Molecular and Pathological Heterogeneity of Synchronous Small and Large Duct Intrahepatic Cholangiocarcinoma—A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Histopathological and Immunohistochemical Analysis
2.3. Molecular Profiling and Genomic Analysis
2.4. Imaging Analysis and Clinical Correlation
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Patient Characteristics
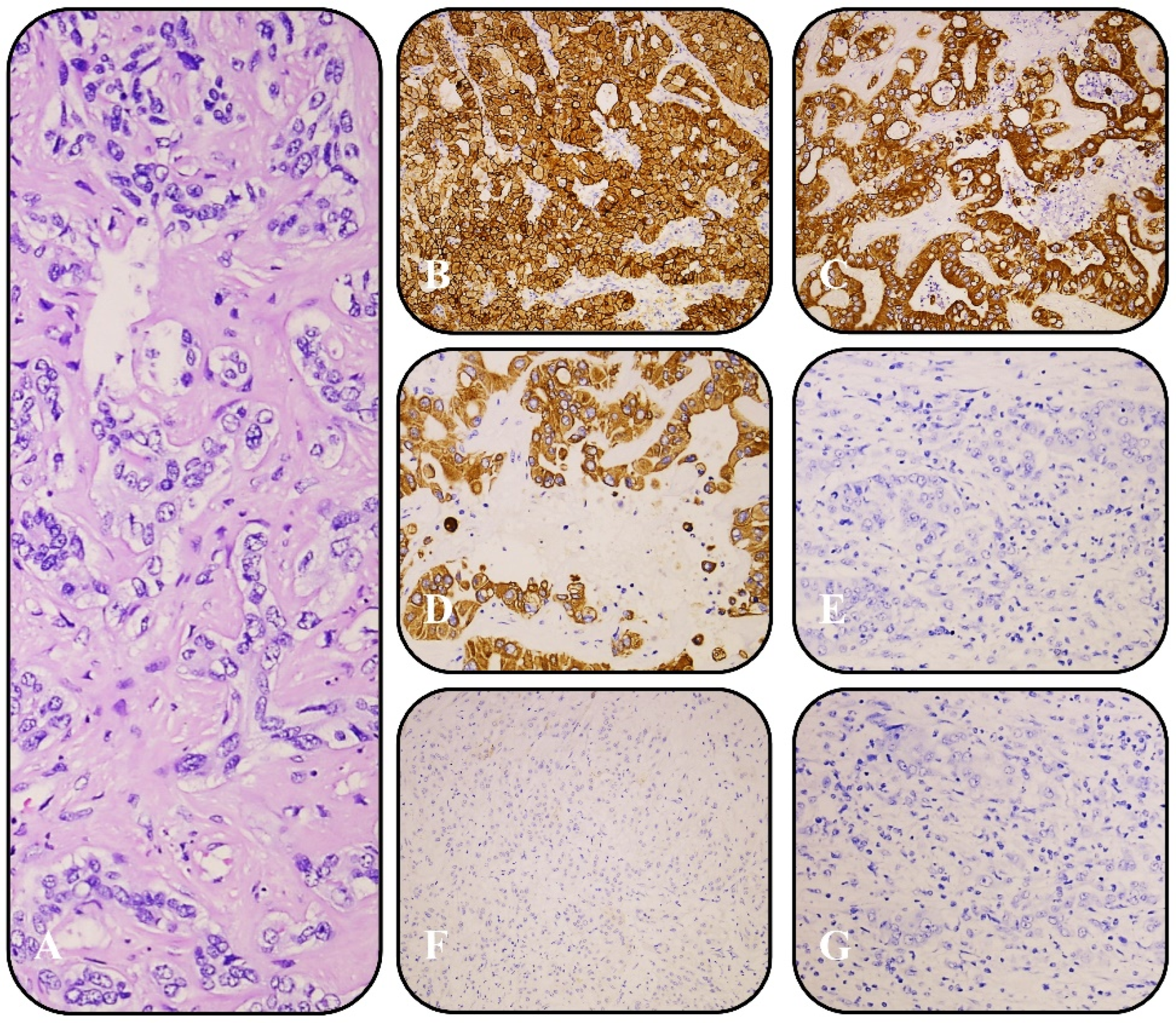
3.2. Histopathological and Immunohistochemical Findings
3.3. Genomic Alterations and Molecular Heterogeneity
3.4. Imaging and Clinical Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, T.; Park, Y.N. Up-to-Date Pathologic Classification and Molecular Characteristics of Intrahepatic Cholangiocarcinoma. Front. Med. 2022, 9, 857140. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, M.N.; Schulze, F.; Bankov, K.; Gretser, S.; Becker, N.; Leichner, R.; Stehle, A.; Abedin, N.; Trojan, J.; Zeuzem, S.; et al. Impact of small duct- and large duct type on survival in patients with intrahepatic cholangiocarcinoma: Results from a German tertiary center. Pathol. Res. Pract. 2022, 238, 154126. [Google Scholar] [CrossRef]
- Zen, Y. Intrahepatic cholangiocarcinoma: Typical features, uncommon variants, and controversial related entities. Hum. Pathol. 2023, 132, 197–207. [Google Scholar] [CrossRef]
- Carotenuto, M.; Sacco, A.; Forgione, L.; Normanno, N. Genomic alterations in cholangiocarcinoma: Clinical significance and relevance to therapy. Explor. Target. Antitumor Ther. 2022, 3, 200–223. [Google Scholar] [CrossRef]
- Lee, S.-H.; Song, S.Y. Recent Advancement in Diagnosis of Biliary Tract Cancer through Pathological and Molecular Classifications. Cancers 2024, 16, 1761. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma with IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef]
- Vogel, A.; Sahai, V.; Hollebecque, A.; Vaccaro, G.M.; Melisi, D.; Al Rajabi, R.M.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. An open-label study of pemigatinib in cholangiocarcinoma: Final results from FIGHT-202. ESMO Open 2024, 9, 103488. [Google Scholar] [CrossRef] [PubMed]
- De Santis, A.; Zhu, L.; Tao, J.; Reißfelder, C.; Schölch, S. Molecular subtypes of intrahepatic cholangiocarcinoma. Trends Mol. Med. 2025. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, J.; Li, Z.; Ma, D.; Wang, Y.; Cheng, Q.; Zhu, J.; Li, Z. Integrative analysis reveals different feature of intrahepatic cholangiocarcinoma subtypes. Liver Int. 2024, 44, 2477–2493. [Google Scholar] [CrossRef]
