |||||
| Southeast Asia | 30,364.63 (26,327.24–34,797.64) | 9.39 (8.12–10.74) | 58,017.08 (49,190.88–67,747.07) | 7.84 (6.68–9.13) | −0.8 |
| (−0.9–−0.69) | |||||
| Ovarian cancer | |||||
| Asia | 54,459.70 (44,299.59–670,05.9) | 2.30 (1.89–2.81) | 149,451.98 (130,021.59–171,706.89) | 2.90 (2.52–3.33) | 0.54 |
| (0.46–0.62) | |||||
| Central Asia | 1219.94 (1112.09–1337.15) | 2.39 (2.17–2.61) | 2998.96 (2602.07–3418.89) | 3.30 (2.88–3.76) | 1.15 |
| (1.00–1.30) | |||||
| East Asia | 21,065.43 (15,069.18–27,403.26) | 2.06 (1.54–2.65) | 44,239.91 (33,123.68–57,358.78) | 2.11 (1.59–2.74) | −0.32 |
| (−0.46–−0.18) | |||||
| High-income Asia–Pacific | 6798.13 (6490.41–7078.81) | 3.36 (3.20–3.50) | 11,227.65 (9782.40–12,235.43) | 3.26 (2.9–3.47) | −0.15 |
| (−0.31–0.00) | |||||
| South Asia | 12,511.24 (9361.05–16,327.09) | 1.79 (1.33–2.31) | 49,664.47 (42,260.25–59,355) | 3.02 (2.59–3.60) | 1.57 |
| (1.49–1.65) | |||||
| Southeast Asia | 10,046.56 (7838.03–14,358.26) | 3.05 (2.43–4.23) | 35,031.98 (26,271.21–45,639.37) | 4.80 (3.61–6.22) | 1.35 |
| (1.24–1.46) | |||||
| Uterine cancer | |||||
| Asia | 49,559.48 (37,592.10–58,190.73) | 2.22 (1.71–2.58) | 152,861.93 (125,810.13–185,975.36) | 2.88 (2.38–3.51) | 0.81 |
| (0.67–0.95) | |||||
| Central Asia | 2930.84 (2758.52–3105.93) | 5.93 (5.58–6.28) | 4813.37 (4249.72–5449.13) | 5.33 (4.72–6.00) | −0.3 |
| (−0.61–0.02) | |||||
| East Asia | 27,189.89 (18,791.97–341,44.00) | 2.80 (1.97–3.48) | 75,543.72 (56,833.53–103,404.09) | 3.39 (2.54–4.64) | 0.52 |
| (0.22–0.83) | |||||
| High-income Asia–Pacific | 4826.00 (4375.92–5235.28) | 2.35 (2.13–2.55) | 13,824.55 (12,300.83–15,052.24) | 4.21 (3.82–4.57) | 2.24 |
| (2.12–2.37) | |||||
| South Asia | 6014.57 (4769.73–7122.42) | 0.97 (0.77–1.15) | 23,668.16 (19,817.78–31,544.04) | 1.52 (1.27–2.02) | 1.26 |
| (1.07–1.45) | |||||
| Southeast Asia | 7221.10 (5143.54–8697.35) | 2.47 (1.79–2.96) | 25,169.01 (17,367.13–30,769.27) | 3.43 (2.39–4.17) | 0.91 |
| (0.85–0.96) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Miao, R.; He, H.; Zhang, N.; Wan, X.; Gao, Y.; Ji, D. The Burden and Trends of Gynecological Cancers in Asia from 1980 to 2021, with Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Curr. Oncol. 2025, 32, 298. https://doi.org/10.3390/curroncol32060298
Yang Y, Miao R, He H, Zhang N, Wan X, Gao Y, Ji D. The Burden and Trends of Gynecological Cancers in Asia from 1980 to 2021, with Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Current Oncology. 2025; 32(6):298. https://doi.org/10.3390/curroncol32060298
Chicago/Turabian StyleYang, Yang, Run Miao, Haoyu He, Ning Zhang, Xingyu Wan, Yuzhou Gao, and Dongmei Ji. 2025. "The Burden and Trends of Gynecological Cancers in Asia from 1980 to 2021, with Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021" Current Oncology 32, no. 6: 298. https://doi.org/10.3390/curroncol32060298
APA StyleYang, Y., Miao, R., He, H., Zhang, N., Wan, X., Gao, Y., & Ji, D. (2025). The Burden and Trends of Gynecological Cancers in Asia from 1980 to 2021, with Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Current Oncology, 32(6), 298. https://doi.org/10.3390/curroncol32060298





