Abstract
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective in non-small-cell lung cancer (NSCLC) with sensitizing mutations. However, patients with uncommon EGFR mutations show variable responses, and resistance often develops. The C797S mutation is a common resistance mechanism after third-generation EGFR-TKI osimertinib therapy, with no standard treatment established. A 37-year-old Chinese woman with advanced NSCLC harboring EGFR G719S/S768I mutations developed an acquired C797S mutation without T790M after second- and third-generation EGFR-TKI therapy. She was treated with a combination of gefitinib and bevacizumab, achieving a partial response, particularly in liver metastases. Her overall survival exceeded 60 months. Gefitinib combined with bevacizumab demonstrates efficacy in managing NSCLC with uncommon EGFR mutations and overcoming acquired C797S resistance. This combination therapy offers a promising treatment strategy for patients with limited options after resistance to second- and third-generation EGFR-TKIs.
1. Introduction
Epidermal growth factor receptor (EGFR) mutations, a common oncogenic driver in non-small-cell lung cancer (NSCLC), can be targeted by EGFR tyrosine kinase inhibitors (TKIs) to improve patient prognosis. The EGFR exon 19 deletion and exon 21 L858R mutations are the most common activating and sensitizing EGFR mutations, accounting for 85–90% of EGFR mutation-positive cases [1]. Additionally, 10–15% of EGFR mutations are uncommon mutations [2], including EGFR 20ins, G719X, S768I, and L861Q. While limited information is available on the efficacy of EGFR-TKIs for these mutations, current clinical data suggest that they are more sensitive to second- and third-generation EGFR-TKIs than to first-generation EGFR-TKIs, excluding EGFR 20ins [3].
However, targeted therapy inevitably leads to acquired resistance. The most significant mechanism of resistance to first- and second-generation EGFR-TKIs is the acquired EGFR exon 20 p.T790M (T790M) [4]. Osimertinib, a third-generation EGFR-TKI, effectively targets the T790M mutation. However, following second-line treatment with osimertinib, 38% of patients developed resistance due to EGFR abnormalities, with the most common being the EGFR C797S mutation [5]. The T790M and C797S mutations in the EGFR gene are classified as either cis or trans based on their allelic relationship. The T790M-trans-C797S mutation responds to combined first- and third-generation EGFR-TKIs, whereas the T790M-cis-C797S mutation is resistant to all generations of EGFR-TKIs [6]. Based on previous research, patients with the C797S mutation may retain some sensitivity to first- or second-generation EGFR-TKI, such as gefitinib and afatinib [7]. The strategy to effectively overcome C797S-based osimertinib resistance involves fourth-generation EGFR-TKIs, most of which are currently in Phase I/II clinical trials. Therefore, there is no standard treatment for NSCLC patients with C797S mutation, particularly for those who develop this mutation without the T790M mutation after first-line therapy.
To date, there have been no reported cases of NSCLC with EGFR G719S/S768I/C797S triple mutations or their treatments. Here, we describe a patient diagnosed with stage IVB lung adenocarcinoma exhibiting uncommon EGFR mutations, G719S/S768I. After the progression of afatinib treatment, osimertinib was administered. Despite the absence of the T790M mutation, the C797S resistance mutation was identified. Ultimately, the combination of gefitinib and bevacizumab resulted in a partial response (PR) in the patient.
2. Case Presentation
In November 2019, a 37-year-old woman with no history of smoking was diagnosed with left lung adenocarcinoma with hilar mediastinal lymph node, left supraspinal lymph node, and extensive bone metastases (cT4N3M1c, stage IVB). Targetable mutation detection in tumor tissue using next-generation sequencing (NGS) by the GeneseeqPrime™ panel (Nanjing Geneseeq Technology Inc., Nanjing, China) [8] revealed EGFR exon 18 p.G719S mutation and EGFR exon 20 p.S768I mutation. The patient was administered with first-line afatinib (40 mg daily [qd] by mouth [po]) and subsequently developed new brain lesions (October 2020, Figure 1) after a 10-month period of stability. And the patient was then treated with second-line osimertinib (80 mg daily [qd] by mouth [po]). However, the patient’s brain lesions continued to deteriorate (February 2021, Figure 1). Due to the effectiveness of the third-generation EGFR-TKI treatment, third-line afatinib was reverted. Both brain and lung lesions remained stable for a duration of eight months. After developing resistance to afatinib, the patient exhibited disease progression in the brain lesions (November 2021, Figure 1), leading to the fourth-line almonertinib (220 mg daily [qd] by mouth [po]). After three months, the patient’s brain lesions showed increased progression (February 2022, Figure 1), prompting the initiation of fifth-line afatinib combined with pemetrexed. The patient expressed concerns regarding potential resistance to afatinib and requested a transition to osimertinib. After thorough evaluation and discussion, the clinical team concurred with proceeding with osimertinib as the subsequent line of therapy. The combination therapy resulted in stable disease (SD) and progression-free survival (PFS) of up to 21 months.