- Ma, B.; Meng, H.; Tian, Y.; Wang, Y.; Song, T.; Zhang, T.; Wu, Q.; Cui, Y.; Li, H.; Zhang, W.; et al. Distinct clinical and prognostic implication of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. BMC Cancer 2020, 20, 318. [Google Scholar] [CrossRef]
- Bernatz, S.; Schulze, F.; Bein, J.; Bankov, K.; Mahmoudi, S.; Grünewald, L.D.; Koch, V.; Stehle, A.; Schnitzbauer, A.A.; Walter, D.; et al. Small duct and large duct type intrahepatic cholangiocarcinoma reveal distinct patterns of immune signatures. J. Cancer Res. Clin. Oncol. 2024, 150, 357. [Google Scholar] [CrossRef]
- Gopal, P.; Robert, M.E.; Zhang, X. Cholangiocarcinoma: Pathologic and Molecular Classification in the Era of Precision Medicine. Arch. Pathol. Lab. Med. 2023, 148, 359–370. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, M.N.; Schulze, F.; Jeroch, J.; Schmitt, C.; Ebner, S.; Gretser, S.; Bein, J.; Finkelmeier, F.; Trojan, J.; Zeuzem, S.; et al. Heterogeneity of small duct- and large duct-type intrahepatic cholangiocarcinoma. Histopathology 2024, 84, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Gao, J.; Phillips, S.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Chen, L.T.; Vogel, A.; Hsu, C.; Chen, M.H.; Fang, W.; Pangarsa, E.A.; Sharma, A.; Ikeda, M.; Park, J.O.; Tan, C.K.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with biliary tract cancer. ESMO Open 2024, 9, 103647. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abrams, T.; Abbott, D.E.; Ahmed, A.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Binder, D.; et al. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J. Natl. Compr. Canc Netw. 2023, 21, 694–704. [Google Scholar] [CrossRef]
- Loeuillard, E.; Gores, G.J.; Ilyas, S.I. MEK Inhibition: A New Ally in Immunotherapy for Intrahepatic Cholangiocarcinoma. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1153–1154. [Google Scholar] [CrossRef] [PubMed]
- Fedele, C.; Ran, H.; Diskin, B.; Wei, W.; Jen, J.; Geer, M.J.; Araki, K.; Ozerdem, U.; Simeone, D.M.; Miller, G.; et al. SHP2 Inhibition Prevents Adaptive Resistance to MEK Inhibitors in Multiple Cancer Models. Cancer Discov. 2018, 8, 1237–1249. [Google Scholar] [CrossRef]
- Bartley, A.N.; Mills, A.M.; Konnick, E.; Overman, M.; Ventura, C.B.; Souter, L.; Colasacco, C.; Stadler, Z.K.; Kerr, S.; Howitt, B.E.; et al. Mismatch Repair and Microsatellite Instability Testing for Immune Checkpoint Inhibitor Therapy: Guideline From the College of American Pathologists in Collaboration With the Association for Molecular Pathology and Fight Colorectal Cancer. Arch. Pathol. Lab. Med. 2022, 146, 1194–1210. [Google Scholar] [CrossRef]
