The Association of OLFM4 with the Progression and Cisplatin Resistance of Head and Neck Squamous Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition and Processing
2.2. Cell Culture
2.3. Overexpression or Knockdown of OLFM4
2.4. Cell Proliferation and Cisplatin Toxicity Assay
2.5. Cell Migration
2.6. Cell Invasion
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Western Blotting
2.9. Flow Cytometry to Detect Cellular Chemoresistance
2.10. Reactive Oxygen Species (ROS) and Antioxidant Capacity Detection
2.11. Measurement of Lipid Peroxidation Levels
2.12. Determination of Intracellular Divalent Iron Ions
2.13. Mitochondrial Membrane Potential Assay
2.14. Statistical Analysis
3. Results
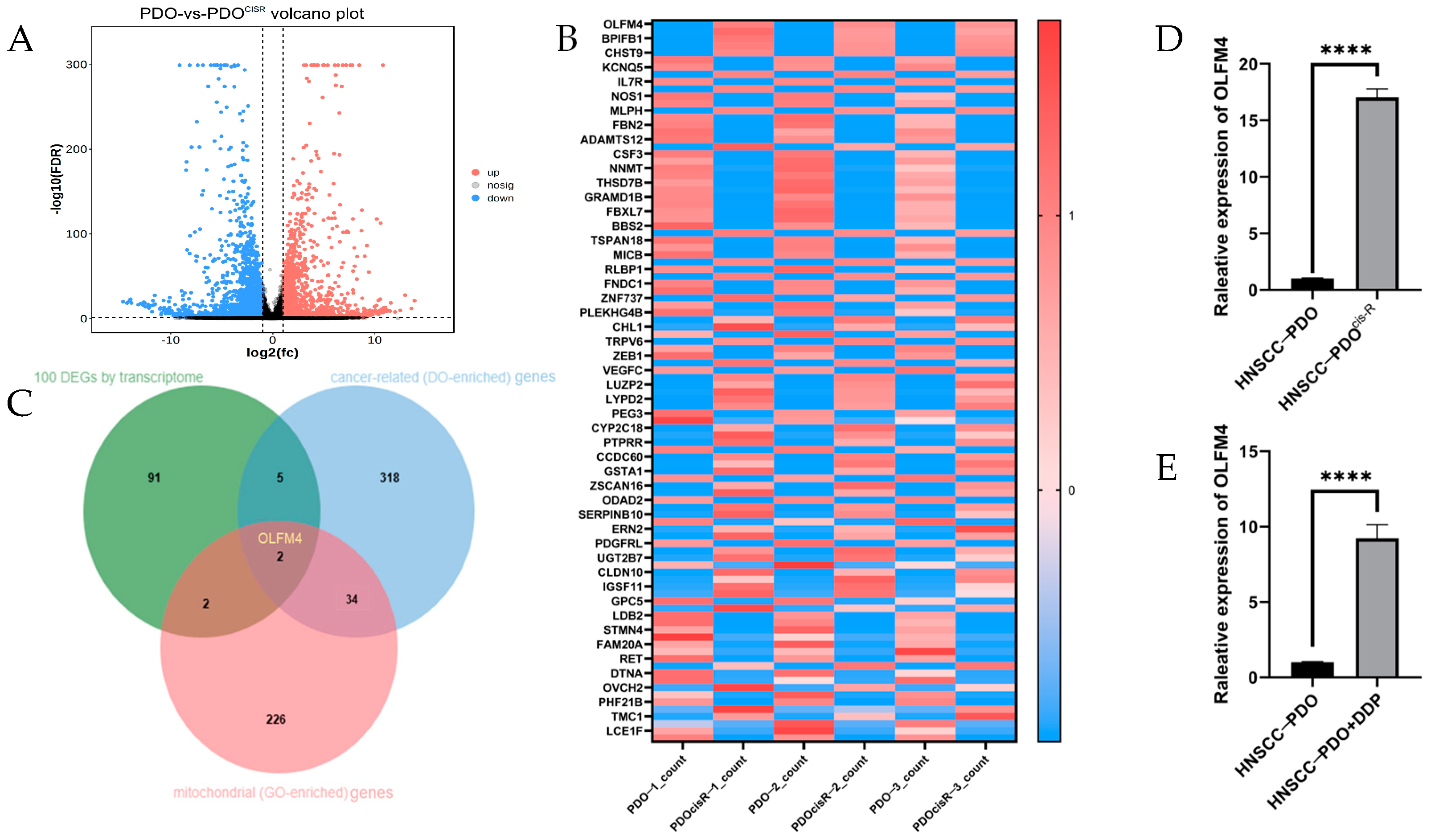
3.1. Elevated OLFM4 Expression in Cisplatin-Resistant HNSCC-PDO Models
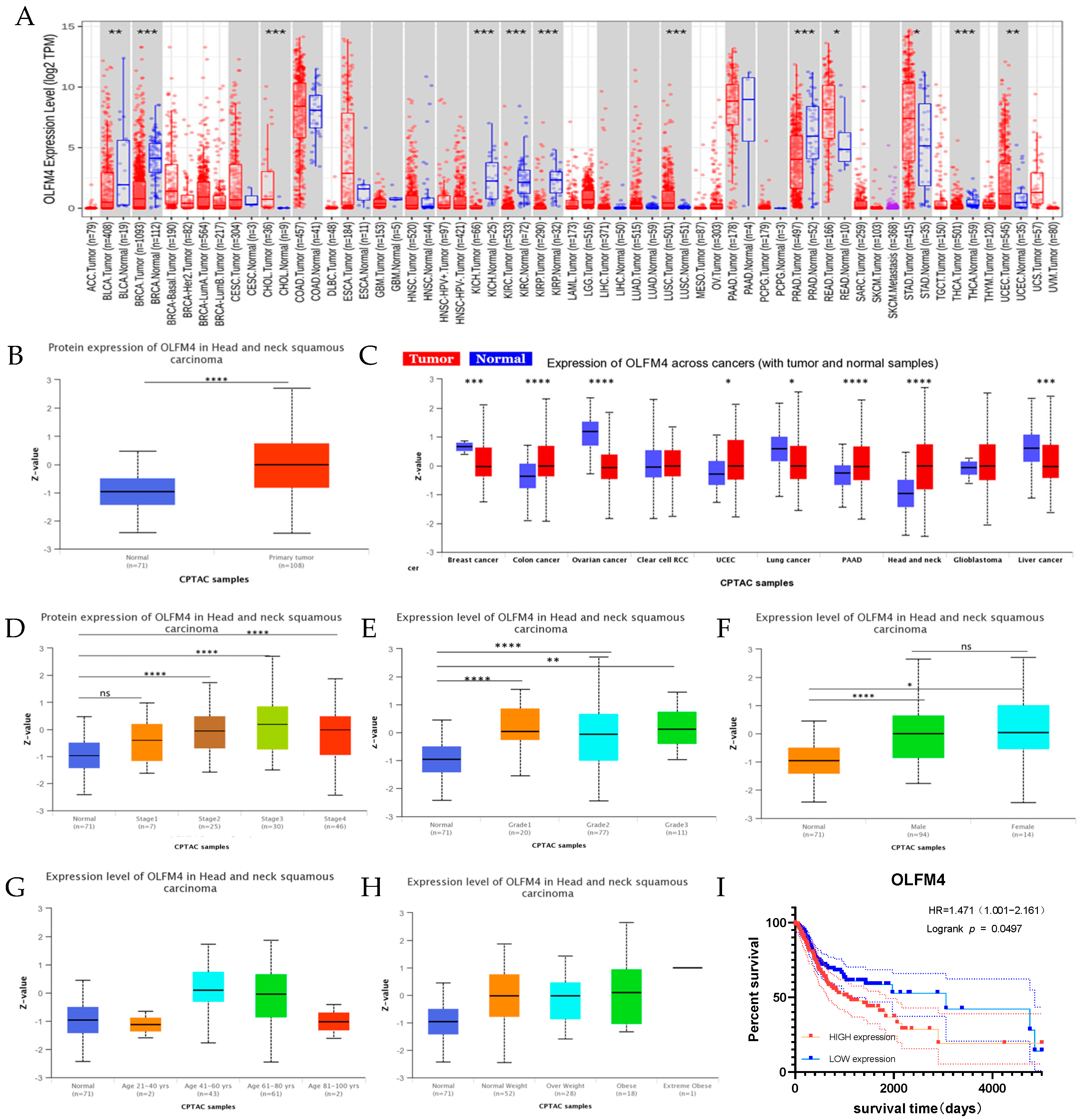
3.2. Clinical Correlation of OLFM4 Expression
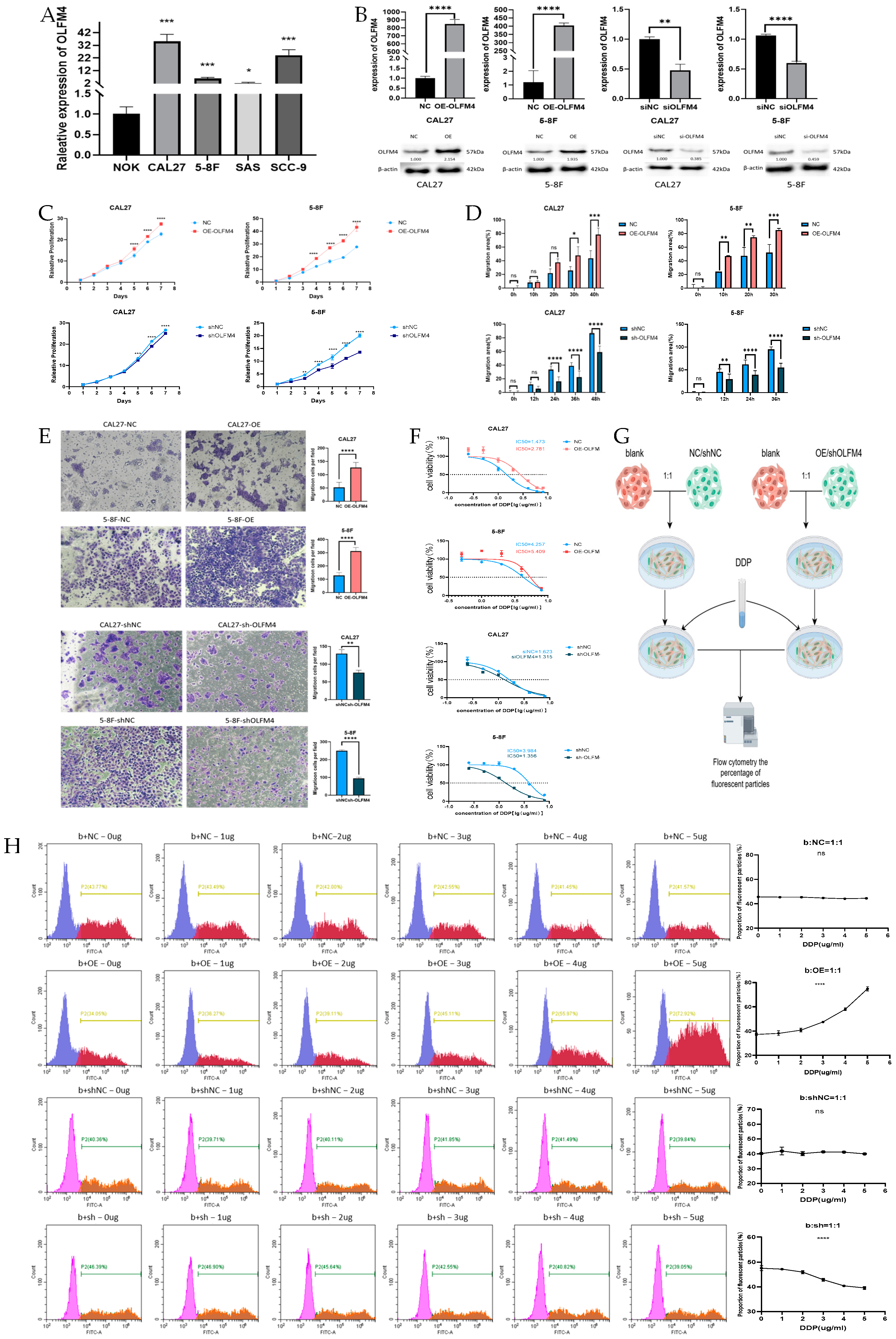
3.3. Functional Characterization of OLFM4 in HNSCC
3.4. OLFM4 Promotes Aggressive HNSCC Phenotypes
3.5. OLFM4 Promoted Cisplatin Resistance of HNSCC Cells
3.6. OLFM4 Modulates Mitochondrial Function- and Ferroptosis-Related Pathways in HNSCC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HNSCC | head and neck squamous carcinoma |
| DEGs | differentially expressed genes |
| PDO | patient-derived organoid |
| PDOcis-R | patient-derived organoid cisplatin-resistant counterpart |
| OLFM4 | olfactomedin 4 |
References
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma—An update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, M.L.; Goh, G.; Chiosea, S.I.; Bauman, J.E.; Freilino, M.L.; Zeng, Y.; Wang, L.; Diergaarde, B.B.; Gooding, W.E.; Lui, V.W.; et al. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J. Clin. Investig. 2016, 126, 169–180. [Google Scholar] [CrossRef]
