Neuropilin-1: A Multifaceted Target for Cancer Therapy
Abstract
1. Introduction
2. VEGFA and NRP1 Signaling
3. Semaphorin-NRP1 Signaling
4. NRP1 in Cancer
5. NRP1 Promotes Angiogenesis, Tumor Proliferation, and Migration
6. Other Regulatory Role of NRP1
7. Role of NRP1 in Immune Cells
8. NRP1 in Various Cancers
8.1. Glioblastoma (GBM) and NRP1
8.2. Pancreatic Cancer and NRP1
8.3. Renal Cancer and NRP1
8.4. Melanoma and NRP1
8.5. Breast Cancer and NRP1
9. Targeting Neuropilin-1 in Cancer Therapy
9.1. Anti-NRP1 Monoclonal Antibodies (mAbs)
9.2. Cell-Penetrating Peptides (CPP)
10. NRP1 as a Biomarker in Cancer Immunotherapy
11. Role of NRP1 in Cancer Stem Cells
12. NRP1 Drug Resistance Mechanism
13. Challenges and Future Directions
13.1. NRP1 Omics in Cancer Biology
13.1.1. Genomics of NRP1 in Cancer
13.1.2. Transcriptomics of NRP1 in Cancer
13.1.3. Proteomics of NRP1 in Cancer
13.1.4. Metabolomics of NRP1 in Cancer
13.2. Integrated Omics Approaches
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| NRP1 | Neuropilin1 |
| NRP’s | Neuropilins |
| VEGF | vascular endothelial growth factor |
| TGF-β | transforming growth factor-beta |
| pDC | plasmacytoid dendritic cells. |
| BMP-1 | bone morphogenetic protein |
| CUB | extracellular complement binding |
| LAP-TGFβ | Latency associated peptide-TGFβ. |
| VEGFR2 | Vascular endothelial growth factor receptor 2. |
| HGF | Hepatic growth factor. |
| PDGF | Platelet-derived growth factor |
| VMSC | Vascular smooth muscle cells |
| FGF2 | Fibroblast growth factor2 |
| PIGF | Placental growth factor |
| PDZ | Postsynaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-1 protein (zo-1) |
| GIPC | GAIP interacting protein, C terminus |
| RAC | Ras-related C3 botulinum toxin substrate 1 |
| GTP | Guanosine triphosphate. |
| MAPK | Map kinase. |
| ABL-1 | Protooncogene 1, non-receptor tyrosine kinase gene |
| CDC 42 | Cell division cycle 42 |
| EMT | Epithelial–Mesenchymal Transition |
| PECAM-1 | Platelet Endothelial Cell Adhesion Molecule 1 |
| MMP9 | Matrix Metalloprotein 9 |
| GO/KEGG | Gene Ontology/Kyoto Encyclopedia of Genes and Genomics |
| IPA | Ingenuity Pathway Analysis. |
| CRISPR/CAS9 | Clustered regularly interspaced palindromic repeats |
| PAAD | Pancreatic adenocarcinoma. |
| PDAC | Pancreatic Ductal Adeno Carcinoma. |
| NKT | Natural Killer cells |
| CAF | Cancer-associated Fibroblasts |
| KIRC | Kidney Renal Cell Carcinoma |
| SKCM | Skin Cutaneous Melanoma |
| RCC | Renal cell Carcinoma. |
| ccRCC | Clear cell Renal Cell Carcinoma |
| mTOR | Rapamycin. |
| TTP | Tumor targeting peptides |
| ER- | negative Breast Cancer—Estrogen Receptor-negative Breast Cancer. |
| ZEB1 | Zinc Finger E-box Binding Homeobox 1 |
| ITGα 6 | Integrin subunit alpha 6 |
| PDGFR | Platelet-derived growth factor receptor |
| NFκB | Nuclear factor kappa B |
| BRCA | Breast Cancer Genes |
| (CTLA-4) | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| PD-1 | Programmed Cell Death Protein 1 |
References
- Tordjman, R.; Lepelletier, Y.; Lemarchandel, V.; Cambot, M.; Gaulard, P.; Hermine, O.; Roméo, P.H. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 2002, 3, 477–482. [Google Scholar] [PubMed]
- Wild, J.R.; Staton, C.A.; Chapple, K.; Corfe, B.M. Neuropilins: Expression and roles in the epithelium. Int. J. Exp. Pathol. 2012, 93, 81–103. [Google Scholar] [PubMed]
- Fang, J.; Lu, Y.; Zheng, J.; Jiang, X.; Shen, H.; Shang, X.; Lu, Y.; Fu, P. Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: New insights and therapeutic implications. Cell Death Dis. 2023, 14, 586. [Google Scholar] [PubMed]
- Raimondi, C.; Brash, J.T.; Fantin, A.; Ruhrberg, C. NRP1 function and targeting in neurovascular development and eye disease. Prog. Retin. Eye Res. 2016, 52, 64–83. [Google Scholar]
- Liu, S.D.; Zhong, L.P.; He, J.; Zhao, Y.X. Targeting neuropilin-1 interactions is a promising anti-tumor strategy. Chin. Med. J. 2020, 134, 508–517. [Google Scholar]
- Chaudhary, B.; Khaled, Y.S.; Ammori, B.J.; Elkord, E. Neuropilin 1: Function and therapeutic potential in cancer. Cancer Immunol. Immunother. 2014, 63, 81–99. [Google Scholar]
- Lampropoulou, A.; Ruhrberg, C. Neuropilin regulation of angiogenesis. Biochem. Soc. Trans. 2014, 42, 1623–1628. [Google Scholar]
- Plein, A.; Fantin, A.; Ruhrberg, C. Neuropilin regulation of angiogenesis, arteriogenesis, and vascular permeability. Microcirculation 2014, 21, 315–323. [Google Scholar]
- Yang, Y.; Liu, X.; Zhang, W.; Qian, Q.; Zhou, L.; Liu, S.; Li, Y.; Hou, X. Stress response proteins NRP1 and NRP2 are pro-survival factors that inhibit cell death during ER stress. Plant Physiol. 2021, 187, 1414–1427. [Google Scholar]
- Mehta, V.; Fields, L.; Evans, I.M.; Yamaji, M.; Pellet-Many, C.; Jones, T.; Mahmoud, M.; Zachary, I. VEGF (Vascular Endothelial Growth Factor) Induces NRP1 (Neuropilin-1) Cleavage via ADAMs (a Disintegrin and Metalloproteinase) 9 and 10 to Generate Novel Carboxy-Terminal NRP1 Fragments That Regulate Angiogenic Signaling. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1845–1858. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Xiong, H.; Wang, Y.; Chen, K.K.; Yang, J.; Xiao, B.; Zhang, R.; Li, S.; Sang, Y. NRP1 promotes cell migration and invasion and serves as a therapeutic target in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 2460–2469. [Google Scholar] [PubMed]
- Rodrigues, E.M.; Giovanini, A.F.; Ribas, C.; Malafaia, O.; Roesler, R.; Isolan, G.R. The Nervous System Development Regulator Neuropilin-1 as a Potential Prognostic Marker and Therapeutic Target in Brain Cancer. Cancers 2023, 15, 4922. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, A.; Allerston, C.K.; Jia, H.; Herzog, B.; Garza-Garcia, A.; Winfield, N.; Ellard, K.; Aqil, R.; Lynch, R.; Chapman, C.; et al. Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction. J. Med. Chem. 2010, 53, 2215–2226. [Google Scholar]