- Fountzilas, E.; Kurzrock, R.; Vo, H.H.; Tsimberidou, A.M. Wedding of Molecular Alterations and Immune Checkpoint Blockade: Genomics as a Matchmaker. J. Natl. Cancer Inst. 2021, 113, 1634–1647. [Google Scholar] [CrossRef]
- Yang, X.; Lian, B.; Zhang, N.; Long, J.; Li, Y.; Xue, J.; Chen, X.; Wang, Y.; Wang, Y.; Xun, Z.; et al. Genomic characterization and immunotherapy for microsatellite instability-high in cholangiocarcinoma. BMC Med. 2024, 22, 42. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Stenzinger, A.; Vogel, A.; Lehmann, U.; Lamarca, A.; Hofman, P.; Terracciano, L.; Normanno, N. Molecular profiling in cholangiocarcinoma: A practical guide to next-generation sequencing. Cancer Treat. Rev. 2024, 122, 102649. [Google Scholar] [CrossRef] [PubMed]
- Keilson, J.M.; Lindsey, S.; Bachini, M.; Medin, C.R.; Berk, A.; Cornew, S.; Maithel, S.K. Patient reported outcomes: Financial toxicity is a barrier to clinical trials and personalized therapy in cholangiocarcinoma. J. Surg. Oncol. 2022, 126, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Cerrito, L.; Ainora, M.E.; Borriello, R.; Piccirilli, G.; Garcovich, M.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. Contrast-Enhanced Imaging in the Management of Intrahepatic Cholangiocarcinoma: State of Art and Future Perspectives. Cancers 2023, 15, 3393. [Google Scholar] [CrossRef]
- Kinoshita, M.; Sato, Y.; Shinkawa, H.; Kimura, K.; Ohira, G.; Nishio, K.; Tanaka, R.; Kurihara, S.; Kushiyama, S.; Tani, N.; et al. Impact of Tumor Subclassifications for Identifying an Appropriate Surgical Strategy in Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2024, 31, 2579–2590. [Google Scholar] [CrossRef]
- Harrison, J.M.; Visser, B.C. Cholangiocarcinoma. Surg. Clin. North. Am. 2024, 104, 1281–1293. [Google Scholar] [CrossRef]

| Case | Sex | Age (Years) | Predominant Variant (%) | IHC | Small-Duct iCCA Mutations | Large-Duct iCCA Mutations | TMB (mut/Mb) | MSI Status |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 72 | Small-duct (65%) | — | MYCN amp, BRCA2 fusion, PIK3R1 | KRAS, TP53 | 7.8 (small)/3.1 (large) | MSI-L (3.2%) |
| 2 | Female | 78 | Small-duct (60%) | EMA+, CK7+, CK19+, Hepatocyte−, Arginase-1− | IDH2, KRAS, EGFR amp | KRAS | 2.3 (small)/3.1 (large) | MSI-L (2.4%) |