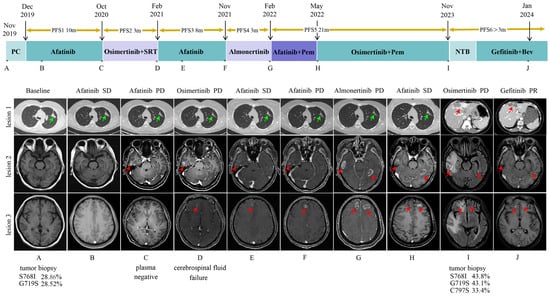
Figure 1.
A summary of the patient’s clinical course. The upper panel shows the various treatments the patient received for EGFR-mutated non-small-cell lung cancer as well as the duration of each treatment. Asterisks indicate the time points for the response assessments. Green arrows indicate the primary lesion, while red arrows indicate the metastatic lesions. Genetic testing results for EGFR of the various tissue biopsies are shown below the corresponding CT images with their minor allele frequency. (A) Baseline chest CT scan and head MRI at diagnosis in November 2019. (B) SD on afitinib treatment in February 2020. (C) Lung lesion SD but head lesions PR on afitinib treatment in October 2020. (D) Lung lesion SD but head lesions PR on osimertinib treatment in February 2021. (E) SD on afitinib treatment in April 2021. (F) Lung lesion SD but head lesions PR on afitinib treatment in November 2021. (G) Lung lesion SD but head lesions PR on almonertinib in February 2022. (H) SD on afitinib treatment in May 2022. (I) Liver lesion and head lesion PD on osimertinib combined with pemetrexed treatment in November 2023. (J) PR on gefitinib and bevacizumab treatment in January 2024. Abbreviations: PFS, progression-free survival; PC, pemetrexed + cisplatin; SRT, stereotactic radiotherapy; Pem, pemetrexed; NTB, nab-paclitaxel + tirellizumab + bevacizumab; Bev, bevacizumab; SD, stable disease; PD, progressive disease; PR, partial response.
In November 2023, the patient developed multiple metastases in the chest wall, lungs, liver, and brain (Figure 1). Subsequently, a biopsy of a mass in the left chest wall confirmed the involvement of lung adenocarcinoma based on the pathological findings. NGS was performed with the patient’s tissue and revealed the retention of EGFR exon 18 p.G719S, EGFR exon 20 p.S768I, and the emergence of EGFR exon 20 p.C797S. Considering that patients may have developed resistance to osimertinib, the treatment was changed to gefitinib (250 mg daily [qd] by mouth [po]) combined with bevacizumab. After two months of treatment, the patient exhibited a significant reduction in the size of lung, brain, and liver metastases compared with their previous dimensions (January 2024, Figure 1). The lesions continued to show PR in June 2024. During combination therapy, the patient exhibited only grade 1 transaminase elevations, which improved with hepatoprotective treatment. No other adverse reactions, such as rash, proteinuria, or bleeding, were observed during this period. The patient continues to receive the combination therapy of gefitinib and bevacizumab. To date, the patient has achieved an overall survival (OS) of more than 60 months, with sustained clinical benefit and stable disease. The whole clinical course of treatment is shown in Figure 1. Antitumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) [9].