- Chuckran, C.A.; Liu, C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A. Neuropilin-1: A checkpoint target with unique implications for cancer immunology and immunotherapy. J. Immunother. Cancer 2020, 8, e000967. [Google Scholar]
- Guo, C.X.; Huang, X.; Xu, J.; Zhang, X.Z.; Shen, Y.N.; Liang, T.B.; Bai, X.L. Combined targeted therapy and immunotherapy for cancer treatment. World J. Clin. Cases 2021, 9, 7643–7652. [Google Scholar]
- Peach, C.J.; Tonello, R.; Damo, E.; Gomez, K.; Calderon-Rivera, A.; Bruni, R.; Bansia, H.; Maile, L.; Manu, A.-M.; Hahn, H.; et al. Neuropilin-1 inhibition suppresses nerve growth factor signaling and nociception in pain models. J. Clin. Investig. 2025, 135, e183873. [Google Scholar]
- Gu, C.; Limberg, B.J.; Whitaker, G.B.; Perman, B.; Leahy, D.J.; Rosenbaum, J.S.; Ginty, D.D.; Kolodkin, A.L. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J. Biol. Chem. 2002, 277, 18069–18076. [Google Scholar]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. BDCA-2, BDCA-3, and BDCA-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000, 165, 6037–6046. [Google Scholar]
- Romeo, P.H.; Lemarchandel, V.; Tordjman, R. Neuropilin-1 in the immune system. Adv. Exp. Med. Biol. 2002, 515, 49–54. [Google Scholar]
- Herzog, Y.; Kalcheim, C.; Kahane, N.; Reshef, R.; Neufeld, G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech. Dev. 2001, 109, 115–119. [Google Scholar]
- Battaglia, A.; Buzzonetti, A.; Monego, G.; Peri, L.; Ferrandina, G.; Fanfani, F.; Scambia, G.; Fattorossi, A. Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by preoperative chemoradiation therapy in cervical cancer. Immunology 2008, 123, 129–138. [Google Scholar] [PubMed]
- Prud'homme, G.J.; Glinka, Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 2012, 3, 921–939. [Google Scholar] [PubMed]
- Matsushita, A.; Sasajima, K.; Yokoyama, T.; Nakamura, Y.; Aimoto, T.; Uchida, E. Neuropilin-1, as a new therapeutic target in human pancreatic cancer. J. Nippon. Med. Sch. 2010, 77, 53–55. [Google Scholar] [PubMed]
- Fujisawa, H.; Kitsukawa, T.; Kawakami, A.; Takagi, S.; Shimizu, M.; Hirata, T. Roles of a neuronal cell-surface molecule, neuropilin, in nerve fiber fasciculation and guidance. Cell Tissue Res. 1997, 290, 465–470. [Google Scholar]
- Shintani, Y.; Takashima, S.; Asano, Y.; Kato, H.; Liao, Y.; Yamazaki, S.; Tsukamoto, O.; Seguchi, O.; Yamamoto, H.; Fukushima, T.; et al. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. EMBO J. 2006, 25, 3045–3055. [Google Scholar]
- Roy, S.; Bag, A.K.; Singh, R.K.; Talmadge, J.E.; Batra, S.K.; Datta, K. Multifaceted Role of Neuropilins in the Immune System: Potential Targets for Immunotherapy. Front. Immunol. 2017, 8, 1228. [Google Scholar]
- Hirata, T.; Takagi, S.; Fujisawa, H. The membrane protein A5, a putative neuronal recognition molecule, promotes neurite outgrowth. Neurosci. Res. 1993, 17, 159–169. [Google Scholar]
- Zachary, I. Neuropilins: Role in signalling, angiogenesis and disease. Chem. Immunol. Allergy 2014, 99, 37–70. [Google Scholar]
- Bernatchez, P.N.; Rollin, S.; Soker, S.; Sirois, M.G. Relative effects of VEGF-A and VEGF-C on endothelial cell proliferation, migration and PAF synthesis: Role of neuropilin-1. J. Cell Biochem. 2002, 85, 629–639. [Google Scholar]
- Iragavarapu-Charyulu, V.; Wojcikiewicz, E.; Urdaneta, A. Semaphorins in Angiogenesis and Autoimmune Diseases: Therapeutic Targets? Front. Immunol. 2020, 11, 346. [Google Scholar]
- Acevedo, L.M.; Barillas, S.; Weis, S.M.; Göthert, J.R.; Cheresh, D.A. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 2008, 111, 2674–2680. [Google Scholar] [PubMed]
- Andriessen, E.; Binet, F.; Fournier, F.; Hata, M.; Dejda, A.; Mawambo, G.; Crespo-Garcia, S.; Pilon, F.; Buscarlet, M.; Beauchemin, K.; et al. Myeloid-resident neuropilin-1 promotes choroidal neovascularization while mitigating inflammation. EMBO Mol. Med. 2021, 13, e11754. [Google Scholar] [PubMed]
- Kwiatkowski, S.C.; Guerrero, P.A.; Hirota, S.; Chen, Z.; Morales, J.E.; Aghi, M.; McCarty, J.H. Neuropilin-1 modulates TGFβ signaling to drive glioblastoma growth and recurrence after anti-angiogenic therapy. PLoS ONE 2017, 12, e0185065. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma'ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar]
- Janssen, B.J.; Malinauskas, T.; Weir, G.A.; Cader, M.Z.; Siebold, C.; Jones, E.Y. Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex. Nat. Struct. Mol. Biol. 2012, 19, 1293–1299. [Google Scholar]
- Lepelletier, Y.; Moura, I.C.; Hadj-Slimane, R.; Renand, A.; Fiorentino, S.; Baude, C.; Shirvan, A.; Barzilai, A.; Hermine, O. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur. J. Immunol. 2006, 36, 1782–1793. [Google Scholar]
- Catalano, A. The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis. J. Immunol. 2010, 185, 6373–6383. [Google Scholar]
- Delgoffe, G.M.; Woo, S.R.; Turnis, M.E.; Gravano, D.M.; Guy, C.; Overacre, A.E.; Bettini, M.L.; Vogel, P.; Finkelstein, D.; Bonnevier, J.; et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013, 501, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Glinka, Y.; Prud'homme, G.J. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 2008, 84, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Soker, S.; Miao, H.Q.; Nomi, M.; Takashima, S.; Klagsbrun, M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J. Cell Biochem. 2002, 85, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Mac Gabhann, F.; Popel, A.S. Differential binding of VEGF isoforms to VEGF receptor 2 in the presence of neuropilin-1: A computational model. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2851–H2860. [Google Scholar] [CrossRef]