- López-Verdín, S.; Lavalle-Carrasco, J.; Carreón-Burciaga, R.G.; Serafín-Higuera, N.; Molina-Frechero, N.; González-González, R.; Bologna-Molina, R. Molecular Markers of Anticancer Drug Resistance in Head and Neck Squamous Cell Carcinoma: A Literature Review. Cancers 2018, 10, 376. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, D. The roles of excision repair cross-complementation group1 in objective response after cisplatin-based concurrent chemoradiotherapy and survival in head and neck cancers: A systematic review and meta-analysis. Oral. Oncol. 2015, 51, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Howell, S.B.; Safaei, R.; Larson, C.A.; Sailor, M.J. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol. Pharmacol. 2010, 77, 887–894. [Google Scholar] [CrossRef]
- Mellish, K.J.; Kelland, L.R.; Harrap, K.R. In vitro platinum drug chemosensitivity of human cervical squamous cell carcinoma cell lines with intrinsic and acquired resistance to cisplatin. Br. J. Cancer 1993, 68, 240–250. [Google Scholar] [CrossRef]
- Kelland, L.R.; Mistry, P.; Abel, G.; Freidlos, F.; Loh, S.Y.; Roberts, J.J.; Harrap, K.R. Establishment and characterization of an in vitro model of acquired resistance to cisplatin in a human testicular nonseminomatous germ cell line. Cancer Res. 1992, 52, 1710–1716. [Google Scholar]
- Brozovic, A.; Fritz, G.; Christmann, M.; Zisowsky, J.; Jaehde, U.; Osmak, M.; Kaina, B. Long-term activation of SAPK/JNK, p38 kinase and fas-L expression by cisplatin is attenuated in human carcinoma cells that acquired drug resistance. Int. J. Cancer 2004, 112, 974–985. [Google Scholar] [CrossRef]
- Wangpaichitr, M.; Theodoropoulos, G.; Nguyen, D.J.M.; Wu, C.; Spector, S.A.; Feun, L.G.; Savaraj, N. Cisplatin Resistance and Redox-Metabolic Vulnerability: A Second Alteration. Int. J. Mol. Sci. 2021, 22, 7379. [Google Scholar] [CrossRef] [PubMed]
- Kleih, M.; Böpple, K.; Dong, M.; Gaißler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef]