3. Discussion
The EGFR T790M mutation alters the affinity of EGFR for ATP, significantly reducing the effectiveness of first- and second-generation EGFR-TKIs in competing for binding [10]. Osimertinib effectively targets both primary activating mutations and T790M resistance mutations [11]. However, resistance inevitably develops during treatment [12]. Common mechanisms of osimertinib resistance include the EGFR C797S mutation, MET amplification, T790M deletion, BRAF mutations, and KRAS mutations [13,14,15]. The EGFR C797S mutation is the primary cause of acquired resistance to osimertinib, often arising after the T790M mutation. Recently, C797S mutations have been documented in a few cases of T790M-negative patients following osimertinib treatment. It is well known that first-generation EGFR-TKIs are ineffective in patients with the T790M mutation. However, their efficacy in patients with C797S-positive and T790M-negative mutations remains uncertain. We summarized previous studies on the clinical outcomes of subsequent treatments in patients who developed C797S resistance mutations following osimertinib therapy [16,17,18,19,20]. In these cases, re-biopsies were performed on lung tissue, pleural effusion, plasma, and cerebrospinal fluid. The characteristics and clinical data of the patients are presented in Table 1. All patients received osimertinib and exhibited EGFR C797S-positive and T790M-negative resistance mutations. Notably, two patients with lung adenocarcinoma harboring the EGFR 19del and T790M mutations acquired the C797S mutation after failing osimertinib treatment and subsequently achieved partial remission with gefitinib.

Table 1.
Clinical characteristics and treatment outcomes of patients with NSCLC with acquired EGFR C797S after osimertinib resistance.
Initially, we selected first-line targeted therapy with afatinib based on a pooled analysis of first-line studies showing favorable responses to afatinib in patients with G719X, S768I, and L861Q mutations, with a PFS comparable to that of common EGFR mutations [21,22]. Additionally, Passaro A et al. [23] demonstrated the promising efficacy of afatinib in a cohort of patients with brain metastases or rare EGFR mutations. In our study, the patient experienced therapeutic failure, as indicated by time to failure, after 10 and 8 months of afatinib treatment, consistent with findings from previous studies [21].
The patient in this case did not exhibit T790M mutation after progressing on afatinib treatment. However, the patient developed a C797S mutation during osimertinib treatment. The C797S mutation affects the cysteine residue at position 797 of the EGFR protein, and osimertinib demonstrated a strong antitumor effect by covalently binding to EGFR 797 cysteine [7,24]. Unfortunately, the C797S mutation blocks this binding, leading to further acquired resistance [25]. The case reported by Rangachari et al. [7,26] showed that in the presence of the C797S mutation alone, without T790M, resistant NSCLC patients may still retain sensitivity to gefitinib. The in vitro results presented by Niederst et al. [6] showed that EGFR Del19/C797S-positive and T790M-negative cell lines were resistant to third-generation EGFR-TKIs but remained sensitive to gefitinib. This may be due to the fact that the first-generation EGFR-TKI is primarily a reversible, non-selective inhibitor with a quinoline–amine structural motif that is not dependent on the cysteine at 797 to inhibit EGFR [24]. Consequently, first-generation EGFR-TKIs, such as gefitinib, still exert an inhibitory effect on the C797S mutation.
Studies have demonstrated that following the development of resistance to EGFR-TKIs, the level of tumor vascular endothelial growth factor (VEGF) increases [27], reducing tumor cell dependence on the EGFR signal and increasing dependence on the VEGF pathway. Preclinical studies have shown that an overactive VEGF/VEGFR pathway and tumor angiogenesis play a crucial role in the development of resistance to EGFR-TKIs. Bevacizumab, a monoclonal antibody that inhibits VEGF, has been utilized in various cancers due to its ability to suppress tumor angiogenesis. Studies indicate that bevacizumab can lead to significant improvements in PFS when used as a single agent, particularly in patients who cannot tolerate other chemotherapy options [28].
A previous Phase II clinical study showed that bevacizumab combined with first-generation EGFR-TKI significantly prolonged PFS in patients with NSCLC carrying EGFR mutations [29]. The combination of bevacizumab with an EGFR-TKI may enhance antitumor efficacy by targeting distinct pathogenic pathways, including angiogenesis and EGFR activation [30]. Additionally, in cases of tertiary C797S mutation, reintroducing a first-generation EGFR-TKI can still target the original sensitive EGFR mutation, even in the absence of T790M [16]. Thus, the dual targeting of the VEGF and EGFR pathways may effectively prevent drug resistance [31]. This is evidenced by our clinical case, which demonstrated the effectiveness of combining gefitinib, a first-generation EGFR-TKI, with bevacizumab in treating the EGFR C797S mutation following progression on the third-generation EGFR-TKI osimertinib.