- Sulpice, E.; Plouët, J.; Bergé, M.; Allanic, D.; Tobelem, G.; Merkulova-Rainon, T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 2008, 111, 2036–2045. [Google Scholar] [CrossRef]
- Matsushita, A.; Götze, T.; Korc, M. Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 2007, 67, 10309–10316. [Google Scholar] [CrossRef]
- Banerjee, S.; Sengupta, K.; Dhar, K.; Mehta, S.; D'Amore, P.A.; Dhar, G.; Banerjee, S.K. Breast cancer cells secreted platelet-derived growth factor-induced motility of vascular smooth muscle cells is mediated through neuropilin-1. Mol. Carcinog. 2006, 45, 871–880. [Google Scholar] [CrossRef]
- West, D.C.; Rees, C.G.; Duchesne, L.; Patey, S.J.; Terry, C.J.; Turnbull, J.E.; Delehedde, M.; Heegaard, C.W.; Allain, F.; Vanpouille, C.; et al. Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J. Biol. Chem. 2005, 280, 13457–13464. [Google Scholar] [CrossRef]
- Snuderl, M.; Batista, A.; Kirkpatrick, N.D.; Ruiz de Almodovar, C.; Riedemann, L.; Walsh, E.C.; Anolik, R.; Huang, Y.; Martin, J.D.; Kamoun, W.; et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 2013, 152, 1065–1076. [Google Scholar] [CrossRef]
- Houck, K.A.; Leung, D.W.; Rowland, A.M.; Winer, J.; Ferrara, N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 1992, 267, 26031–26037. [Google Scholar] [CrossRef]
- Nishijima, K.; Ng, Y.S.; Zhong, L.; Bradley, J.; Schubert, W.; Jo, N.; Akita, J.; Samuelsson, S.J.; Robinson, G.S.; Adamis, A.P.; et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007, 171, 53–67. [Google Scholar] [PubMed]
- Erskine, L.; Reijntjes, S.; Pratt, T.; Denti, L.; Schwarz, Q.; Vieira, J.M.; Alakakone, B.; Shewan, D.; Ruhrberg, C. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron 2011, 70, 951–965. [Google Scholar] [PubMed]
- Lanahan, A.; Zhang, X.; Fantin, A.; Zhuang, Z.; Rivera-Molina, F.; Speichinger, K.; Prahst, C.; Zhang, J.; Wang, Y.; Davis, G.; et al. The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev. Cell 2013, 25, 156–168. [Google Scholar]
- Bovenkamp, D.E.; Goishi, K.; Bahary, N.; Davidson, A.J.; Zhou, Y.; Becker, T.; Becker, C.G.; Zon, L.I.; Klagsbrun, M. Expression and mapping of duplicate neuropilin-1 and neuropilin-2 genes in developing zebrafish. Gene Expr. Patterns 2004, 4, 361–370. [Google Scholar] [CrossRef]
- Fantin, A.; Schwarz, Q.; Davidson, K.; Normando, E.M.; Denti, L.; Ruhrberg, C. The cytoplasmic domain of neuropilin 1 is dispensable for angiogenesis, but promotes the spatial separation of retinal arteries and veins. Development 2011, 138, 4185–4191. [Google Scholar] [CrossRef]
- Fantin, A.; Lampropoulou, A.; Senatore, V.; Brash, J.T.; Prahst, C.; Lange, C.A.; Liyanage, S.E.; Raimondi, C.; Bainbridge, J.W.; Augustin, H.G.; et al. VEGF165-induced vascular permeability requires NRP1 for ABL-mediated SRC family kinase activation. J. Exp. Med. 2017, 214, 1049–1064. [Google Scholar]
- Cao, Y.; E, G.; Wang, E.; Pal, K.; Dutta, S.K.; Bar-Sagi, D.; Mukhopadhyay, D. VEGF Exerts an Angiogenesis-Independent Function in Cancer Cells to Promote Their Malignant Progression. Cancer Res. 2012, 72, 3912–3918. [Google Scholar]
- Kolodkin, A.L.; Ginty, D.D. Steering clear of semaphorins: Neuropilins sound the retreat. Neuron 1997, 19, 1159–1162. [Google Scholar]
- Castro-Rivera, E.; Ran, S.; Thorpe, P.; Minna, J.D. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc. Natl. Acad. Sci. USA 2004, 101, 11432–11437. [Google Scholar]
- Loginov, V.I.; Dmitriev, A.A.; Senchenko, V.N.; Pronina, I.V.; Khodyrev, D.S.; Kudryavtseva, A.V.; Krasnov, G.S.; Gerashchenko, G.V.; Chashchina, L.I.; Kazubskaya, T.P.; et al. Tumor Suppressor Function of the SEMA3B Gene in Human Lung and Renal Cancers. PLoS ONE 2015, 10, e0123369. [Google Scholar]
- Varshavsky, A.; Kessler, O.; Abramovitch, S.; Kigel, B.; Zaffryar, S.; Akiri, G.; Neufeld, G. Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res. 2008, 68, 6922–6931. [Google Scholar] [PubMed]
- Karayan-Tapon, L.; Wager, M.; Guilhot, J.; Levillain, P.; Marquant, C.; Clarhaut, J.; Potiron, V.; Roche, J. Semaphorin, neuropilin and VEGF expression in glial tumours: SEMA3G, a prognostic marker? Br. J. Cancer 2008, 99, 1153–1160. [Google Scholar]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [PubMed]
- Toledano, S.; Nir-Zvi, I.; Engelman, R.; Kessler, O.; Neufeld, G. Class-3 Semaphorins and Their Receptors: Potent Multifunctional Modulators of Tumor Progression. Int. J. Mol. Sci. 2019, 20, 556. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Fournier, A.; Nakamura, F.; Wang, L.H.; Murakami, Y.; Kalb, R.G.; Fujisawa, H.; Strittmatter, S.M. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 1999, 99, 59–69. [Google Scholar]
- Sharma, S.; Ehrlich, M.; Zhang, M.; Blobe, G.C.; Henis, Y.I. NRP1 interacts with endoglin and VEGFR2 to modulate VEGF signaling and endothelial cell sprouting. Commun. Biol. 2024, 7, 112. [Google Scholar]
- Adams, R.H.; Lohrum, M.; Klostermann, A.; Betz, H.; Püschel, A.W. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997, 16, 6077–6086. [Google Scholar]
- Guo, H.F.; Li, X.; Parker, M.W.; Waltenberger, J.; Becker, P.M.; Vander Kooi, C.W. Mechanistic basis for the potent anti-angiogenic activity of semaphorin 3F. Biochemistry 2013, 52, 7551–7558. [Google Scholar]
- Takahashi, T.; Strittmatter, S.M. Plexina1 autoinhibition by the plexin sema domain. Neuron 2001, 29, 429–439. [Google Scholar]
- Liu, H.; Juo, Z.S.; Shim, A.H.; Focia, P.J.; Chen, X.; Garcia, K.C.; He, X. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell 2010, 142, 749–761. [Google Scholar]
- Nogi, T.; Yasui, N.; Mihara, E.; Matsunaga, Y.; Noda, M.; Yamashita, N.; Toyofuku, T.; Uchiyama, S.; Goshima, Y.; Kumanogoh, A.; et al. Structural basis for semaphorin signalling through the plexin receptor. Nature 2010, 467, 1123–1127. [Google Scholar] [PubMed]