- Li, J.; Zheng, C.; Wang, M.; Umano, A.D.; Dai, Q.; Zhang, C.; Huang, H.; Yang, Q.; Yang, X.; Lu, J.; et al. ROS-regulated phosphorylation of ITPKB by CAMK2G drives cisplatin resistance in ovarian cancer. Oncogene 2022, 41, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qiao, Y.; Liu, Y.; Zhou, J.; Wang, X.; Zheng, H.; Xu, Z.; Zhang, J.; Zhou, Y.; Qian, L.; et al. ent-Kaurane diterpenoids induce apoptosis and ferroptosis through targeting redox resetting to overcome cisplatin resistance. Redox Biol. 2021, 43, 101977. [Google Scholar] [CrossRef]
- Harada, K.; Sakamoto, N.; Kitaoka, T.; Nakamura, Y.; Kondo, R.; Morisue, R.; Hashimoto, H.; Yamamoto, Y.; Ukai, S.; Maruyama, R.; et al. PI3 expression predicts recurrence after chemotherapy with DNA-damaging drugs in gastric cancer. J. Pathol. 2025, 265, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Cao, D.; Li, Y.; Wang, Z.; Dong, R.; Zhu, M.; Gao, P.; Wang, X.; Cai, J.; Zuo, X. Circ_0008315 promotes tumorigenesis and cisplatin resistance and acts as a nanotherapeutic target in gastric cancer. J. Nanobiotechnol. 2024, 22, 519. [Google Scholar] [CrossRef]
- Liang, C.; Shan, D.; Wei, Z. PDO-Based Drug Screening in Advanced Pancreatic Cancer. Gastroenterology 2025, 168, 627–628. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, C.; Yao, X.I.; Su, J.; Lu, W.; Wei, Z.; Xie, Y. CES1 is associated with cisplatin resistance and poor prognosis of head and neck squamous cell carcinoma. Oncol. Res. 2024, 32, 1935–1948. [Google Scholar] [CrossRef]
- Huang, Z.; Yao, X.; Liu, Q.; Xie, Y.; Wei, Z. Optimization of Culture Medium and Transfection Method for Head and Neck Squamous Cell Carcinoma Organoids. Med. Plant 2023, 14, 100–104. [Google Scholar]
- Zeng, F.; Nijiati, S.; Tang, L.; Ye, J.; Zhou, Z.; Chen, X. Ferroptosis Detection: From Approaches to Applications. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300379. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Zhai, D.; Xie, C.; Kuang, X.; Lin, Y.; Shao, N. Quantification of ferroptosis pathway status revealed heterogeneity in breast cancer patients with distinct immune microenvironment. Front. Oncol. 2022, 12, 956999. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Yang, M.H.; Xiang, H.; Bao, L.M.; Yang, H.A.; Yue, L.W.; Jiang, X.; Ang, N.; Wu, L.Y.; Huang, Y. Depletion of OLFM4 gene inhibits cell growth and increases sensitization to hydrogen peroxide and tumor necrosis factor-alpha induced-apoptosis in gastric cancer cells. J. Biomed. Sci. 2012, 19, 38. [Google Scholar] [CrossRef]