Although gefitinib is typically well tolerated, it is associated with several adverse effects, including rash, diarrhea, and liver function abnormalities [32]. In addition, bevacizumab may lead to potential complications such as hypertension, bleeding, and gastrointestinal perforation [33]. The concomitant use of these two agents could increase the incidence of adverse events. Nevertheless, data from a Phase II clinical trial conducted in Japan indicated that serious adverse events associated with the combination of gefitinib and bevacizumab included grade 3 rash, hypertension, elevated aspartate aminotransferase and alanine aminotransferase, proteinuria, intracranial bleeding, and grade 4 gastrointestinal perforation [34]. Notably, no treatment-related deaths were reported. Fortunately, the patient in this case did not experience any of the serious adverse reactions, but close monitoring is still necessary during the combination therapy.
The EGFR C797S mutation is a key driver of resistance to third-generation EGFR-TKIs, making the development of next-generation inhibitors crucial for NSCLC treatment. Several fourth-generation EGFR-TKIs are currently in clinical trials. Although BLU-945 was one of the most advanced fourth-generation EGFR-TKIs [35,36], it was discontinued due to its dose-limiting toxicity and limited clinical benefit. EAI045, which targets the EGFR activator subunit, has proven ineffective as a monotherapy in blocking EGFR-driven cell proliferation, though it demonstrates significant antitumor activity in vitro and in animal models when combined with cetuximab [37,38]. Additionally, BDTX-1535, an orally bioavailable, potent, selective, and irreversible allosteric EGFR inhibitor, has shown promising preliminary efficacy and durability in Phase II trials in patients with relapsed or refractory EGFR-mutant NSCLC [39,40]. These agents offer novel therapeutic options with the potential to overcome resistance to third-generation EGFR-TKIs in NSCLC and other lung cancers.
For patients who develop a C797S mutation without a concurrent T790M mutation following treatment with EGFR-TKIs, the range of effective therapeutic options remains limited. In this case, while the patient exhibited PR to the combination of gefitinib and bevacizumab, the assessment of efficacy and the duration of treatment were relatively brief. It is therefore not possible to ascertain with certainty the precise efficacy of this combination therapy. Further studies with extended follow-up periods, along with overall survival and quality of life data, are required to evaluate the potential of this combination therapy in such cases.
4. Conclusions
In summary, we reported the clinical benefit of gefitinib combined with bevacizumab in a case characterized by EGFR G719S and S768I mutations/T790M negativity/C797S mutation-mediated resistance to osimertinib. Currently, treatment options for osimertinib-induced C797S mutation resistance are limited. This combination treatment may offer a potential new strategy.
Author Contributions
Conceptualization, W.L. and Y.L.; methodology, W.L., P.T. and Y.L.; software, W.L.; validation, P.T. and Y.L.; formal analysis, W.L., P.T. and Y.L.; investigation, W, L., J.S., Y.J., J.X., C.H. and Y.D.; resources, P.T. and Y.L.; data curation, W.L., J.S., Y.J., J.X., C.H. and Y.D.; writing—original draft preparation, W.L. and Y.L.; writing—review and editing, W.L., J.S., Y.J., J.X., C.H., Y.D., P.T. and Y.L.; visualization, W.L. and Y.L.; supervision, P.T. and Y.L.; project administration, W.L., P.T. and Y.L.; funding acquisition, P.T. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82470099 to Y Li, No. 82473213 to P Tian, 92159302 to W Li), Noncommunicable Chronic Diseases-National Science and Technology Major Project (No. 2024ZD0522806/2024ZD0522800 to P Tian, 2024ZD0522801 to Y Li, 2024ZD0522806 to W Lu).
Institutional Review Board Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the patient provided written informed consent. IRB approval is not required at our institution for case-report studies.
Informed Consent Statement
Written informed consent was obtained from the patient for the anonymized information and the accompanying images to be published in this article.
Data Availability Statement
All data underlying the findings of this study are available within this publication. Patient data from the West China Hospital in Sichuan University have been anonymized to ensure confidentiality. Due to ethical and legal restrictions related to data protection regulations, raw patient data cannot be shared publicly. Requests for further information may be directed to the corresponding author.
Acknowledgments
The authors kindly thank the physicians, nurses, and other staff at the hospital who assisted with the study.