- Barnkob, M.B.; Michaels, Y.S.; André, V.; Macklin, P.S.; Gileadi, U.; Valvo, S.; Rei, M.; Kulicke, C.; Chen, J.L.; Jain, V.; et al. Semmaphorin 3 A causes immune suppression by inducing cytoskeletal paralysis in tumour-specific CD8(+) T cells. Nat. Commun. 2024, 15, 3173, Erratum in Nat. Commun. 2024, 15, 3448. [Google Scholar] [PubMed]
- Cao, Y.; Wang, L.; Nandy, D.; Zhang, Y.; Basu, A.; Radisky, D.; Mukhopadhyay, D. Neuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axes. Cancer Res. 2008, 68, 8667–8672. [Google Scholar]
- Chu, W.; Song, X.; Yang, X.; Ma, L.; Zhu, J.; He, M.; Wang, Z.; Wu, Y. Neuropilin-1 promotes epithelial-to-mesenchymal transition by stimulating nuclear factor-kappa B and is associated with poor prognosis in human oral squamous cell carcinoma. PLoS ONE 2014, 9, e101931. [Google Scholar]
- Miao, H.Q.; Lee, P.; Lin, H.; Soker, S.; Klagsbrun, M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000, 14, 2532–2539. [Google Scholar]
- Wang, Y.; Wang, E.; Anany, M.; Füllsack, S.; Huo, Y.H.; Dutta, S.; Ji, B.; Hoeppner, L.H.; Kilari, S.; Misra, S.; et al. The crosstalk between neuropilin-1 and tumor necrosis factor-α in endothelial cells. Front. Cell Dev. Biol. 2024, 12, 1210944. [Google Scholar]
- Vivekanandhan, S.; Yang, L.; Cao, Y.; Wang, E.; Dutta, S.K.; Sharma, A.K.; Mukhopadhyay, D. Genetic status of KRAS modulates the role of Neuropilin-1 in tumorigenesis. Sci. Rep. 2017, 7, 12877. [Google Scholar]
- Pal, K.; Madamsetty, V.S.; Dutta, S.K.; Wang, E.; Angom, R.S.; Mukhopadhyay, D. Synchronous inhibition of mTOR and VEGF/NRP1 axis impedes tumor growth and metastasis in renal cancer. npj Precis. Oncol. 2019, 3, 31. [Google Scholar]
- Vivekanandhan, S.; Mukhopadhyay, D. Genetic status of KRAS influences Transforming Growth Factor-beta (TGF-β) signaling: An insight into Neuropilin-1 (NRP1) mediated tumorigenesis. Semin. Cancer Biol. 2019, 54, 72–79. [Google Scholar]
- Pan, Q.; Chanthery, Y.; Liang, W.C.; Stawicki, S.; Mak, J.; Rathore, N.; Tong, R.K.; Kowalski, J.; Yee, S.F.; Pacheco, G.; et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 2007, 11, 53–67. [Google Scholar]
- Feldman, D.R.; Baum, M.S.; Ginsberg, M.S.; Hassoun, H.; Flombaum, C.D.; Velasco, S.; Fischer, P.; Ronnen, E.; Ishill, N.; Patil, S.; et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009, 27, 1432–1439. [Google Scholar] [PubMed]
- Xie, C.; He, J.; Meng, C.; Chen, X.; Liu, H.; Sun, B. Red emissive N-doped carbon dots encapsulated within molecularly imprinted polymers for optosensing of pyrraline in fatty foods. Mikrochim. Acta 2023, 190, 88. [Google Scholar] [PubMed]
- Bica, C.; Tirpe, A.; Nutu, A.; Ciocan, C.; Chira, S.; Gurzau, E.S.; Braicu, C.; Berindan-Neagoe, I. Emerging roles and mechanisms of semaphorins activity in cancer. Life Sci. 2023, 318, 121499. [Google Scholar] [PubMed]
- Casazza, A.; Laoui, D.; Wenes, M.; Rizzolio, S.; Bassani, N.; Mambretti, M.; Deschoemaeker, S.; Van Ginderachter, J.A.; Tamagnone, L.; Mazzone, M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 2013, 24, 695–709. [Google Scholar]
- Gao, P.; Ren, G.; Liang, J.; Liu, J. STAT6 Upregulates NRP1 Expression in Endothelial Cells and Promotes Angiogenesis. Front. Oncol. 2022, 12, 823377. [Google Scholar]
- Zhang, M.; Zhou, K.; Wang, Z.; Liu, T.; Stevens, L.E.; Lynce, F.; Chen, W.Y.; Peng, S.; Xie, Y.; Zhai, D.; et al. A Subpopulation of Luminal Progenitors Secretes Pleiotrophin to Promote Angiogenesis and Metastasis in Inflammatory Breast Cancer. Cancer Res. 2024, 84, 1781–1798. [Google Scholar]
- Lyu, Z.; Jin, H.; Yan, Z.; Hu, K.; Jiang, H.; Peng, H.; Zhuo, H. Effects of NRP1 on angiogenesis and vascular maturity in endothelial cells are dependent on the expression of SEMA4D. Int. J. Mol. Med. 2020, 46, 1321–1334. [Google Scholar]
- El Baba, N.; Farran, M.; Khalil, E.A.; Jaafar, L.; Fakhoury, I.; El-Sibai, M. The Role of Rho GTPases in VEGF Signaling in Cancer Cells. Anal. Cell. Pathol. 2020, 2020, 2097214. [Google Scholar]
- Yoshida, A.; Shimizu, A.; Asano, H.; Kadonosono, T.; Kondoh, S.K.; Geretti, E.; Mammoto, A.; Klagsbrun, M.; Seo, M.K. VEGF-A/NRP1 stimulates GIPC1 and Syx complex formation to promote RhoA activation and proliferation in skin cancer cells. Biol. Open 2015, 4, 1063–1076. [Google Scholar]
- Dong, Y.; Ma, W.M.; Shi, Z.D.; Zhang, Z.G.; Zhou, J.H.; Li, Y.; Zhang, S.Q.; Pang, K.; Li, B.B.; Zhang, W.D.; et al. Role of NRP1 in Bladder Cancer Pathogenesis and Progression. Front. Oncol. 2021, 11, 685980. [Google Scholar]
- Peng, H.; Yang, M.; Feng, K.; Lv, Q.; Zhang, Y. Semaphorin 3C (Sema3C) reshapes stromal microenvironment to promote hepatocellular carcinoma progression. Signal Transduct. Target. Ther. 2024, 9, 169. [Google Scholar] [PubMed]
- Yin, L.; Li, J.; Wang, J.; Pu, T.; Wei, J.; Li, Q.; Wu, B.J. MAOA promotes prostate cancer cell perineural invasion through SEMA3C/PlexinA2/NRP1-cMET signaling. Oncogene 2021, 40, 1362–1374. [Google Scholar] [PubMed]
- Zhang, P.; Chen, L.; Zhou, F.; He, Z.; Wang, G.; Luo, Y. NRP1 promotes prostate cancer progression via modulating EGFR-dependent AKT pathway activation. Cell Death Dis. 2023, 14, 159. [Google Scholar]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Yin, H.; Gu, S.; Li, G.; Yu, H.; Zhang, X.; Zuo, Y. Long noncoding RNA PVT1 predicts poor prognosis and promotes the progression of colorectal cancer through the miR-24-3p/NRP1 axis in zebrafish xenografts. Neoplasma 2023, 70, 500–513. [Google Scholar]
- Li, X.; Fan, S.; Pan, X.; Xiaokaiti, Y.; Duan, J.; Shi, Y.; Pan, Y.; Tie, L.; Wang, X.; Li, Y.; et al. Nordihydroguaiaretic acid impairs prostate cancer cell migration and tumor metastasis by suppressing neuropilin 1. Oncotarget 2016, 7, 86225–86238. [Google Scholar]