- Xu, K.; Zheng, P.; Zhao, S.; Wang, J.; Feng, J.; Ren, Y.; Zhong, Q.; Zhang, H.; Chen, X.; Chen, J.; et al. LRFN5 and OLFM4 as novel potential biomarkers for major depressive disorder: A pilot study. Transl. Psychiatry 2023, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Klaas, M.; Mäemets-Allas, K.; Heinmäe, E.; Lagus, H.; Arak, T.; Eller, M.; Kingo, K.; Kankuri, E.; Jaks, V. Olfactomedin-4 improves cutaneous wound healing by promoting skin cell proliferation and migration through POU5F1/OCT4 and ESR1 signalling cascades. Cell Mol. Life Sci. 2022, 79, 157. [Google Scholar] [CrossRef]
- Yao, R.Q.; Shen, Z.; Ma, Q.M.; Ling, P.; Wei, C.R.; Zheng, L.Y.; Duan, Y.; Li, W.; Zhu, F.; Sun, Y.; et al. Combination of transcriptional biomarkers and clinical parameters for early prediction of sepsis indued acute respiratory distress syndrome. Front. Immunol. 2022, 13, 1084568. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Li, H.; Rodgers, G.P. Olfactomedin 4 contributes to hydrogen peroxide-induced NADPH oxidase activation and apoptosis in mouse neutrophils. Am. J. Physiol. Cell Physiol. 2018, 315, C494–C501. [Google Scholar] [CrossRef]
- Kobayashi, D.; Koshida, S.; Moriai, R.; Tsuji, N.; Watanabe, N. Olfactomedin 4 promotes S-phase transition in proliferation of pancreatic cancer cells. Cancer Sci. 2007, 98, 334–340. [Google Scholar] [CrossRef]
- Liu, W.; Lee, H.W.; Liu, Y.; Wang, R.; Rodgers, G.P. Olfactomedin 4 is a novel target gene of retinoic acids and 5-aza-2′-deoxycytidine involved in human myeloid leukemia cell growth, differentiation, and apoptosis. Blood 2010, 116, 4938–4947. [Google Scholar] [CrossRef]