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Abbreviations
The following abbreviations are used in this manuscript:
| EGFR | Epidermal growth factor receptor |
| EGFR-TKIs | Epidermal growth factor receptor tyrosine kinase inhibitors |
| NSCLC | Non-small-cell lung cancer |
| PR | Partial response |
| NGS | Next-generation sequencing |
| SD | Stable disease |
| PFS | Progression-free survival |
| OS | Overall survival |
| VEGF | Vascular endothelial growth factor |
References
- He, Q.; Qu, M.; Bao, H.; Xu, Y.; Shen, T.; Tan, D.; Barkat, M.Q.; Xu, C.; Zeng, L.H.; Wu, X. Multiple post-translational modifications ensure EGFR functionality: Potential therapeutic targets to overcome its drug-resistance mutations. Cytokine Growth Factor Rev. 2023, 70, 41–53. [Google Scholar] [PubMed]
- Kobayashi, Y.; Mitsudomi, T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci. 2016, 107, 1179–1186. [Google Scholar] [PubMed]
- Borgeaud, M.; Parikh, K.; Banna, G.L.; Kim, F.; Olivier, T.; Le, X.; Addeo, A. Unveiling the Landscape of Uncommon EGFR Mutations in NSCLC-A Systematic Review. J. Thorac. Oncol. 2024, 19, 973–983. [Google Scholar]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [PubMed]
- Schoenfeld, A.J.; Chan, J.M.; Kubota, D.; Sato, H.; Rizvi, H.; Daneshbod, Y.; Chang, J.C.; Paik, P.K.; Offin, M.; Arcila, M.E.; et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin. Cancer Res. 2020, 26, 2654–2663. [Google Scholar]
- Niederst, M.J.; Hu, H.; Mulvey, H.E.; Lockerman, E.L.; Garcia, A.R.; Piotrowska, Z.; Sequist, L.V.; Engelman, J.A. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin. Cancer Res. 2015, 21, 3924–3933. [Google Scholar]
- Ercan, D.; Choi, H.G.; Yun, C.H.; Capelletti, M.; Xie, T.; Eck, M.J.; Gray, N.S.; Jänne, P.A. EGFR Mutations and Resistance to Irreversible Pyrimidine-Based EGFR Inhibitors. Clin. Cancer Res. 2015, 21, 3913–3923. [Google Scholar]
- Zheng, Q.; Lin, X.; Qi, W.; Yin, J.; Li, J.; Wang, Y.; Wang, W.; Li, W.; Liang, Z. NGS and FISH for MET amplification detection in EGFR TKI resistant non-small cell lung cancer (NSCLC) patients: A prospective, multicenter study in China. Lung Cancer 2024, 194, 107897. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar]
- Pao, W.; Miller, V.A.; Politi, K.A.; Riely, G.J.; Somwar, R.; Zakowski, M.F.; Kris, M.G.; Varmus, H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005, 2, e73. [Google Scholar]
- Remon, J.; Steuer, C.E.; Ramalingam, S.S.; Felip, E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann. Oncol. 2018, 29 (Suppl. S1), i20–i27. [Google Scholar] [PubMed]
- Chmielecki, J.; Gray, J.E.; Cheng, Y.; Ohe, Y.; Imamura, F.; Cho, B.C.; Lin, M.C.; Majem, M.; Shah, R.; Rukazenkov, Y.; et al. Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nat. Commun. 2023, 14, 1070. [Google Scholar] [PubMed]
- Ho, C.C.; Liao, W.Y.; Lin, C.A.; Shih, J.Y.; Yu, C.J.; Yang, J.C. Acquired BRAF V600E Mutation as Resistant Mechanism after Treatment with Osimertinib. J. Thorac. Oncol. 2017, 12, 567–572. [Google Scholar]
- Ortiz-Cuaran, S.; Scheffler, M.; Plenker, D.; Dahmen, L.; Scheel, A.H.; Fernandez-Cuesta, L.; Meder, L.; Lovly, C.M.; Persigehl, T.; Merkelbach-Bruse, S.; et al. Heterogeneous Mechanisms of Primary and Acquired Resistance to Third-Generation EGFR Inhibitors. Clin. Cancer Res. 2016, 22, 4837–4847. [Google Scholar]