- Nerlakanti, N.; McGuire, J.J.; Bishop, R.T.; Nasr, M.M.; Li, T.; Reed, D.R.; Lynch, C.C. Histone deacetylase upregulation of neuropilin-1 in osteosarcoma is essential for pulmonary metastasis. Cancer Lett. 2024, 606, 217302. [Google Scholar]
- Vintonenko, N.; Pelaez-Garavito, I.; Buteau-Lozano, H.; Toullec, A.; Lidereau, R.; Perret, G.Y.; Bieche, I.; Perrot-Applanat, M. Overexpression of VEGF189 in breast cancer cells induces apoptosis via NRP1 under stress conditions. Cell Adhes. Migr. 2011, 5, 332–343. [Google Scholar]
- Meier, P.; Legrand, A.J.; Adam, D.; Silke, J. Immunogenic cell death in cancer: Targeting necroptosis to induce antitumour immunity. Nat. Rev. Cancer 2024, 24, 299–315. [Google Scholar]
- Li, J.; Yu, T.; Sun, J.; Zeng, Z.; Liu, Z.; Ma, M.; Zheng, Z.; He, Y.; Kang, W. Comprehensive analysis of cuproptosis-related immune biomarker signature to enhance prognostic accuracy in gastric cancer. Aging 2023, 15, 2772–2796. [Google Scholar]
- Sarris, M.; Andersen, K.G.; Randow, F.; Mayr, L.; Betz, A.G. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 2008, 28, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Bruder, D.; Probst-Kepper, M.; Westendorf, A.M.; Geffers, R.; Beissert, S.; Loser, K.; von Boehmer, H.; Buer, J.; Hansen, W. Neuropilin-1: A surface marker of regulatory T cells. Eur. J. Immunol. 2004, 34, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Lepelletier, Y.; Smaniotto, S.; Hadj-Slimane, R.; Villa-Verde, D.M.; Nogueira, A.C.; Dardenne, M.; Hermine, O.; Savino, W. Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions. Proc. Natl. Acad. Sci. USA 2007, 104, 5545–5550. [Google Scholar] [CrossRef] [PubMed]
- Renand, A.; Milpied, P.; Rossignol, J.; Bruneau, J.; Lemonnier, F.; Dussiot, M.; Coulon, S.; Hermine, O. Neuropilin-1 expression characterizes T follicular helper (Tfh) cells activated during B cell differentiation in human secondary lymphoid organs. PLoS ONE 2013, 8, e85589. [Google Scholar] [CrossRef]
- Liu, C.; Somasundaram, A.; Manne, S.; Gocher, A.M.; Szymczak-Workman, A.L.; Vignali, K.M.; Scott, E.N.; Normolle, D.P.; John Wherry, E.; Lipson, E.J.; et al. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat. Immunol. 2020, 21, 1010–1021. [Google Scholar] [CrossRef]
- Singh, K.; Hjort, M.; Thorvaldson, L.; Sandler, S. Concomitant analysis of Helios and Neuropilin-1 as a marker to detect thymic derived regulatory T cells in naïve mice. Sci. Rep. 2015, 5, 7767. [Google Scholar] [CrossRef]
- Leclerc, M.; Voilin, E.; Gros, G.; Corgnac, S.; de Montpréville, V.; Validire, P.; Bismuth, G.; Mami-Chouaib, F. Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat. Commun. 2019, 10, 3345. [Google Scholar] [CrossRef]
- Philip, M.; Fairchild, L.; Sun, L.; Horste, E.L.; Camara, S.; Shakiba, M.; Scott, A.C.; Viale, A.; Lauer, P.; Merghoub, T.; et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017, 545, 452–456. [Google Scholar] [CrossRef]
- Chi, H.; Deng, S.; Xu, K.; Zhang, Y.; Song, T.; Yu, J.; Wang, Y.; Liu, J.; Zhang, Y.; Shi, J.; et al. SEMA3G-NRP1 Signaling Functions as an Immune Checkpoint That Enables Tumor Immune Evasion by Impairing T-cell Cytotoxicity. Cancer Res. 2025, 85, 912–924. [Google Scholar] [CrossRef]
- Zhang, H.; Tomar, V.S.; Li, J.; Basavaraja, R.; Yan, F.; Gui, J.; McBrearty, N.; Costich, T.L.; Beiting, D.P.; Blanco, M.A.; et al. Protection of Regulatory T Cells from Fragility and Inactivation in the Tumor Microenvironment. Cancer Immunol. Res. 2022, 10, 1490–1505. [Google Scholar] [CrossRef]
- He, L.H.; Zhang, X.Z.; Lao, M.Y.; Zhang, H.J.; Yang, H.S.; Bai, X.L. Immune Checkpoint Neuropilins as Novel Biomarkers and Therapeutic Targets for Pancreatic Cancer. Cancers 2023, 15, 2225. [Google Scholar] [CrossRef] [PubMed]
- Schellenburg, S.; Schulz, A.; Poitz, D.M.; Muders, M.H. Role of neuropilin-2 in the immune system. Mol. Immunol. 2017, 90, 239–244. [Google Scholar]
- Curreli, S.; Arany, Z.; Gerardy-Schahn, R.; Mann, D.; Stamatos, N.M. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J. Biol. Chem. 2007, 282, 30346–30356. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O'Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar]
- Stamatos, N.M.; Zhang, L.; Jokilammi, A.; Finne, J.; Chen, W.H.; El-Maarouf, A.; Cross, A.S.; Hankey, K.G. Changes in polysialic acid expression on myeloid cells during differentiation and recruitment to sites of inflammation: Role in phagocytosis. Glycobiology 2014, 24, 864–879. [Google Scholar]
- Campos-Mora, M.; Contreras-Kallens, P.; Gálvez-Jirón, F.; Rojas, M.; Rojas, C.; Refisch, A.; Cerda, O.; Pino-Lagos, K. CD4+Foxp3+T Regulatory Cells Promote Transplantation Tolerance by Modulating Effector CD4+ T Cells in a Neuropilin-1-Dependent Manner. Front. Immunol. 2019, 10, 882. [Google Scholar]
- Hansen, W.; Hutzler, M.; Abel, S.; Alter, C.; Stockmann, C.; Kliche, S.; Albert, J.; Sparwasser, T.; Sakaguchi, S.; Westendorf, A.M.; et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med. 2012, 209, 2001–2016. [Google Scholar] [CrossRef]
- Dumond, A.; Pagès, G. Neuropilins, as Relevant Oncology Target: Their Role in the Tumoral Microenvironment. Front. Cell Dev. Biol. 2020, 8, 662. [Google Scholar]
- Sun, S.; Lei, Y.; Li, Q.; Wu, Y.; Zhang, L.; Mu, P.P.; Ji, G.Q.; Tang, C.X.; Wang, Y.Q.; Gao, J.; et al. Neuropilin-1 is a glial cell line-derived neurotrophic factor receptor in glioblastoma. Oncotarget 2017, 8, 74019–74035. [Google Scholar]
- Angom, R.S.; Mondal, S.K.; Wang, F.; Madamsetty, V.S.; Wang, E.; Dutta, S.K.; Gulani, Y.; Sarabia-Estrada, R.; Sarkaria, J.N.; Quiñones-Hinojosa, A.; et al. Ablation of neuropilin-1 improves the therapeutic response in conventional drug-resistant glioblastoma multiforme. Oncogene 2020, 39, 7114–7126. [Google Scholar]