| 3 | Female | 77 | Small-duct (85%) | CK7+, TTF1−, GATA3−, Hepatocyte− | IDH1, EGFR amp, MDM2 amp | KRAS | 1.6 (small)/3.1 (large) | MSI-L (2.9%) |
| 4 | Female | 72 | Large-duct (75%) | CK7+, CK20 (focal)+, ER−, TTF1−, GATA3−, CDX2− | BAP1 mutation | TP53 | 7.9 (small)/23.5 (large) | MSI-L (1.2%) |
| 5 | Male | 64 | Large-duct (90%) | — | ARID1A, ATM | NF1 | 3.1 (small)/23.5 (large) | MSI-H (14.8%) |
| 6 | Female | 65 | Large-duct (55%) | CK7+, CK19+, CK8/18+, EMA+, Villin+, MOC-31+, Hepatocyte−, Glypican 3−, Arginase−, HMWCK− | FGFR2 fusion | KRAS, SMAD4 | 2.9 (small)/7.8 (large) | MSI-L (2.9%) |
| Case | Localization | Biliary Obstruction | Vascular Invasion | Lymph Node Involvement | Grade and Stage | Adjuvant Treatment | PFS (Months) |
|---|---|---|---|---|---|---|---|
| 1 | Poorly defined right hepatic lobe lesion | No | No | No | G2; pT4N0M0/IIIB | Capecitabine | 12, 10 |
| 2 | Left hepatic lobe lesion (5.24 × 3.7 cm) | No | No | No | G3; pT2aN0M0/II | - | 14, 50 |
| 3 | Multiple lesions in segments IVb, V and VII | No | No | No | G2; pT2bN0M0/II | - | 13, 80 |
| 4 | Mass in segments VII–VIII (9.74 × 8.3 cm) | Yes | Yes | Yes | G2; pT4N1M0/IIIB | Capecitabine | 5, 40 |
| 5 | Hypervascular lesions in segments II-V and VIII | Yes | Yes | Yes | G3; pT2N1M0/IIIB | - | 3, 70 |
| 6 | Poorly defined lesion in segment IV with left hepatic duct invasion | Yes | Yes | Yes | G2; pT2N1M0/IIIB | Capecitabine | 6, 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovska, S.; Nankov, V.; Ilcheva, B.; Dimitrov, G. Molecular and Pathological Heterogeneity of Synchronous Small and Large Duct Intrahepatic Cholangiocarcinoma—A Case Series. Curr. Oncol. 2025, 32, 255. https://doi.org/10.3390/curroncol32050255
Popovska S, Nankov V, Ilcheva B, Dimitrov G. Molecular and Pathological Heterogeneity of Synchronous Small and Large Duct Intrahepatic Cholangiocarcinoma—A Case Series. Current Oncology. 2025; 32(5):255. https://doi.org/10.3390/curroncol32050255
Chicago/Turabian StylePopovska, Savelina, Vladislav Nankov, Boriana Ilcheva, and George Dimitrov. 2025. "Molecular and Pathological Heterogeneity of Synchronous Small and Large Duct Intrahepatic Cholangiocarcinoma—A Case Series" Current Oncology 32, no. 5: 255. https://doi.org/10.3390/curroncol32050255
APA StylePopovska, S., Nankov, V., Ilcheva, B., & Dimitrov, G. (2025). Molecular and Pathological Heterogeneity of Synchronous Small and Large Duct Intrahepatic Cholangiocarcinoma—A Case Series. Current Oncology, 32(5), 255. https://doi.org/10.3390/curroncol32050255