- Mayama, A.; Takagi, K.; Suzuki, H.; Sato, A.; Onodera, Y.; Miki, Y.; Sakurai, M.; Watanabe, T.; Sakamoto, K.; Yoshida, R.; et al. OLFM4, LY6D and S100A7 as potent markers for distant metastasis in estrogen receptor-positive breast carcinoma. Cancer Sci. 2018, 109, 3350–3359. [Google Scholar] [CrossRef]
- Ashizawa, Y.; Kuboki, S.; Nojima, H.; Yoshitomi, H.; Furukawa, K.; Takayashiki, T.; Takano, S.; Miyazaki, M.; Ohtsuka, M. OLFM4 Enhances STAT3 Activation and Promotes Tumor Progression by Inhibiting GRIM19 Expression in Human Hepatocellular Carcinoma. Hepatol. Commun. 2019, 3, 954–970. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Gao, M.; Chen, Z.; Lu, J.; Li, Y.; Di, Y.; Zhao, Y.; Liu, B.; Tang, R. Lipocalin-2 promotes CKD vascular calcification by aggravating VSMCs ferroptosis through NCOA4/FTH1-mediated ferritinophagy. Cell Death Dis. 2024, 15, 865. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhong, B.; Zhao, L.; Hou, Y.; Ai, N.; Lu, J.J.; Ge, W.; Chen, X. Fighting drug-resistant lung cancer by induction of NAD(P)H:quinone oxidoreductase 1 (NQO1)-mediated ferroptosis. Drug Resist. Updat. 2023, 70, 100977. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, Y.; Jiang, Y.; Zhang, L.; Cheng, W. GPX4: The hub of lipid oxidation, ferroptosis, disease and treatment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188890. [Google Scholar] [CrossRef]
- Ding, K.; Liu, C.; Li, L.; Yang, M.; Jiang, N.; Luo, S.; Sun, L. Acyl-CoA synthase ACSL4: An essential target in ferroptosis and fatty acid metabolism. Chin. Med. J. (Engl.) 2023, 136, 2521–2537. [Google Scholar] [CrossRef]
- Yue, X.; Zhao, P.; Wu, K.; Huang, J.; Zhang, W.; Wu, Y.; Liang, X.; He, X. GRIM-19 inhibition induced autophagy through activation of ERK and HIF-1α not STAT3 in Hela cells. Tumour Biol. 2016, 37, 9789–9796. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, Y.; Lu, H.; Cao, X. Coupling mitochondrial respiratory chain to cell death: An essential role of mitochondrial complex I in the interferon-beta and retinoic acid-induced cancer cell death. Cell Death Differ. 2007, 14, 327–337. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, R.; Huang, P.; Chen, M.; Chen, H.; Zeng, X.; Liu, J.; Zhang, J.; Huang, D.; Lao, L. Ferroptotic Neutrophils Induce Immunosuppression and Chemoresistance in Breast Cancer. Cancer Res. 2025, 85, 477–496. [Google Scholar] [CrossRef]