- Matkar, P.N.; Jong, E.D.; Ariyagunarajah, R.; Prud'homme, G.J.; Singh, K.K.; Leong-Poi, H. Jack of many trades: Multifaceted role of neuropilins in pancreatic cancer. Cancer Med. 2018, 7, 5036–5046. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Wey, J.S.; Belcheva, A.; McCarty, M.F.; Trevino, J.G.; Evans, D.B.; Ellis, L.M.; Gallick, G.E. Neuropilin-1 suppresses tumorigenic properties in a human pancreatic adenocarcinoma cell line lacking neuropilin-1 coreceptors. Cancer Res. 2005, 65, 3664–3670. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.G.; Akter, S.; Uddin, M.J. Emerging Role of Neuropilin-1 and Angiotensin-Converting Enzyme-2 in Renal Carcinoma-Associated COVID-19 Pathogenesis. Infect. Dis. Rep. 2021, 13, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Morin, E.; Lindskog, C.; Johansson, M.; Egevad, L.; Sandström, P.; Harmenberg, U.; Claesson-Welsh, L.; Sjöberg, E. Perivascular Neuropilin-1 expression is an independent marker of improved survival in renal cell carcinoma. J. Pathol. 2020, 250, 387–396. [Google Scholar] [CrossRef]
- Grandclement, C.; Borg, C. Neuropilins: A new target for cancer therapy. Cancers 2011, 3, 1899–1928. [Google Scholar] [CrossRef]
- Hansel, D.E.; Wilentz, R.E.; Yeo, C.J.; Schulick, R.D.; Montgomery, E.; Maitra, A. Expression of neuropilin-1 in high-grade dysplasia, invasive cancer, and metastases of the human gastrointestinal tract. Am. J. Surg. Pathol. 2004, 28, 347–356. [Google Scholar] [CrossRef]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef]
- Bachelder, R.E.; Crago, A.; Chung, J.; Wendt, M.A.; Shaw, L.M.; Robinson, G.; Mercurio, A.M. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001, 61, 5736–5740. [Google Scholar]
- Pellet-Many, C.; Frankel, P.; Evans, I.M.; Herzog, B.; Jünemann-Ramírez, M.; Zachary, I.C. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem. J. 2011, 435, 609–618. [Google Scholar] [CrossRef]
- Jia, H.; Cheng, L.; Tickner, M.; Bagherzadeh, A.; Selwood, D.; Zachary, I. Neuropilin-1 antagonism in human carcinoma cells inhibits migration and enhances chemosensitivity. Br. J. Cancer 2010, 102, 541–552. [Google Scholar] [CrossRef]
- Lacal, P.M.; Failla, C.M.; Pagani, E.; Odorisio, T.; Schietroma, C.; Falcinelli, S.; Zambruno, G.; D'Atri, S. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J. Investig. Dermatol. 2000, 115, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Lacal, P.M.; Ruffini, F.; Pagani, E.; D’Atri, S. An autocrine loop directed by the vascular endothelial growth factor promotes invasiveness of human melanoma cells. Int. J. Oncol. 2005, 27, 1625–1632. [Google Scholar] [PubMed]
- Ruffini, F.; Graziani, G.; Levati, L.; Tentori, L.; D’Atri, S.; Lacal, P.M. Cilengitide downmodulates invasiveness and vasculogenic mimicry of neuropilin 1 expressing melanoma cells through the inhibition of αvβ5 integrin. Int. J. Cancer 2015, 136, E545–E558. [Google Scholar] [PubMed]
- Ruffini, F.; Tentori, L.; Dorio, A.S.; Arcelli, D.; D'Amati, G.; D’Atri, S.; Graziani, G.; Lacal, P.M. Platelet-derived growth factor C and calpain-3 are modulators of human melanoma cell invasiveness. Oncol. Rep. 2013, 30, 2887–2896. [Google Scholar]
- Tang, Y.H.; Rockstroh, A.; Sokolowski, K.A.; Lynam, L.R.; Lehman, M.; Thompson, E.W.; Gregory, P.A.; Nelson, C.C.; Volpert, M.; Hollier, B.G. Neuropilin-1 is over-expressed in claudin-low breast cancer and promotes tumor progression through acquisition of stem cell characteristics and RAS/MAPK pathway activation. Breast Cancer Res. 2022, 24, 8. [Google Scholar] [CrossRef]
- Abdullah, A.; Akhand, S.S.; Paez, J.S.P.; Brown, W.; Pan, L.; Libring, S.; Badamy, M.; Dykuizen, E.; Solorio, L.; Andy Tao, W.; et al. Epigenetic targeting of neuropilin-1 prevents bypass signaling in drug-resistant breast cancer. Oncogene 2021, 40, 322–333. [Google Scholar] [CrossRef]
- Luo, M.; Hou, L.; Li, J.; Shao, S.; Huang, S.; Meng, D.; Liu, L.; Feng, L.; Xia, P.; Qin, T.; et al. VEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-κB and β-catenin. Cancer Lett. 2016, 373, 1–11. [Google Scholar]
- Powell, J.; Mota, F.; Steadman, D.; Soudy, C.; Miyauchi, J.T.; Crosby, S.; Jarvis, A.; Reisinger, T.; Winfield, N.; Evans, G.; et al. Small Molecule Neuropilin-1 Antagonists Combine Antiangiogenic and Antitumor Activity with Immune Modulation through Reduction of Transforming Growth Factor Beta (TGFβ) Production in Regulatory T-Cells. J. Med. Chem. 2018, 61, 4135–4154. [Google Scholar]
- Liang, W.C.; Dennis, M.S.; Stawicki, S.; Chanthery, Y.; Pan, Q.; Chen, Y.; Eigenbrot, C.; Yin, J.; Koch, A.W.; Wu, X.; et al. Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J. Mol. Biol. 2007, 366, 815–829. [Google Scholar]
- Xin, Y.; Li, J.; Wu, J.; Kinard, R.; Weekes, C.D.; Patnaik, A.; Lorusso, P.; Brachmann, R.; Tong, R.K.; Yan, Y.; et al. Pharmacokinetic and pharmacodynamic analysis of circulating biomarkers of anti-NRP1, a novel antiangiogenesis agent, in two phase I trials in patients with advanced solid tumors. Clin. Cancer Res. 2012, 18, 6040–6048. [Google Scholar] [CrossRef]
- Barr, M.P.; Byrne, A.M.; Duffy, A.M.; Condron, C.M.; Devocelle, M.; Harriott, P.; Bouchier-Hayes, D.J.; Harmey, J.H. A peptide corresponding to the neuropilin-1-binding site on VEGF(165) induces apoptosis of neuropilin-1-expressing breast tumour cells. Br. J. Cancer 2005, 92, 328–333. [Google Scholar] [PubMed]
- Teesalu, T.; Sugahara, K.N.; Ruoslahti, E. Tumor-penetrating peptides. Front. Oncol. 2013, 3, 216. [Google Scholar] [CrossRef] [PubMed]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar]
- Cackowski, F.C.; Xu, L.; Hu, B.; Cheng, S.Y. Identification of two novel alternatively spliced Neuropilin-1 isoforms. Genomics 2004, 84, 82–94. [Google Scholar]
- Uniewicz, K.A.; Cross, M.J.; Fernig, D.G. Exogenous recombinant dimeric neuropilin-1 is sufficient to drive angiogenesis. J. Biol. Chem. 2011, 286, 12–23. [Google Scholar]
