A Rare Malignant Case of a Primary Pseudomyogenic Haemangioendothelioma of the Bone
Abstract
1. Introduction
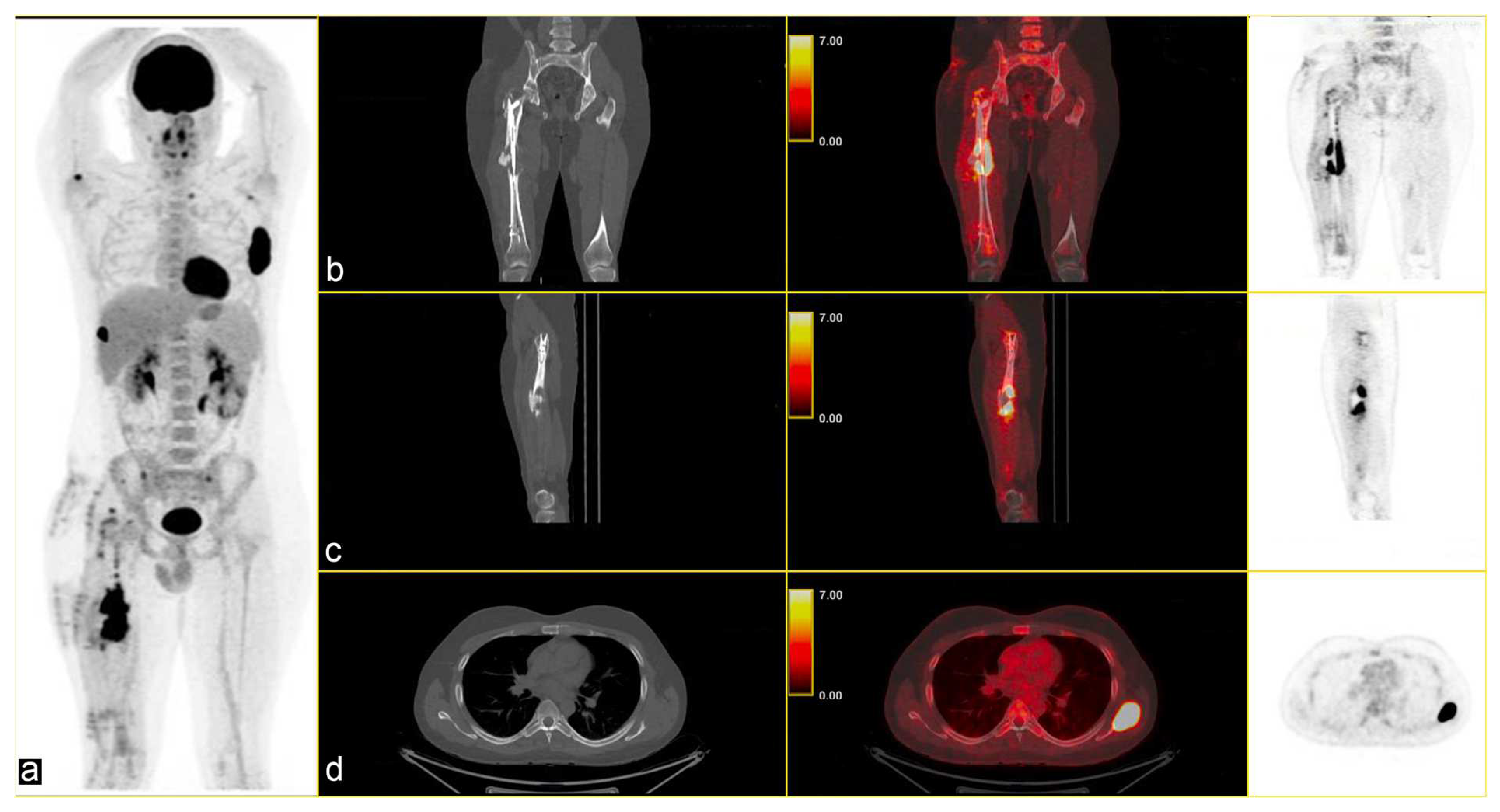
2. Case Presentation
3. Discussion
4. Material and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Billings, S.D.; Folpe, A.L.; Weiss, S.W. Epithelioid sarcoma-like hemangioendothelioma. Am. J. Surg. Pathol. 2003, 27, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L.; Fletcher, C.D. Pseudomyogenic hemangioendothelioma: A distinctive, often multicentric tumor with indolent behavior. Am. J. Surg. Pathol. 2011, 35, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Schoedel, K.; McGough, R.L.; Ranganathan, S.; Rao, U.N.M. Pseudomyogenic hemangioendothelioma of skin, bone and soft tissue-a clinicopathological, immunohistochemical, and fluorescence in situ hybridization study. Hum. Pathol. 2018, 71, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Inyang, A.; Mertens, F.; Puls, F.; Sumathi, V.; Inwards, C.; Folpe, A.; Lee, C.H.; Zhang, Y.; Symmans, P.; Rubin, B.; et al. Primary Pseudomyogenic Hemangioendothelioma of Bone. Am. J. Surg. Pathol. 2016, 40, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Kosemehmetoglu, K.; Rekhi, B.; Wakely, P.E., Jr.; Pant, V.; Dervisoglu, S.; Aydingoz, U. Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma of bone: Clinicopathologic features of 5 cases. Ann. Diagn. Pathol. 2019, 41, 116–123. [Google Scholar] [CrossRef]
- Gabor, K.M.; Sapi, Z.; Tiszlavicz, L.G.; Fige, A.; Bereczki, C.; Bartyik, K. Sirolimus therapy in the treatment of pseudomyogenic hemangioendothelioma. Pediatr. Blood Cancer 2018, 65, e26781. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L.; Fletcher, C.D.M.; Mertens Fletcher, C.D.; Bridge, J.A.; Hogendoorn, P.C.W.; Mertens, F. Psudomyogenic Haemangioendothelioma WHO Classification of Tumours of Soft Tissue and Bone; 2014th Lyon (France) International Agency for Research on Cancer: Lyon, France, 2014; pp. 153–154. [Google Scholar]
- Fan, C.; Yang, L.; Lin, X.; Wang, E. Pseudomyogenic hemangioendothelioma/epithelioid sarcoma-like hemangioendothelioma of the lower limb: Report of a rare case. Diagn. Pathol. 2015, 10, 150. [Google Scholar] [CrossRef]