- Yu, H.A.; Tian, S.Z.K.; Drilon, A.E.; Borsu, L.; Riely, G.J.; Arcila, M.E.; Ladanyi, M. Acquired Resistance of EGFR-Mutant Lung Cancer to a T790M-Specific EGFR Inhibitor: Emergence of a Third Mutation (C797S) in the EGFR Tyrosine Kinase Domain. JAMA Oncol. 2015, 1, 981–983. [Google Scholar]
- Enrico, D.; Tsou, F.; Catani, G.; Pupareli, C.; Girotti, M.R.; Ulloa Alvarez, D.E.; Waisberg, F.; Rodríguez, A.; Reyes, R.; Chacón, M.; et al. Overcoming Resistance to Osimertinib by T790M Loss and C797S Acquisition Using Gefitinib in a Patient With EGFR-Mutant NSCLC: A Case Report. JTO Clin. Res. Rep. 2023, 4, 100456. [Google Scholar]
- Goldberg, M.E.; Montesion, M.; Young, L.; Suh, J.; Greenbowe, J.; Kennedy, M.; Giaccone, G.; Akerley, W.L.; Dowlati, A.; Creelan, B.C.; et al. Multiple configurations of EGFR exon 20 resistance mutations after first- and third-generation EGFR TKI treatment affect treatment options in NSCLC. PLoS ONE 2018, 13, e0208097. [Google Scholar]
- Russo, A.; Scilla, K.A.; Mehra, R.; Gittens, A.; McCusker, M.G.; de Miguel-Perez, D.; Gomez, J.E.; Peleg, A.; Del Re, M.; Rolfo, C.D. Tracking Clonal Evolution of EGFR-Mutated Non-Small Cell Lung Cancer Through Liquid Biopsy: Management of C797S Acquired Mutation. Clin. Lung Cancer 2023, 24, 660–665. [Google Scholar] [PubMed]
- Wang, M.; Zhu, F.; Luo, N.; Li, M.; Qi, Y.; Wang, M. Erlotinib combined with bevacizumab and chemotherapy in first line osimertinib-resistant NSCLC patient with leptomeningeal metastasis: A case report. Medicine 2021, 100, e27727. [Google Scholar]
- Chic, N.; Mayo-de-Las-Casas, C.; Reguart, N. Successful Treatment with Gefitinib in Advanced Non-Small Cell Lung Cancer after Acquired Resistance to Osimertinib. J. Thorac. Oncol. 2017, 12, e78–e80. [Google Scholar]
- Yang, J.C.; Schuler, M.; Popat, S.; Miura, S.; Heeke, S.; Park, K.; Märten, A.; Kim, E.S. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J. Thorac. Oncol. 2020, 15, 803–815. [Google Scholar] [PubMed]
- Yang, J.C.; Sequist, L.V.; Geater, S.L.; Tsai, C.M.; Mok, T.S.; Schuler, M.; Yamamoto, N.; Yu, C.J.; Ou, S.H.; Zhou, C.; et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015, 16, 830–838. [Google Scholar] [CrossRef]
- Passaro, A.; de Marinis, F.; Tu, H.Y.; Laktionov, K.K.; Feng, J.; Poltoratskiy, A.; Zhao, J.; Tan, E.H.; Gottfried, M.; Lee, V.; et al. Afatinib in EGFR TKI-Naïve Patients with Locally Advanced or Metastatic EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Pooled Analysis of Three Phase IIIb Studies. Front. Oncol. 2021, 11, 709877. [Google Scholar]
- Godin-Heymann, N.; Ulkus, L.; Brannigan, B.W.; McDermott, U.; Lamb, J.; Maheswaran, S.; Settleman, J.; Haber, D.A. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol. Cancer Ther. 2008, 7, 874–879. [Google Scholar] [PubMed]
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Cho, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 2015, 21, 560–562. [Google Scholar]
- Rangachari, D.; To, C.; Shpilsky, J.E.; VanderLaan, P.A.; Kobayashi, S.S.; Mushajiang, M.; Lau, C.J.; Paweletz, C.P.; Oxnard, G.R.; Jänne, P.A.; et al. EGFR-Mutated Lung Cancers Resistant to Osimertinib through EGFR C797S Respond to First-Generation Reversible EGFR Inhibitors but Eventually Acquire EGFR T790M/C797S in Preclinical Models and Clinical Samples. J. Thorac. Oncol. 2019, 14, 1995–2002. [Google Scholar] [CrossRef]
- Naumov, G.N.; Nilsson, M.B.; Cascone, T.; Briggs, A.; Straume, O.; Akslen, L.A.; Lifshits, E.; Byers, L.A.; Xu, L.; Wu, H.K.; et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin. Cancer Res. 2009, 15, 3484–3494. [Google Scholar] [CrossRef]