- Narazaki, M.; Tosato, G. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood 2006, 107, 3892–3901. [Google Scholar]
- Schuch, G.; Machluf, M.; Bartsch, G., Jr.; Nomi, M.; Richard, H.; Atala, A.; Soker, S. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood 2002, 100, 4622–4628. [Google Scholar]
- Bartsch, G., Jr.; Eggert, K.; Soker, S.; Bokemeyer, C.; Hautmann, R.; Schuch, G. Combined antiangiogenic therapy is superior to single inhibitors in a model of renal cell carcinoma. J. Urol. 2008, 179, 326–332. [Google Scholar]
- Hong, T.M.; Chen, Y.L.; Wu, Y.Y.; Yuan, A.; Chao, Y.C.; Chung, Y.C.; Wu, M.H.; Yang, S.C.; Pan, S.H.; Shih, J.Y.; et al. Targeting neuropilin 1 as an antitumor strategy in lung cancer. Clin. Cancer Res. 2007, 13, 4759–4768. [Google Scholar]
- Bergé, M.; Bonnin, P.; Sulpice, E.; Vilar, J.; Allanic, D.; Silvestre, J.S.; Lévy, B.I.; Tucker, G.C.; Tobelem, G.; Merkulova-Rainon, T. Small interfering RNAs induce target-independent inhibition of tumor growth and vasculature remodeling in a mouse model of hepatocellular carcinoma. Am. J. Pathol. 2010, 177, 3192–3201. [Google Scholar]
- Xin Yu, J.; Hubbard-Lucey, V.M.; Tang, J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 2019, 18, 899–900. [Google Scholar] [PubMed]
- Rad, H.S.; Rad, H.S.; Shiravand, Y.; Radfar, P.; Arpon, D.; Warkiani, M.E.; O'Byrne, K.; Kulasinghe, A. The Pandora's box of novel technologies that may revolutionize lung cancer. Lung Cancer 2021, 159, 34–41. [Google Scholar] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Overacre-Delgoffe, A.E.; Chikina, M.; Dadey, R.E.; Yano, H.; Brunazzi, E.A.; Shayan, G.; Horne, W.; Moskovitz, J.M.; Kolls, J.K.; Sander, C.; et al. Interferon-γ Drives T(reg) Fragility to Promote Anti-tumor Immunity. Cell 2017, 169, 1130–1141.e1111. [Google Scholar]
- Jung, K.; Kim, J.A.; Kim, Y.J.; Lee, H.W.; Kim, C.H.; Haam, S.; Kim, Y.S. A Neuropilin-1 Antagonist Exerts Antitumor Immunity by Inhibiting the Suppressive Function of Intratumoral Regulatory T Cells. Cancer Immunol. Res. 2020, 8, 46–56. [Google Scholar]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar]
- Douyère, M.; Chastagner, P.; Boura, C. Neuropilin-1: A Key Protein to Consider in the Progression of Pediatric Brain Tumors. Front. Oncol. 2021, 11, 665634. [Google Scholar]
- Cojoc, M.; Mäbert, K.; Muders, M.H.; Dubrovska, A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin. Cancer Biol. 2015, 31, 16–27. [Google Scholar]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; ME, L.L. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar]
- Grun, D.; Adhikary, G.; Eckert, R.L. VEGF-A acts via neuropilin-1 to enhance epidermal cancer stem cell survival and formation of aggressive and highly vascularized tumors. Oncogene 2016, 35, 4379–4387. [Google Scholar]
- Elaimy, A.L.; Amante, J.J.; Zhu, L.J.; Wang, M.; Walmsley, C.S.; FitzGerald, T.J.; Goel, H.L.; Mercurio, A.M. The VEGF receptor neuropilin 2 promotes homologous recombination by stimulating YAP/TAZ-mediated Rad51 expression. Proc. Natl. Acad. Sci. USA 2019, 116, 14174–14180. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Pursell, B.; Chang, C.; Shaw, L.M.; Mao, J.; Simin, K.; Kumar, P.; Vander Kooi, C.W.; Shultz, L.D.; Greiner, D.L.; et al. GLI1 regulates a novel neuropilin-2/α6β1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol. Med. 2013, 5, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Grun, D.; Adhikary, G.; Eckert, R.L. NRP-1 interacts with GIPC1 and α6/β4-integrins to increase YAP1/∆Np63α-dependent epidermal cancer stem cell survival. Oncogene 2018, 37, 4711–4722. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Van Buren, G.; Dallas, N.A.; Xia, L.; Wang, X.; Yang, A.D.; Somcio, R.J.; Lin, Y.G.; Lim, S.; Fan, F.; et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J. Natl. Cancer Inst. 2008, 100, 109–120. [Google Scholar] [CrossRef]
- Cao, Y.; Hoeppner, L.H.; Bach, S.; E, G.; Guo, Y.; Wang, E.; Wu, J.; Cowley, M.J.; Chang, D.K.; Waddell, N.; et al. Neuropilin-2 promotes extravasation and metastasis by interacting with endothelial α5 integrin. Cancer Res. 2013, 73, 4579–4590. [Google Scholar] [CrossRef]
- Mitra, A.; Mishra, L.; Li, S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015, 6, 10697–10711. [Google Scholar] [CrossRef]
- Rizzolio, S.; Cagnoni, G.; Battistini, C.; Bonelli, S.; Isella, C.; Van Ginderachter, J.A.; Bernards, R.; Di Nicolantonio, F.; Giordano, S.; Tamagnone, L. Neuropilin-1 upregulation elicits adaptive resistance to oncogene-targeted therapies. J. Clin. Investig. 2018, 128, 3976–3990. [Google Scholar] [CrossRef]
- Napolitano, V.; Tamagnone, L. Neuropilins Controlling Cancer Therapy Responsiveness. Int. J. Mol. Sci. 2019, 20, 2049. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jung, K.; Baek, D.S.; Hong, S.S.; Kim, Y.S. Co-targeting of EGF receptor and neuropilin-1 overcomes cetuximab resistance in pancreatic ductal adenocarcinoma with integrin β1-driven Src-Akt bypass signaling. Oncogene 2017, 36, 2543–2552. [Google Scholar] [CrossRef]
- Kim, Y.J.; Baek, D.S.; Lee, S.; Park, D.; Kang, H.N.; Cho, B.C.; Kim, Y.S. Dual-targeting of EGFR and Neuropilin-1 attenuates resistance to EGFR-targeted antibody therapy in KRAS-mutant non-small cell lung cancer. Cancer Lett. 2019, 466, 23–34. [Google Scholar] [CrossRef]
- Naik, A.; Al-Yahyaee, A.; Abdullah, N.; Sam, J.E.; Al-Zeheimi, N.; Yaish, M.W.; Adham, S.A. Neuropilin-1 promotes the oncogenic Tenascin-C/integrin β3 pathway and modulates chemoresistance in breast cancer cells. BMC Cancer 2018, 18, 533. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar]
- Fuh, G.; Garcia, K.C.; de Vos, A.M. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J. Biol. Chem. 2000, 275, 26690–26695. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Nakayama, A.; van Lessen, M.; Yamamoto, H.; Hoffmann, S.; Drexler, H.C.; Itoh, N.; Hirose, T.; Breier, G.; Vestweber, D.; et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat. Cell Biol. 2013, 15, 249–260. [Google Scholar]