- Trombetta, D.; Magnusson, L.; von Steyern, F.V.; Hornick, J.L.; Fletcher, C.D.; Mertens, F. Translocation t(7;19)(q22;q13)—A recurrent chromosome aberration in pseudomyogenic hemangioendothelioma? Cancer Genet. 2011, 204, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.; Tayebwa, J.; Lilljebjörn, H.; Magnusson, L.; Nilsson, J.; von Steyern, F.V.; Øra, I.; Domanski, H.A.; Fioretos, T.; Nord, K.H.; et al. A novel SERPINE1-FOSB fusion gene results in transcriptional up-regulation of FOSB in pseudomyogenic haemangioendothelioma. J. Pathol. 2014, 232, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Benayed, R.; Ho, C.; Mullaney, K.; Sukhadia, P.; Rios, K.; Berry, R.; Rubin, B.P.; Nafa, K.; Wang, L.; et al. Diagnosis of known sarcoma fusions and novel fusion partners by targeted RNA sequencing with identification of a recurrent ACTB-FOSB fusion in pseudomyogenic hemangioendothelioma. Mod. Pathol. 2019, 32, 609–620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panagopoulos, I.; Lobmaier, I.; Gorunova, L.; Heim, S. Fusion of the Genes WWTR1 and FOSB in Pseudomyogenic Hemangioendothelioma. Cancer Genom. Proteom. 2019, 16, 293–298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugita, S.; Hirano, H.; Kikuchi, N.; Kubo, T.; Asanuma, H.; Aoyama, T.; Emori, M.; Hasegawa, T. Diagnostic utility of FOSB immunohistochemistry in pseudomyogenic hemangioendothelioma and its histological mimics. Diagn. Pathol. 2016, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Fletcher, C.D.; Hornick, J.L. FOSB is a Useful Diagnostic Marker for Pseudomyogenic Hemangioendothelioma. Am. J. Surg. Pathol. 2017, 41, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Kallen, M.E.; Hornick, J.L. The 2020 WHO Classification: What’s new in soft tissue tumor pathology? Am. J. Surg. Pathol. 2021, 45, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaderi, A.; Mansour, A.T. Pseudomyogenic Hemangioendothelioma. Arch. Pathol. Lab. Med. 2019, 143, 763–767. [Google Scholar] [CrossRef]
- Dianat, S.; Yousaf, H.; Murugan, P.; Marette, S. Pseudomyogenic hemangioendothelioma-A case report and review of the literature. Radiol. Case Rep. 2019, 14, 1228–1232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasricha, S.; Sharma, A.; Pruthi, M.; Durga, G.; Jajodia, A.; Gupta, G.; Kamboj, M.; Gupta, M.; Mehta, A. Multifocal primary pseudomyogenic hemangioendothelioma of bone managed with denosumab: A rare case with diagnostic and therapeutic challenge. J. Cancer Res. Ther. 2022, 18, 817–819. [Google Scholar] [CrossRef]