| Gene | Primer Sequences (5′ → 3′) |
|---|---|
| GAPDH-F | TGCAACCGGGAAGGAAATGA |
| GAPDH-R | GTGGAATTTGCCATGGGTGGA |
| OLFM4-F | CATCTGCTTCTAACGCCTTCAT |
| OLFM4-R | TAGTTTGCCCTCTTTCCCTGTG |
| LCN2-F | CCCCATCTCTGCTCACTGTC |
| LCN2-R | TTTTTCTGGACCGCATTG |
| ACSL4-F | GCTACTTGCCTTTGGCTCATGTGC |
| ACSL4-R | GTGTGGGCTTCAGTACAGTACAGTCTCC |
| LPCAT3-F | TCAGGATACCTGATTTGCTTCCA |
| LPCAT3-R | GGATGGGTCTGTTGCACCAAGTAG |
| TfR1-F | ACCATTGTCATATACCCGGTTCA |
| TfR1-R | GGCCTTTGTGTTATTGTCAGCAT |
| SLC7A11-F | TGCTGGGCTGATTTTATCTTCG |
| SLC7A11-R | GAAAGGGCAACCATGAAGAGG |
| GPX4-F | GAAGCAGGAGCCAGGGAGTA |
| GPX4-R | GGTGAAGTTCCACTTGATGGC |
| FTH1-F | AATTTCTTGACCCACTGGTGCACT |
| FTH1-R | TCGAATCGAGAGTAGTGGCACA |
| NDUFA13-F | CTTGATCGGGGAGCTGTACG |
| NDUFA13-R | GGCCTACGTGTACCACATGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Yao, X.; Pang, K.; Chen, X.; Wei, Z.; Xie, Y. The Association of OLFM4 with the Progression and Cisplatin Resistance of Head and Neck Squamous Carcinoma. Curr. Oncol. 2025, 32, 276. https://doi.org/10.3390/curroncol32050276
He X, Yao X, Pang K, Chen X, Wei Z, Xie Y. The Association of OLFM4 with the Progression and Cisplatin Resistance of Head and Neck Squamous Carcinoma. Current Oncology. 2025; 32(5):276. https://doi.org/10.3390/curroncol32050276
Chicago/Turabian StyleHe, Xinlu, Xi Yao, Keling Pang, Xulin Chen, Zhengbo Wei, and Ying Xie. 2025. "The Association of OLFM4 with the Progression and Cisplatin Resistance of Head and Neck Squamous Carcinoma" Current Oncology 32, no. 5: 276. https://doi.org/10.3390/curroncol32050276
APA StyleHe, X., Yao, X., Pang, K., Chen, X., Wei, Z., & Xie, Y. (2025). The Association of OLFM4 with the Progression and Cisplatin Resistance of Head and Neck Squamous Carcinoma. Current Oncology, 32(5), 276. https://doi.org/10.3390/curroncol32050276