- Weekes, C.D.; Beeram, M.; Tolcher, A.W.; Papadopoulos, K.P.; Gore, L.; Hegde, P.; Xin, Y.; Yu, R.; Shih, L.M.; Xiang, H.; et al. A phase I study of the human monoclonal anti-NRP1 antibody MNRP1685A in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 653–660. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Yu, K.H.; Snyder, M. Omics Profiling in Precision Oncology. Mol. Cell. Proteom. 2016, 15, 2525–2536. [Google Scholar]
- He, X.; Liu, X.; Zuo, F.; Shi, H.; Jing, J. Artificial intelligence-based multi-omics analysis fuels cancer precision medicine. Semin. Cancer Biol. 2023, 88, 187–200. [Google Scholar] [CrossRef]
- Menyhárt, O.; Győrffy, B. Multi-omics approaches in cancer research with applications in tumor subtyping, prognosis, and diagnosis. Comput. Struct. Biotechnol. J. 2021, 19, 949–960. [Google Scholar]
- Zhuo, Y.J.; Shi, Y.; Wu, T. NRP-1 and KDR polymorphisms are associated with survival time in patients with advanced gastric cancer. Oncol. Lett. 2019, 18, 4629–4638. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.L.; Bielenberg, D.R.; Gechtman, Z.; Miao, H.Q.; Takashima, S.; Soker, S.; Klagsbrun, M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc. Natl. Acad. Sci. USA 2000, 97, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative splicing and cancer: A systematic review. Signal Transduct. Target. Ther. 2021, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Kim, Y.; Gewirtz, A.D.H.; Jo, B.; Gao, C.; McDowell, I.C.; Engelhardt, B.E.; Battle, A. Co-expression networks reveal the tissue-specific regulation of transcription and splicing. Genome Res. 2017, 27, 1843–1858. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Zhang, X.; Wang, J.; Gu, D.; Wang, Y. Prognostic implication of neuropilin-1 upregulation in human nasopharyngeal carcinoma. Diagn. Pathol. 2013, 8, 155. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]


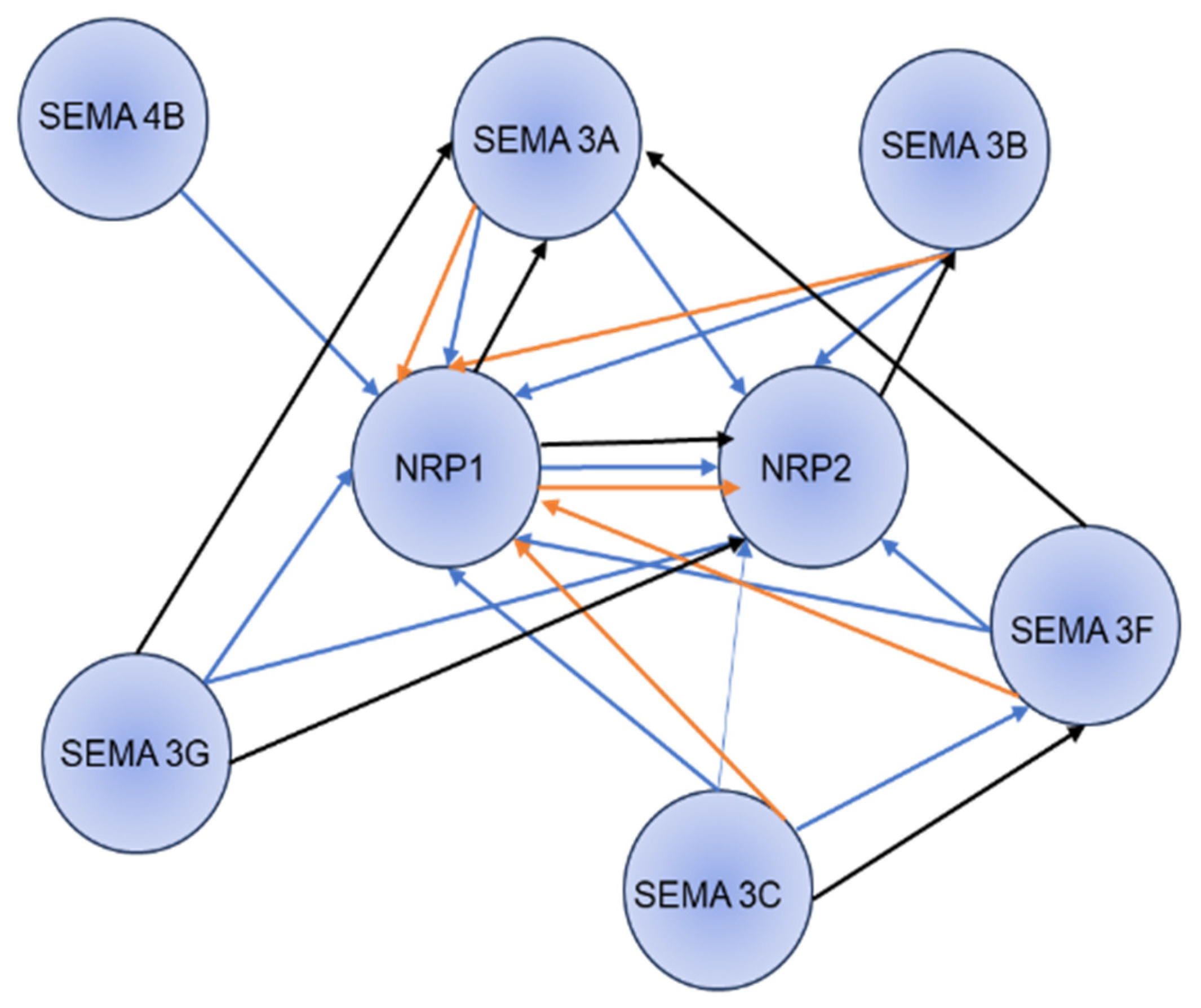
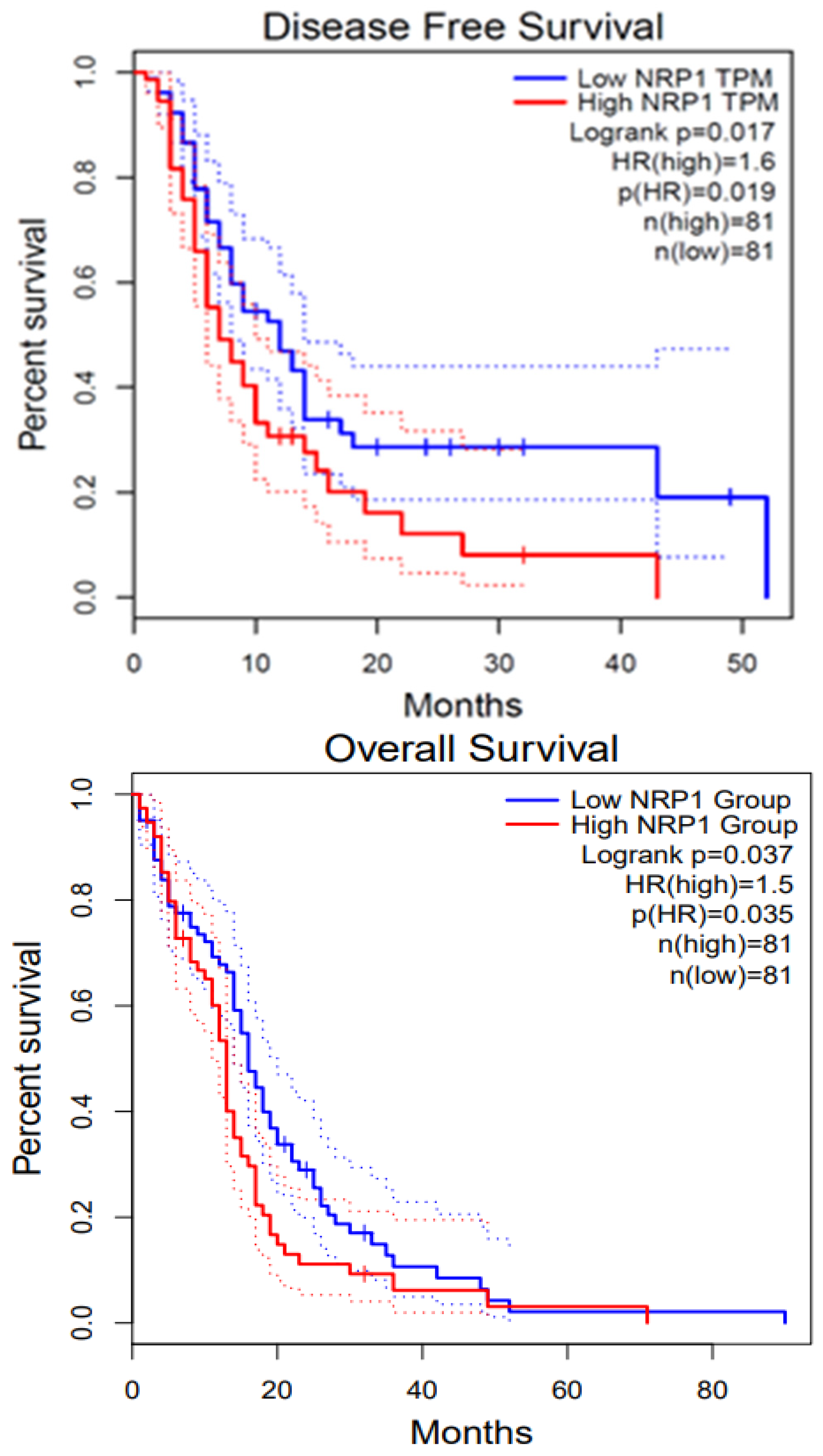


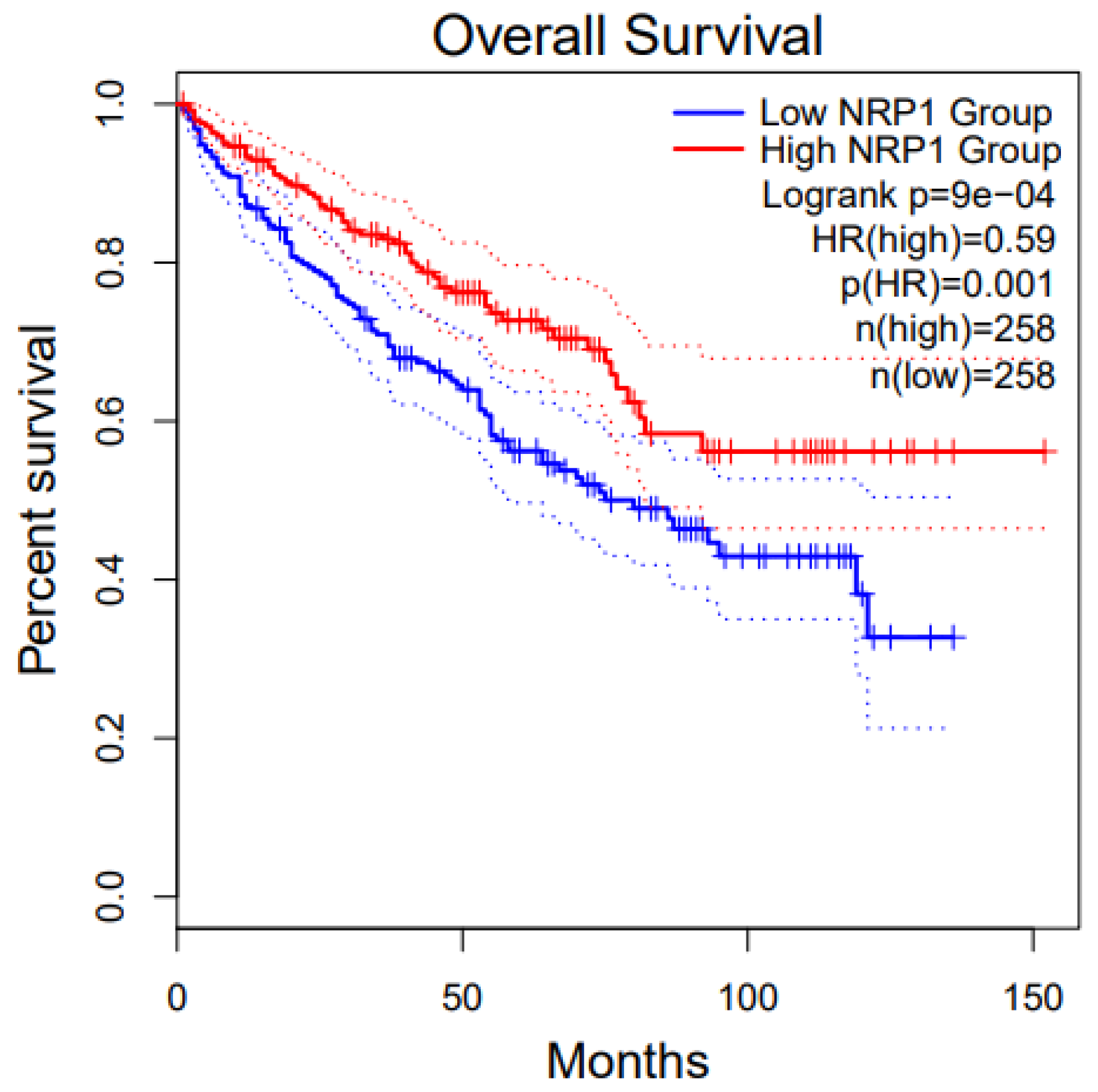
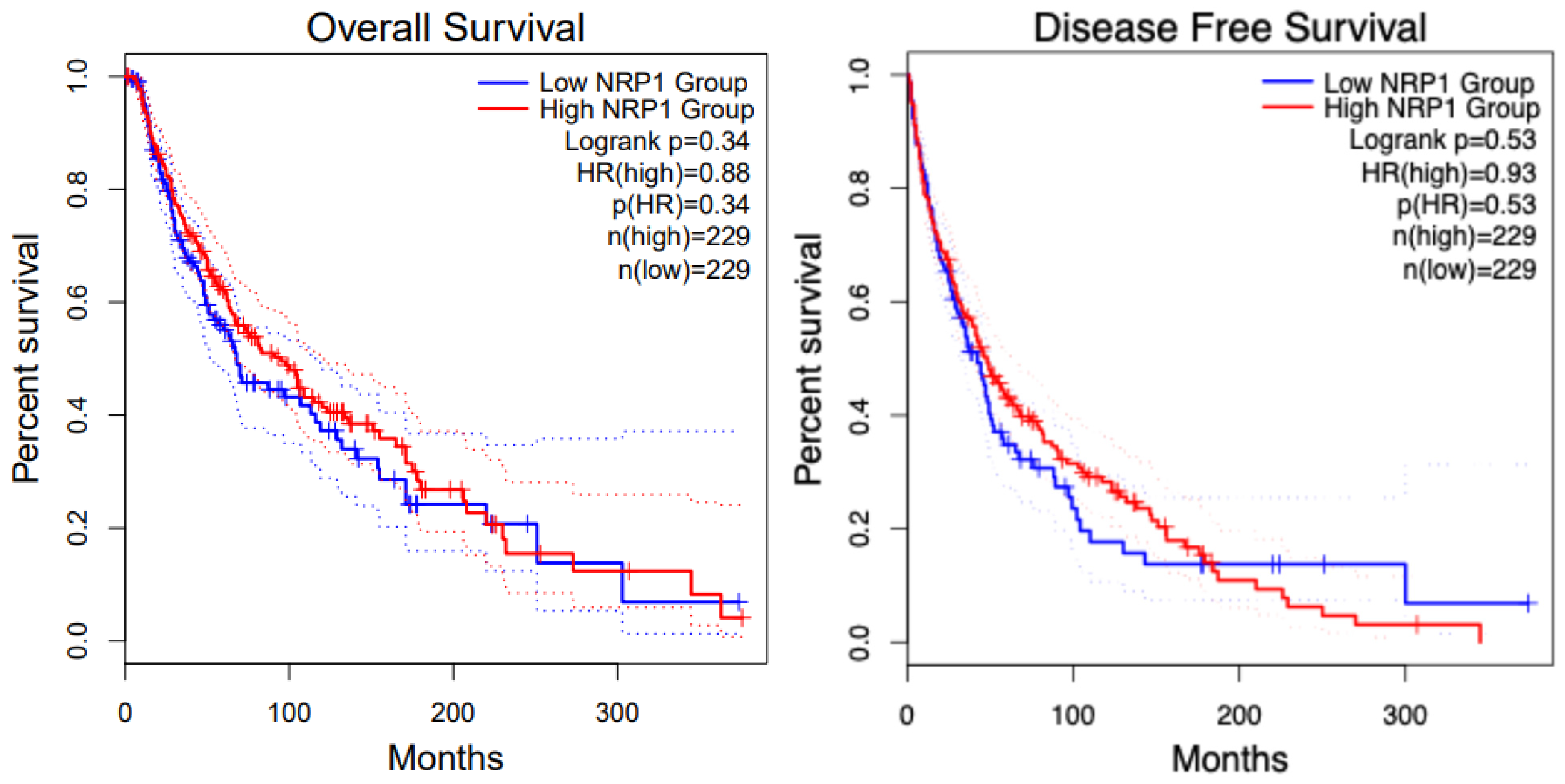
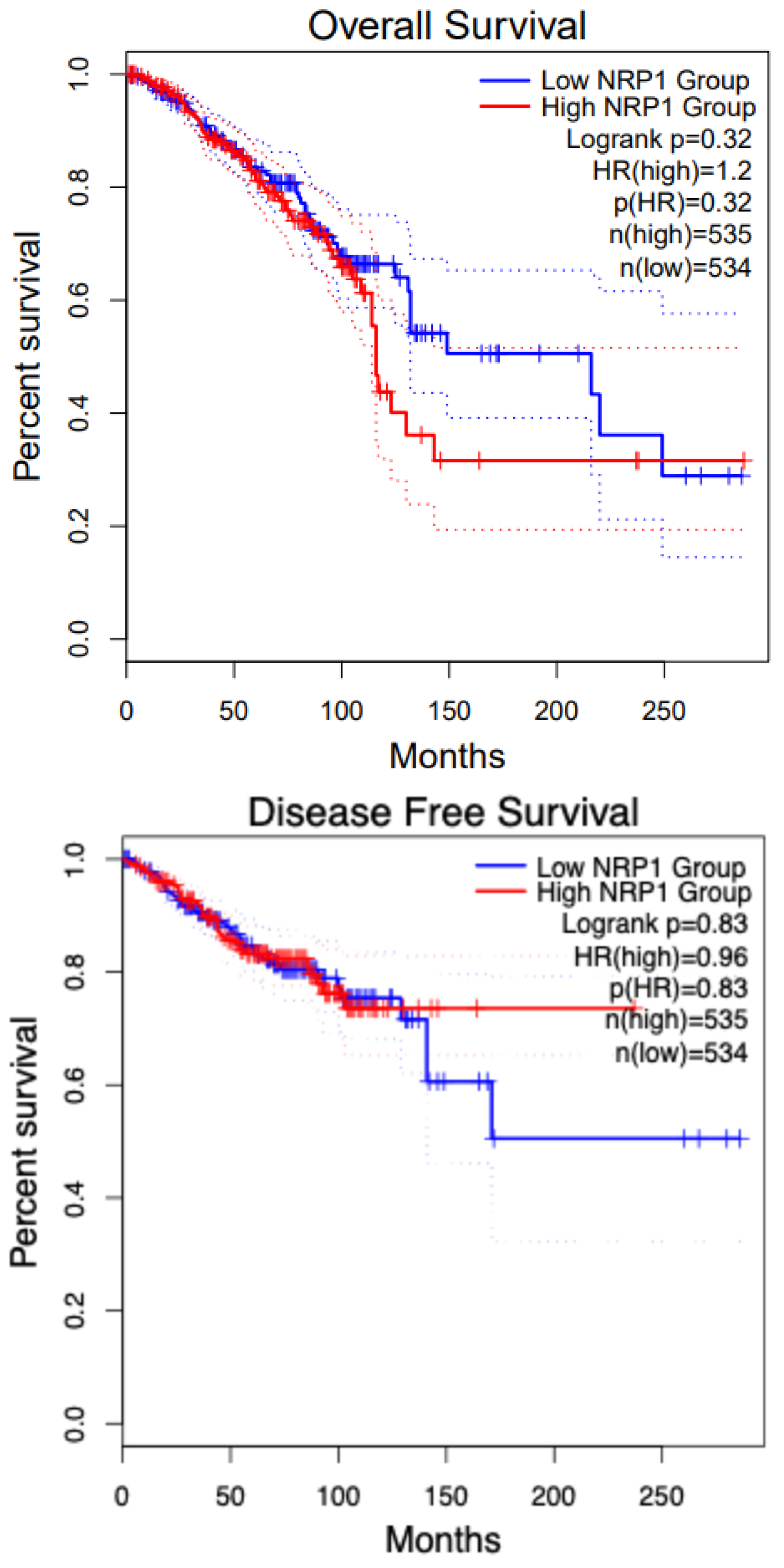

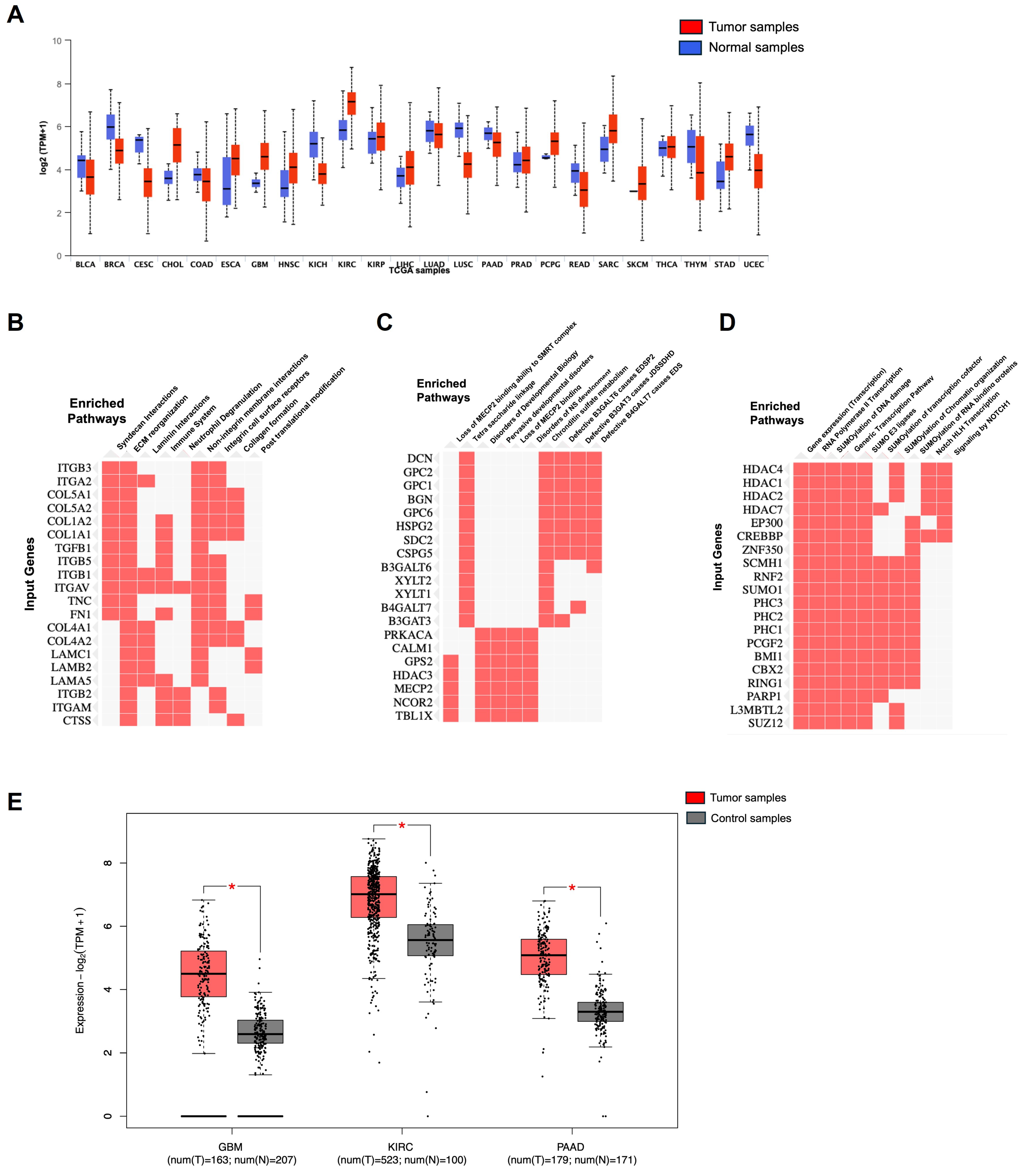
| Ligand | NRP1 Domain | Effect | References |
|---|---|---|---|
| SEMA3A | CUB (a1/a2) extracellular domain | Promotes prolonged T cell–DC interaction and T cell activation and IL-10 secretion | [38,39,40] |
| SEMA4A | CUB (a1/a2) extracellular domain | Promotes contact-independent Treg function (via IL-10 and IL-35) and maintains Treg stability in vivo | [41] |
| TGF-β | b1/b2 extracellular domain | Activates latent LAP–TGF-β enhancing TGF-β immune suppression and TGF-β mediated Treg generation. | [42] |
| VEGF165/145 | b1/b2 extracellular domain | VEGF165 enhances VEGFR2–NRP1 complex formation by acting as a ‘bridging molecule’—this enhances the proangiogenic effects of VEGF165 | [43,44] |
| HGF | b1/b2 extracellular domain | NRP1–HGF binding enhances c-Met signaling, promoting endothelial cell proliferation and angiogenesis | [45,46] |
| PDGF | Unconfirmed physical interaction with NRP1; possibly b1/b2 domain | PDGF upregulates NRP1 expression, promoting VSMC mobilization and angiogenesis | [47] |
| FGF2 | Unconfirmed physical interaction with NRP1; possibly b1/b2 domain | NRP1 binding of FGF2 enhances the FGF2 growth stimulatory functions and proangiogenic activity | [48] |
| PIGF | b1/b2 extracellular domain | PlGF signals through its receptor, NRP1, promoting angiogenesis and tumor growth | [49] |
| Neuropilins Role in Immune Cells | |||
|---|---|---|---|
| Immune Cells | Neuropilin Type | Function | References |
| Dendritic cells | NRP1 | Mediates Primary IR activation by antigen processing and presentation by DCs | [39] |
| NRP2 | Differentiation from Monocytes to DCs, protecting their migration by sialyation where DC activates T-cells | [112,113] | |
| Macrophages | NRP1 | Promotes immune suppressive role and induces a protumoral response | [114] |
| NRP2 | Differentiation of Monocytes to macrophages to induce phagocytosis, NRP2 sialyation reduces phagocytosis capacity | [39,115] | |
| T cells | |||
| Cytotoxic T cells | NRP1 | Promotes antigen recognition, a biomarker to determine the efficacy of anti-PD-1 immunotherapies | [26,107] |
| Helper T cells | NRP1 | On CD4+, T cells promote B cell differentiation | [26,102,116] |
| NRP1 | On Treg cells and CD4+ T cells induce Immunosuppressive function | ||
| NKT cells | NRP1/NRP2 | Unknown | [26] |
| T regulatory cells | NRP1 | Attracts to VEGF where NRP1 acts as a co-receptor enhancing infiltration of tumors and Immunosuppressive response | [117] |
| NRP2 | Interaction between NRP2, SEMA3A, PlexinA1 inhibit immature T cell migration | [118] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varanasi, S.M.; Gulani, Y.; Rachamala, H.K.; Mukhopadhyay, D.; Angom, R.S. Neuropilin-1: A Multifaceted Target for Cancer Therapy. Curr. Oncol. 2025, 32, 203. https://doi.org/10.3390/curroncol32040203
Varanasi SM, Gulani Y, Rachamala HK, Mukhopadhyay D, Angom RS. Neuropilin-1: A Multifaceted Target for Cancer Therapy. Current Oncology. 2025; 32(4):203. https://doi.org/10.3390/curroncol32040203
Chicago/Turabian StyleVaranasi, Sai Manasa, Yash Gulani, Hari Krishnareddy Rachamala, Debabrata Mukhopadhyay, and Ramcharan Singh Angom. 2025. "Neuropilin-1: A Multifaceted Target for Cancer Therapy" Current Oncology 32, no. 4: 203. https://doi.org/10.3390/curroncol32040203
APA StyleVaranasi, S. M., Gulani, Y., Rachamala, H. K., Mukhopadhyay, D., & Angom, R. S. (2025). Neuropilin-1: A Multifaceted Target for Cancer Therapy. Current Oncology, 32(4), 203. https://doi.org/10.3390/curroncol32040203