- Frustaci, S.; Foladore, S.; Buonadonna, A.; De Paoli, A.; Crivellari, D.; Carbone, A.; Sorio, R.; Morassut, S.; Monfardini, S. Epirubicin and ifosfamide in advanced soft tissue sarcomas. Ann. Oncol. 1993, 4, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Tursz, T.; Mouridsen, H.; Verweij, J.; Steward, W.; Somers, R.; Buesa, J.; Casali, P.; Spooner, D.; Rankin, E.; et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: A randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J. Clin. Oncol. 1995, 13, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.R.; Fernando, M.; Musson, R.; Kotnis, N. An aggressive case of pseudomyogenic haemangioendothelioma of bone with pathological fracture and rapidly progressive pulmonary metastatic disease: Case report and review of the literature. Skelet. Radiol. 2015, 44, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.; Gambarotti, M.; Picci, P.; Dei Tos, A.P.; Vanel, D. Primary pseudomyogenic haemangioendothelioma of bone: Report of two cases. Skelet. Radiol. 2015, 44, 727–731. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mauro, A.; Tafuto, S.; Cannella, L.; Collina, F.; Neri, G.; Clemente, O.; D’Arbitrio, I.; Ricci, F.; Lastoria, S.; Ferrara, G.; et al. A Rare Malignant Case of a Primary Pseudomyogenic Haemangioendothelioma of the Bone. Curr. Oncol. 2025, 32, 219. https://doi.org/10.3390/curroncol32040219
Di Mauro A, Tafuto S, Cannella L, Collina F, Neri G, Clemente O, D’Arbitrio I, Ricci F, Lastoria S, Ferrara G, et al. A Rare Malignant Case of a Primary Pseudomyogenic Haemangioendothelioma of the Bone. Current Oncology. 2025; 32(4):219. https://doi.org/10.3390/curroncol32040219
Chicago/Turabian StyleDi Mauro, Annabella, Salvatore Tafuto, Lucia Cannella, Francesca Collina, Giovanni Neri, Ottavia Clemente, Imma D’Arbitrio, Francesca Ricci, Secondo Lastoria, Gerardo Ferrara, and et al. 2025. "A Rare Malignant Case of a Primary Pseudomyogenic Haemangioendothelioma of the Bone" Current Oncology 32, no. 4: 219. https://doi.org/10.3390/curroncol32040219
APA StyleDi Mauro, A., Tafuto, S., Cannella, L., Collina, F., Neri, G., Clemente, O., D’Arbitrio, I., Ricci, F., Lastoria, S., Ferrara, G., & De Chiara, A. (2025). A Rare Malignant Case of a Primary Pseudomyogenic Haemangioendothelioma of the Bone. Current Oncology, 32(4), 219. https://doi.org/10.3390/curroncol32040219