- Seiwerth, F.; Bitar, L.; Samaržija, M.; Jakopović, M. Long-term progression-free survival in non-small cell lung cancer patients: A spotlight on bevacizumab and its biosimilars. Expert Opin. Biol. Ther. 2024, 24, 1017–1024. [Google Scholar] [CrossRef]
- Planchard, D.; Loriot, Y.; André, F.; Gobert, A.; Auger, N.; Lacroix, L.; Soria, J.C. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann. Oncol. 2015, 26, 2073–2078. [Google Scholar]
- Zhang, Y.; Zeng, L.; Zhang, X.; Li, Y.; Liu, L.; Xu, Q.; Yang, H.; Jiang, W.; Lizaso, A.; Qiu, L.; et al. Clinical and molecular feature-based nomogram model for predicting benefit from bevacizumab combined with first-generation EGFR-tyrosine kinase inhibitor (TKI) in EGFR-mutant advanced NSCLC. BMC Med. 2021, 19, 245. [Google Scholar]
- Yu, Y.; Wang, Y.; Wu, L.; Xu, X.; Zhou, H.; Wang, Q.; Zhou, J. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) combined with bevacizumab for advanced non-squamous non-small-cell lung cancer patients with gradual progression on EGFR-TKI treatment: A cohort study. Medicine 2021, 100, e23712. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, V. Emerging safety data for bevacizumab in advanced non-small-cell lung cancer. Clin. Lung Cancer 2008, 9 (Suppl. S2), S62–S70. [Google Scholar] [PubMed]
- Ichihara, E.; Hotta, K.; Nogami, N.; Kuyama, S.; Kishino, D.; Fujii, M.; Kozuki, T.; Tabata, M.; Harada, D.; Chikamori, K.; et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: The Okayama Lung Cancer Study Group Trial 1001. J. Thorac. Oncol. 2015, 10, 486–491. [Google Scholar] [CrossRef]
- Elamin, Y.Y.; Nagasaka, M.; Shum, E.; Bazhenova, L.; Camidge, D.R.; Cho, B.C.; Felip, E.; Goto, K.; Lin, C.-C.; Piotrowska, Z.; et al. BLU-945 monotherapy and in combination with osimertinib (OSI) in previously treated patients with advanced EGFR-mutant (EGFRm) NSCLC in the phase 1/2 SYMPHONY study. J. Clin. Oncol. 2023, 41, 9011. [Google Scholar]
- Eno, M.S.; Brubaker, J.D.; Campbell, J.E.; De Savi, C.; Guzi, T.J.; Williams, B.D.; Wilson, D.; Wilson, K.; Brooijmans, N.; Kim, J.; et al. Discovery of BLU-945, a Reversible, Potent, and Wild-Type-Sparing Next-Generation EGFR Mutant Inhibitor for Treatment-Resistant Non-Small-Cell Lung Cancer. J. Med. Chem. 2022, 65, 9662–9677. [Google Scholar]
- Jia, Y.; Yun, C.H.; Park, E.; Ercan, D.; Manuia, M.; Juarez, J.; Xu, C.; Rhee, K.; Chen, T.; Zhang, H.; et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016, 534, 129–132. [Google Scholar]
- Wang, S.; Song, Y.; Liu, D. EAI045: The fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett. 2017, 385, 51–54. [Google Scholar]
- Wen, P.Y.; Johnson, M.L.; Henry, J.T.; Spira, A.; Battiste, J.; Alnahhas, I.; Nam, D.-H.; Patel, J.D.; Edenfield, W.J.; Hormigo, A.; et al. Phase 1 study of BDTX-1535, an oral 4th generation covalent EGFR inhibitor, in patients with recurrent glioblastoma: Preliminary dose escalation results. J. Clin. Oncol. 2024, 42, 2068. [Google Scholar]
- Patel, J.; Spira, A.; Stevenson, J.; Chen, H.; Baik, C.; Gordon, S.; Henry, J.T.; Ahluwalia, M.; Barve, M.; Huang, C.; et al. EP.12H.01 A Phase 2 Study to Assess BDTX-1535, An Oral EGFR Inhibitor, in Patients with Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2024, 19, S657. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).